Introduction
Antimicrobial peptides of cathelicidin family are
found in many mammalian species, such as human being, bovine and
swine (1). As the role of
endogenous hCAP18/LL-37 (human cationic antimicrobial peptide of 18
kDa), the innate immunity of this peptide to prevent the infection
was previously reported (2). The
antimicrobial peptide, hCAP18/LL-37 is expressed in various types
of organs, such as leucocytes (3),
myelocytes (4), testis (5), skin (6), nasal mucosa (7), saliva (8), and the gastrointestinal tract
(9). The protein expression and
following secretion of the hCAP18/LL-37 result in the involvement
of this peptide in the body fluids, such as serum, urine and sweat.
The existence of hCAP18/LL-37 in these body fluids is expected to
have a biological significance to prevent the bacterial
proliferation, and following infection at the local tissues.
In addition to the anti-infectious effect, the
anti-proliferative effect of analogue peptide of hCAP18/LL-37 was
reported to induce apoptosis against the human squamous cell
carcinoma cell line, SAS-H1 (10).
Although the antimicrobial peptide is expected to induce the pore
formation on the cellular membrane (11,12),
the detailed mechanism is poorly understood as to how hCAP18/LL-37
induces the anti-proliferative effect to the malignant cancer
cells. In a previous study, the analogue peptide of hCAP18/LL-37,
which is removed 5 residues from each ends
(hCAP18109–135) and has two amino acid substitutions
from the original hCAP18/LL-37, was reported to have stronger
anti-proliferative effect, compared with hCAP18109–135
(10). The anti-proliferative
effect of hCAP18 and its analogue peptide against the squamous cell
carcinoma cell line (SAS-H1) led us to form the hypothesis that
these peptides might be effective in other types of cancer, such as
colon cancer.
Colon cancer is classified as one of the major
cancers in recent years. The 5-year relative survival rate is
around 90%, when detected at early stage of carcinogenesis (stage
I). In the late stage, such as metastatic stage, the average of
median survival duration is 5–6 months, indicating the high
lethality of colon cancer (13).
The effectiveness of chemotherapeutic regents is very different
among the multiple types of cancers (14,15).
This situation guided us to evaluate whether hCAP18/LL-37 and its
analogue peptides are effective in preventing cell proliferation,
in the colon cancer derived cell line HCT116. The specific reason
for selecting HCT116 as the material is the existence of its
genetic mutant of p53 tumor suppressor gene.
The p53 gene product is known to have a central role
for apoptosis, cell cycle arrest, DNA repair, tumor suppression and
sensitivity to chemotherapy. Due to the huge impact of the p53
protein for the protection against carcinogenesis, p53 is called
the ‘guardian of genome’ (16).
Bunz et al established the p53 null mutant with the
homologous recombination technique from the wild-type HCT116
(17). Although wild-type of HCT116
still keeps the role of the p53, the null mutant lost the function
of the p53 (17). Therefore, we can
address the association of the p53 gene to the specific phenomenon,
when we compared the response of the p53 wild and null mutant
(p53−/−) cells.
In this study, we exposed the hCAP18/LL-37 and its
analogue peptides to HCT116 cells, and evaluated the efficacy to
prevent proliferation. Moreover, we compared the effects of
peptides among the p53 wild and p53−/− HCT116 cells. Our
study would explore the effectiveness of the anti-microbial
peptide, hCAP18/LL-37, in colon cancer, and addresses the role of
p53 gene on its anti-proliferative effect.
Materials and methods
Cell culture and reagents
The human colon cancer derived cell line, HCT116,
and the isogenic HCT116 p53−/− cells were kindly
provided by Dr Bert Vogelstein (The Johns Hopkins University,
Baltimore, MD, USA) (17). Cells
were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Nakarai
Tesque, Inc., Kyoto, Japan) with 10% PBS (Invitrogen, Carlsbad, CA,
USA), 1% antibiotic-antimycotic mixed stock solution (Nakarai
Tesque, Inc., Kyoto, Japan) at 37°C in 5% CO2. All cells
were kept under the exponential growth condition, and used as the
experimental materials.
Peptides
Primary structure of the original hCAP18/LL-37 is
represented as LLGDFFRKSKEKIGKEFKRIVQRIKDFL- RNLVPRTES (18). The hCAP18109–135 is the
peptide of 27mer, which resulted from the removal of the first and
last five amino acids from hCAP18/LL-37. To enhance the
anti-microbial activity, FF/CAP18 was designed by replacement of
glutamic acid and lysine residue with phenylalanine
(FRKSKEKIGKFFKRIVQRIFDFLRNLV) of hCAP18109–135.
Cell growth assay
HCT116 and HCT116 p53−/− cells were
seeded at 5.0×105 cells per 48-well plate (Falcon,
Franklin Lakes, NJ, USA) with complete culture medium, and
incubated at 37°C in 5% CO2. Twenty-four hours later,
medium was changed with or without FF/CAP18 peptide (total
concentration was 10 μg/ml or 40 μg/ml) in the 250 μl complete
culture medium. The cells were stained with trypan blue
(Invitrogen), and the cell concentration was counted by Countess
Automatic Cell Counter (Invitogen) at 24, 48, 72 and 96 h after the
exposure of the peptides.
MitoCapture™ assay
Disruption of mitochondrial transmembrane potential
is one of the earliest intracellular events that occur following
induction of apoptosis (19). This
transmembrane change can be visualized using MitoCapture
(Mitochondrial Apoptosis Detection kit, MBL, Nagoya, Japan), under
the unfixed cell condition. Mitocapture™ assay was performed
according to manufacturer’s instructions. The signal of the
MitoCapture was detected by a fluorescence microscope (FSX100,
Olympus, Tokyo, Japan).
Immunohistochemistry for single strand
(ss) DNA
The genomic DNA causes fragmentation at the time of
apoptosis due to the cleavage of the DNase I, which is activated by
caspases. To detect the DNA fragmentation and following apoptosis,
we conducted immunohistochemical staining of single strand DNA
(ss-DNA) for peptide treated cells. The immunohistochemical study
was performed on cells prepared by 4% paraformaldehyde solution in
PBS after incubation in a 48-well plate with or without FF/CAP18.
The fixed cells were washed with PBS, incubated with 0.5% Triton
X-PBS solution for 30 min on ice for permeabilization. As the
positive control for the DNA fragmentation, the cells were treated
with the recombinant DNaseI (Takara Bio Inc., Shiga, Japan) at 37°C
in 5% CO2 for 1 h. As the negative control, cells
treated with the same volume of PBS was used. After PBS wash, the
cells were treated with 2% normal goat serum in PBS as blocking
buffer for 1 h at room temperature. Anti ss-DNA Rabbit IgG Affinity
Purify (Immuno-Biological Laboratories Co., Ltd., Gunma, Japan) was
used as first antibody. The samples were exposed to 0.6 μg/ml of
antibody at 4°C overnight. Cells were washed with PBS for 5 min 3
times, treated with Alexa Fluor 488 (Invitrogen) as second antibody
for 2 h at room temperature under protection from light. The nuclei
were visualized by staining with 1 μg/ml of the
4′,6-diamidino-2-phenylindole (DAPI) (Dojindo Laboratories,
Kumamoto, Japan). The staining feature was detected by a
fluorescence microscope system (FSX100, Olympus).
Statistical test
In this study, all of the experiments were carried
out with at least triplicated samples. Means and standard errors
were calculated. The statistical significance was evaluated with
the Student’s t-test. A P-value <0.05 was considered
statistically significant.
Results
Effect of LL-37 and FF/CAP18 on HCT116
growth
To investigate the effect of cathelicidin family
anti-microbial peptides, we exposed LL-37 and the analogue
FF/CAP18, to the colon cancer HCT116 cells. The effect to the cell
growth was evaluated at 24, 48, 72 and 92 h at 40 μg/ml
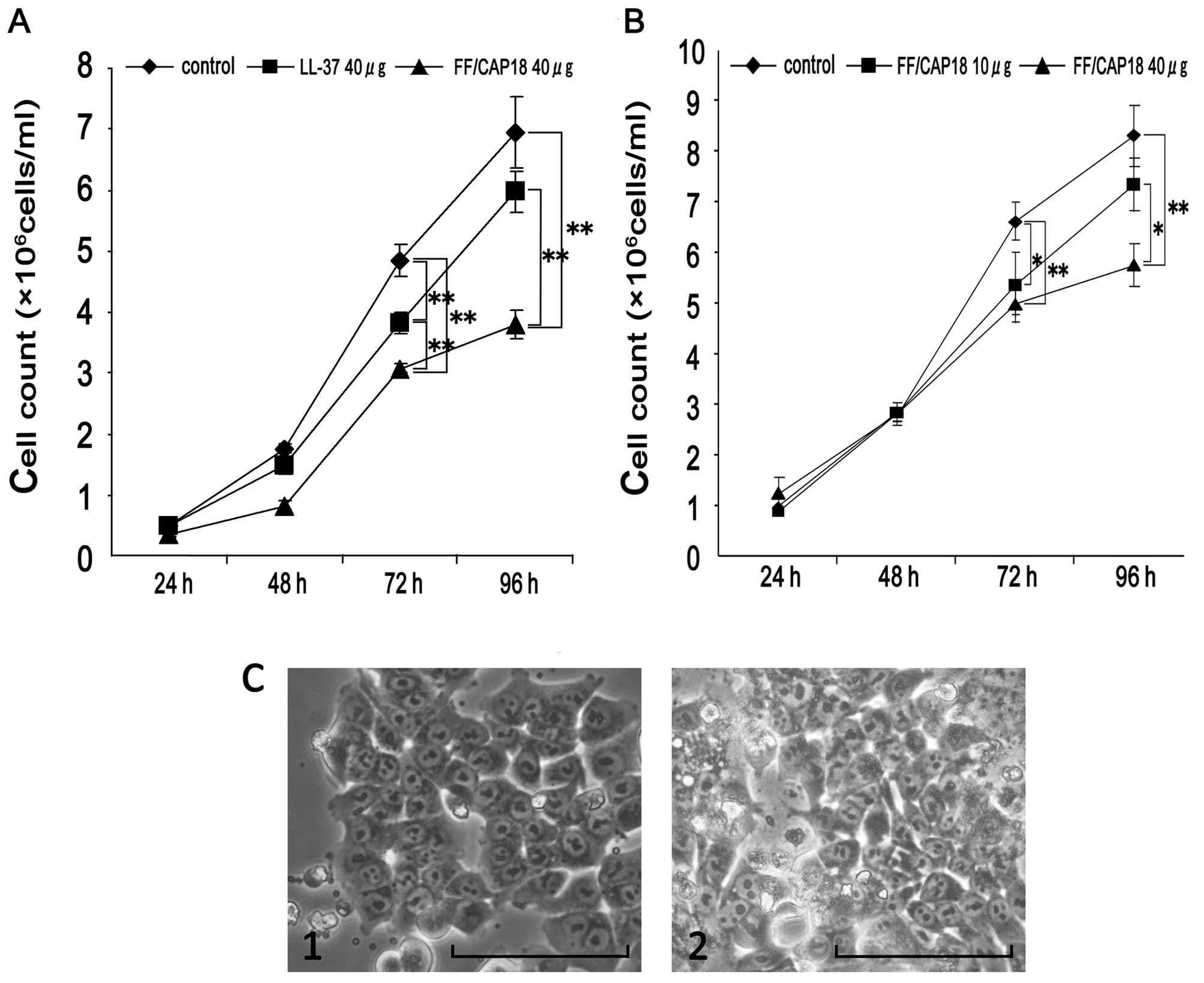
concentration of the peptide. As shown in Fig. 1A, a relatively slower cell growth
(FF/CAP18 treated group) was observed at both 72 and 96 h. However,
the effect of the LL-37 was relatively less when it is compared
with that of FF/CAP18 (Fig. 1A). We
considered that cathelicidin peptides have a potential to suppress
the cell growth of the colon cancer cells.
As the next experiments, we detected the detailed
anti-proliferative effect of FF/CAP18 to the HCT116 cell line, at
the multiple dose and time points. We could not see any
anti-proliferative effect of FF/CAP18 until 48 h (Fig. 1B). At 72 h cell number treated with
10 and 40 μg/ml FF/CAP18 was significantly low (P<0.05), when
compared with that of non-treated cells (control). At 96 h, the
cell number treated with 40 μg/ml of FF/CAP18 was significantly low
(P<0.05). From these results, we concluded that FF/CAP18 has
growth inhibition ability to the HCT116 from 10 μg/ml. Furthermore,
the effect of the FF/CAP18 to HCT116 was dose-dependent to the
peptide concentration. To estimate the cell condition, the cell
morphology of the peptide treated cells was compared with that of
the control cells (Fig. 1C).
Although the growth suppression was obvious in the peptide treated
cells, we did not see remarkable change on the morphology of the
cells (Fig. 1C).
Loss of mitochondrial membrane
potential
Loss of mitochondrial membrane potential is
associated with early stage of apoptosis. In present study,
MitoCapture™ is used for detection of this stage. MitoCapture™ is a
cationic dye that enables the accumulation of red fluorescence
aggregation in the corresponding area of the mitochondria, due to
its transmembrane potential. In contrast, in the early stage of
apoptosis, the disruption of the mitochondrial trans-membrane
potential is one of the intracellular events, therefore, the
aggregation of the red fluorescence does not occurs in the cells,
which stay in the early stage of apoptosis. Furthermore,
mitochondrial depolarization causes a regression in red
fluorescence and an increase in green fluorescence due to the
presence of the monomeric form of the dye in the cytoplasm.
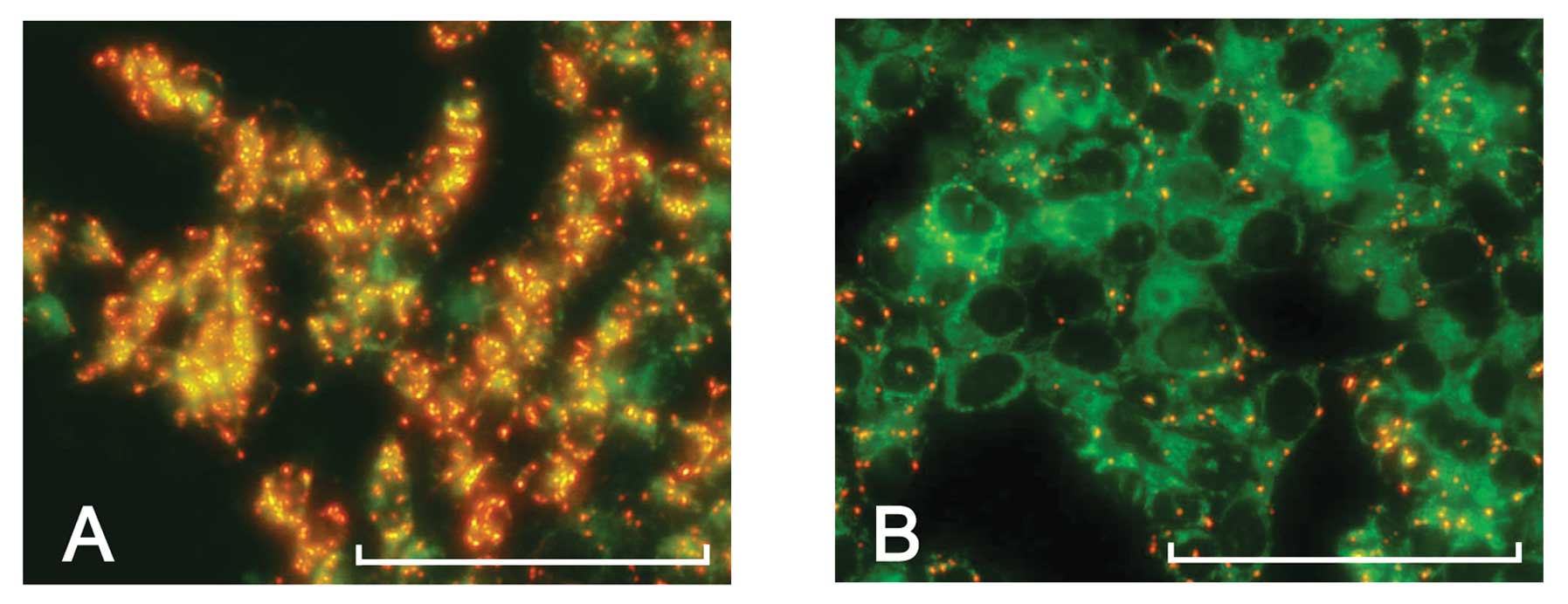
To address the cell status of the HCT116 cells
treated with the FF/CAP18, the MitoCapture™ was applied to the
analysis. As shown in Fig. 2, the
control HCT116 cells showed the intense red signals, which indicate
that the mitochondria of control HCT116 cell is intact (Fig. 2A). In contrast, the cells treated
with FF/CAP18 showed decreased signal intensity when compared with
the control (Fig. 2B). Furthermore,
although it signal was faint, certain cell population at high cell
density showed green fluorescence, compared with the control (data
not shown). These results showed that FF/CAP18 causes loss of
mitochondrial membrane potential.
DNA fragmentation by FF/CAP18
As the next step from the mitochondrial disruption,
the activation of DNase I and fragmentation of the genomic DNA
occurs in the process of apoptosis. To detect the DNA
fragmentation, we carried out the detection with the anti ss-DNA
antibody. In a previous study, the detection of ss-antibody was
more sensitive in detecting apoptotic cells, and with low
background, and high specificity was indicated, when compared with
the TUNEL method (20).
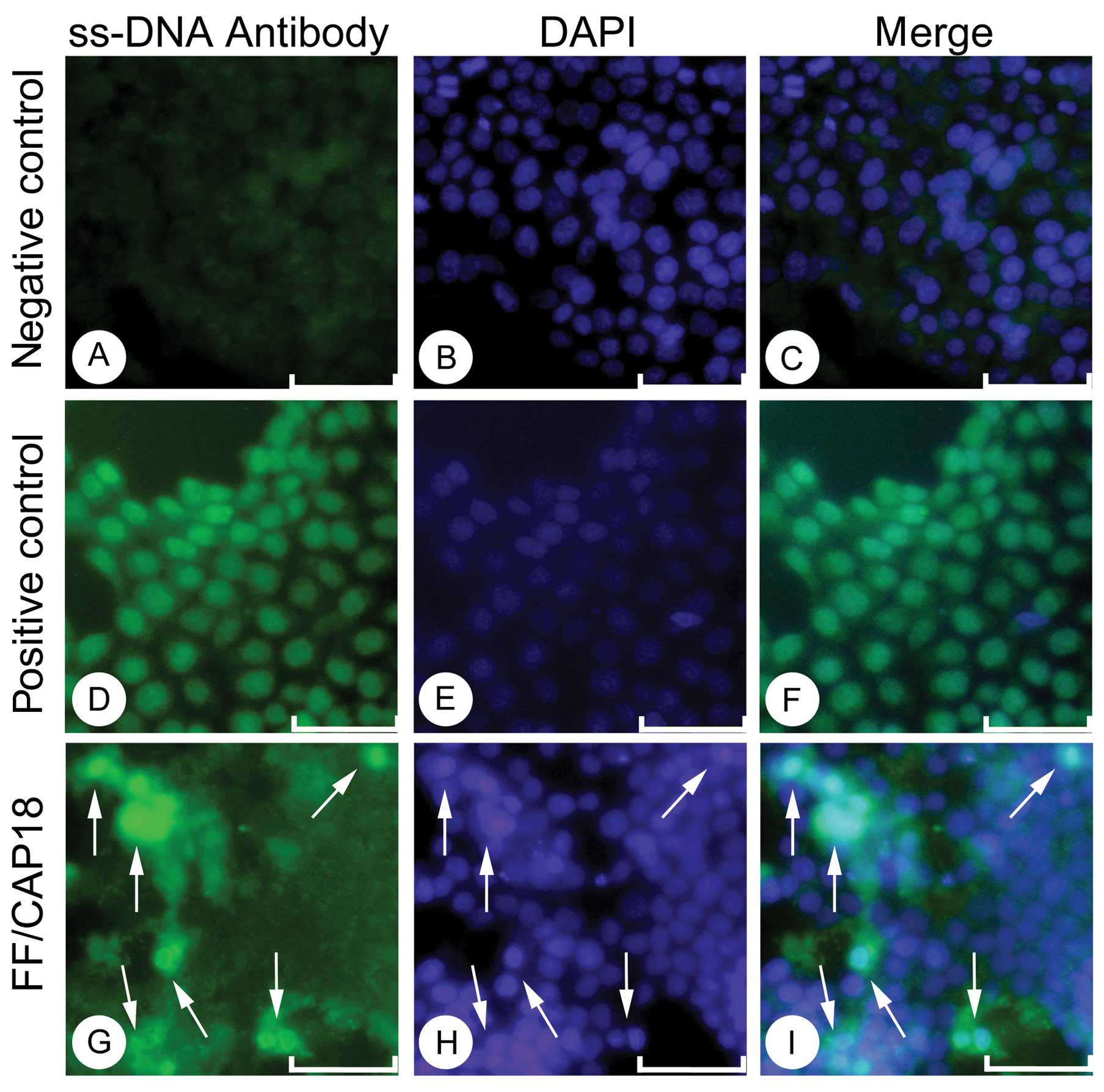
The immunostaining of anti ss-DNA antibody showed
the intense positive staining on the nuclei in the positive control
cells. For the positive control, we used the HCT116 cells treated
with DNase I. The almost no signal in the nuclei in the
non-treatment cells (Fig. 3A-C),
and the intense staining in the positive control (Fig. 3D-F) facilitate that our detection of
the single strand DNA in the nucleus is specific. HCT116 cells,
treated with FF/CAP18, showed intense signals in a limited number
of cells. The cells showed relatively condensed nuclear, and
intense positive reactivity for the anti ss-DNA antibody (Fig. 3G-I, arrows). From these data,
although based on a limited number of population, we concluded that
FF/CAP18 treatment induces apoptotic cell death in HCT116 cells,
which possibly explained the anti-proliferative effect.
The anti-proliferative effect of FF/CAP18
in the p53−/− HCT116 cells
The p53 is classified as the genomic guardian due to
its nature as the tumor suppressor gene, and loss of function of
p53 is commonly observed in around 50% in various types of cancers.
The results in the previous section showed that the addition of 10
or 40 μg/ml of FF/CAP18 peptide suppresses the HCT116 cells. We
expected that the cellular growth data in p53 mutant of HCT116
would reveal whether p53 tumor suppressor pathways is involved in
the anti-proliferative effects of FF/CAP18.
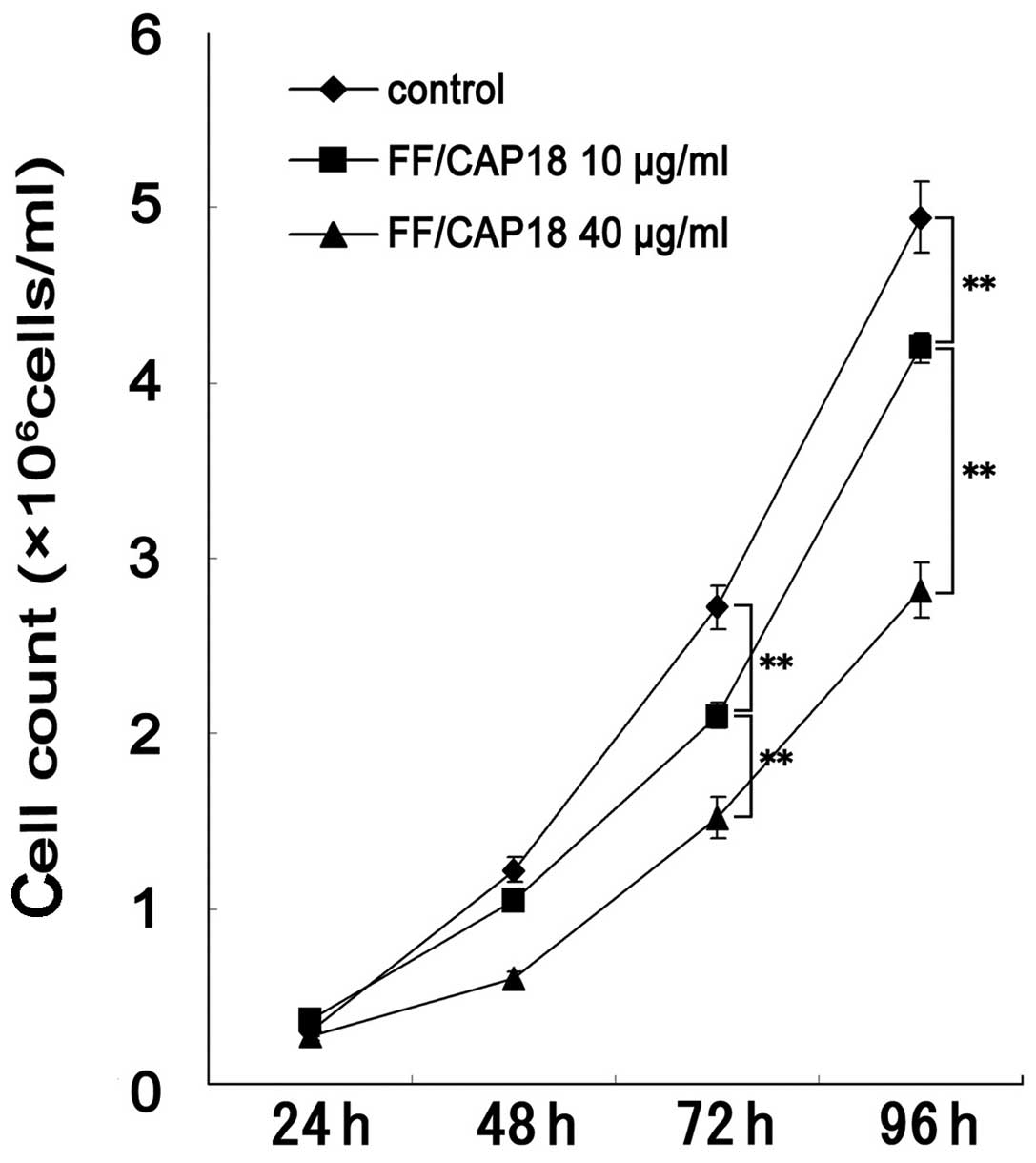
As shown in Fig. 4,
the exposure of 10 or 40 μg/ml of FF/CAP18 peptide significantly
suppressed the cell growth of p53−/− HCT116, which is
exactly same result as in p53 wild-type HCT116 cells. The growth of
the p53−/− cells was suppressed at 72 and 96 h in the 10
or 40 μg/ml treated groups, in a dose-dependent fashion. Therefore,
we could not detect any difference in the growth suppression
effects of FF/CAP18 peptide among the p53−/− and p53
wild-type HCT116. We also evaluated the difference among wild-type
and p53 mutant of HCT116, in the the MitoCapture assay, and
immuno-histochemistry of anti ss-DNA antibody, but no differene was
detected (data not shown).
Discussion
In this study, we showed that the hCAP18/LL-37
analogue peptide, FF/CAP18 has anti-proliferative effect on human
colon cancer cell HCT116 via inducing depolarization of the partial
mitochondrial membrane, which is the earlier stage of apoptosis.
The further key finding in this study is that the
anti-proliferative effect of this peptide is independent from the
p53 signaling pathway.
The cationic antimicrobial peptides, such as
BMAP-27, BMAP-28, and α-difensin, are known to induce apoptosis of
tumor cells through mitochondrial damage (21–23).
The hCAP18/LL-37 belongs to the cathelicidine anti-microbial
peptide family, which includes BMAP-27 and -28. The cytoplasmic
membranes of bacteria contain many anionic phospholipids, causing a
negative charge on the cell surface. The cationic antimicrobial
peptides, such as hCAP18/LL-37, and BMAP-27 are expected to have a
positive charge. The positive charge of the peptide and negative
charge of the bacterial membrane is one of the possible
explanations, how these peptides preferably bind to the bacteria.
After the binding of these peptide to the bacteria, the peptides
are expected to form the pore on the cell membrane (11,12) or
induce the leaking out of the intracellular molecules, resulting in
cell death (12,18). In general, there are several reports
that the cancer cells have a high contents of anionic phospholipids
in their cell membranes, when compared with that of normal cells
(24,25). Based on this concept, the
cathelicidine anti-microbial peptides might preferably bind to
cancer cells, when compared with normal cells.
The genetic diversity of the malignant cancer is one
of the important characteristics. Okumura et al reported
that hCAP18109–135 (active domain of hCAP18 synthesized
as C-terminal 27 amino acids) and its modified peptide, FF/CAP18
efficiently suppress cell growth of the squamous cell carcinoma
SAS-H1 (10). In brief, Okamura
et al reported that both hCAP18109–135 and
FF/CAP18 are effective in suppressing the growth of the SAS-H1, but
its effect was more obvious in FF/CAP18 (10). In this study, we observed the
anti-proliferative effect of FF/CAP18, and almost no effect on cell
growth in case of LL-37. FF/CAP18 has modified amino acids to
enhance the negative charge of the peptide. The difference of the
anti-proliferative effect among the LL-37 and FF/CAP18 might be
explained by the grade of the negative charge of the peptide.
Furthermore, the results of the current study showed that there is
diversity among the multiple cancer cell lines for the
anti-proliferative effect of FF/CAP18.
FF/CAP18 treated HCT116 cells showed the slower
growth, and showed disruption of the mitochondrial membrane, which
can be observed at early stage of apoptosis. Until this stage,
squamous cell carcinoma cell line, SAS-H1 and colon cancer derived
cell line HCT116, showed very similar results. However, in case of
SAS-H1, the DNA fragmentation was detected after gel
electrophoresis, indicating that SAS-H1 entered into the late stage
of apoptosis. In contrast, in this study, we could not detect the
massive fragmentation of the genomic DNA, which was detected by
electrophoresis of the genomic DNA. These results suggest that the
sensitivity to the peptide is different depending on the nature of
each cancer cell line.
For the detection of the apoptotic cells in HCT116,
we first tried the detection of the apoptosis cells with the
standard TUNEL method. However, an obvious high background in
FF/CAP18 treated cells were observed (data not shown) and specific
detection with the TUNEL method was difficult. Although the
detailed situation is unknown, the non-specific binding of the
terminal transferase might occur due to the peptide treatment (data
not shown). In contrast, fluorescence staining of anti ss-DNA
antibody works well, due to the simple immunostaining. The staining
of the ss-DNA antibody was previously reported as the effective
method to detect apoptotic cells in cancer tissues (20).
The disruption of mitochondrial membrane in the
process of apoptosis is largely affected by the function of the p53
gene. The p53 gene is activated after ultra violet and/or radiation
exposure (26,27). Although numerous p53 functions have
been studied on carcinogenesis, the induction of the cell cycle
arrest at G1S and G2M phase of the cell cycle, is when the cell is
exposed to damage (28,29). In this study, the wild-type and p53
mutant of HCT116 cells did not show any difference in response to
the FF/CAP18 treatment. Our results are in good agreement with the
previous published results in SAS-H1. In brief, Okumura et
al reported that the cell death induced by the
hCAP18109–135 or FF/CAP18 is independent of the
activation of the caspase, which is the major downstream molecule
of the p53 dependent apoptosis (10). Although the detailed molecular
pathway of the cell death induced by the LL-37 or FF/CAP18 is not
clear, we revealed that the anti-proliferative effect of LL-37 or
FF/CAP18 is independent from p53 tumor suppressor pathway, with the
genetic mutant of HCT116 colon cancer cell line.
In recent years, the anticancer effects of several
natural compound-derived peptides or toxin have been reported, such
as defensin peptide family from a beetle, Allomyrina dichotoma
(30) or the toxin like peptide
piericin from cabbage butterfly (31). The anticancer effect of these
naturally-derived peptides or toxins is not as strong as the
chemotherapeutic reagents, however, the anti-proliferative effect
might increase the sensitivity of the chemotherapeutic reagents,
which might relate to the reduction of the side effects.
Acknowledgements
This study was supported by the grant from the Japan
Science and Technology Agency (AS231Z0188E). We are grateful for
the support and encouragement of all members of the Laboratory of
Animal Microbiology, Graduate School of Agricultural Science,
Tohoku University, all through this study.
References
|
1
|
Zanetti M, Gennaro R, Scocchi M and
Skerlavaj B: Structure and biology of cathelicidins. Adv Exp Med
Biol. 479:203–218. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zanetti M: The role of cathelicidins in
the innate host defenses of mammals. Curr Issues Mol Biol.
7:179–196. 2005.PubMed/NCBI
|
|
3
|
Cowland JB, Johnsen AH and Borregaard N:
hCAP-18, a cathelin/pro-bactenecin-like protein of human neutrophil
specific granules. FEBS Lett. 368:173–176. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sorensen O, Arnljots K, Cowland JB,
Bainton DF and Borregaard N: The human antibacterial cathelicidin,
hCAP-18, is synthesized in myelocytes and metamyelocytes and
localized to specific granules in neutrophils. Blood. 90:2796–2803.
1997.PubMed/NCBI
|
|
5
|
Agerberth B, Gunne H, Odeberg J, Kogner P,
Boman HG and Gudmundsson GH: FALL-39, a putative human peptide
antibiotic, is cysteine-free and expressed in bone marrow and
testis. Proc Natl Acad Sci USA. 92:195–199. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Frohm M, Agerberth B, Ahangari G, et al:
The expression of the gene coding for the antibacterial peptide
LL-37 is induced in human keratinocytes during inflammatory
disorders. J Biol Chem. 272:15258–15263. 1997. View Article : Google Scholar
|
|
7
|
Chen PH and Fang SY: The expression of
human antimicrobial peptide LL-37 in the human nasal mucosa. Am J
Rhinol. 18:381–385. 2004.PubMed/NCBI
|
|
8
|
Murakami M, Ohtake T, Dorschner RA and
Gallo RL: Cathelicidin antimicrobial peptides are expressed in
salivary glands and saliva. J Dent Res. 81:845–850. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bals R, Wang X, Zasloff M and Wilson JM:
The peptide antibiotic LL-37/hCAP-18 is expressed in epithelia of
the human lung where it has broad antimicrobial activity at the
airway surface. Proc Natl Acad Sci USA. 95:9541–9546. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Okumura K, Itoh A, Isogai E, et al:
C-terminal domain of human CAP18 antimicrobial peptide induces
apoptosis in oral squamous cell carcinoma SAS-H1 cells. Cancer
Lett. 212:185–194. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Henzler Wildman KA, Lee DK and Ramamoorthy
A: Mechanism of lipid bilayer disruption by the human antimicrobial
peptide, LL-37. Biochemistry. 42:6545–6558. 2003.PubMed/NCBI
|
|
12
|
Zasloff M: Antimicrobial peptides of
multicellular organisms. Nature. 415:389–395. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Van Cutsem E and Geboes K: The
multidisciplinary management of gastrointestinal cancer. The
integration of cytotoxics and biologicals in the treatment of
metastatic colorectal cancer. Best Pract Res Clin Gastroenterol.
21:1089–1108. 2007.PubMed/NCBI
|
|
14
|
Li Z, Song H, He W, Tian Y and Huang T: In
vitro chemosensitivity testing of primary and recurrent breast
carcinomas and its clinical significance. J Huazhong Univ Sci
Technolog Med Sci. 28:683–687. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yanagawa E, Nishiyama M, Saeki T, et al:
Chemosensitivity tests in colorectal cancer patients. Jpn J Surg.
19:432–438. 1989. View Article : Google Scholar
|
|
16
|
Lane DP and Lain S: Therapeutic
exploitation of the p53 pathway. Trends Mol Med. 8:S38–S42. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bunz F, Dutriaux A, Lengauer C, et al:
Requirement for p53 and p21 to sustain G2 arrest after DNA damage.
Science. 282:1497–1501. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Johansson J, Gudmundsson GH, Rottenberg
ME, Berndt KD and Agerberth B: Conformation-dependent antibacterial
activity of the naturally occurring human peptide LL-37. J Biol
Chem. 273:3718–3724. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zamzami N, Marchetti P, Castedo M, et al:
Sequential reduction of mitochondrial transmembrane potential and
generation of reactive oxygen species in early programmed cell
death. J Exp Med. 182:367–377. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Watanabe I, Toyoda M, Okuda J, et al:
Detection of apoptotic cells in human colorectal cancer by two
different in situ methods: antibody against single-stranded DNA and
terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick
end-labeling (TUNEL) methods. Jpn J Cancer Res. 90:188–193. 1999.
View Article : Google Scholar
|
|
21
|
Aarbiou J, Ertmann M, van Wetering S, et
al: Human neutrophil defensins induce lung epithelial cell
proliferation in vitro. J Leukoc Biol. 72:167–174. 2002.PubMed/NCBI
|
|
22
|
Risso A, Braidot E, Sordano MC, et al:
BMAP-28, an antibiotic peptide of innate immunity, induces cell
death through opening of the mitochondrial permeability transition
pore. Mol Cell Biol. 22:1926–1935. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Risso A, Zanetti M and Gennaro R:
Cytotoxicity and apoptosis mediated by two peptides of innate
immunity. Cell Immunol. 189:107–115. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Utsugi T, Schroit AJ, Connor J, Bucana CD
and Fidler IJ: Elevated expression of phosphatidylserine in the
outer membrane leaflet of human tumor cells and recognition by
activated human blood monocytes. Cancer Res. 51:3062–3066.
1991.PubMed/NCBI
|
|
25
|
Van Blitterswijk WJ, De Veer G, Krol JH
and Emmelot P: Comparative lipid analysis of purified plasma
membranes and shed extracellular membrane vesicles from normal
murine thymocytes and leukemic GRSL cells. Biochim Biophys Acta.
688:495–504. 1982.
|
|
26
|
Morgan SE and Kastan MB: p53 and ATM: cell
cycle, cell death, and cancer. Adv Cancer Res. 71:1–25. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Westphal CH: Cell-cycle signaling: Atm
displays its many talents. Curr Biol. 7:R789–R792. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lowe SW and Sherr CJ: Tumor suppression by
Ink4a-Arf: progress and puzzles. Curr Opin Genet Dev. 13:77–83.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Vousden KH and Lu X: Live or let die: the
cell’s response to p53. Nat Rev Cancer. 2:594–604. 2002.
|
|
30
|
Miyanoshita A, Hara S, Sugiyama M, et al:
Isolation and characterization of a new member of the insect
defensin family from a beetle, Allomyrina dichotoma. Biochem
Biophys Res Commun. 220:526–531. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nagaoka I, Hirota S, Yomogida S, Ohwada A
and Hirata M: Synergistic actions of antibacterial neutrophil
defensins and cathelicidins. Inflamm Res. 49:73–79. 2000.
View Article : Google Scholar : PubMed/NCBI
|