Introduction
Breast cancer is a devastatingly common disease in
women worldwide and there is a considerable need to improve
approaches for prevention, diagnosis and treatment. Despite
conventional treatments, including surgical resection, radiation
therapy and chemotherapy, frequent tumor recurrence results in poor
prognosis (1).
The phosphatidylinositol 3-kinase (PI3K)/AKT pathway
plays an important role in the biology of human cancers. Components
of this pathway are frequently deregulated in a wide range of
tumors, making them attractive targets for cancer therapy (2,3).
Inhibitors of PI3K and AKT have undergone preclinical evaluation
with encouraging results (4,5).
PTEN, one of the most frequently mutated tumor
suppressor genes in human cancer, shows a very high frequency of
mutations in tumor cells (6).
Recent studies have shown that the frequencies of breast cancer
cases associated with a loss of PTEN expression are 30% in primary
tumors and 25% in metastatic tumors (7). Loss of PTEN function leads to
increased concentrations of PIP3, resulting in
constitutive activation of downstream components of the PI3K
pathway, including AKT and mTOR (8).
LY294002, a classic PI3K inhibitor, is widely used
to study the PI3K/AKT signaling pathway, and has strong antitumor
activity (9–11). However, most clinical trials of
low-molecular-weight kinase inhibitors as monotherapies have failed
to demonstrate survival benefits in cancer patients. In response,
combination therapy is considered a promising therapeutic model in
overcoming therapeutic resistance and enhancing treatment
efficacy.
In this study, adenovirus-mediated gene transfer of
PTEN (AD-PTEN) was combined with LY294002 treatment and was
utilized to evaluate its effect on the cell proliferation, cell
cycle, invasion and migration in MCF-7 breast cancer cells. The
addition of AD-PTEN reduced the 50% inhibitory concentration
(IC50) value and enhanced the antitumor effect of
LY294002. In addition, combination therapy resulted in lower levels
of phosphorylated AKTSer473 and GSK-3βSer9
than single treatment with LY294002. Several members of the
Wnt/β-catenin pathway, including Tcf-4, Fra-1, c-Myc, and cyclin
D1, were dysregulated in combination therapy. Furthermore,
intratumoral administration of LY294002 to subcutaneous LN229
xenograft tumors delayed tumor growth. Our results indicated that
the synergistic cytotoxic effect of AD-PTEN and LY294002 is
achieved by the inhibition of Wnt/β-catenin signaling pathway.
Materials and methods
Cell culture
Human breast adenocarcinoma MCF-7 cells were
provided by Wuhan University Cell Center (Wuhan, China). Cell
cultures were incubated at 37°C in a 5% CO2 atmosphere
and routinely maintained in Dulbecco’s modified Eagle’s medium
(DMEM) supplemented with 10% fetal bovine serum (FBS) and 2 mM
L-glutamine (Invitrogen, Carlsbad, CA).
Reagents
Recombinant adenoviral vectors containing wild-type
PTEN (PTEN sequence, AACTGCTCACCGGAAT) were constructed by the
Chinese National Human Genome Center (Beijing, China), at a
concentration of lx1010 IU/ml. LY294002 was purchased by
Promega. Antibodies against PTEN, P13K, AKT,
p-AKTSer473, GSK-3β, p-GSK-3βSer9, β-catenin,
p-β-cateninSer33, Fra-1, Tcf-4, c-myc, PCNA, Bcl-2,
cyclin Dl, MMP-2 and MMP-9 were from Santa Cruz Biotechnology, Inc.
β-actin antibody was purchased from Cell Signaling Technology,
Inc.
Cell viability assays
Cell viability was determined by MTT
(3-(4,5-dimethylthiazol-2-yl)-3,5-diphenyltetrazolium bromide)
assays. Briefly, 96-well plates were seeded with 104
cells/well 24 h prior to drug treatment. Cells were treated with
LY294002 and/or AD-PTEN at various concentrations for two days.
Each experiment was carried out for eight wells. Quantification
measurements were obtained at a wavelength of 570 nm using
spectrophotometric analysis. IC50 values were calculated
from a linear regression line of the plot of percentage inhibition
vs. log inhibitor concentration. The IC50 of LY294002 in
MCF-7 cells was used to detect cell viability.
Cell cycle analysis
Cells were grown for 24 h in normal growth media
followed by drug treatment for additional 72 h. After
centrifugation, the cell pellets were fixed in 70% cold ethanol
overnight at 4°C, and then incubated with RNase at 37°C for 30 min.
The nuclei of cells were stained with propidium iodide for
additional 30 min. A total of 104 nuclei were examined
using a FACSCalibur flow cytometer (BD Biosciences, USA). Samples
were analyzed by flow cytometry for the FL-2 area, and DNA
histograms were analyzed by Modifit software.
Transwell invasion assays
Cell invasion chambers were prepared by placing 100
μl matrigel diluted 1 in 5 onto the filter, and incubating at 37°C
for 30 min to allow matrigel polymerization. Cells were removed
from culture flasks by trypsinization and resuspended at a
concentration of 5×105 cells/ml in serum-free medium. Of
each cell suspension, 2 ml was added to the upper chambers. The
chambers were incubated for 48 h, and then fixed and stained with
hematoxylin. Five fields of vision were viewed under a light
microscope and the cell number under the chamber membrane was
counted.
Migration analysis
Briefly, MCF-7 cells (1×106/well) were
seeded in six-well plates, cultured overnight, and transfected with
LY294002 and/or AD-PTEN and the negative control. Upon reaching
confluency, the cell layer was scratched with a sterile plastic tip
and then immediately washed with growth medium twice and cultured
again in DMEM medium (including 10% FBS) for up to 24 h. At
different time points, photographic images of the plates were taken
under a microscope.
Western blotting
After treatment, cells were lysed in a buffer
composed of 50 mM Tris-HCl, pH 7.4, 0.1 mM phenylmethylsulfonyl
fluoride (PMSF) and 5 mM EGTA for extraction of cellular proteins.
The concentration of total proteins was determined by a NanoDrop
2000D (Thermo Scientific, USA). Polyacrylamide gel (SDS-PAGE)
electrophoresis was performed on 10% gels and then transferred onto
PVDF membranes. The blots were then immunoblotted with a specific
primary IgG antibody overnight at 4°C, followed by incubation with
alkaline horseradish peroxidase-conjugated secondary IgG antibody
for 1 hour at room temperature. Blots were developed using enhanced
chemiluminescence (ECL) reagents (Amersham Pharmacia,
Buckinghamshire, UK) and visualized using the GeneGenius Imaging
System (Syngene, Frederick, MD, USA).
In vivo experiments
BALB/C nu 4-week-old female mice were purchased from
the Cancer Institute and Hospital, Chinese Academy of Medical
Sciences (Beijing, China), and the animal studies were conducted
according to institutional ethics guidelines. All mice were
maintained under specific pathogen-free conditions. Mice were
injected with 10 μ LY294002 once every 4 days. Mice were randomized
into five groups (n=6 mice/group) to receive either vehicle
(control, DMSO), LY294002, AD-PTEN or LY294002 and AD-PTEN
combined. Mice were sacrificed on Day 28 after tumor cell
injections. Tumor growth was assessed weekly by measuring the two
greatest perpendicular tumor dimensions. Tumor volume was
calculated as follows: tumor volume (mm3) = [tumor
length (mm) × tumor width (mm)2]/2.
Immunohistochemistry
Following sacrifice, tumor tissue was removed from
the mice and fixed with 4% formaldehyde for 24 h. Immunostaining
was performed on paraffin sections of tumor specimens using the
avidin-biotin complex (ABC) method. Briefly, sections were
incubated with primary antibody (dilution, 1:200) overnight at 4°C,
then incubated with a biotinylated secondary antibody (dilution,
1:100) at room temperature for 1 h, followed by incubation with
ABC-peroxidase reagent and DAB (diaminobenzidine), counterstaining
with hematoxylin and visualization under the microscope.
Statistical analysis
Data are expressed as the mean ± SE. Statistical
significance was determined using ANOVA, χ2 test, or
Student’s t-test using SPSS13.0. Statistical significance was
determined at P<0.05.
Results
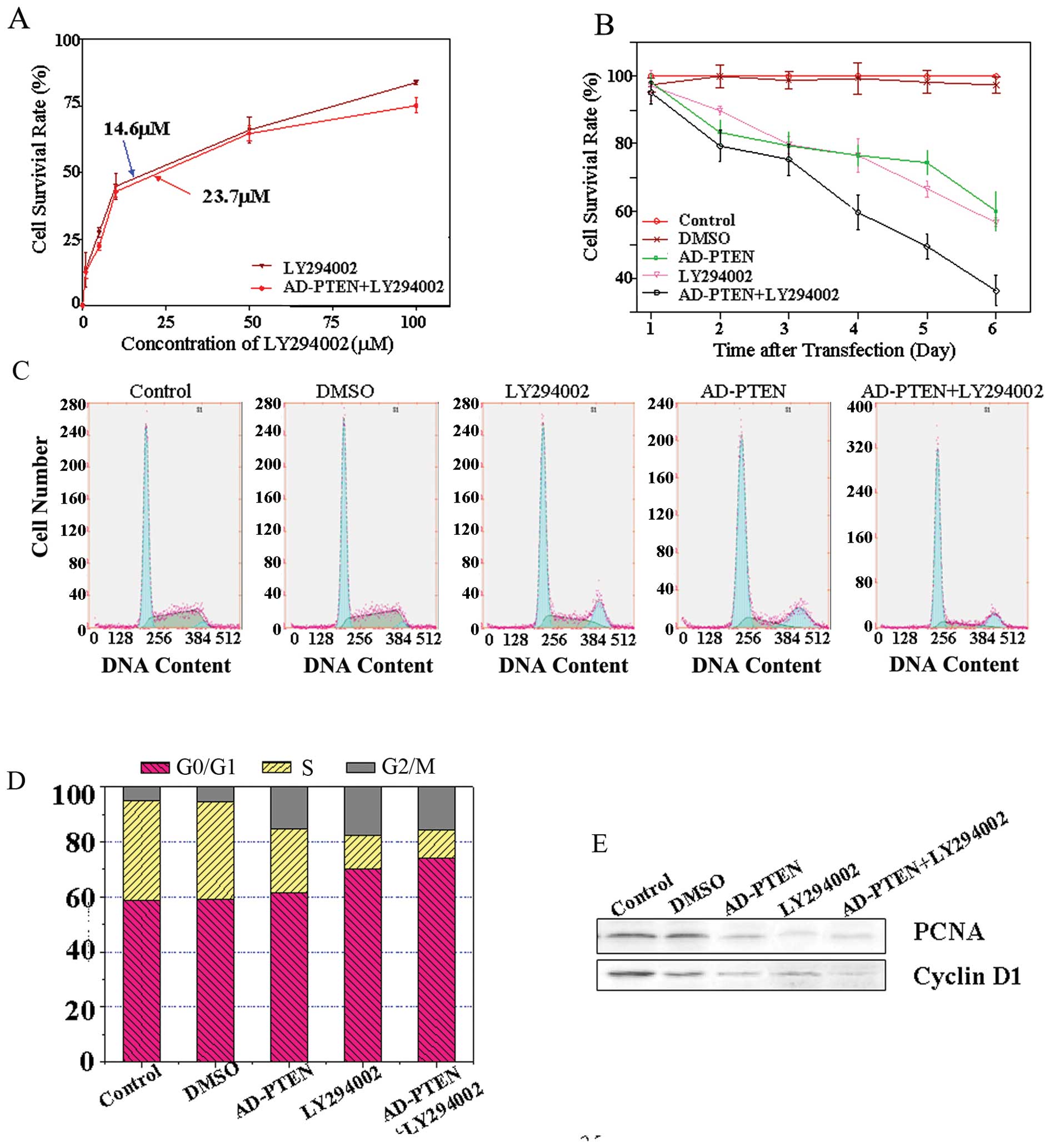
AD-PTEN increases LY294002 cytotoxicity
in MCF-7 cells
The results indicated that LY294002 can decrease the
proliferation of MCF-7 cells, and addition of AD-PTEN can increase
the sensitivity of cells to LY294002 treatment. Fig. 1A shows that the LY294002
concentration causing 50% growth inhibition (IC50) of
MCF-7 cells is 23.7 μmol/l; whereas, in combination with AD-PTEN
the IC50 was 14.6 μmol/l.
To evaluate the synergistic effect of LY294002 and
AD-PTEN on cell proliferation, we performed MTT assays in MCF-7
cells transfected with AD-PTEN or treated with LY294002 alone, or
both transfected with AD-PTEN and treated with LY294002.
Measurements were taken 48 h after transfection. The data from
triplicate samples were analyzed for differences by unpaired,
two-tailed t-tests. As indicated, LY294002 alone exhibited a
moderate suppressive effect in the first three days of the MTT
assay, resulting in maximal inhibition of 60% in MCF-7 cells
(Fig. 1B). The combination of
LY294002 and AD-PTEN appeared to show increased suppressive effects
in MTT assays. Co-delivery of LY294002 and AD-PTEN consistently
maintained the best suppressive effect during the entire MTT assay
and resulted in maximal inhibition of 38% during the six days.
To better understand the synergistic effects on cell
cycle progression, we exposed the cells to LY294002 and AD-PTEN
alone or in combination, and evaluated changes in cell cycle
distribution by flow cytometry analysis (Fig. 1C). Untreated cells served as
negative controls. Fig. 1C shows a
representative experiment in which 61% of cells treated with
LY29004 were in G0/G1-phase, whereas
treatment with LY294002 and AD-PTEN resulted in 74% of cells in
G0/G1 phase.
Subsequently, we would like to further explore
whether the effects of LY294002 combined with AD-PTEN had any
effect on the expression of a set of proteins that are important
for cell proliferation and survival. Proliferating cell nuclear
antigen (PCNA) is a critical event in growth regulation of breast
cancer cells, which acted as a molecular platform to recruit
proteins involved in DNA synthesis, cell cycle control, and
DNA-damage response and repair. In the current study, a significant
decrease in the level of PCNA expression could be observed in the
combination treatment group (Fig.
1E). The protein level of PCNA revealed a 5.8-fold reduction in
the LY294002-alone-treated cells, and a 7.6-fold reduction in cells
treated with LY294002 combined with AD-PTEN-treated cells.
Cyclin D1 is a critical mitogen-regulated cell cycle
control element whose transcriptional modulation plays a crucial
role in breast cancer growth and progression. The protein level of
cyclin D1 revealed a 4.2-fold reduction in single LY294002-treated
cells and a 5.8-fold decrease in the combined treatment group,
respectively (Fig. 1E). These
findings indicate that, at least in vitro, AD-PTEN
sensitizes breast cancer cells to LY294002 cytotoxicity.
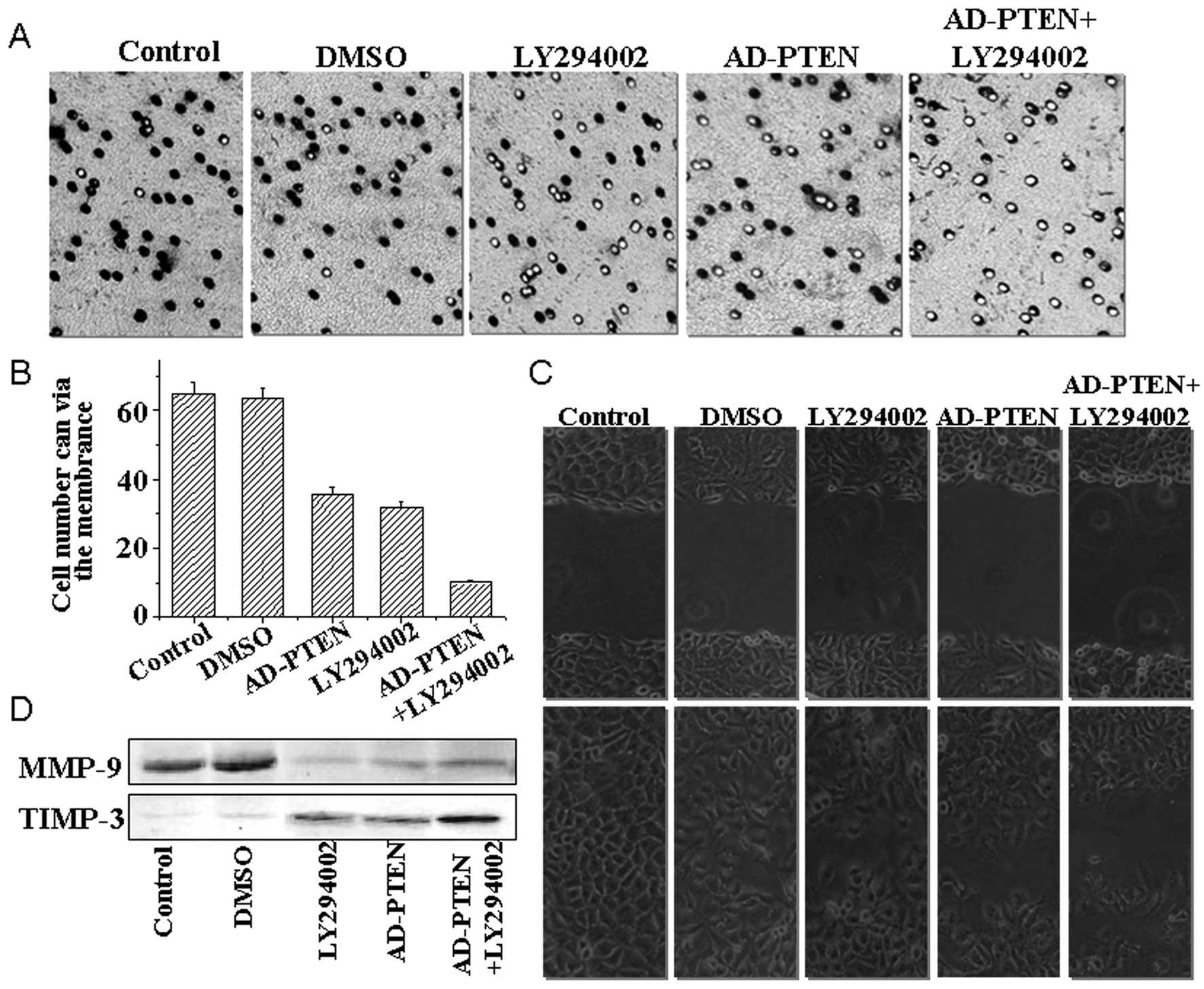
LY294002 and AD-PTEN combination therapy
regulates cell invasion and migration
To measure the effects of LY294002 combined with
AD-PTEN on breast cancer cell invasiveness and migration, we
employed the transwell invasion assay and a xenograft model, which
are better indicators of cancer migratory and invasive properties
in vitro. Transwell assays consist of two fluid-filled,
stacked compartments separated by a porous membrane filter coated
with matrigel. Cells were grown in the upper chamber and assessed
for invasion through the matrigel toward a chemo-attractant (10%
serum) in the lower chamber. The number of invasive cells in
cultures treated with LY294002 and AD-PTEN was reduced relative to
the control cells without any treatment (Fig. 2A); a decrease of 65 to 31–35 MCF-7
cells. The number of invasive cells in cultures with combination
treatment was significantly reduced relative to the cells treated
with LY294002 or AD-PTEN alone (Fig.
2B); a decrease from 31–35 to 10 MCF-7 cells.
The number of migrating cells in LY294002 and
AD-PTEN combination treatment group were 43 (Fig. 2C), which was significantly fewer
than the LY294002 treated group (n=134), the AD-PTEN treated group
(n=102) and the control group (n=352).
Proteins of the matrix metalloproteinase (MMP)
family are involved in the breakdown of extracellular matrix, which
contributes to tumor cell invasion of normal tissues and
metastasis. In this respect, the levels of expression of TIMP-3 and
MMP-9 proteins after combination treatments were evaluated by
western blotting (Fig. 2D).
Significantly decreased expression of was observed after treatment
with LY294002 combined with AD-PTEN, while the level of TIMP-3
protein was dramatically enhanced in the combination treatment
group compared to the single LY294002-treated group.
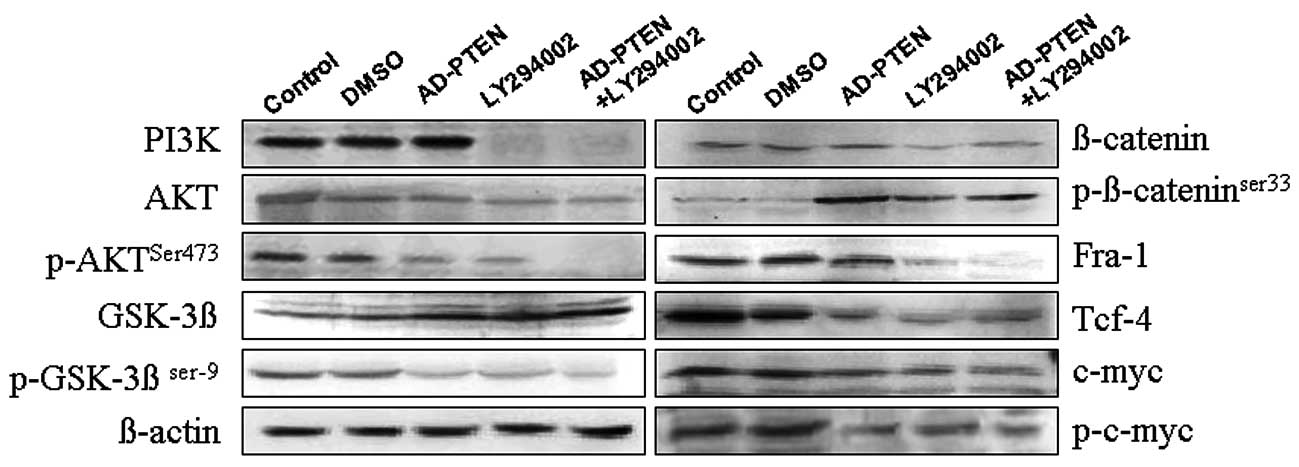
Combination treatment with LY294002 and
AD-PTEN significantly decreases AKTSer473 and
GSK-3βSer9 phosphorylation in breast cancer cells
To understand the molecular mechanisms by which
LY294002 combined with AD-PTEN resulted in decreased proliferation,
invasion and migration in MCF-7 breast cancer cells, the levels of
phosphorylated AKTSer473 and GSK-3βSer9 were
compared after 12-h single or combined treatment with LY294002
or/and AD-PTEN. As shown in Fig. 3,
both phosphorylated AKTSer473 and GSK-3βSer9
were decreased by single LY294002 treatment or single AD-PTEN
treatment. After combination treatment with LY294002 and AD-PTEN,
both phosphorylated AKTSer473 and GSK-3βSer9
were significantly decreased.
To detect whether decreased proliferation, invasion
and migration in MCF-7 breast cancer cells caused by combined
treatment of LY294002 and AD-PTEN was modulated by GSK-3β/β-catenin
signaling pathway, cell cycle regulators such as c-Myc and cyclin
D1, and transcription factors such as Fra-1 and Tcf-4, were
measured by western blotting. As shown in Fig. 3, combined treatment could
significantly inhibit downstream β-catenin signal transduction
pathways, compared with LY294002 or AD-PTEN single treatments.
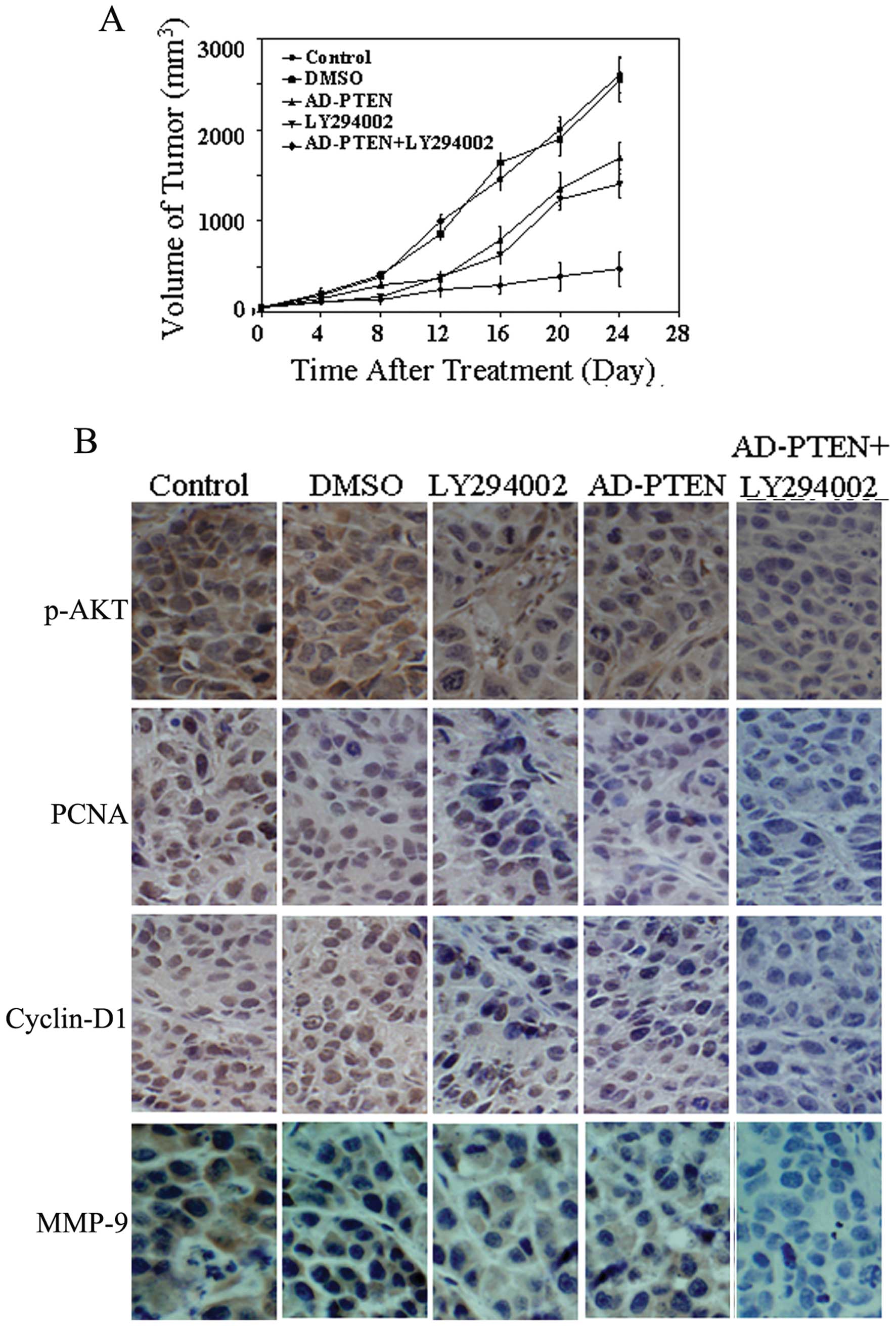
LY294002 combined with AD-PTEN suppresses
breast cancer growth in the xenograft model
LY294002 combined with AD-PTEN demonstrated an
excellent tumor suppressive effect and may be a potential therapy
for human breast cancer. To further confirm this hypothesis, four
experimental groups were examined in an MCF-7 xenograft model: i)
control, ii) LY294002, iii) AD-PTEN and iv) combined AD-PTEN and
LY294002 therapy groups. At the beginning of treatment, the mean
tumor volumes of the mice in LY294002 and AD-PTEN treated groups
alone or in combination were 120, 127 and 136 mm3,
respectively, with no statistically significant differences among
these three groups. The mice were monitored every 4 days for 4
weeks, and the tumor volume of mice in each group was measured and
compared. At the end of the experiment, significant decreases in
tumor volume was only observed in the combination treatment group;
the tumor volume was greatly reduced compared with the LY294002
group (763 mm3 vs. 1789 mm3 and 1433
mm3, P<0.01). Consistent with the in vitro
results, we observed decreased expression of phosphorylated AKT as
well as PCNA, cyclin D1 and MMP-9 in the combination-treated tumors
(Fig. 4B).
Discussion
Selection of appropriate combinations of anticancer
agents that can exert synergistic cytotoxic interactions has been
widely adopted and utilized in preclinical and clinical studies.
Yang et al(12) revealed
that temsirolimus treatment combined with either BEZ235 (a dual
PI3K/mTOR inhibitor) or ZSTK474 (a pan PI3K inhibitor) inhibited
phosphorylation of both 4E-BP1 and the substrate for S6K, ribosomal
S6 (rS6), which ultimately resulted in synergistic cell death of
six endometrial cancer cell lines. Morelli et al(13) demonstrated that dual inhibition of
MAPK signaling and HDAC activity resulted in inhibition of several
colorectal cancer biological processes, which was mediated by an
increased level of caspase 3/7 activity, cleaved PARP and perhaps
increased acetylated histone H3 levels. Recent studies in human
gastric cancer indicated that DLL4 inhibitor combined with
Jagged1-siRNA gene therapy mediated by adenovirus can enhance
inhibition of SGC7901 human gastric cancer cell proliferation and
invasion through the DLL4/Jagged1-Notch1 pathway (14).
Despite the availability of several active
combination regimens for breast cancer, the 5-year survival rate
remains poor, supporting the development of novel therapeutic
approaches. The PI3K/AKT signaling pathway is almost universally
dysregulated in breast cancer, with specific occurrence of PTEN
mutations, which results in overactivation of PI3K/AKT signaling
and its downstream signal transduction pathways (15,16).
Therefore, it has become an attractive target for cancer treatment
and as a result, inhibition of this pathway is a major strategy for
cancer therapy.
However, single-targeted therapeutics against the
PI3K/AKT pathway have demonstrated only modest clinical benefits.
In the present study, we compared the pro-apoptotic effect of
treatment with LY294002, a specific PI3K inhibitor, and AD-PTEN, a
recombinant adenovirus-PTEN, alone or in combination. Our results
revealed that AD-PTEN significantly reduced the IC50 of
LY294002 concentration by ~2-fold compared to LY294002 alone. Cell
proliferation, migration, and invasion ability were also
dramatically inhibited by combination treatment compare to
individual LY294002 or AD-PTEN treatment.
To understand the underlying molecular mechanisms,
we then investigated the activation of downstream effectors of the
PI3K signaling pathway. Expression of total AKT was not changed,
while p-AKTSer473 levels were dramatically decreased in
combination therapy compared to LY294002 alone. Importantly,
combined treatment resulted in lower levels of phosphorylation
GSK-3βSer9, β-catenin, c-Myc and Tcf-4 (Fig. 3). GSK-3β is the main target for AKT
and is inhibited by AKT phosphorylation. In the Wnt/β-catenin
signaling pathway, GSK-3β directly determines the stabilization of
β-catenin by phosphorylation (17).
As a result, there is an accumulation of stabilized
hypophosphorylated β-catenin, which then translocates to the
nucleus and associates with transcription factors of the Lef/Tcf
family to initiate the expression of a broad range of genes, such
as c-Myc and cyclin D1. Thus, our data indicated that the
Wnt/β-catenin signaling pathway may be responsible for the
synergistic cytotoxic effect mechanism of LY294002 and AD-PTEN.
We showed for the first time that inactivation of
PI3K/AKT represses β-catenin-mediated transcription in breast
cancer. Indeed, this hypothesis of cross-talk between the β-catenin
and PI3K/AKT signaling pathways might have supportive evidence.
Damsky et al(18)
demonstrated that β-catenin is a central mediator of melanoma
metastasis to the lymph nodes and lungs, controls tumor
differentiation and regulates both MAPK/ERK and PI3K/AKT signaling.
In U87MG cells, Zhang et al(19) also showed that the activation of
Notch1 by DLL4 stimulation or by the overexpression of Notch
intracellular domain (NICD) resulted in AKT activation, thereby
promoting β-catenin activity. It has also been reported that the
cooperation between Wnt/β-catenin and PTEN/PI3K/AKT signaling
promotes primitive hematopoietic stem cell self-renewal and
expansion (20).
Taken together, our data demonstrate that AD-PTEN
significantly enhances the sensitization of breast cancer cells to
LY294002. We also showed that increased GSK-3β activity resulting
in the inhibition of β-catenin signaling pathway may be responsible
for the synergistic cytotoxic effect of LY294002 and AD-PTEN. Thus,
inhibition of PI3K was proven to be an effective strategy for
inactivation of the Wnt/β-catenin pathway. Furthermore, we also
showed for the first time that cross-talk between PI3K/AKT and
Wnt/β-catenin pathway occurs in breast cancer. The findings of the
present study might help guide the development of potential
therapeutic targets for the treatment of breast cancer
patients.
Acknowledgements
This study was financially supported by the 973
Programme Grant (2009CB918903, 2011CB933100) from the Ministry of
Science and Technology of China, the China National Natural
Scientific Fund (51073118, 81101916, 51103107), the Tianjin Science
and Technology Committee (10JCYBJC12500,10JCZDJC19700), and the
Program for New Century Excellent Talents in University
(NCET-08-0393).
References
|
1
|
Ferté C, André F and Soria JC: Molecular
circuits of solid tumors: prognostic and predictive tools for
bedside use. Nat Rev Clin Oncol. 7:367–380. 2010.PubMed/NCBI
|
|
2
|
Sun CH, Chang YH and Pan CC: Activation of
the PI3K/Akt/mTOR pathway correlates with tumour progression and
reduced survival in patients with urothelial carcinoma of the
urinary bladder. Histopathology. 58:1054–1063. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mazzoletti M, Bortolin F, Brunelli L,
Pastorelli R, Di Giandomenico S, Erba E, Ubezio P and Broggini M:
Combination of PI3K/mTOR inhibitors: antitumor activity and
molecular correlates. Cancer Res. 71:4573–4584. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen Y, Wang BC and Xiao Y: PI3K: A
potential therapeutic target for cancer. J Cell Physiol.
227:2818–2821. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ghayad SE and Cohen PA: Inhibitors of the
PI3K/Akt/mTOR pathway: new hope for breast cancer patients. Recent
Pat Anticancer Drug Discov. 5:29–57. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu W, Zhou Y, Reske SN and Shen C: PTEN
mutation: many birds with one stone in tumorigenesis. Anticancer
Res. 28:3613–3619. 2008.PubMed/NCBI
|
|
7
|
Heikkinen T, Greco D, Pelttari LM,
Tommiska J, Vahteristo P, Heikkila P, Blomqvist C, Aittomaki K and
Nevanlinna H: Variants on the promoter region of PTEN affect breast
cancer progression and patient survival. Breast Cancer Res.
13:R1302011. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Carraway H and Hidalgo M: New targets for
therapy in breast cancer: mammalian target of rapamycin (mTOR)
antagonists. Breast Cancer Res. 6:219–224. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lai JP, Sandhu DS, Yu C, Moser CD, Hu C,
Shire AM, Aderca I, Murphy LM, Adjei AA, Sanderson S and Roberts
LR: Sulfatase 2 protects hepatocellular carcinoma cells against
apoptosis induced by the PI3K inhibitor LY294002 and ERK and JNK
kinase inhibitors. Liver Int. 30:1522–1528. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shin JY, Kim JO, Lee SK, Chae HS and Kang
JH: LY294002 may overcome 5-FU resistance via down-regulation of
activated p-AKT in Epstein-Barr virus-positive gastric cancer
cells. BMC Cancer. 13:4252010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fujiwara M, Izuishi K, Sano T, Hossain MA,
Kimura S, Masaki T and Suzuki Y: Modulating effect of the
PI3-kinase inhibitor LY294002 on cisplatin in human pancreatic
cancer cells. J Exp Clin Cancer Res. 27:76–84. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang S, Xiao X, Meng X and Leslie KK: A
mechanism for synergy with combined mTOR and PI3 kinase inhibitors.
PLoS One. 6:e263432011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Morelli MP, Tentler JJ, Kulikowski GN, Tan
AC, Bradshaw-Pierce EL, Pitts TM, Brown AM, Nallapareddy S,
Arcaroli JJ, Serkova NJ, et al: Preclinical activity of the
rational combination of Selumetinib (AZD6244) in combination with
Vorinostat in KRAS mutant colorectal cancer models. Clin Cancer
Res. 18:1051–1062. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sun HW, Wu C, Tan HY and Wang QS:
Combination DLL4 with Jagged1-siRNA can enhance inhibition of the
proliferation and invasiveness activity of human gastric carcinoma
by Notch1/VEGF pathway. Hepatogastroenterology. 59:115–116.
2011.PubMed/NCBI
|
|
15
|
Adamo B, Deal AM, Burrows E, Geradts J,
Hamilton E, Blackwell KL, Livasy C, Fritchie K, Prat A, Harrell JC,
et al: Phosphatidylinositol 3-kinase (PI3K) pathway activation in
breast cancer brain metastases. Breast Cancer Res. 13:R1252011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Miller TW, Rexer BN, Garrett JT and
Arteaga CL: Mutations in the phosphatidylinositol 3-kinase pathway:
role in tumor progression and therapeutic implications in breast
cancer. Breast Cancer Res. 13:2242011. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dozza B, Smith MA, Perry G, Tabaton M and
Strocchi P: Regulation of glycogen synthase kinase-3beta by
products of lipid peroxidation in human neuroblastoma cells. J
Neurochem. 89:1224–1232. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Damsky WE, Curley DP, Santhanakrishnan M,
Rosenbaum LE, Platt JT, Gould Rothberg BE, Taketo MM, Dankort D,
Rimm DL, McMahon M and Bosenberg M: β-catenin signaling controls
metastasis in Braf-activated Pten-deficient melanomas. Cancer Cell.
20:741–754. 2011.
|
|
19
|
Zhang X, Chen T, Zhang J, Mao Q, Li S,
Xiong W, Qiu Y, Xie Q and Ge J: Notch1 promotes glioma cell
migration and invasion by stimulating β-catenin and NF-κB signaling
via AKT activation. Cancer Sci. 103:181–190. 2011.PubMed/NCBI
|
|
20
|
Perry JM, He XC, Sugimura R, Grindley JC,
Haug JS, Ding S and Li L: Cooperation between both Wnt/β-catenin
and PTEN/PI3K/Akt signaling promotes primitive hematopoietic stem
cell self-renewal and expansion. Genes Dev. 25:1928–1942. 2011.
|