Introduction
The antitumor effect of oxaliplatin (L-OHP) is
achieved through inhibition of DNA synthesis and duplication.
Clinically, oxaliplatin, in combination with other antitumor drugs,
has demonstrated significant activity against advanced colorectal
cancer. However, all cytotoxic drugs pose significant toxic
activity to the human body, leading to conditions such as
neurotoxicity, gastrointestinal reaction and cardiotoxicity
(1). Moreover, the non-selectivity
of cytotoxic drugs between normal tissue and the pathological site
poses a challenge for the treatment strategy of tumors.
Targeting of drugs specifically to the colon is
advantageous for the treatment, but a chemotherapy drug may not
reach its target site in effective concentrations (2,3). Thus,
effective treatment may demand an increased dose, which may lead to
side effects. To overcome these limitations, new delivery systems
with alternative drug release mechanisms have been suggested.
Liposomes were shown as good carriers of antineoplastic
chemotherapeutics by changing the distribution characteristics of
drugs and facilitating extended drug release (3–5). A few
reports have shown that liposomes were one of the first
nanomolecular drug delivery systems to show increased delivery of
small molecular weight anticancer drugs to solid tumors by altering
the bio-distribution of associated drugs (6,7). It
was repeatedly demonstrated that liposomes could improve the
therapeutic index of a variety of drugs. In a recent study,
DSPE-PEG modification of the surface of the liposomes may have
prevented interactions with the biological in vivo
environment. Thus, the circulation lifetime of the liposomes was
extended (8–10). This, in turn, resulted in extensive
extravasation of the liposomes due to the tumor selective enhanced
permeability and retention effect, ultimately leading to enhanced
accumulation of the liposomes in the tumor interstitium (11). According to these theories, the
liposome containing drugs will show markedly enhanced antitumor
activity and induce tumor cells apoptosis.
Recent studies have shown that the inhibitor of
apoptosis protein (IAP) and Bcl families of proteins are closely
associated with the anti-apoptotic effect. X-linked inhibitor of
apoptosis protein (XIAP) is a member of the IAP family and inhibits
apoptosis by directly inhibiting the activity of caspase-3,
caspase-7 and caspase-9 (12).
Bcl-2, Bcl-XL, Bax and Bad are members of the Bcl-2 family. An
increase in the expression of Bcl-2 and Bcl-XL genes and a decrease
in the expression of Bax and Bad genes lead to a reduction in the
permeability of the mitochondrial membrane, inhibition of both
mitochondrial depolarization and release of cytochrome c, resulting
in further inhibition of caspase-9 (13,14).
Caspase-9 can activate caspase-7 and caspase-3, the latter being
the final executor of apoptosis (15,16).
Various proteins are involved in cell division/cell cycle control.
Cyclins comprise a family of regulatory proteins that are
classified as either G1-, S- or M-phase, depending upon when their
levels reach maximum abundance and when they are thought to be
functional (17,18). Cyclin D1 is the main protein in the
G1-phase, whereas Cyclin A is classified as an S-phase and mitotic
cyclin. Interference with cell cycle-related proteins and induction
of apoptosis is an important treatment strategy for cancer
(19,20). Therefore, cell cycle regulation and
apoptosis play a key role in cancer therapy.
In this study, we prepared PEG-modified oxaliplatin
liposomes to treat human colorectal cancer SW480 cells, in order to
investigate the therapeutic activity of PEG-liposomal oxaliplatin
and its effect on the expression of cyclins.
Materials and methods
Reagents
Oxaliplatin was purchased from Sigma Co.
1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[maleimide(polyethyleneglycol)-2000]
(DSPE-PEG2000) was obtained from Avanti Polar Lipids, Inc.
DIOC18(3), 3,3′-dioctadecyloxacarbocyanine perchlorate (DiO) was
obtained from Vigorous Biotech Co., Ltd. Rabbit polyclonal
anti-β-actin, goat anti-rabbit IgG, and peroxidase conjugated
secondary antibodies were obtained from Bioscience Co., USA. Rabbit
polyclonal antibodies for Bcl-2 and Bax were purchased from Santa
Cruz Biotechnology, Inc. Rabbit polyclonal anti-XIAP,
anti-caspase-9, anti-caspase-7, anti-activated-caspase-3 (P17),
anti-Bcl-XL, anti-Bad, anti-Cyclin A and anti-Cyclin D antibodies
were obtained from Bioworld Technology, Inc. The TUNEL kit was
purchased from Promega. Life Science Academy of Chongqing Medical
University provided the human colorectal cancer SW480 cells.
Preparation of PEG-liposomes, cellular
uptake PEG-liposomes and MTT assay
PEG-liposomes were prepared, then cell uptake and
cell viability were measured using the MTT assay, as described
previously (21,22).
Colony formation assay for long-term cell
survival
Human colorectal cancer SW480 cells were trypsinized
and cultured in 6-well plates (103/well). After the
cells were grown for 14 days, cells were treated with free
oxaliplatin (28 μg/ml), PEG-liposomal oxaliplatin (containing 28
μg/ml oxaliplatin) or empty PEG-liposomes (2.6 μmol/ml
phospholipids) for 24 h, with no treatment as a control. Then cells
were washed twice in PBS, fixed with 70% ethanol and stained with
crystal violet (0.5% in ethanol). The plates were rinsed with
water, air-dried, photographed and evaluated for colony
estimation.
Terminal deoxynucleotidyl
transferase-mediated dUTP-fluorescein nick-end-labeling (TUNEL)
assay
SW480 cells (5×103/slide) were incubated
on glass slides for 24 h, treated with empty PEG-liposome, free
oxaliplatin and PEG-liposomal oxaliplatin for 12 h, respectively.
After removal of culture solution, cells were fixed with freshly
prepared 4% paraformaldehyde (dissolved in pH 7.4 PBS) for 30 min
at room temperature and washed with PBS. The slides were blocked
with 0.3% H2O2 MeOH solution for 30 min at
room temperature to block endogenous peroxidase activity. Then
cells were incubated with permeation mixture (0.1% Triton X-100
dissolved in 0.1% sodium citrate solution) for 2 min in an ice
bath. The cells were subsequently incubated with 50 μl TUNEL
reaction mixture for 1 h prior to addition of 50 μl transforming
agent-POD for 30 min. Stained cells were visualized by
3,3′-diaminobenzidine (DAB) coloration and analyzed under a light
microscope. Cells treated with deoxyribonuclease I served as
positive control for DNA fragmentation.
Flow cytometric analysis of cell
cycle
The empty PEG-liposome, free oxaliplatin and
PEG-liposomal oxali-platin-treated cells were collected and fixed
with 70% ice-cold ethanol overnight at 4°C. Cells were centrifuged,
resuspended in 400 μl 1X binding buffer (>1×106/ml)
and incubated with Annexin V-FITC (5 μl) in the dark for 15 min.
Cells were subsequently treated with PI (10 μl) and incubated in
the dark for 5 min prior to detection and analyzed using a BD FACS
Calibur (BD Biosciences).
Western blot analysis
After 12 h of treatment, cells were collected,
disrupted using cell lysis solution, and protein was extracted as
previously reported (23,24). The protein concentration was
determined with the Bradford assay. Fifty micrograms of protein
were separated by 12 or 15% SDS-PAGE, and the proteins were
transferred to a PVDF membrane. The membrane was incubated
overnight at 4°C using Bcl-2, Bax, Bcl-XL, Bad, XIAP, caspase-9,
caspase-7, activated-caspase-3 (P17), Cyclin A, Cyclin D and
β-actin antibodies. The membrane was subsequently incubated at room
temperature for 1 h with a HRP-labeled secondary antibody (goat
anti-rabbit IgG) and developed using an ECL chemiluminescent
reagent for visualization on a BIO-Rad imaging system. β-actin was
used as an internal standard to normalize protein expression. Band
intensities of target proteins are expressed as the percentage of
the β-actin band intensity, which was set at 100%.
Data analysis
All the data are presented as the mean ± SD.
Treatment group comparisons were performed using Student’s t-test
analysis, with P<0.05 being considered statistically
significant. The statistical data were analyzed using SPSS17.0
software.
Results
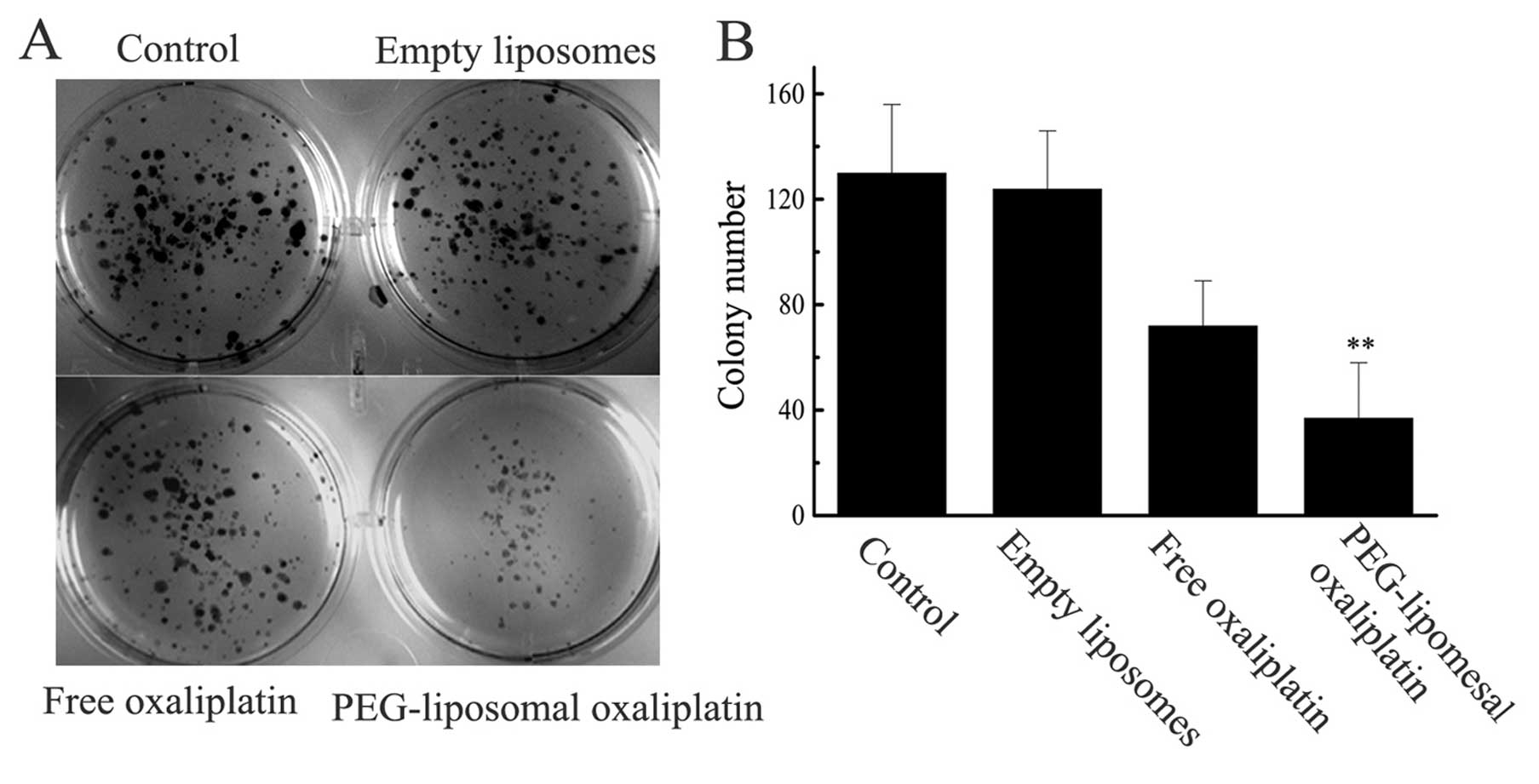
PEG-liposomal oxaliplatin induced growth
inhibition
The sensitizing effects of the treatment of SW480
cells with PEG-liposomal oxaliplatin were further confirmed using
the colony forming assay (Fig. 1).
The colony formation of cultures exposed to empty PEG-liposomes was
indistinguishable from the untreated controls, while the free
oxaliplatin (28 μg/ml) and PEG-liposomal oxaliplatin (containing 28
μg/ml oxaliplatin) treatments showed a decrease in colony
formation. However, after treatment of cells with PEG-liposomal
oxaliplatin there was a drastic reduction in the colony formation
owing to the severe synergistic effects on the survival of SW480
cells.
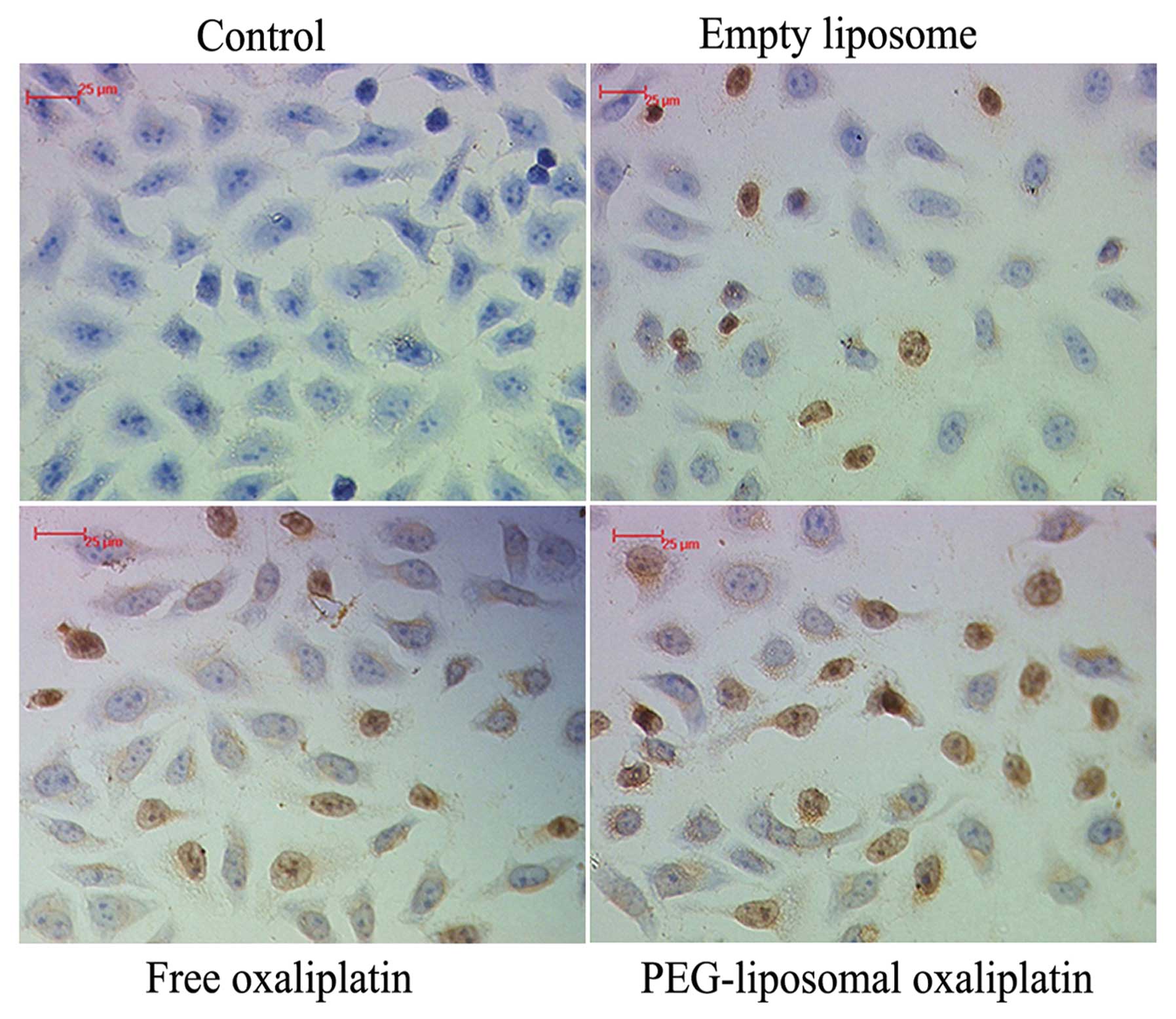
Analysis of apoptosis
During apoptosis, genomic DNA is disrupted, which
results in the presence of DNA fragments. Terminal deoxynucleotidyl
transferase-catalyzed polymerization of tagged deoxynucleotides at
the free 3′-terminal in a non-template-dependent manner allows for
labeling the gaps at the broken ends of the DNA. DNA fragments can
be detected using an HRP conjugated anti-Fab fragment. The karyons
assumed a yellowish-brown color after the DAB reaction. The results
of the TUNEL assay confirmed the flow cytometry data with regard to
the change in the levels of apoptosis (Fig. 2).
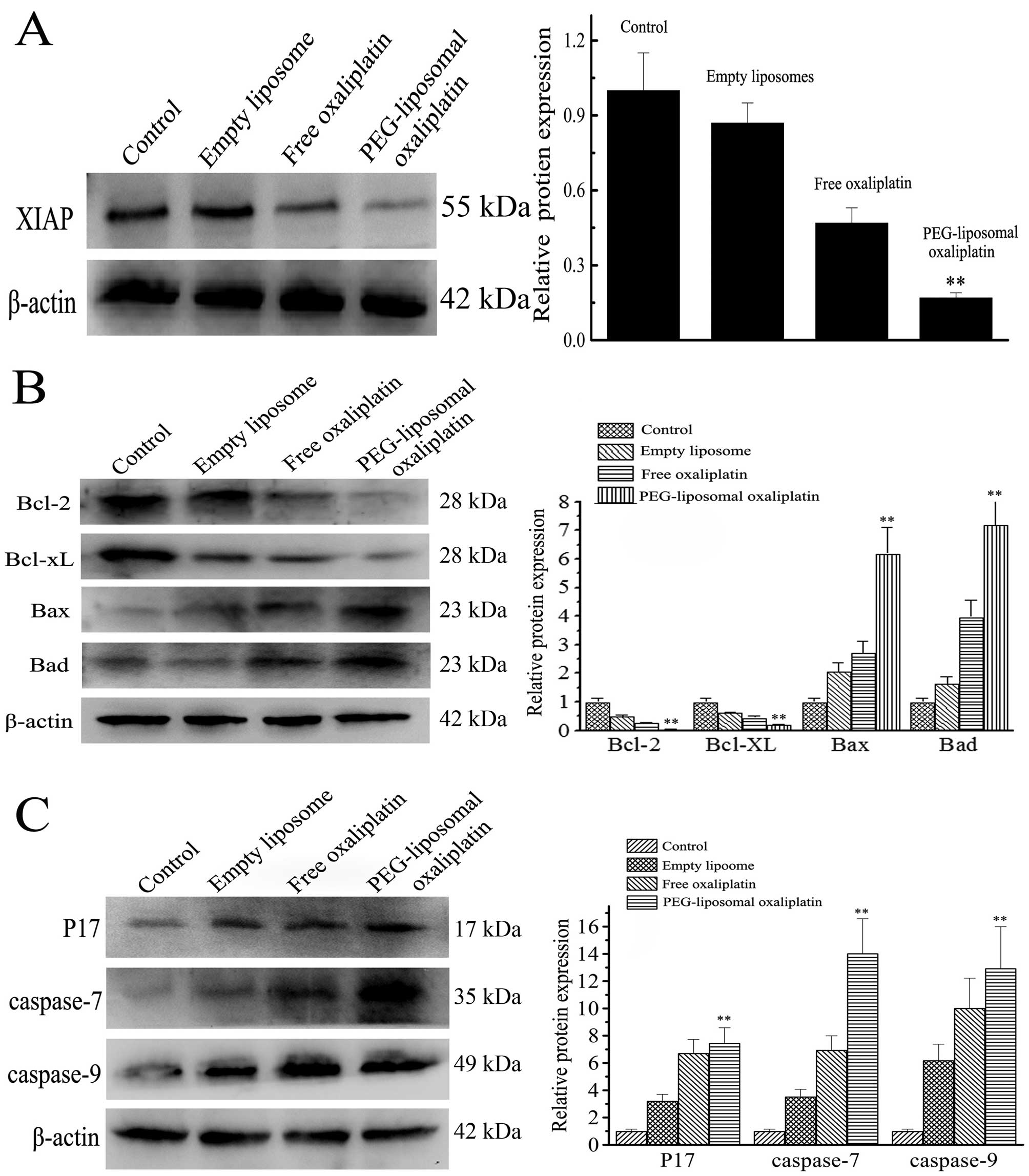
Expression levels of pro-apoptotic and
anti-apoptotic proteins
Apoptotic death was further confirmed by western
blot analysis. The IAP and Bcl families of protein are associated
with apoptosis. XIAP, a member of the IAP family, is the most
potent inhibitor of caspase activity. After treatment with
PEG-liposomal oxaliplatin, there was not only increased apoptosis,
but also the expression of XIAP was markedly decreased (Fig. 3A). Expression of relative protein
was lower (0.16±0.06) as compared with the other treatments
(P<0.01). However, expression of caspase-9, caspase-7 and
activated-caspase-3 were increased after treatment with
PEG-liposomal oxaliplatin (Fig.
3B). Expression of caspase-9, caspase-7 and activated-caspase-3
was 13.61±4.19, 7.63±2.75 and 14.05±3.16, respectively. We also
detected protein expression levels of Bcl family members. After
treatment of cells with PEG-liposomal oxaliplatin, expression of
anti-apoptotic Bcl-2 and Bcl-XL decreased to 0.32±0.07 and
0.31±0.05, respectively, whereas pro-apoptotic Bax and Bad proteins
increased to 6.10±1.02 and 7.30±0.94, respectively (Fig. 3C). These results indicate that
apoptosis was strongly induced by PEG-liposomal oxaliplatin.
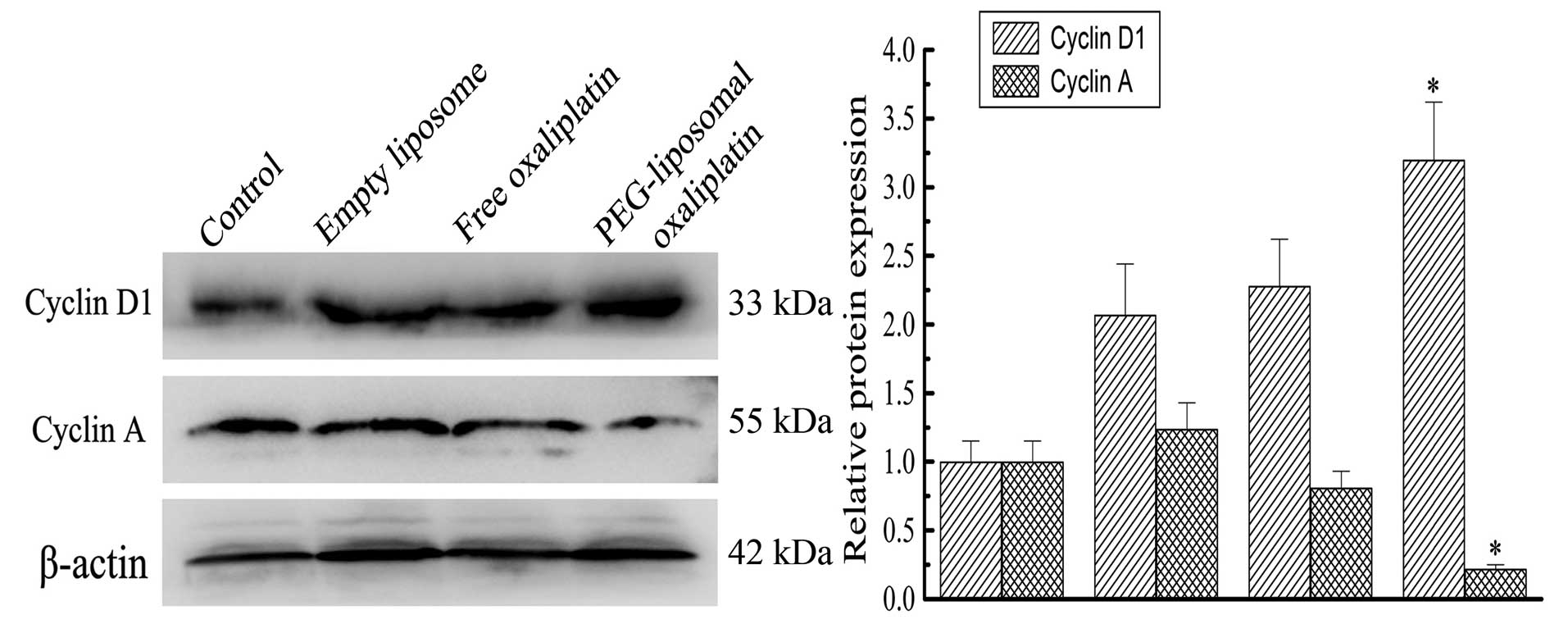
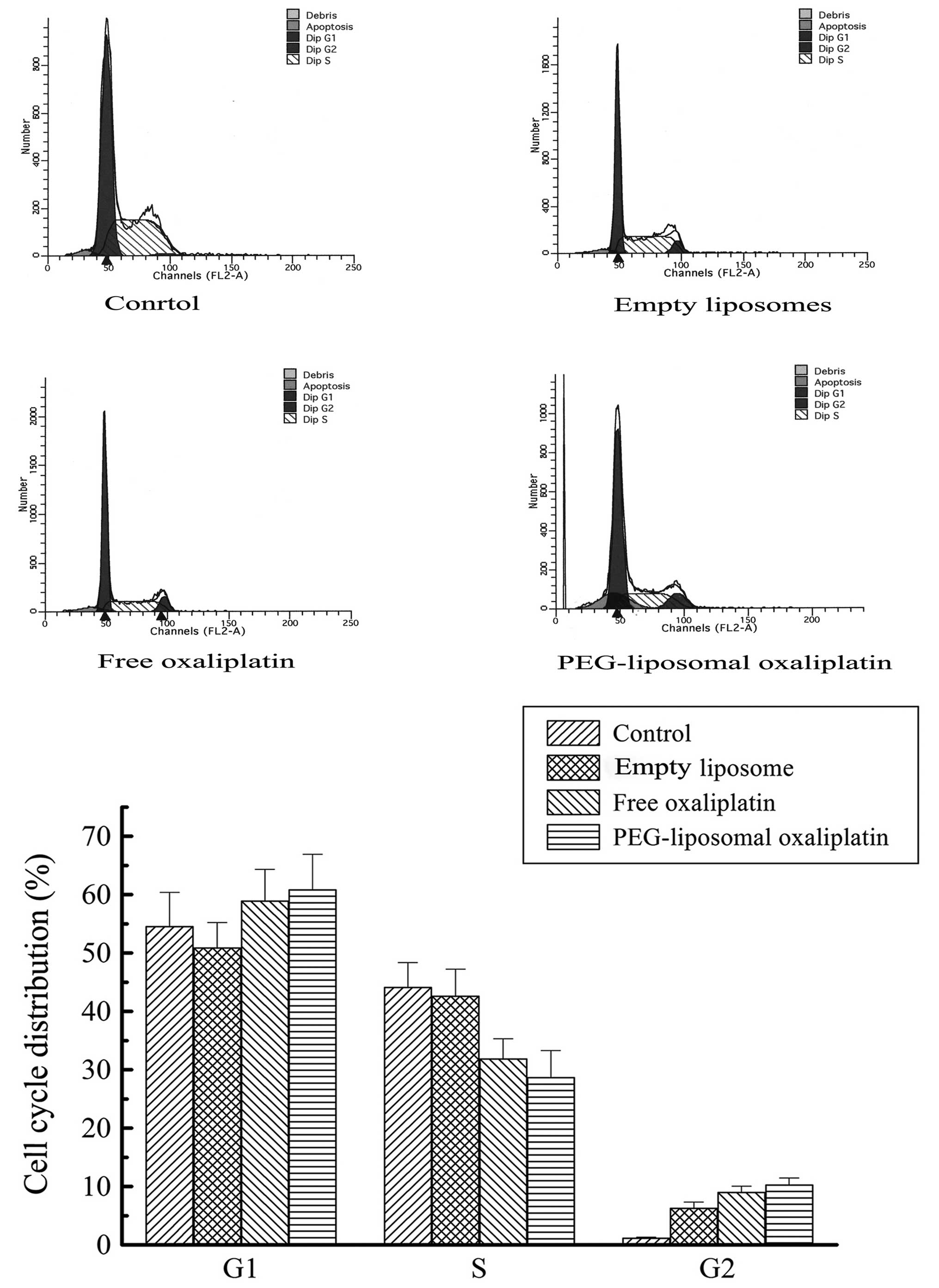
Expression levels of Cyclin A, Cyclin D
and cell cycle arrest
PEG-liposomal oxaliplatin treatment dramatically
reduced the levels of Cyclin A (0.22±0.03), compared with other
treatments, (P<0.01). We also found that the Cyclin D1 level was
elevated in these cells (3.2±0.42), compared with other treatments
(P<0.01) (Fig. 4). This analysis
indicates that altered expression of Cyclin A and Cyclin D1 was
associated with changes in the cell cycle. Progression of the cell
cycle was examined using flow cytometry. As expected, there were a
high proportion of G1-phase cells after treatment with
PEG-liposomal oxaliplatin. We found that the cell cycle was
distinctly arrested in the G1-phase, with the percentage of cells
greater in those treated with PEG-liposomal oxaliplatin
(60.91±3.24%), than in control cells (54.59±1.04%) (P<0.05). In
contrast, the number of cells in S-phase was less after treatment
with PEG-liposomal oxaliplatin (28.73±0.29%), as compared to
control cells (P<0.01) (Fig.
5).
Discussion
We carried out the present studies to provide
further evidence to support the use of PEG-liposome containing
drugs in therapy trials and in cancer prevention. Cytotoxic drugs
have no target selectivity between normal tissues and pathological
sites (25). The dose escalation
necessary to overcome even a small increase in cellular resistance
can cause severe cytotoxicity to dose-limiting normal tissue. The
ideal therapy would deliver the drugs directly to the pathological
sites. Thus, strategies containing agents that act through distinct
molecular mechanisms, rather than using single agents, represent
the most useful alternatives for achieving higher curability with
the least toxicity during cancer chemotherapy.
Recently, there has been an increasing interest in
evaluating synergistic cancer cell cytotoxicity from
chemotherapeutic agents with highly promising results. PEG-modified
liposomes have been used as carriers of anticancer drugs to enhance
the affinity and uptake of anticancer drugs in cancer cells
(26). It has been found that
PEG-liposomes are not readily taken up by the macrophages in the
reticuloendothelial system (RES) and hence stays in the circulation
for a relatively long period of time. Therapeutic studies and
pharmacokinetic analysis with tumor bearing mice revealed that
PEG-liposomes have considerable potential as drug carriers for
cancer therapy (27–29).
Our in vitro study revealed the uptake of
liposomes by cancer cells, and resulted in accumulation of
liposomes within the cells, similar to that reported by Gabizon and
Goren et al(27,30). As reported previously, we observed
PEG-liposome intracellular distribution (21). This suggests that cells may undergo
endocytosis of more than a single liposome, which may be related to
the electric potential of the liposomes and cells. This study
revealed a time-dependent decrease in SW480 cell viability after
treatment with PEG-liposomal oxaliplatin. Previous literature was
less expansive in explaining the above phenomenon.
Using a flow cytometer and TUNEL technology, it was
shown that apoptosis significantly increased after treatment with
PEG-liposomal oxaliplatin. We also analyzed the expression levels
of pro-apoptotic and anti-apoptotic proteins in the PEG-liposomal
oxaliplatin-treated cells. The anti-apoptotic proteins XIAP, Bcl-2
and Bcl-XL decreased in expression. In contrast, the expression of
the pro-apoptotic proteins caspase-9, caspase-7,
activated-caspase-3, Bax and Bad increased (Fig. 3). The oxaliplatin alone or empty
PEG-lioposome treatment alone did not appreciably alter the
cellular levels of these cell survival regulators. However, these
changes were significant in the PEG-liposomal oxaliplatin group.
During apoptosis, activated caspase-9 further activates caspase-7
and caspase-3 and augments the apoptotic pathway (31,32).
Increased apoptosis induced by oxaliplatin is another feature of
cellular response to PEG-liposomal oxaliplatin treatment, which
manifests as the synergetic rowth inhibitory effect on SW480 cells.
Taken together, these results indicate that PEG-liposomal
oxaliplatin-induces apoptosis.
Further investigation elucidated the potential
mechanism of PEG-liposomal oxaliplatin-induced apoptosis. The
antiproliferative effects of PEG-liposomal oxaliplatin have been
attributed to changes in the cell cycle and expression levels of
cyclins. However, determining the mechanisms regulating the
passaging of these cells is important to the understanding of
diverse cellular processes including cellular proliferation, DNA
repair-mediated cell cycle arrest, terminal differentiation and
apoptosis (33,34).
In this study, we observed an increase in the
G1-phase cell population in PEG-liposomal oxaliplatin treated cells
(P<0.05), whereas the S-phase population was reduced
(P<0.01). We hypothesize that DNA synthesis was blocked, and
thus, cells proliferation was inhibited. Further analyses indicated
that the effects on SW480 cell proliferation were dependent on the
expression of cyclins. The precise regulation of G1 phase events is
mediated by the G1 phase cyclins. Cyclin A is a necessary
regulatory protein for a cell to move from G1-phase to S-phase, and
is a rate-limiting factor (35,36).
Cyclin D1 governs a G1/S checkpoint by blocking unregulated S-phase
entry in the presence of DNA damaging agents (37,38).
Thus, Cyclin D1 accelerates transit through G1 but inhibits
transition into S-phase (39,40).
Our results show that expression of Cyclin A was decreased and
Cyclin D1 was increased when cells were treated with PEG-liposomal
oxaliplatin. PEG-liposomal oxaliplatin decreased Cyclin A
expression, blocked DNA synthesis, and resulted in a significantly
shortened S-phase. Similar to Cyclin A, the expression of the
anti-apoptotic proteins XIAP, Bcl-2 and Bcl-xl decreased. Similar
to Cyclin D1, the expression of the pro-apoptotic proteins
caspase-9, caspase-7, activated-caspase-3, Bax and Bad increased.
These results were coincident with cell cycle arrest in the
G1-phase. In addition, we found that the number of cells in the
G1-phase decreased after treatment with empty PEG-liposomes, but
this was not significant when compared with control.
In summary, these data from human colorectal cancer
SW480 cells show that liposome entrapment of oxaliplatin enhanced
the anticancer potency of the chemotherapeutic agent. Moreover,
PEG-liposomal oxaliplatin effect on apoptosis may be through the
regulation of expression of Cyclin A or Cyclin D1, as well as
pro-apoptotic and anti-apoptotic proteins.
Acknowledgements
This study was supported by a grant (No. 09-2-12)
from the Health Administration of Chongqing. We thank Xin-Hui Jiang
for expert technical assistance of HLPC, we are grateful to the
Central Laboratories, the Ophthalmological Laboratory of Chongqing
Medical University First Affiliated Hospital and the College of
Life Science of Chongqing Medical University for their technical
supports.
References
|
1
|
Pasetto LM, D’Andrea MR, Rossi E and
Monfardini S: Oxaliplatin-related neurotoxicity: how and why? Crit
Rev Oncol Hematol. 59:159–168. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lammers T, Hennink WE and Storm G:
Tumour-targeted nanomedicines: principles and practice. Br J
Cancer. 99:392–397. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Michor FJ, Iwasa Y, Lengauer C and Nowak
MA: Dynamics of colorectal cancer. Semin Cancer Biol. 15:484–493.
2005. View Article : Google Scholar
|
|
4
|
Hong M, Zhu S, Jiang Y, Tang G and Pei Y:
Efficient tumor targeting of hydroxycamptothecin loaded PEGylated
niosomes modified with transferrin. J Control Release. 133:96–102.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hussain S, Plückthun A, Allen TM and
Zangemeister-Wittke U: Antitumor activity of an epithelial cell
adhesion molecule targeted nanovesicular drug delivery system. Mol
Cancer Ther. 6:3019–3027. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pietrangeli A, Leandri M, Terzoli E,
Jandolo B and Garufi C: Persistence of high-dose
oxaliplatin-induced neuropathy at long-term follow-up. Eur Neurol.
56:13–16. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stern ST, Hall JB, Yu LL, Wood LJ,
Paciotti GF, Tamarkin L, Long SE and McNeil SE: Translational
considerations for cancer nanomedicine. J Control Release.
146:164–174. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sun W, Zou W, Huang G, Li A and Zhang N:
Pharmacokinetics and targeting property of TFu-loaded liposomes
with different sizes after intravenous and oral administration. J
Drug Target. 16:357–365. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Allen TM, Hansen C, Martin F, Redemann C
and Yau-Young A: Liposomes containing synthetic lipid derivatives
of poly(ethylene glycol) show prolonged circulation half-lives in
vivo. Biochim Biophys Acta. 1066:29–36. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Klibanov AL, Maruyama K, Beckerleg AM,
Torchilin VP and Huang L: Activity of amphipathic poly(ethylene
glycol) 5000 to prolong the circulation time of liposomes depends
on the liposome size and is unfavorable for immunoliposome binding
to target. Biochim Biophys Acta. 1062:142–148. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tsumura Y and Maeda H: A new concept for
macromolecular therapeutics in cancer chemotherapy: mechanism of
tumoritropic accumulation of proteins and the antitumor agent
smancs. Cancer Res. 46:6387–6392. 1986.PubMed/NCBI
|
|
12
|
Hao Z and Mak TW: Type I and type II
pathways of fas-mediated apoptosis are differentially controlled by
XIAP. J Mol Cell Biol. 2:63–64. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Luo X, Budihardjo I, Zou H, Slaughter C
and Wang X: Bid, a Bcl2 interacting protein, mediates cytochrome c
release from mitochondria in response to activation of cell surface
death receptors. Cell. 94:481–490. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Adams JM and Cory S: The Bcl-2 protein
family: arbiters of cell survival. Science. 281:1322–1326. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jaattela M and Tschopp J:
Caspase-independent cell death in T lymphocytes. Nat Immunol.
4:416–423. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Walters J, Pop C, Scott FL, Drag M, Swartz
P, Mattos C, Salvesen GS and Clark AC: A constitutively active and
uninhibitable caspase-3 zymogen efficiently induces apoptosis.
Biochem J. 424:335–345. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bortner DM and Rosenberg MP:
Overexpression of cyclin A in the mammary glands of transgenic mice
results in the induction of nuclear abnormalities and increased
apoptosis. Cell Growth Differ. 6:1579–1589. 1995.PubMed/NCBI
|
|
18
|
Owa T, Yoshino H, Yoshimatsu K and Nagasu
T: Cell cycle regulation in the Gl phase: a promising target for
the development of new chemotherapeutic anticancer agents. Curr Med
Chem. 8:1487–1503. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Buolamwini JK: Cell cycle molecular
targets in hovel anticancer drug discovery. Curr Pharm Des.
6:379–392. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
McDonald ER and El-Deiry WS: Cell cycle
control as a basis for cancer drug development. Int J Oncol.
16:871–886. 2000.PubMed/NCBI
|
|
21
|
Yang C, Liu HZ, Fu ZX and Lu WD:
Oxaliplatin long-circulating liposomes improved therapeutic index
of colorectal carcinoma. BMC Biotechnol. 11:1–8. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang C, Liu HZ, Lu WD and Fu ZX:
PEG-liposomal oxaliplatin potentialization of antitumor efficiency
in a nude mouse tumor-xenograft model of colorectal carcinoma.
Oncol Rep. 25:1621–1628. 2011.PubMed/NCBI
|
|
23
|
Jost PJ, Grabow S, Gray D, McKenzie MD,
Nachbur U, Huang DC, Bouillet P, Thomas HE, Borner C, Silke J, et
al: XIAP discriminates between type I and type II FAS-induced
apoptosis. Nature. 460:1035–1039. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Harikumar KB, Kunnumakkara AB, Ahn KS,
Anand P, Krishnan S, Guha S and Aggarwal BB: Modification of the
cysteine residues in IκBα kinase and NF-κB(p65) by xanthohumol
leads to suppression of NF-κB-regulated gene products and
potentiation of apoptosis in leukemia cells. Blood. 113:2003–2013.
2009.
|
|
25
|
Nobili S, Checcacci D, Filippelli F, Del
Buono S, Mazzocchi V, Mazzei T and Mini E: Bimonthly chemotherapy
with oxaliplatin, irinotecan, infusional 5-fluorouracil/folinic
acid in patients with metastatic colorectal cancer pretreated with
irinotecan- or oxaliplatin-based chemotherapy. J Chemother.
20:622–631. 2008. View Article : Google Scholar
|
|
26
|
Abizon D and Papahadjopoulos D: Liposome
formulations with prolonged circulation time in blood and enhanced
uptake by tumors. Proc Natl Acad Sci USA. 85:6949–6953. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gabizon AA: Stealth liposomes and tumor
targeting: a step further in the quest for the magic bullet. Clin
Cancer Res. 7:223–225. 2001.PubMed/NCBI
|
|
28
|
Zalipsky S, Brandeis E, Newman MS and
Woodle MC: Long circulating, cationic liposomes containing
amino-PEG-phosphatidylethanolamine. FEBS Lett. 53:71–74. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Panwar P, Pandey B, Lakhera PC and Singh
KP: Preparation, characterization, and in vitro release study of
albendazole-encapsulated nanosize liposomes. Int J Nanomed.
5:101–108. 2010.PubMed/NCBI
|
|
30
|
Goren D, Horowitz AT, Tzemach D, Tarshish
M, Zalipsky S and Gabizon A: Nuclear delivery of doxorubicin via
folate-targeted liposomes with bypass of multidrug-resistance
efflux pump. Clin Cancer Res. 6:1949–1957. 2000.PubMed/NCBI
|
|
31
|
Yuan Z, Wang F, Zhao Z, Zhao X, Qiu J, Nie
C and Wei Y: BIM-mediated AKT phosphorylation is a key modulator of
arsenic trioxide-induced apoptosis in cisplatin-sensitive and
-resistant ovarian cancer cells. PLoS One. 6:e205862011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pramanik KC, Boreddy SR and Srivastava SK:
Role of mitochondrial electron transport chain complexes in
capsaicin mediated oxidative stress leading to apoptosis in
pancreatic cancer cells. PLoS One. 6:e201512011. View Article : Google Scholar
|
|
33
|
Hunter T and Pines J: Cyclins and cancer
II: Cyclin D and CDK inhibitors come of age. Cell. 79:573–582.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Santamaria D and Ortega S: Cyclins and
CDKS in development and cancer: lessons from genetically modified
mice. Front Biosci. 11:1164–1188. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Viallard JF, Lacombe F, Belloc F,
Pellegrin JL and Reiffers J: Molecular mechanisms controlling the
cell cycle: fundamental aspects and implications for oncology.
Cancer Radiother. 5:109–129. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hsia DA, Tepper CG, Pochampalli MR, Hsia
EY, Izumiya C, Huerta SB, Wright ME, Chen HW, Kung HJ and Izumiya
Y: KDM8, a H3K36me2 histone demethylase that acts in the Cyclin A1
coding region to regulate cancer cell proliferation. Proc Natl Acad
Sci USA. 107:9671–9676. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sun PC, Tzao C, Chen BH, Liu CW, Yu CP and
Jin JS: Suberoylanilide hydroxamic acid induces apoptosis and
sub-G1 arrest of 320 HSR colon cancer cells. J Biomed Sci.
17:762010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tung JN, Chiang CC, Tsai YY, Chou YY, Yeh
KT, Lee H and Cheng YW: CyclinD1 protein expressed in pterygia is
associated with β-catenin protein localization. Mol Vis.
16:2733–2738. 2010.PubMed/NCBI
|
|
39
|
Prall OW, Sarcevic B, Musgrove EA, Watts
CK and Sutherland RL: Estrogen-induced activation of Cdk4 and Cdk2
during G1-S phase progression is accompanied by increased Cyclin D1
expression and decreased cyclin-dependent kinase inhibitor
association with cyclin E-Cdk2. J Biol Chem. 272:10882–10894. 1997.
View Article : Google Scholar
|
|
40
|
Zhu X, Ohtsubo M, Böhmer RM, Roberts JM
and Assoian RK: Adhesion-dependent cell cycle progression linked to
the expression of Cyclin D1, activation of cyclin E-cdk2, and
phosphorylation of the retinoblastoma protein. J Cell Biol.
33:391–403. 1996. View Article : Google Scholar : PubMed/NCBI
|