Introduction
Turner syndrome (TS) is one of the most common human
chromosomal abnormalities occurring in approximately 1:2,500 live
female births. It is characterized by short stature, gonadal
dysgenesis, typical visible dysmorphic stigmata, and urinary,
cardiovascular, and skeletal abnormalities (1,2). This
syndrome has been associated with a wide range of chromosomal
karyotypes. The most frequent is 45, X monosomy, however, a variety
of other anomalies have been found, including mosaicism, Xp or Xq
deletion, and isochromosome of the long arm of chromosome X
(2). In addition, a cell line with
a Y-chromosome is present in 5% of patients, and an additional 3%
of cases have an unidentifiable marker sex chromosome, presumably
derived from a Y-chromosome in pure or mosaic form (3). It has been proposed that all female
patients with TS and 45, X karyotypes carry a cell line containing
2 sex chromosomes at a low level of mosaicism (4), which is undetectable using standard
cytogenetics analysis. Theoretically, this hidden mosaicism may
have a Y-chromosome. The gonadal dysgenesis seen in patients with
TS is associated with gonadoblastoma in cases where the
Y-chromosome-derived material is present in the genome (5).
The presence or absence of the Y-encoded
male-determining sex-determining region Y (SRY) gene directs the
developing gonad to differentiate into either a testis or an ovary,
which in turn determines the sex development of the remainder of
the embryo (6). The SRY gene
increases the risk of developing gonadoblastoma and/or non-tumoral
gonadal lesions in TS. It may be useful to perform molecular
analyses in these subjects, to rule out the presence of
Y-chromosome sequences (7,8).
The expression analysis of genes SRY,
octamer-binding transcription factor 4 (OCT4), and testis-specific
protein Y encoded gene (TSPY) in TS patients with gonadal
dysgenesis may allow introducing modifications in the
microenviroment that could contributed to a malignant
transformation (9).
A systematic search for hidden Y-chromosome
mosaicism, especially SRY, in TS patients is justified by the
possibility of evaluating the risk of development of germ cell
tumor (10–13).
In this report, the aims of the study were to use
PCR coupled with FISH, to determine the presence, and incidence of
cryptic Y-chromosome material in patients with TS.
Materials and methods
Population under study
Thirty-two unrelated TS patients of Mexican mestizo
ethnic origin aged 9.9±3.4 years (mean ± SD; range, 4–15 years)
that were examined at the Department of Endocrinology, High
Specialty Medical Unit No. 25 (UMAE), Mexican Institute of Social
Security (IMSS) in Monterrey, Mexico, between 2005 and 2009 were
enrolled in this study. The clinical diagnosis of the patients was
set upon the medical history and presented clinical features at 1
of 3 major stages of maturation, namely at birth, when typical
signs of TS appeared (congenital lymphedema: puffy hands and feet
or redundant nuchal skin), at mid-childhood, when TS appeared as
growth retardation with or without TS phenotypic finding, and in
adolescence, when they failed to enter puberty (primary
amenorrhea). The exclusion criteria were: ambiguous genitalia or
enlargement of the clitoris.
The diagnosis of TS was verified by cytogenetic
analysis (standard karyotyping) in all cases.
The Ethics Committee of the UMAE No. 25, IMSS,
approved the study. The parents provided informed consent for the
participation of their daughters in this study.
All women were evaluated by gynecologists via a
physical examination, sonogram of the pelvis, and an
endocrinological test. The samples were sent to the
Cytogenetic-Molecular Laboratory of the Biomedical Research Center
of Northeast (CIBIN), IMSS, for chromosomal and molecular
studies.
Cytogenetic analysis
Karyotyping was performed from blood lymphocyte
cultures using G-banding by trypsin/Giemsa (GTG) analysis (14). Thirty cells were counted per patient
(this was increased to 100 in cases of mosaicism).
Fluorescence in situ hybridization
(FISH)
FISH was performed according to the manufacturer's
protocol (VYSIS, Inc., Downers Grove, IL, USA) on lymphocytes using
a CEP-Y (Spectrum Orange) probe, which hybridizes to the centromere
region of the Y-chromosome (Yp11.1-q11.1). FISH analysis was also
performed on gonadal tissues from the 3 patients who underwent
gonadectomy.
The slides were viewed under a Zeiss Axiophot
photomicroscope equipped with an epifluorescence system and a CCD
camera. All samples were analyzed in duplicate, in a blinded
manner. Five hundred interphase nuclei, and 30 metaphases were
analyzed per patient.
Molecular genetic analysis
Peripheral blood samples were collected from 32 TS
patients. DNA was isolated using a DNA isolation kit for mammalian
blood (Roche Diagnostics, Mannheim, Germany). In 3 patients which
underwent gonadectomy, DNA was also extracted from
paraffin-embedded gonadal tissues using the QIAmp Tissue kit
(Qiagen Inc., Hilden, Germany).
The SRY gene, which is located in the short arm of
the Y-chromosome (Yp11.3), was used for the detection of cryptic
chromosome material using PCR analysis. PCR reactions were
performed in a volume of 25 μl, which contained extracted DNA,
primers (20 pmol; SRY1, 5′-ATAAGTATCGACCTCGTCGGAAG-3′; and SRY2,
3′-GCACTTCGCTGCAGAGTACCGAAG-3′), DNA polymerase (2.5 units), 1X PCR
buffer containing 1.5 mM MgCl2, and 200 μM of each dNTP.
Thermal cycling was performed as follows: initial activation of
TaqDNA polymerase at 94°C for 5 min, followed by 30 cycles of
denaturation at 94°C for 30 sec and annealing at 56°C for 30 sec. A
final extension at 72°C for 5 min was performed. The PCR
amplification products were separated using 2% agarose gel
electrophoresis and were visualized by exposure to ultraviolet
light after ethidium bromide staining.
For the FISH and molecular analysis, 5 healthy
females and 5 healthy males were included as negative and positive
control groups, respectively. All DNA extractions and PCR reactions
were performed by a female technician, to avoid the risk of
contamination with male DNA.
Results
Clinical
The referral data revealed that short stature (100%)
and the presence of streak-like or invisible gonads (28.1%) on
ultrasonography were the most common findings. Additionally, 6
patients (18.7%) had cardiac anomalies and 2 patients (6.2%) had
renal anomalies (renal agenesis or ectopic kidneys). All patients
had clinical features of TS and did not show signs of
virilization.
The hormonal profiles were as follows: LH=9.3 UI/l
(range, 0.74–28.9), FSH=66.2 UI/l (range, 1.64–172.41), and
TSH=2.26±0.59 UI/l (range, 0.55–3.43).
Cytogenetics (G-banding)
The cytogenetic findings of the cases analyzed in
the present study are summarized in Table I. As shown, most of the TS cases had
a 45, X karyotype, 75% with a pure line and 25% with mosaicism; 4
cases had a 46, XX karyotype, 2 patients had Y-chromosome [45,
X/46, X,mar (case 1) and 45, X/46, XY (case 2)], 1 case had an Xq
deletion. One patient (3.1%) presented isochromosome Xq in pure
form.
 | Table IDistribution of karyotypes among 30
Mexican patients with Turner syndrome. |
Table I
Distribution of karyotypes among 30
Mexican patients with Turner syndrome.
| Karyotype | N (%) |
|---|
| 45, X | 24 (75) |
| 45, X/46, XX | 4 (12.5) |
| 45, X/46, X, del
(Xq) | 1 (3.1) |
| 46, Xi (Xq) | 1 (3.1) |
| 45, X/46, X, mar | 1 (3.1) |
| 45, X/46, XY | 1 (3.1) |
| Total | 32 (100) |
FISH analysis
Using a specific probe for the Y-chromosome, signals
of appreciable intensity were detected in lymphocytes from male
controls, whereas no signals were observed in cells obtained from
female controls.
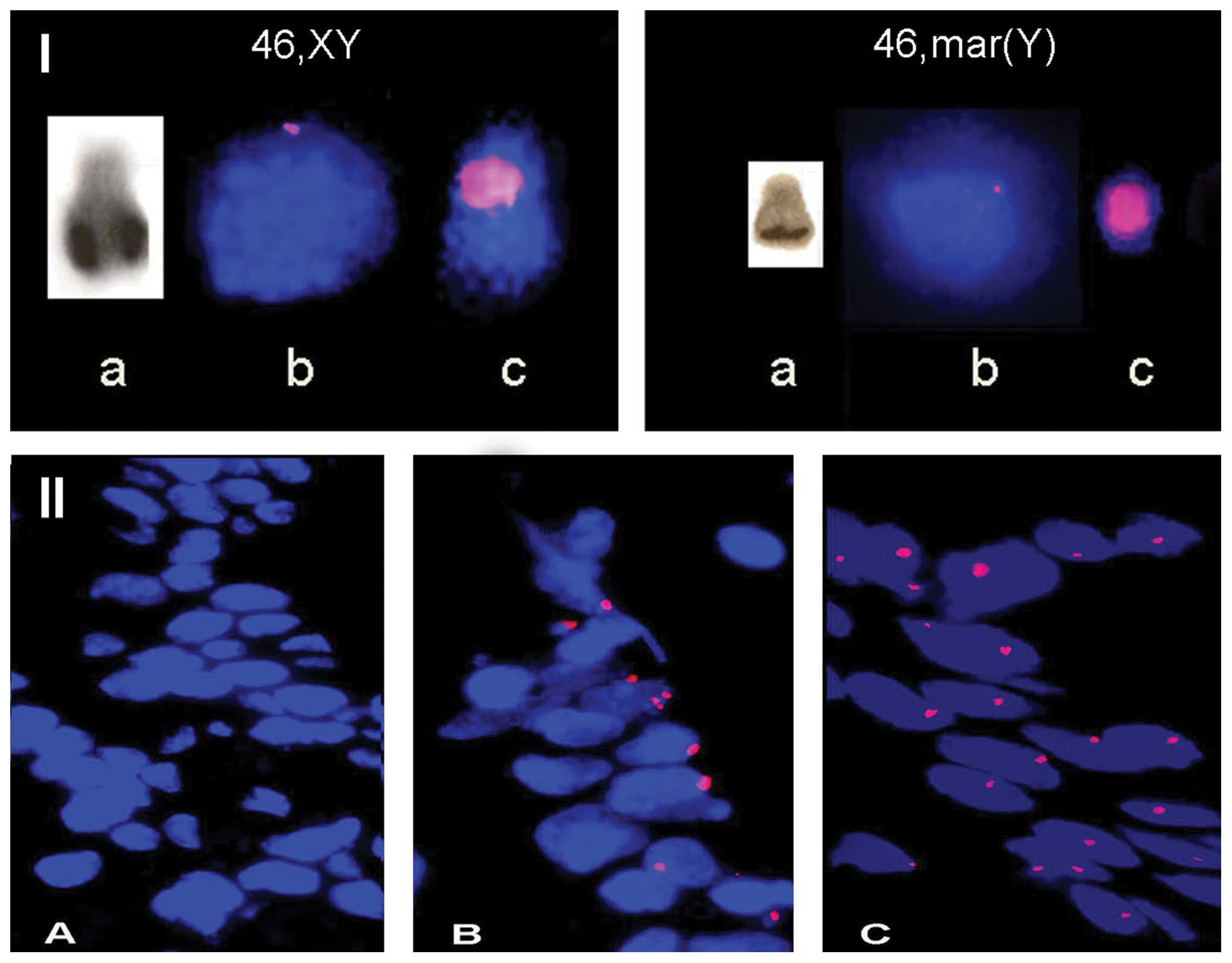
FISH analysis in lymphocytes confirmed Y-chromosome
positivity in case 2, and the marker chromosome was shown to be of
Y-chromosome origin (case 1) (Fig.
1I). No Y-chromosome was detected in case 3 (Table II). FISH analysis on gonadal
tissues revealed that Y-chromosome material was present in 57, 46
and 26% of cells, respectively (Table
II, and Fig. 1IIB).
 | Table IIResults of karyotyping (GTG), FISH,
PCR-SRY, and surgical reports of Turner patients with Y-chromosome
positive. |
Table II
Results of karyotyping (GTG), FISH,
PCR-SRY, and surgical reports of Turner patients with Y-chromosome
positive.
| Case 1 | Case 2 | Case 3 |
|---|
| Age (years) | 7 | 10 | 13 |
| Karyotype (GTG) | 45, X/46, mar | 45, X/46, XY | 45, X |
| FISH
(lymphocytes) | 45, X/46,
mar(Y)(57/43) | 45, X/46, XY
(62/38) | 45, X |
| FISH (gonads) |
cepY-[43]/cepY+[57] |
cepY-[54]/cepY+[46] |
cepY-[74]/cepY+[26] |
| PCR-SRY
(lymphocytes) | Positive | Positive | Positive |
|
PCR-SRY(gonads) | Positive | Positive | Positive |
| Histology | Streak gonads | Gonodoblastoma | Streak gonads |
PCR of the SRY gene
PCR of the SRY gene was performed using DNA obtained
from peripheral blood cells and gonadal tissues. An internal
control was used to confirm the presence and availability of DNA in
the sample; this control consisted of a region of the
glyceraldehyde-phosphate dehydrogenase (GADPH) gene for peripheral
blood and β-globin gene for gonadal tissues, which were amplified
simultaneously, yielding a PCR product of 980 and 260 bp
respectively. The β-globin gene was used in gonadal tissues because
the DNA extracted by QIAmp Tissue kit did not amplify a PCR product
of 980 pb. All samples showed amplification bands of the expected
size.
To determine the sensitivity of the SRY gene PCR, a
serial dilution of male genomic DNA with female genomic DNA was
used as a template. We were able to detect Y-specific products up
to 1:1,000 dilution of male positive control DNA with female DNA.
This method is capable of detecting Y-chromosome fragments in the
samples, if at least 0.1% of the cells contained Y-chromosome
material (Fig. 2I).
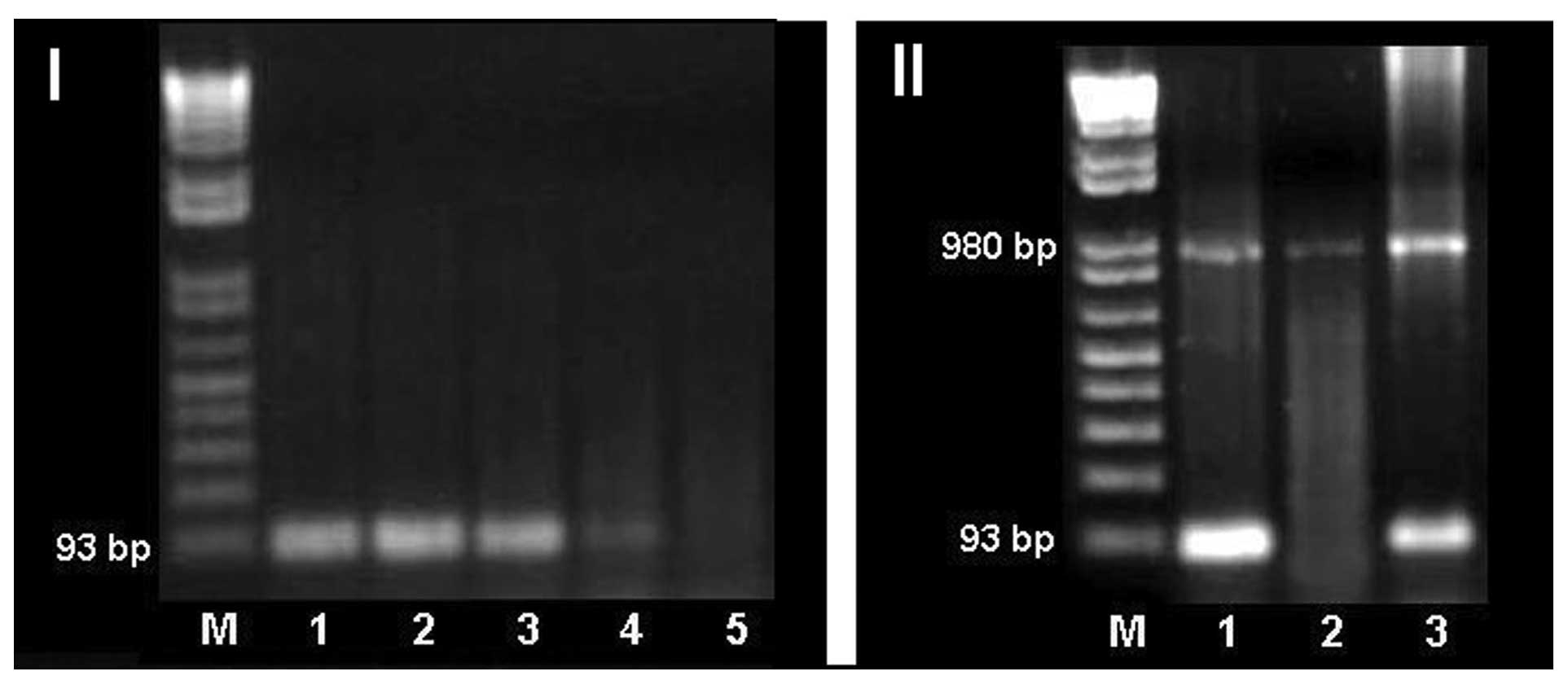 | Figure 2(I) PCR detection of SRY in serial
dilution of male genomic DNA with female genomic. M, molecular
weight marker; lane 1, SRY male control; lanes 2–5 correspond to
dilutions 1:10, 1:100, 1:1,000 and 1:2,000 respectively. (II) PCR
detection of SRY. M, molecular weight marker; lane 1, SRY male
control; lane 2, SRY female control and lane 3, case 3. |
PCR of the SRY gene, as expected, generated a band
of 93 bp when male genomic DNA was used as a template; in contrast,
no amplification products were detected when female genomic DNA was
used as a template.
PCR of SRY was performed on genomic DNA obtained
from blood cells of all 32 patients. We confirmed the presence of
the Y-chromosome in cases 1 and 2, and detected 1 patient (case 3)
with a Y-chromosome, who was initially considered as Y-negative
(Table II and Fig. 2II).
PCR-SRY gonadal analysis was positive in all 3
cases. The prophylactic operation reveled than 1 of the cases (case
2) had bilateral gonadoblastoma without any clinical signs
(Table II).
Discussion
Standard karyotyping is a routinely applied method
for testing Y-chromosome positivity in TS. However, this frequently
applied method may miss cases if the Y-chromosome is present only
in a small proportion of cells or very small parts of the
Y-chromosome or even marker chromosomes containing Y-specific
regions are present (15).
FISH highlights the differences between the initial
diagnosis based on G-banding, and final diagnoses, determined by
specific probes for the X and Y-chromosome. FISH (using a large
number of cells) is a useful tool in the detection of low frequency
cell lines and identification of the nature and origin of
derivative chromosomes and unknown chromosome markers that have
important implications for the treatment of patients with TS
(15).
Recombinant DNA technology has enabled the analysis
of sex chromosome abnormalities at the molecular level in patients
with TS. In addition, it has helped the identification of the
parental origin of the abnormality, the existence of
cytogenetically hidden mosaicism, and the correlation between
genotypes and phenotypes. Molecular analytical techniques as PCR
are useful in complementing the genetic approach used in TS
(16,17). This analysis has advantages over the
cytogenetic analysis, as it is rapid and several samples can be
analyzed in parallel; it could be applied to the broader screening
of a larger number of patients, and to the achievement of greater
sensitivity in the recognition of mosaicism (18–26).
On the basis of clinical experience, it is generally
accepted that mixed gonadal dysgenesis increases the risk for germ
cell tumor when Y-chromosome is present. Recently, the importance
of detecting the presence of the Y-chromosome and significance of
the ratio of mosaicism in gonadal tissues have been raised
(11–13,27).
Previous studies in TS patients with different
karyotypes have demonstrated the presence of Y-chromosome-derived
sequences (0–61%), and emphasize the clinical necessity of
molecular analysis of hidden Y-mosaicism in TS for the evaluated
risk of developing gonadoblastoma (Table III). This variability may be
caused by molecular methods used, selection criteria of the
patients, racial variation, different tissues examined, and the
selection of the Y-chromosome-specific primers (5,18–20,23,26,28–33).
 | Table IIIPrevalence of cryptic Y-chromosomal
material in patients with TS in previous studies. |
Table III
Prevalence of cryptic Y-chromosomal
material in patients with TS in previous studies.
| Reference | N | Methods | Cryptic
Y-chromosomal material (%) | Conclusion |
|---|
| Gravholt et
al (5) | 114 | PCR: ZFY, SRY,
DYZ3, DY132 and DYZ1 | 12.2 | Future studies
should be undertaken to focus on the incidence of gonadoblastoma in
the presence of Y-chromosome material in all diagnosed females with
Turner syndrome. This study emphasizes the need for prospective
unbiased studies. |
| Binder et al
(18) | 53 | Nested PCR: SRY,
TSP, Y-encoded, DYZ3 | 3.7 | The data in this
study exclude low level Y-mosaicism in almost all TS cases
tested. |
| Coto et al
(19) | 18 | PCR: SRY, DYZ3 | 61.1 | Sensitive method
for the detection of Y as PCR has important implications for UTS
patients to evaluate the risk of gonadoblastoma. |
| Fernández et
al (20) | 41 | FISH: CEP-X, CEP-Y,
WCP-X WCP-Y, XIST, DXZ4, SCPL 116, SCPL102. PCR: SRY | 4.9 | This study support
the hypothesis of ‘the necessity of mosaicism for survival’, and
thus, a mitotic origin for this syndrome. |
| Álvarez-Nava et
al (28) | 52 | FISH: CEP-X, CEP-Y,
WCP-X WCP-Y, PCR: PABY, SRY, ZFY, DYS19, DYZ3. Amelogenin, Kal-Y,
DYZ1, TSPY, YRRM | 7.7 | Detection of the
Y-chromosome material should be carried out in all patients with
TS. |
| Larsen et al
(30) | 40 | FISH: SRY, ZFY,
DYZ3, DYZ1 and DYS132 PCR: microsatellite | 0 | Contrary to other
reports using the PCR technique to unravel ‘hidden’ Y-chromosome
mosaics, we did not find any positive cases. |
| Mendes et al
(31) | 36 | PCR: SRY, ZFY,
DYZ3 | 5.5 | The molecular study
was sensitive and useful in the evaluation of patients at risk of
developing gonadoblastoma. |
| Nishi et al
(32) | 122 | Nested PCR: SRY,
TSPY | 25 | Nested PCR
overestimated the frequency of Y-sequences in patients with
TS. |
| Canto et al
(33) | 107 | PCR: SRY, ZFY, Yc
and Yq | 9.3 | Because of the high
proportion of gonadal tumors in patients with Y-chromosome
sequences, adequate counseling regarding a gonadectomy should be
given. |
| López et al
(23) | 50 | Southern blotting:
ZFY, SRY, Yqh, Ycen. PCR: ZFY, SRY, Yqh, Ycen | 12 | This study
discusses the presence of Y-sequences in patients with UTS and
compares the frequency with that previously reported. |
| Sallai et al
(16) | 130 | FISH: SRY, DEAD/H
box polypeptide, DDX3Y, HSFY1, TSPY1. RT-PCR | 6.9 | A routine molecular
screening for hidden Y-chromosome sequences in TS is recommended in
order to calculate the risk of developing gonadoblastoma. |
| Semerci et
al (35) | 40 | PCR: PABY, SRY,
DYS14, AMGY, DYZ3, DYS273, DYS280, DYS218, DYS224, DYS209, DYS231,
DYS1, YRRM, DYZ1 | 5 | The patients with a
45, X karyotype should be analyzed for the presence of Y-chromosome
derivatives by sensitive methods, such as PCR, in order to
calculate the future risk of developing gonadoblastoma. |
| Bianco et al
(11) | 20 | PCR: SRY, DYZ3 | 35 | A systematic search
for hidden Y-chromosome mosaicism in patients with TS and 45, X
karyotype is justified by the possibility of developing
gonadoblastoma. |
| Araujo et al
(36) | 42 | PCR: SRY, DYZ3,
ZFY, DYZ1, DYS1 and PABY | 4.8 | PCR method should
be included in the routine assistance of TS patients. |
| Kim et al (37) | 32 | FISH: DXZ1 and DYZ3
PCR: ZFY, TSPY, DYZ3, DYF49S1, RBM and DYZ1 | 12.5 | It may be
reasonable to consider using a PCR method to screen for Y-specific
sequences in all patients with TS. |
We found that 9.4% (3 out of 32) Mexican patients
with TS had Y-chromosome material; 2 patients showed Y-chromosome
by conventional cytogenetic analysis, and 1 patient had no
Y-chromosome on initial karyotype analysis (45, X) but was positive
when lymphocyte DNA was analyzed by Y-sequence-specific gene
analysis.
Page (34) suggested
that FISH or PCR analysis should be performed only in patients with
clitoromegaly, signs of virilization, and those that show marker
chromosomes using conventional analysis. In contrast, using PCR, we
identified 1 patient (3.1%) that was a carrier of cryptic
Y-chromosome material with neither virilization nor marker
chromosome positivity. Other authors have demonstrated that
Venezuelan patients with a 45, X karyotype had cryptic Y-specific
sequences (3.8%) (28); similar
results were reported in Turkish patients (5%) (35), in Brazilian patients (4.8%)
(36), in Hungarian patients (4.7%)
(16), and in Korean patients
(3.2%) (37).
The results of our study favor the idea that all
patients with TS should be assessed using DNA analysis. In our
study, 1 patient with a 45, X karyotype had hidden Y-chromosome
material that was detectable by DNA analysis in blood cells. FISH
analysis in gonadal tissue revelead than Y-chromosome material was
present in 26% of cells. She was 13-years-old, had no virilization,
and no gonadoblastoma was found on the gonads.
Similar findings have been reported by other
researchers (29,38). These studies have documented the
presence of Y-chromosomes on gonadal fibroblasts, but not on
leukocytes. This could be due to erratic behavior during the
mitosis of this abnormal Y-chromosome, which may lead to an uneven
distribution of cells bearing the Y-chromosome.
It has been shown that the detection of chromosomal
mosaicism can be enhanced substantially if at least 2 kinds of
tissue and a large number of cells are examined (39). It is easier and more practical to
test chromosomal mosaicism using 2 methods (karyotype, FISH, or PCR
analyses) compared with the testing of 2 tissues (e.g., peripheral
blood, oral epithelial cells, hair root, skin, and muscle). Thus,
we suggest that all patients with TS should be screened using
chromosomal analysis of blood cells and PCR analysis, to rule out
the possibility of hidden Y-chromosome mosaicism in cases where the
karyotype did not identify Y-chromosome material.
The more expensive and time consuming FISH technique
is helpful to evaluate Y-chromosome rearrangements, but, on the
basis of our results, we suggest it is use only after a positive
PCR results.
In the present study, using PCR coupled with FISH,
the analysis was more sensitive and method for the assessment of
hidden Y-chromosome mosaicism in patients with TS with or without
an a priori risk (i.e., presence of a Y-chromosome that is
identifiable cytogenetically) of development of gonadoblastoma or
neonatal or postpuberal virilization.
From an ethical point of view, it is impossible to
establish a prospective study to directly measure gonadoblastoma
incidence in humans, as prophylactic gonadectomy is the common
procedure in cases of Y-chromosome material carriers (40). In our study, the prophylactic
operation was carried out in all 3 patients, and 1 (3.1%) of them
had bilateral gonadoblastoma without any clinical signs. This
prevalence is in agreement to previous studies (8,33).
In further studies we recommend the use of primers
than analyze a wider region of the SRY gene than includes the HMG
(high mobility group) box, and other genes, such as TSPY1, which
have recently been identified as gonadoblastoma candidate genes and
will be tested (40,41).
In conclusion, our data support the suggestion that
these patients be analyzed for the presence of Y-chromosome
derivatives using sensitive methods, such as PCR coupled with FISH,
to predict the future risk of development of gonadoblastoma.
However larger studies are needed to determine the most appropriate
clinical management of these patients.
Acknowledgements
The authors are grateful to the Department of
Pediatrics of UMAE No. 25, IMSS for the use of facilities to sample
and interview participants in the study. We are grateful to
Sanjuana Guardado Limón for excellent technical assistance.
References
|
1
|
Ranke MB and Saenger P: Turner's syndrome.
Lancet. 358:309–314. 2001. View Article : Google Scholar
|
|
2
|
Saenger P, Wikland KA, Conway GS, et al:
Recommendations for the diagnosis and management of Turner
syndrome. J Clin Endocrinol Metab. 86:3061–3069. 2001.PubMed/NCBI
|
|
3
|
Nagafuchi S, Tamura T, Nakahori Y, et al:
The majority of the marker chromosomes in Japanase patients with
stigmata of Turner syndrome are derived from Y chromosome. Hum
Genet. 89:590–592. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Held KR, Kerber S, Kaminsky E, et al:
Mosaicism in 45, X Turner syndrome: does survival in early
pregnancy depend on the presence of two sex chromosomes? Hum Genet.
88:288–294. 1992.PubMed/NCBI
|
|
5
|
Gravholt CH, Fedder J, Naeraa RW and
Müller J: Occurence of gonadoblastoma in females with Turner
syndrome and Y chromosome material: a population study. J Clin
Endocrinol Metab. 85:3199–3202. 2000.PubMed/NCBI
|
|
6
|
Sekido R: SRY A transcriptional activator
of mammalian testis determination. Int J Biochem Cell Biol.
42:417–420. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chu CE, Connor JM, Donaldson MD, Kelnar
CJ, Smail PJ and Greene SA: Detection of Y mosaicism in patients
with Turner's syndrome. J Med Genet. 32:578–580. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mazzanti L, Cicognani A, Baldazzi L, et
al: Gonadoblastoma in Turner syndrome and Y-chromosome-derived
material. Am J Med Genet A. 135:150–154. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bianco B, Oliveira KC, Guedes AD, Barbosa
CP, Lipay MV and Verreschi IT: OCT4 gonadal gene expression related
to the presence of Y-chromosome sequences in Turner syndrome.
Fertil Steril. 94:2347–2349. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Brant WO, Rajimwale A, Lovell MA, et al:
Gonadoblastoma and Turner syndrome. J Urol. 175:1858–1860. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bianco B, Lipay MV, Melaragno MI, et al:
Detection of hidden Y mosaicism in Turner's syndrome: importance in
the prevention of gonadoblastoma. J Pediatr Endocrinol Metab.
19:1113–1117. 2006.PubMed/NCBI
|
|
12
|
Bianco B, Nunes Lipay MV, Guedes AD and
Verreschi IT: Clinical implications of the detection of
Y-chromosome mosaicism in Turner's syndrome: report of 3 cases.
Fertil Steril. 90:e17–e20. 2008. View Article : Google Scholar
|
|
13
|
Bianco B, Lipay M, Guedes A, Oliveira K
and Verreschi IT: SRY gene increases the risk of developing
gonadoblastoma and/or nontumoral gonadal lesions in Turner
syndrome. Int J Gynecol Pathol. 28:197–202. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Verma RS and Babu A: Human Chromosomes:
Manual of Basic Techniques. Pergamon Press; New York: 5–11. pp.
47–49. 1989
|
|
15
|
Cortés-Gutiérrez EI, Cerda-Flores RM,
Silva-Cudish JB, Dávila Rodríguez MI, Hernández-Herrera R and
Leal-Garza CH: Evaluation of sex chromosome aneuploidies in women
with Turner's syndrome by G-banding and FISH. A serial case study.
J Reprod Med. 48:804–808. 2003.
|
|
16
|
Sallai A, Sólyom J, Dobos M, et al:
Y-chromosome markers in Turner syndrome: screening of 130 patients.
J Endocrinol Invest. 33:222–227. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Modi D and Bhartiya D: Y chromosome
mosaicism and occurrence of gonadoblastoma in cases of Turner
syndrome and amenorrhoea. Reprod Biomed Online. 15:547–553. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Binder G, Koch A, Wajs E and Ranke MB:
Nested polymerase chain reaction study of 53 cases with Turner's
syndrome: is cytogenetically undetected Y mosaicism common? J Clin
Endocrinol Metab. 80:3532–3536. 1995. View Article : Google Scholar
|
|
19
|
Coto E, Toral JF, Menéndez MJ, et al:
PCR-based study of the presence of Y-chromosome in patients with
Ullrich-Turner syndrome. Am J Med Genet. 57:393–396. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ferández-García R, García-Doval S, Costoya
S and Pásaro E: Analysis of sex chromosome aneuploidy in 41
patients with Turner syndrome: a study of ‘hidden’ mosaicism. Clin
Genet. 58:201–208. 2000.
|
|
21
|
Gicquel C, Gaston V, Cabrol S and Le Bouc
Y: Assessment of Turner's syndrome by molecular analysis of the X
chromosome in growth-retarded girls. J Clin Endocrinol Metab.
83:1472–1476. 1998.
|
|
22
|
Jacobs P, Dalton P, James R, Mosse K,
Power M, Robinson D, et al: Turner syndrome: a cytogenetics and
molecular study. Ann Hum Genet. 61:471–483. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
López M, Canto P, Aguinaga M, et al:
Frequency of Y chromosomal material in Mexican patients with
Ullrich-Turner syndrome. Am J Med Genet. 76:120–124.
1998.PubMed/NCBI
|
|
24
|
Medlej R, Lobaccaro JM, Berta P, et al:
Screening for Y-derived sex determining gene (SRY) in 40 patients.
J Clin Endocrinol Metab. 75:1289–1292. 1992.PubMed/NCBI
|
|
25
|
Monroy N, López M, Cervantes A, et al:
Microsatellite analysis in Turner syndrome: parental origin of X
chromosomes and possible mechanism of formation of abnormal
chromosomes. Am J Med Genet. 107:181–189. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Osipova GR, Karmanov ME, Kozlova SI and
Evgrafov OV: PCR detection of Y-specific sequences in patients with
Ullrich-Turner syndrome: clinical implications and limitations. Am
J Med Genet. 76:283–287. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vodicka R, Vrtel R, Scheinost O, et al:
Refined quantitative fluorescent PCR of Y-chromosome DNA sequences
mosaics in Turner's syndrome patients alternative to real-time PCR.
J Biochem Biophys Methods. 60:151–162. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Alvarez-Nava F, Soto M, Sanchez MA,
Fernández E and Lanes R: Molecular analysis in Turner syndrome. J
Pediatr. 142:336–340. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kocova M, Siegel SF, Wenger SL, Lee PA,
Nalesnik M and Trucco M: Detection of Y chromosome sequences in a
45,X/46,XXq-patient by Southern blot analysis of PCR-amplified DNA
and fluorescent in situ hybridization (FISH). Am J Med Genet.
55:483–488. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Larsen T, Gravholt CH, Tillebeck A, et al:
Parental origin of the X chromosome, X chromosome mosaicism and
screening for ‘hidden’ Ychromosome in 45, X Turner syndrome
ascertained cytogenetically. Clin Genet. 48:6–11. 1995.
|
|
31
|
Mendes JR, Strufaldi MW, Delcelo R, et al:
Y-chromosome identification by PCR and gonadal histopathology in
Turner's syndrome without overt Y-mosaicism. Clin Endrocrinol
(Oxf). 50:19–26. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nishi MY, Domenice S, Medeiros MA,
Mendonca BB and Billerbeck AE: Detection of Y-specific sequences in
122 patients with Turner syndrome: nested PCR is not a reliable
method. Am J Med Genet. 107:299–305. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Canto P, Kofman-Alfaro S, Jiménez AL, et
al: Gonadoblastoma in Turner syndrome patients with nonmosaic 45, X
karyotype and Y chromosome sequences. Cancer Genet Cytogenet.
150:70–72. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Page DC: Y chromosome sequences in
Turner's syndrome and risk of gonadoblastoma or virilisation.
Lancet. 343:2401994. View Article : Google Scholar
|
|
35
|
Semerci CN, Satiroglu-Tufan NL, Turan S,
et al: Detection of Y chromosomal material in patients with a 45, X
karyotype by PCR method. Tohoku J Exp Med. 211:243–249. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Araujo C, Galera MF, Galera BB, Silvestre
FG and Medeiros SF: Molecular identification of chromosome Y
sequences in Brazilian patients with Turner syndrome. Gynecol
Endocrinol. 24:713–717. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kim HR, Shin JH, Jung WY and Lee JN:
Identification of Y-chromosome by molecular analysis in patients
with Turner syndrome. Korean J Lab Med. 26:131–136. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bisat T, May K, Litwer S and Broecker B: Y
chromosome mosaicism in the gonads, but not in the blood, of a girl
with the Turner phenotype and virilized external genitalia. Clin
Genet. 44:142–145. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tejada MI, Mornet E, Tizzano E, Molina M,
Baiget M and Boue A: Identification by molecular analysis of mosaic
Turner's syndrome in an obligate carrier female for fragile X
syndrome. J Med Genet. 31:76–78. 1994.
|
|
40
|
Bianco B, Lipay M, Guedes A, Oliveira K
and Verreschi IT: SRY gene increases the risk of developing
gonadoblastoma and/or nontumoral gonadal lesions in Turner
syndrome. Int J Gynecol Pathol. 28:197–201. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Delbridge ML, Longepied G, Depetris D, et
al: TSPY, the candidate gonadoblastoma gene on the human Y
chromosome, has a widely expressed homologue on the X-implications
for Y chromosome evolution. Chromosome Res. 12:345–356. 2004.
View Article : Google Scholar : PubMed/NCBI
|