Introduction
Cervical carcinoma is one of the most common cancers
and the second-leading cause of cancer deaths in women worldwide
(1). Substantial research has been
performed to identify the causative agents for development of
cervical cancer and now it is generally accepted that human
papilloma virus (HPV) is the principal etiological agent of
cervical cancer (2). Although the
virus infecting these tumors can immortalize human cells, it does
not result in transformation. Therefore, HPV infection is likely to
be necessary, but insufficient for developing cervical cancers. It
might mean there are factors epigenetic, genetic, cellular, and
environmental that can influence carcinogenesis (3). Although the precise molecular
mechanisms are still unclear, the most possible signaling pathways
that is considered as the second hit in the multistep process of
cervical carcinogenesis caused by HPV is the Wingless-type
(Wnt)/β-catenin pathway (4,5).
The Wnt pathway is an important regulator in the
control of several biological processes such as proliferation and
differentiation in embryogenesis, regulation of the cell cycle,
tissue homeostasis in adult tissue and tumor progression (6). Wnt ligands binding to its receptor
complex comprised of Frizzled/low-density lipoprotein
receptor-related protein (Fz/LRP) trigger a canonical pathway. In
this pathway, β-catenin was stabilized by inhibition of its
phosphorylation and subsequent proteosomal degradation. Stabilized
β-catenin translocates to the nucleus and forms a complex with
T-cell factor/lymphoid enhancer factor (TCF/LEF) to activate target
genes (7). In contrast, Wnt
inhibition caused by its antagonists leads to decreased
accumulation of cytosolic and nuclear β-catenin with consequent
downregulation of Wnt reactive genes.
Aberrant activation of the Wnt/β-catenin signaling
pathway contributes to the progression of several major human
cancers (6). Cytoplasmic and
nuclear accumulation of β-catenin is the main hallmark of Wnt
activation and is observed in most cervical cancer specimens
(8). However, mutations of APC and
β-catenin genes that are usually responsible for the deregulated
Wnt/β-catenin pathway in other tumors are rare in human cervical
cancer (8,9). It suggests that Wnt activation is the
main regulator of β-catenin in cervical cancer. Supporting this
hypothesis, promoter hypermethylation of characteristic Wnt
antagonists Dickkopf-3, secreted Frizzled related protein-1, -2, -4
and Klotho has been identified in cervical carcinoma (10,11).
Klotho was first identified as a potent suppressor
of aging, so loss of Klotho can result in multiple aging-like
phenotypes (12). Klotho is a
1012-amino acid single pass transmembrane protein, and its
extracellular domain can be cleaved, shed into the serum, and it
can act as a circulating hormone (13,14).
The intracellular domain is short and no known functional domains
exist. Besides of its principle β-glucosidase activity (12,15),
Klotho is involved in multiple biological processes. It is now
generally accepted that Klotho inhibits insulin and insulin-like
growth factor (IGF-1) signaling and acts as a co-receptor for
fibroblast growth factor 23 (FGF23) (16). Multiple lines of evidence proposed
that the expression level of Klotho influences human breast, and
lung cancers via intervention of IGF-1 and insulin pathway
(17,18). However, there are not sufficient
studies on the role of Klotho in cervical cancer progression. In
our previous study (19), we showed
epigenetic silencing of Klotho in HPV16-positive cervical cancer
cell lines (CasKi and SiHa) and human cervical carcinoma.
In the present study, we demonstrated that in
vivo Klotho expression in cervical carcinoma tissues was
decreased as invasiveness became acute compare to its normal
counterparts. In vitro studies revealed that Klotho
restoration causes a dramatic downregulation of the Wnt/β-catenin
target gene expression and demonstrate that Klotho significantly
inhibit tumor proliferation and invasion by reversal of EMT
markers. Based on this, our study indicated that Klotho shows
clinical importance in Wnt/β-catenin pathway activating
HPV16-infected cervical cancer.
Materials and methods
Cell culture
The human cervical cancer cell line SiHa, was
purchased from the American Type Culture Collection (Manassas, VA)
and was grown in Dulbecco’s modified Eagle’s medium (WelGene,
Seoul) supplemented with 10% fetal bovine serum, 100 μg/ml
penicillin and 100 U/ml streptomycin. Culture was grown at 37°C in
a 5% CO2 atmosphere.
Patient specimens
All uterine cervical tissues were obtained from
patients under protocols approved by the institutional review board
of Soonchunhyang University (Cheonan, Korea). Tumor tissue (n=20)
and adjacent matched normal tissue (n=20) were obtained from women
diagnosed with invasive cervical cancer. Histological diagnosis,
tumor stage and grade were followed by the Union for International
Cancer Control (UICC) classification schemes.
Immunohistochemistry
The uterine cervical tissue was fixed in 10% neutral
buffered formalin for 8–12 h in room temperature and the paraffin
blocks was made with standard methods. For immunohistochemical
staining of Klotho, the paraffin block sections (4 mm thick) were
deparaffinised, rehydrated, placed in 0.01 mol/l citrate buffer (pH
6.0), and treated by microwave heating for 15 min. The sections
were then preincubated with 0.3% H2O2 in
methanol for 20 min at room temperature to quench endogenous
peroxidase activity. Subsequently, the sections were immunostained
with an UltraTech kit (Immunotech, Marseille, France) according to
the manufacturer’s instructions. The sections were pretreated with
1% bovine serum albumin in phosphate-buffered saline (PBS), and
then incubated with anti-Klotho antibody (Sigma, MO, USA) by
dilution 1:60 for 1 h at room temperature. Thereafter, the sections
were incubated with biotinylated secondary antibody for 15 min,
washed with PBS, and treated with peroxidase-conjugated
streptavidin for 20 min. Finally the sections were incubated in
3,3-diaminobenzidine tetrahydrochloride with 0.05%
H2O2 for 5 min and then counterstained with
haematoxylin. Sections of kidney formalin-fixed paraffin-embedded
tissue that had been confirmed to overexpress this protein was used
as positive control. The PBS was applied instead of the primary
antibody to negative controls.
Reverse transcription-PCR
Total RNA was isolated from vector- or
Klotho-transfected SiHa using the Qiagen RNeasy kit (Qiagen) and
then reverse transcribed with ImProm-II™ Reverse Transcription
System (Promega), according to the manufacturer’s instructions. The
cDNA was amplified by PCR using the AccuPower PCR premix (Bioneer,
South Korea) with the following primers: Klotho-specific primers
were: forward, 5′-ACTCCCCCAGTCAGGTGGCGGTA-3′ and reverse,
5′-TGGGCCCGGGAAACCATTGCTGTC-3′. C-myc primers: forward,
5′-ACCAGCAGCGACTCTGAGGAGG AAC-4′ and reverse,
5′-TGACCCTCTTGGCAGCAGGATAG TCC-3′; cyclin D1 primers: forward,
5′-ACCTTCGTTGCCC TCTGTGCCACAGATG-3′ and reverse, 5′-AGGCCCGGAGG
CAGTCCGGGT-3′; E-cadherin primers: forward, 5′-TCCCA
TCAGCTGCCCAGAAA-3′ and reverse, 5′-TGACTCCTGT GTTCCTGTGTA-3′;
N-cadnerin primers: forward, 5′-CAC TGCTCAGGACCCAGAT-3′ and
reverse, 5′-TAAGCCGAG TGATGGTCC-3′; MMP7 primers: forward,
5′-CGGATGGT AGCAGTCTAGGGATTAAC-3′ and reverse, 5′-GGAGTG
GAGGAACAGTGCTTATCAATTC-3′; MMP9 primers: forward,
5′-CTTCTCTGGGCGCCAGGT-3′ and reverse,
5′-AGGCTTTCTCTCGGTACTGGAAGAC-3′. Human ACTB forward,
5′-CTCGGTGAGGATCTTCATGAGGT AGT-3′ and reverse,
5′-CCATCGAGCACGGCATCGTCA CCA-3′ was amplified as an endogenous
control.
Modification of Klotho expression
The secreted form of human Klotho (sKL) cDNA cloned
into pcDNA3.1/V5-His expression vector (Invitrogen) was a generous
gift from Michael J. Econs (Indiana University School of Medicine,
Indianapolis, IN, USA). SiHa cells were plated at 1×106
cells/60-mm3 dishes 24 h prior to transfection and were
transfected with 3 μg of either sKL expression vector or an empty
vector control for 5 h in serum-free medium using Lipofectamine
2000 (Invitrogen) following the manufacturer’s instructions. After
replacing the DNA-Lipofectamine complex-containing medium with
complete growth medium, transfected cells were incubated for 72 h.
After modification of sKL, the altered expression of sKL and
Wnt/β-catenin signal-related genes was also examined by RT-PCR and
immunoblotting.
Immunoblotting
Cells transfected or non-transfected with sKL
plasmid were harvested and extracts formed by the addition of lysis
buffer. After boiling with 2X sample buffer, proteins were resolved
on 10% SDS-polyacrylamide gels and electrotransferred to PVDF
membranes (Millipore). Modified sKL expression level and influenced
proteins was examined using the antibodies: Polyclonal anti-sKL
(T-19, Santa Cruz Biotechnology), cyclin D1 (M-20, Santa Cruz
Biotechnology), total β-catenin (6B3, Cell Signaling), active
β-catenin (no. 9582, Cell Signaling), GSK3β (no. 2199, Epitomics),
phospho-GSK3β (no. 2435, Epitomics), E-cadherin (610181, BD
Biosciences), N-cadherin (610920, BD), MMP7 (J-22, Santa Cruz
Biotechnology), and MMP9 (no. 3852, Cell Signaling). As a loading
control, blots were probed with γ-tubulin (sc7396, Santa Cruz
Biotechnology). Immunoreactivity of each protein was visualized
using chemiluminescence and recorded on X-ray film.
Wound healing migration
Alteration of cell migration induced by
overexpressed sKL was estimated by means of wound-healing migration
to measure alteration of two-dimensional cellular movement.
pcDNA3.1/HA vector and sKL-transfected SiHa cells were cultured to
confluence in 60-mm3 dishes. A scratch was made on the
monolayer using a sterile pipette tip. At the initiation of the
experiment, a microscopic image of the scratch wound was taken at
×50 magnification. At 36 h, the same region was imaged. The width
of the scratch wounds was measured in Photoshop 7.0. The relative
fold change of the scratch wound width at 36 h after introduction
of the scratch compared to the control which control the fold
change was calculated as the average of 6 fields.
Matrigel invasion assay and extracellular
matrix transition
To examine invasiveness, 2.5×104 of
vector- or sKL-transfected SiHa cells per well in serum-free DMEM
were placed in the upper chamber. DMEM plus 10% FBS was placed in
the lower chamber as a chemoattractant. Cells were allowed to
migrate through a uncoated membrane for 24 h at 37°C.
Chemoattractant was removed and 4 μM Calcein AM Fluorescent Dye (BD
Biosciences) was mixed to media or PBS, 0.5 ml dye was added to
bottom chamber of 24-well for 1 h at 37°C. The amount of migrating
cells was determined by measured fluorescence at 494/517 nm
(Abs/Em).
Results
Expression of Klotho in human cervical
carcinoma
It has been reported that the decreased expression
of Klotho was related to carcinogenesis in breast cancer cells
(20), but not in cervical cancer.
Our previous study firstly reported that Klotho expression was
downregulated in cervical cancer by epigenetic silencing on
promoter region (19). According to
our previous epigenetic study, Klotho mRNA in human cervical cancer
cell lines did not or were slightly expressed, especially in those
having higher metastatic potential (CasKi and SiHa). In addition,
the Klotho mRNA level in human cervical cancer tissues was also
downregulated compare to its normal tissues. From this study, we
hypothesized that the level of Klotho expression may be in inverse
proportion to invasiveness of cervical cancer.
To examine the Klotho expression level and cervical
cancer invasiveness, we first examined Klotho expression in a human
cervical cancer with different grades by immunohistochemistry. In
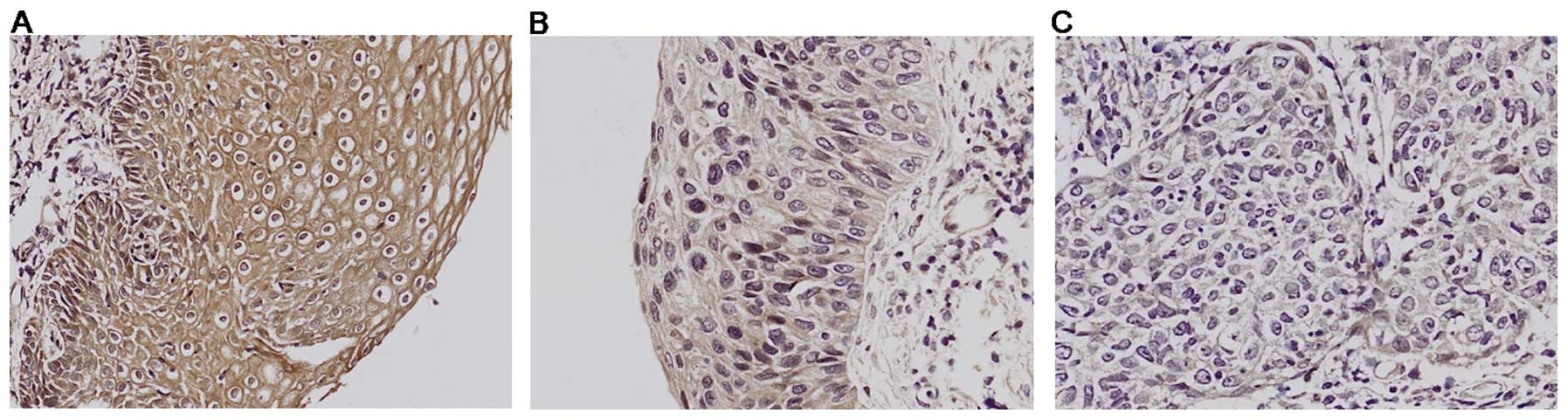
the uterine cervical normal stratified squamous epithelium, strong
Klotho expression was observed in the cytoplasm (Fig. 1A). All normal cervical samples were
obtained from women undergoing surgery expressing high Klotho
levels. Klotho expression in cervical intraepithelial neoplasia III
(CIN III), which is cancer but has not yet invaded deeper tissues,
was decreased about half compare to normal counterparts (Fig. 1B). In contrast, there was no Klotho
expression detected in invasive cervical cancer tissues (Fig. 1C). Our immunohistochemistry results
clearly demonstrate a significant decrease in Klotho protein
expression and correlated well with our previous epigenetic
silencing of Klotho mRNA as shown by RT-PCR in higher grade of
cervical cancer when compared with the normal tissue. It suggests
that Klotho downregulation is closely related in cervical
invasiveness.
Reversal of epithelial-to-mesenchymal
transition by Klotho expression in SiHa cervical cancer cells
The immunohistochemistry data indicated that
downregulated Klotho expression might be one of the important
factors to increase cervical cancer invasiveness. In addition, our
previous study (19) showed that
ectopic expression of Klotho with CasKi cells were more compact and
adherent to adjacent cells than vector-transfected ones, indicating
that Klotho can influence the EMT of cervical cancer cells. Tumor
invasion into surrounding tissues requires epithelial cells to lose
their polarity and intercellular adhesion (21). E-cadherin is the prime mediator of
intercellular adhesion (22) and
its downregulation is a hallmark of tumor invasion (23). Fig.
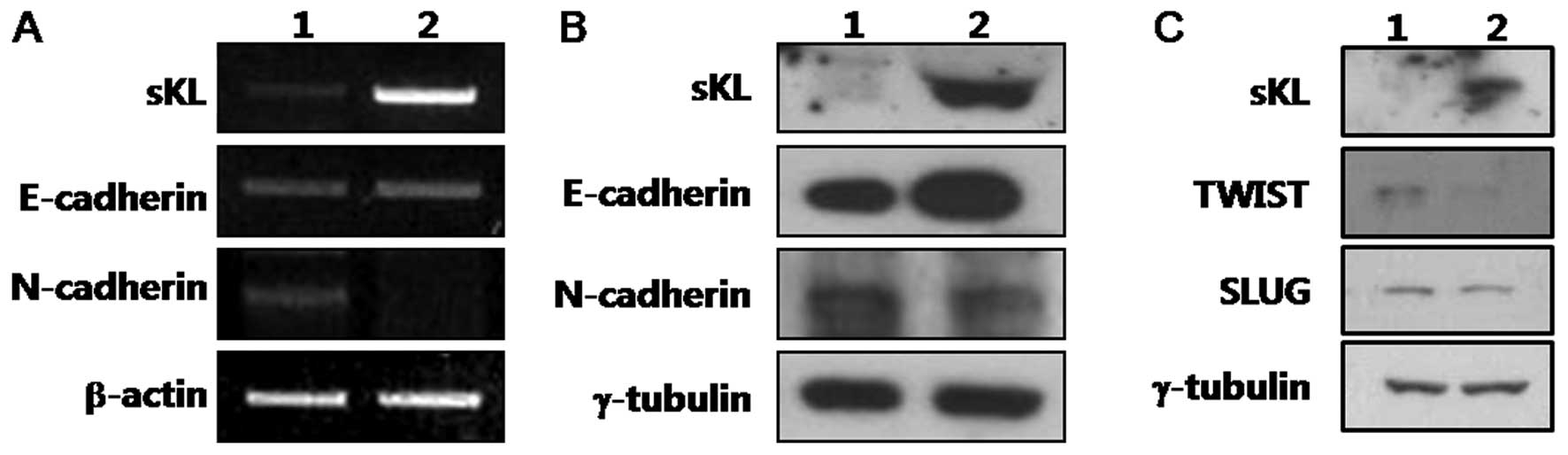
2B shows that ectopic expression of Klotho in SiHa cells
results in a dramatic increase in the protein levels of E-cadherin
and a decrease in N-cadherin. This result suggests that Klotho
expression causes a reversal of EMT in cervical cancer cells.
Association with downregulating
Slug/Twist expression in EMT modulation by Klotho
Compared to vector control, RT-PCR analysis also
shows that Klotho expression in SiHa cells causes upregulation of
E-cadherin, and leads to downregulation of N-cadherin (Fig. 2A). It suggests that the effect of
Klotho on the alteration of EMT in SiHa cells may be associated
with transcriptional regulation.
Most representative transcription factors involving
in EMT regulation are Slug, Snail and Twist which are repressors
for E-cadherin gene transcription (24). Furthermore, Wnt/β-catenin signaling
has been reported to cause upregulation of the expression of Slug
and Twist (25,26) and Klotho can influence its
expression acting as Wnt antagonist (19). Fig.
2C demonstrates that the protein levels of Slug and Twist are
decreased by Klotho expression. It means that the Klotho-induced
reversal of EMT in SiHa cells is associated with downregulation of
transcriptional factor Slug/Twist and resultant upregulation of
E-cadherin.
Suppression of cellular motility,
invasive capacity via downregulation of MMP7 and -9 by Klotho
expression
To examine the effect of Klotho on EMT reversal, we
studied the effect of Klotho expression on migration of SiHa cells
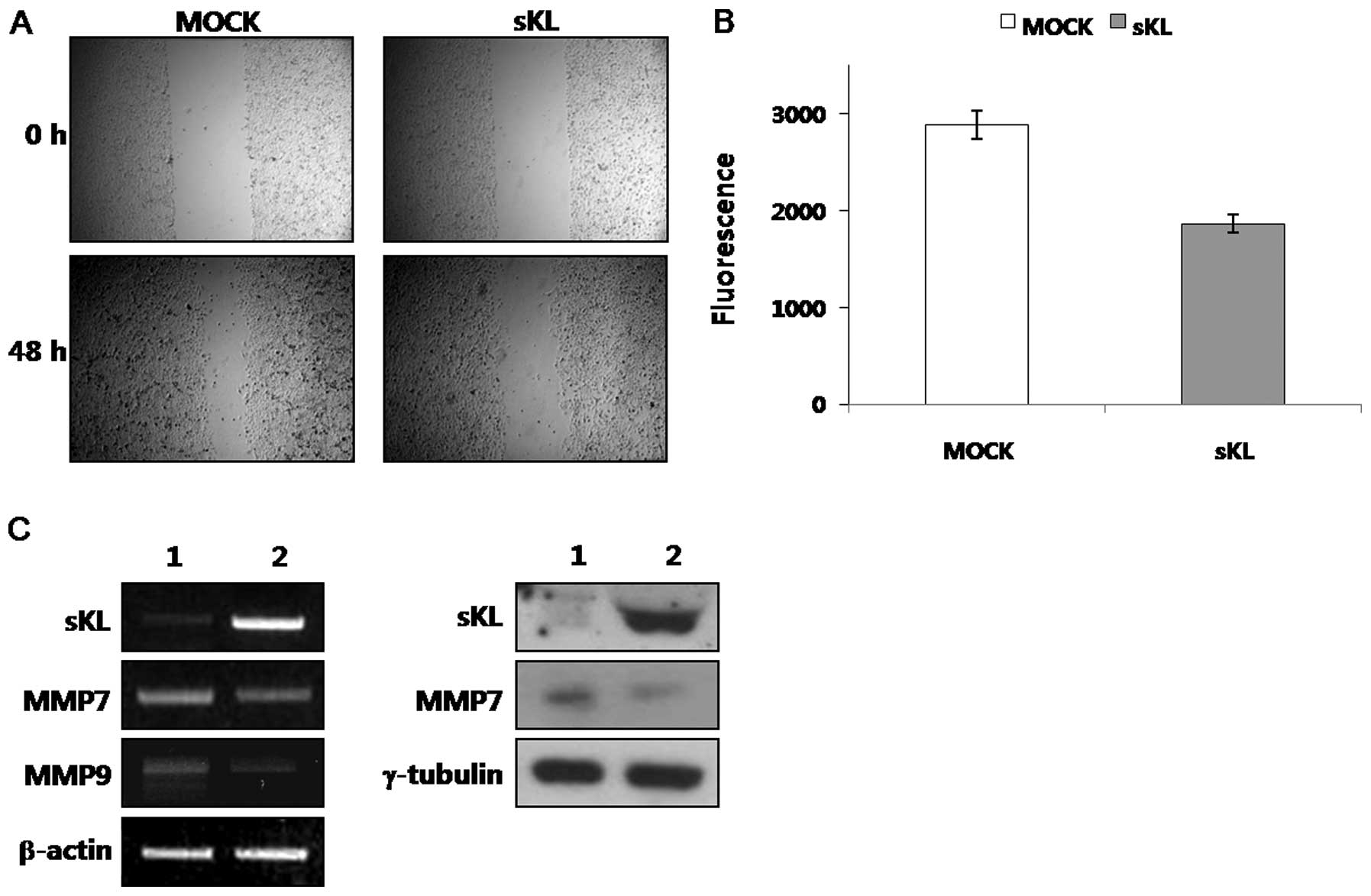
using a wound healing assay. Fig.
3A shows that Klotho-transfected SiHa cells exhibited slower
migration into the wounded area comparing with vector-transfected
ones.
We also examined the in vitro invasiveness of
SiHa cells overexpressing Klotho, or vector transfected cells in a
Matrigel invasion assay. Cell motility was measured by average
fluorescence of cells migrating through a control, uncoated insert.
Klotho-transfected cells exhibited a significant decrease in
invasive capacity compared with control cells (Fig. 3B). These data suggest that EMT
reversal caused by Klotho expression contributes to the
invasiveness of cervical cancer cells.
Matrix metalloproteinases (MMPs) play an important
role in cell-matrix interaction and tumor invasion. We therefore
studied the effect of Klotho on MMPs expression. Fig. 3C shows that ectopic expression of
Klotho in SiHa cells resulted in decreased level of MMP7 and -9. In
addition, transcripts of MMP7 and -9 also are decreased in
Klotho-transfected SiHa cells similar to the modulation of protein
expression levels. It has been reported that MMP activities are
correlated with protein expression level of each MMP (27,28).
Based on this, Klotho-induced downregulation of MMPs suggests that
its activity is also decreased. Altogether, ectopic expression of
Klotho suppresses the invasiveness of cervical cancer cells via EMT
reversal and downregulation of MMP expression.
Inhibition of canonical Wnt/β-catenin
pathway by Klotho
Our data indicates that loss of Klotho expression
can relate to increased invasiveness through alteration of EMT,
cell motility, and MMPs activities. It has been reported that Wnt
pathway is involved in cell proliferation, invasion and metastasis
through regulation of Wnt target gene expression (29). Altered gene and cellular
characteristics by Klotho expression during enhancement of cervical
cancer was observed mostly in the Wnt pathway. In addition, we
previously found Klotho is Wnt antagonist, so we analyzed the
expression of Klotho, GSK3β, β-catenin and specific Wnt/β-catenin
direct transcriptional target genes with representative roles in
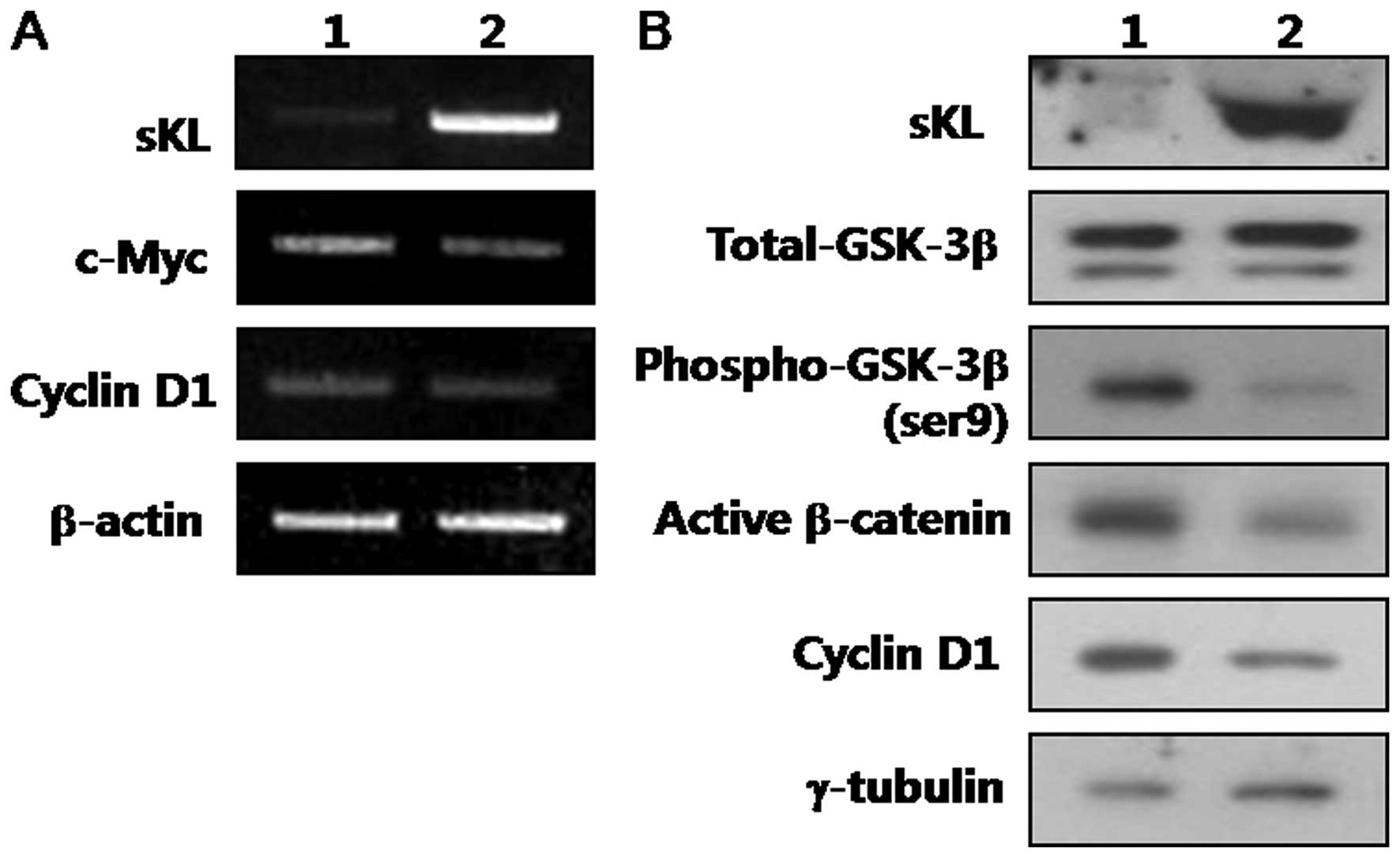
tumor progression and invasion. Fig.
4 shows that ectopic expression of Klotho in SiHa cells caused
a decreased expression of phospho-GSK3β at serine-9 without
changing the levels of its total protein. In addition, alteration
of GSK3β reduced the active form of β-catenin (ABC), which is
dephosphorylated on S37 or T41 residues.
c-Myc seems to be essential for sustaining
proliferation of human tumor cells (30). Importantly, Klotho overexpression
resulted in a marked downregulation of c-Myc mRNA being a direct
transcriptional Wnt/β-catenin target (Fig. 4A). The expression of cyclin D1,
another direct transcriptional Wnt/β-catenin target with major
roles in cell proliferation (31,32)
was also markedly downregulated in Klotho-transfected SiHa cells
(Fig. 4). Together, these results
indicate that Klotho expression inhibits human cervical cancer
invasion by having a major regulatory role on the expression of
specific β-catenin direct transcriptional targets.
Discussion
It has been reported that the first etiology of
cervical cancer is HPV infection and it potentially activates
Wnt/β-catenin signaling pathway to promote tumorigenesis. Klotho is
known as a Wnt antagonist and it is downregulated mainly by
promoter hypermethylation in several human tumors, including lung,
breast, pancreatic, colon and cervical cancer (19,33–35).
Although it has been reported that Klotho acts as tumor suppressor
and inhibits cancer cell proliferation in many cancers (36), little is known about its potential
effect on metastatic cervical tumor and the process of this tumor
metastasis. We reported here that Klotho expression resulted in a
decreased capacity of cell migration and invasion. This was
confirmed by immunohistochemistry data using human cervical cancer
tissues. Normal cervical tissues have very strong Klotho expression
and it was decreased to about half in CIN III grade. In contrast,
no or very low level of Klotho was observed in invasive cervical
cancer tissues. This suggests that the loss of Klotho in cervical
cancer may contribute to its metastatic potential. Based on our
study, the action mechanism of Klotho is associated with a reversal
of the EMT process via downregulation of E-cadherin expression,
upregulation of N-cadherin expression and decreased activity of
MMP7 and -9.
EMT is characterized by increased migratory
features, decreased epithelial cell adhesion, loss of cytoskeleton
components and acquisition of mesenchymal components (37). A hallmark of EMT is the loss of
E-cadherin expression (37). Our
study showed that overexpression of Klotho in SiHa cells changed
EMT by both increase of E-cadherin and decrease of N-cadherin. The
increase of E-cadherin with ectopic expression of Klotho was
associated with downregulation of the transcriptional repressors
Slug and Twist. In a recent study Doi et al(38) demonstrated that in renal fibrosis
and metastatic cells restoring the expression of Klotho leads to
inhibition of TGF-β1-induced EMT. It means that Wnt/β-catenin
pathway may also participate in regulation of the EMT process in
cancer progression and that Klotho may interfere with it.
Downregulation of E-cadherin leads to the loss of
intercellular adhesion (23). SiHa
cells transfected with Klotho showed a marked decrease in the
expression of active β-catenin, c-Myc and cyclin D1. These data
suggest that Klotho blocks Wnt/β-catenin pathway and consequently
inhibits cervical cancer cell proliferation. In addition, it can
alter the invasive behavior of cervical cancer cells. According to
our wound healing assay, Klotho-transfected SiHa cells which have
increased E-cadherin moved slower into wound lesions. E-cadherin
binds directly to β-catenin (22)
and this binding is prerequisite for formation of cell-cell
adhesion, which prevents tumor invasion. Concurring with this,
E-cadherin expression correlates negatively with progression of
cervical intraepithelial neoplasia (39). Overall, it strongly support the
hypothesis that Klotho blocks human cervical cancer progression,
and its re-expression has the possibility to change patient’s
prognosis. Taken together, Klotho is a potent invasion suppressor
as strong as tumor suppressor. Based on our results, the effect of
Klotho can be a result of synergistic convergence of multiple
regulatory pathways, including induction of apoptosis, decrease in
proliferation, and angiogenesis, due to the Wnt/β-catenin signaling
pathway being immensely influential. Therefore, our further studies
in progress will determine which Klotho could cause concomitant
suppression of tumor progression. However, it still remains unknown
that the inhibitory mechanism of Klotho in Wnt/β-catenin signaling
especially in cervical cancer. It has been reported that Klotho can
interact with Wnt ligands such as Wnt5a, and Wnt3 to suppress
metastasis in melanoma or aging (36,40).
Therefore, our future study will be focused on the processes of
Wnt/β-catenin pathway by Klotho.
In conclusion, we report for the first time that
loss of Klotho leads to aberrant activation of Wnt/β-catening
signaling in human cervical cancer. It was also shown that
re-expression of Klotho causes a remarkable inhibition of the
Wnt/β-catenin pathway blocking tumor invasion. Thus, our findings
revealed more information on the unknown and novel
invasion-suppressive signaling mechanisms of Klotho, and also
emphasize the potential therapeutic value of Klotho in human
cervical cancer treatment.
Acknowledgements
This study was supported by a grant funded by
Sookmyung Women’s University (2011).
Abbreviations:
|
HPV
|
human papilloma virus
|
|
Fz/LRP
|
Frizzled/low-density lipoprotein
receptor-related protein
|
|
TCF/LEF
|
T-cell factor/lymphoid enhance
factor
|
|
IGF-1
|
insulin-like growth factor-1
|
|
FGF23
|
fibroblast growth factor 23
|
|
EMT
|
epithelial-to-mesenchymal
transition
|
|
MMPs
|
matrix metalloproteinases
|
|
ABC
|
active form of β-catenin
|
References
|
1
|
Parkin DM and Bray F: The burden of
HPV-related cancers. Vaccine. 24(Suppl 3): Chapter 2. S3/11–25.
2006. View Article : Google Scholar
|
|
2
|
Woodman CB, Collins SI and Young LS: The
natural history of cervical HPV infection: unresolved issues. Nat
Rev Cancer. 7:11–22. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Estimating the world cancer burden: Globocan 2000. Int J Cancer.
94:153–156. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Uren A, Fallen S, Yuan H, Usubutun A,
Kucukali T, Schlegel R, et al: Activation of the canonical Wnt
pathway during genital keratinocyte transformation: a model for
cervical cancer progression. Cancer Res. 65:6199–6206. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Perez-Plasencia C, Duenas-Gonzalez A and
Alatorre-Tavera B: Second hit in cervical carcinogenesis process:
involvement of wnt/beta catenin pathway. Int Arch Med. 1:102008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
MacDonald BT, Tamai K and He X:
Wnt/β-catenin signaling: components, mechanisms, and diseases. Dev
Cell. 17:9–26. 2009.
|
|
7
|
Paul S and Dey A: Wnt signaling and cancer
development: therapeutic implication. Neoplasma. 55:165–176.
2008.PubMed/NCBI
|
|
8
|
Shinohara A, Yokoyama Y, Wan X, Takahashi
Y, Mori Y, Takami T, et al: Cytoplasmic/nuclear expression without
mutation of exon 3 of the β-catenin gene is frequent in the
development of the neoplasm of the uterine cervix. Gynecol Oncol.
82:450–455. 2001.
|
|
9
|
Ueda M, Gemmill RM, West J, Winn R, Sugita
M, Tanaka N, et al: Mutations of the β- and γ-catenin genes are
uncommon in human lung, breast, kidney, cervical and ovarian
carcinomas. Br J Cancer. 85:64–68. 2001.
|
|
10
|
Chung MT, Sytwu HK, Yan MD, Shih YL, Chang
CC, Yu MH, et al: Promoter methylation of SFRPs gene family in
cervical cancer. Gynecol Oncol. 112:301–306. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee EJ, Jo M, Rho SB, Park K, Yoo YN, Park
J, et al: Dkk3, downregulated in cervical cancer, functions
as a negative regulator of β-catenin. Int J Cancer. 124:287–297.
2009. View Article : Google Scholar
|
|
12
|
Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi
H, Suga T, Utsugi T, et al: Mutation of the mouse klotho
gene leads to a syndrome resembling ageing. Nature. 390:45–51.
1997. View Article : Google Scholar
|
|
13
|
Imura A, Iwano A, Tohyama O, Tsuji Y,
Nozaki K, Hashimoto N, et al: Secreted Klotho protein in sera and
CSF: implication for post-translational cleavage in release of
Klotho protein from cell membrane. FEBS Lett. 565:143–147. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen CD, Podvin S, Gillespie E, Leeman SE
and Abraham CR: Insulin stimulates the cleavage and release of the
extracellular domain of Klotho by ADAM10 and ADAM17. Proc Natl Acad
Sci USA. 104:19796–19801. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mian IS: Sequence, structural, functional,
and phylogenetic analyses of three glycosidase families. Blood
Cells Mol Dis. 24:83–100. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kurosu H, Ogawa Y, Miyoshi M, Yamamoto M,
Nandi A, Rosenblatt KP, et al: Regulation of fibroblast growth
factor-23 signaling by klotho. J Biol Chem. 281:6120–6123. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tao Y, Pinzi V, Bourhis J and Deutsch E:
Mechanisms of disease: signaling of the insulin-like growth factor
1 receptor pathway - therapeutic perspectives in cancer. Nat Clin
Pract Oncol. 4:591–602. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mattarocci S, Abbruzzese C, Mileo AM,
Visca P, Antoniani B, Alessandrini G, et al: Intracellular presence
of insulin and its phosphorylated receptor in non-small cell lung
cancer. J Cell Physiol. 221:766–770. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee J, Jeong DJ, Kim J, Lee S, Park JH,
Chang B, et al: The anti-aging gene KLOTHO is a novel target
for epigenetic silencing in human cervical carcinoma. Mol Cancer.
9:1092010.
|
|
20
|
Wolf I, Levanon-Cohen S, Bose S, Ligumsky
H, Sredni B, Kanety H, et al: Klotho: a tumor suppressor and a
modulator of the IGF-1 and FGF pathways in human breast cancer.
Oncogene. 27:7094–7105. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Polyak K and Weinberg RA: Transitions
between epithelial and mesenchymal states: acquisition of malignant
and stem cell traits. Nat Rev Cancer. 9:265–273. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huber AH and Weis WI: The structure of the
β-catenin/E-cadherin complex and the molecular basis of diverse
ligand recognition by β-catenin. Cell. 105:391–402. 2001.
|
|
23
|
Laux H, Tomer R, Mader MT, Smida J,
Budczies J, Kappler R, et al: Tumor-associated E-cadherin mutations
do not induce Wnt target gene expression, but affect E-cadherin
repressors. Lab Invest. 84:1372–1386. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang J and Weinberg RA:
Epithelial-mesenchymal transition: at the crossroads of development
and tumor metastasis. Dev Cell. 14:818–829. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vallin J, Thuret R, Giacomello E, Faraldo
MM, Thiery JP and Broders F: Cloning and characterization of three
Xenopus slug promoters reveal direct regulation by
Lef/β-catenin signaling. J Biol Chem. 276:30350–30358.
2001.PubMed/NCBI
|
|
26
|
Howe LR, Watanabe O, Leonard J and Brown
AM: Twist is up-regulated in response to Wnt1 and inhibits mouse
mammary cell differentiation. Cancer Res. 63:1906–1913.
2003.PubMed/NCBI
|
|
27
|
Marchenko GN, Marchenko ND, Leng J and
Strongin AY: Promoter characterization of the novel human matrix
metalloproteinase-26 gene: regulation by the T-cell factor-4
implies specific expression of the gene in cancer cells of
epithelial origin. Biochem J. 363:253–262. 2002. View Article : Google Scholar
|
|
28
|
Wu B, Crampton SP and Hughes CC: Wnt
signaling induces matrix metalloproteinase expression and regulates
T cell transmigration. Immunity. 26:227–239. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yee DS, Tang Y, Li X, Liu Z, Guo Y,
Ghaffar S, et al: The Wnt inhibitory factor 1 restoration in
prostate cancer cells was associated with reduced tumor growth,
decreased capacity of cell migration and invasion and a reversal of
epithelial to mesenchymal transition. Mol Cancer. 9:1622010.
View Article : Google Scholar
|
|
30
|
Wang H, Mannava S, Grachtchouk V, Zhuang
D, Soengas MS, Gudkov AV, et al: c-Myc depletion inhibits
proliferation of human tumor cells at various stages of the cell
cycle. Oncogene. 27:1905–1915. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fu M, Wang C, Li Z, Sakamaki T and Pestell
RG: Minireview: Cyclin D1: normal and abnormal functions.
Endocrinology. 145:5439–5447. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kainz C, Kohlberger P, Tempfer C, Sliutz
G, Gitsch G, Reinthaller A, et al: Prognostic value of CD44 splice
variants in human stage III cervical cancer. Eur J Cancer.
31A:1706–1709. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang L, Wang X, Jie P, Lu H, Zhang S, Lin
X, et al: Klotho is silenced through promoter hypermethylation in
gastric cancer. Am J Cancer Res. 1:111–119. 2011.PubMed/NCBI
|
|
34
|
King GD, Rosene DL and Abraham CR:
Promoter methylation and age-related downregulation of Klotho in
rhesus monkey. Age (Dordr). Sep 16–2011.(Epub ahead of print).
|
|
35
|
Pan J, Zhong J, Gan LH, Chen SJ, Jin HC,
Wang X, et al: Klotho, an anti-senescence related gene, is
frequently inactivated through promoter hypermethylation in
colorectal cancer. Tumour Biol. 32:729–735. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu H, Fergusson MM, Castilho RM, Liu J,
Cao L, Chen J, et al: Augmented Wnt signaling in a mammalian model
of accelerated aging. Science. 317:803–806. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kang Y and Massague J:
Epithelial-mesenchymal transitions: twist in development and
metastasis. Cell. 118:277–279. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Doi S, Zou Y, Togao O, Pastor JV, John GB,
Wang L, et al: Klotho inhibits transforming growth factor-beta1
(TGF-beta1) signaling and suppresses renal fibrosis and cancer
metastasis in mice. J Biol Chem. 286:8655–8665. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Branca M, Giorgi C, Ciotti M, Santini D,
Di Bonito L, Costa S, et al: Down-regulation of E-cadherin is
closely associated with progression of cervical intraepithelial
neoplasia (CIN), but not with high-risk human papillomavirus (HPV)
or disease outcome in cervical cancer. Eur J Gynaecol Oncol.
27:215–223. 2006.
|
|
40
|
Camilli TC, Xu M, O’Connell MP, Chien B,
Frank BP, Subaran S, et al: Loss of Klotho during melanoma
progression leads to increased filamin cleavage, increased Wnt5A
expression, and enhanced melanoma cell motility. Pigment Cell
Melanoma Res. 24:175–186. 2011. View Article : Google Scholar : PubMed/NCBI
|