Introduction
Cancer, a multi-step and systematic disease, is a
leading cause of mortality worldwide. In 2008, 7.6 million deaths
due to cancer were recorded (approximately 13% of the total number
of deaths) (1). As one of the main
types of cancer, breast cancer accounted for 458,000 deaths in 2008
(1). There were almost 230,480 new
cases of invasive breast cancer and 39,520 breast cancer deaths
among women in the US 2011 (2). In
developing countries, breast cancer occupies approximately half of
the total number of breast cancer cases worldwide and 60% of
deaths, and is the leading cause of cancer mortality among women in
this area (3). In China, the
incidence of breast cancer increased rapidly from 126,227 cases in
2002 (4) to 169,452 in 2008
(5). However, currently available
chemotherapy treatments provide little benefit, combined with
serious side-effects and dose-limiting toxicities (6). Therefore, agents which can interfere
with the essential steps of cancer development, such as
angiogenesis, are being increasingly used in the treatment of human
cancer (7,8).
Angiogenesis is a complex process, referring to the
formation of new blood vessels from pre-existing ones (9). During angiogenesis, several steps are
involved: degradation of the extracellular matrix, migration,
proliferation, sprouting, elongation and tube formation of
endothelial cells (10). It is well
known that physiological angiogenesis has a great contribution to
embryonic development, wound healing and tissue regeneration
(11–13), and it is tightly controlled by the
balance between the pro-angiogenic factors, such as vascular
endothelial cell growth factor (VEGF) and anti-angiogenic factors,
such as endostatin. Forming new blood vessels is an essential step
in tumor development (14–17). Without blood supply, the tumor
volume will not exceed 1–2 mm3(18). Tumor development can only continue
with the formation of new blood vessels (19). Therefore, anti-angiogenesis is a
promising strategy for cancer treatment.
Since angiostatin and endostatin were recognized as
endogenous anti-angiogenic factors, a number of phytochemicals,
such as Salvia officinalis(20), cinnamon extract (21), and koetjapic acid from Sandoricum
koetjaoe Merr. (22), have been
proven to possess anti-angiogenesis activities. Trametes
robiniophila Murr. (Huaier extract) a traditional Chinese
medicine (TCM), has been widely used in China for many years.
Previous studies have reported that Huaier extract inhibits the
growth of hepatocellular carcinoma cells (23,24),
and our previous study showed that Huaier aqueous extract inhibited
the proliferation of breast cancer cells by inducing apoptosis
(25). Although the antitumor
activity of Huaier extract has been revealed, the exact underlying
mechanisms remain largely unknown. In the present study, we
evaluated the anti-angiogenic effect of Huaier extract, in
combination with its antitumor effects.
Materials and methods
Reagents
The human umbilical vein endothelial cell line
(HUVEC) and mouse mammary tumor cell line, 4T1, were purchased from
the American Type Culture Collection (ATCC, Manassas, VA, USA), and
were routinely cultured in DMEM medium (Gibco-BRL, Rockville, IN,
USA) containing 10% FBS (Haoyang Biological Manufacturer Co., Ltd.,
Tianjin, China), 100 U/ml penicillin and 100 μg/ml streptomycin in
5% CO2 at 37°C. Anti-p21, anti-extracellular
signal-regulated kinase (ERK), anti-phosphorylated (p)-ERK,
anti-c-Jun N-terminal kinase (JNK), anti-p-JNK, anti-p65,
anti-p-p65, anti-signal transducer and activator of transcription 3
(STAT3) and anti-p-STAT3 (ser727) antibodies were obtained from
Cell Signaling Technology, (Beverly, MA, USA). Anti-VEGF antibody
was provided by Abcam, (Cambridge, MA, USA). Anti-β-actin (1:5000)
antibody was obtained from Sigma-Aldrich (St. Louis, MO, USA).
Anti-mouse and rabbit IgG horseradish peroxidase (HRP) antibodies
(1:5000) was from ZhongShan Goldenbridge Biotechnology Co., Ltd.
(Beijing, China). The pro-lighting HRP agent for western blot
analysis was supplied by Tiangen Biotech Co., Ltd., (Beijing,
China).
Preparation of Huaier aqueous
extract
Electuary ointment of Huaier extract was kindly
provided by Gaitianli Medicine Co., Ltd. (Jiangsu, China). The
electuary ointment (2 g) was soaked in 20 ml of DMEM. The solid
residue of the above dissolved herbs was filtered and discarded
through a 0.22-μm filter. The final 100 mg/ml stock solution was
kept at −20°C for long storage.
Effect of Huaier extract on cell
morphology
HUVECs were seeded in a 24-well plate. After 12 h,
the HUVECs were exposed to various concentrations of Huaier extract
for an additional 24 h. Finally, the morphological changes caused
by Huaier extract were observed under an Olympus light microscope
and photomicrographs were taken with an Olympus digital camera
(Olympus, Tokyo, Japan).
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
MTT assay was performed to measure the viability of
the HUVECs and 4T1 cells after treatment with Huaier extract. In
brief, the HUVECs (103 cells/well) and 4T1 cells (700
cells/well) were seeded in cultured medium in 96-well plates and
incubated in 5% CO2 at 37°C. After 12 h, the medium in
each well was replaced with the vehicle or different concentrations
(2, 4 and 8 mg/ml) of Huaier extract and incubated for another 48
or 72 h. Subsequently, 20 μl of MTT (5 mg/ml in PBS) were added
into each well. After 4 h of incubation at 37°C, the supernatants
were aspirated carefully and 100 μl of dimethyl sulfoxide (DMSO)
were added to each well. Absorbance values at 490 nm were
determined by the Microplate Reader (Bio-Rad, Hercules, CA,
USA).
Cell cycle analysis
After 24 h of starvation in serum-free medium at
37°C, the HUVECs were treated with various concentrations of Huaier
extract or complete medium as the negative control. After 24 h, the
treated cells were harvested, washed with 1X cold PBS and fixed
with 70% ice-cold ethanol overnight. After the ethanol was removed
by centrifugation at 1,200 × g for 1 min, the fixed cells were
washed with PBS twice. The pellets were then resuspended with 1 ml
of DNA staining solution (MultiSciences Biotech Co., Ltd.). After
incubation for 30 min at room temperature in the dark, cells were
analyzed in the presence of the dye by FACScan flow cytometry
(Becton-Dickinson, Franklin Lakes, NJ, USA) and the data were
analyzed by ModFitLT V2.0 software (Becton-Dickinson).
Propidium iodide (PI)-Annexin-V staining
analysis
The BD Pharmingen™ PE Annexin V Apoptosis Detection
Kit (BD Biosciences, Franklin Lakes, NJ, USA) was used to detect
the proportion of apoptotic cells, according to the instructions of
the manufacturer. Briefly, after treatment with various
concentrations of Huaier extract for the indicated times, the
HUVECs were harvested and washed with PBS twice. The cells
(1×105) were then resuspended in 100 μl of binding
buffer, followed by adding 5 μl Annexin V-FITC and 5 μl PI. After
incubation for 15 min in the dark, another 400 μl of binding buffer
were added before the cells were analyzed by FACScan flow
cytometry.
In vitro scratch assay
Scratch assay was applied to determine cell
mortality caused by Huaier extract. This assay was performed using
a standard method (26) with some
modifications. Briefly, 2.5×104 HUVECs were seeded on a
12-well plate in complete medium overnight to obtain a full
confluent monolayer. After 24 h of starvation, a 20-μl pipette tip
was used to create a straight cell-free wound. Each well was washed
twice with PBS to remove debris. The cells were then cultured in
serum-free medium in the absence or presence of various
concentrations of Huaier extract. The distances between the 2 edges
of the scratch were analyzed quantitatively.
Cell migration assay
In vitro cell migration assay was performed
using the Transwell system (24-wells, 8-μm pore size with
polycarbonate membrane; Corning Costar, Lowell, MA, USA). Cells
were starved in serum-free medium for 24 h at 37°C. The HUVECs were
then harvested and resuspended in various concentrations of Huaier
extract diluted in serum-free medium. The conditional medium from
the NIH3T3 fibroblasts and the complete medium were then mixed (v/v
1:1). Subsequently, 1 ml of the mixture was added to the lower well
of each chamber, and 100 μl of cell solutions containing
1×104 HUVECs were added to the upper wells. After
treatment for 24 h, the cells attached to the lower surface were
fixed with methanol and stained with 0.2% Giemsa. The successfully
migrated cells were counted on 5 random fields using an Olympus
light microscope.
Tube formation assay
The ability of the HUVECs to form network structures
was tested on Matrigel basement membrane matrix (BD Biosciences,
San Jose, CA, USA). Firstly, 50 μl of Matrigel were plated per well
on 96-well plates and allowed to polymerize at 37°C for 30 min.
Subsequently, 100 μl of HUVECs suspended in complete medium at a
density of 1×105/ml were added to each well in the
absence or presence of Huaier extract. After 9 h, tube-like
structures were photographed with an Olympus digital camera.
Chick embryo chorioallantoic membrane
(CAM) assay
CAM assay was performed as described previously
(27) with small modifications.
Briefly, 40 fertilized chicken eggs were incubated at 37°C at
constant humidity and randomly divided into 4 groups. On the 9th
day of incubation, a square window (1×1 cm2) was opened
in the shell. The following day, filter discs loaded with 20 μl
complete medium or various concentrations of Huaier extract were
placed on the top of the growing CAMs under sterile conditions.
Afterwards, the window was sealed with sterilized surgical tape and
the eggs were returned to the incubator. After 24 h of incubation,
the CAMs were photographed using an Olympus Live View Digital SLR
camera.
Rat aortic ring assay
Angiogenesis ex vivo was also studied by rat
aortic ring assay (28). Briefly, a
48-well plate was first covered with Matrigel and incubated for 30
min at 37°C. Subsequently, 2-month old BALB/c mice were sacrificed
by cervical dislocation, and the thoracic aortas were dissected and
cut into 1–2-mm long sections. Afterwards, aortic rings were placed
into wells pre-coated with Matrigel, and then covered with another
layer of Matrigel. After 30 min of polymerization, DMEM
supplemented with 20% FBS was added into each well. The following
day, the supernatants were replaced with medium in the absence or
presence of various concentrations of Huaier extract. On day 6, the
fields covered by the sprouting from the aortic rings were measured
by an Olympus digital camera.
Western blot analysis
In brief, the HUVECs were allowed to grow to 60–70%
confluence in 25 cm2 cell culture flasks, and then
incubated with gradient concentrations of Huaier extract at 37°C
under 5% CO2. After 2 h of treatment, the cells were
harvested, and the proteins were lysed in lysis buffer (1X PBS, 1%
NP40, 0.1% sodium dodecyl sulfate, 5 mM EDTA, 0.5% sodium
deoxycholate and 1 mM sodium orthovanadate) with protease
inhibitors. Subsequently, 50 μg of total cellular protein from each
sample were separated by 10% SDS-PAGE and electrotransferred onto a
polyvinylidene fluoride (PVDF) membranes by using a semi-dry
blotting apparatus (Bio-Rad). After blocking with 5% non-fat milk,
the PVDF membranes were covered with specific primary antibodies,
followed by incubation with secondary antibodies. The protein bands
were then visualized by using Pro-lighting HRP agent and their
densities were analyzed using ImageJ software. β-actin was used as
the loading control.
Animals and tumor model
Twenty BALB/c female mice, 4–5 weeks old, were
purchased from the Center for New Drugs Evaluation of Shandong
University, and housed under pathogen-free conditions. All the
experiments were approved by the institutional guidelines of the
Animal Care and Use Committee at Shandong University. 4T1 cells
(1×106) were subcutaneously injected into the left flank
of each mouse. After 2 days, each mouse was given 100 μl solution
containing 50 mg Huaier extract by gavage daily. After 21 days, the
mice were sacrificed, and the xenografts were removed for
immunohistochemical staining.
Histology and immunohistochemistry
Immediately after excision, the tumor tissues were
stored in 10% neutral-buffered formalin. After 24 h, the samples
were paraffin-embedded and then sliced into 4-μm section for
hematoxylin and eosin (H&E) staining according to the standard
techniques. The relative areas of necrosis in tumors were
analyzed.
To quantify the microvessel density (MVD), the
SP-9000 Histostain™-Plus Kits (ZhongShan Goldenbridge Biotechnology
Co.) were used to detect CD34 expression using the standard steps.
Briefly, the sections were deparaffinized and rehydrated, followed
by antigen retrieval with pH 6.0 citrate buffer. Endogenous
peroxidase activity was inhibited with 3%
H2O2 for 15 min and the sections were
incubated with 10% normal goat serum to block non-specific binding.
After incubation with anti-CD34 antibody (Santa Cruz Biotechnology,
Santa Cruz, CA, USA) at 4°C overnight, the sections were washed,
treated with biotinylated anti-immunoglobulin antibody for 20 min
and reacted with horseradish peroxidase-conjugated streptavidin.
Then the liquid DAB substrate/chromogen system (Maixin Bio, Fuzhou,
China) was used, followed by counterstaining with hematoxylin. The
representative images of tumor tissues were taken by an Olympus
light microscope.
Terminal deoxynucleotidyl transferase
(TdT)-mediated dUTP nick end-labeling (TUNEL) assay
TUNEL assay was performed to identify the apoptotic
cells in the paraffin-embedded sections using the One Step TUNEL
Apoptosis Assay kit (Beoytime, Beijing, China) according to the
manufacturer’s instructions. TUNEL-positive cells were visualized
with red fluorescent staining observed by a fluorescence microscope
(Olympus).
Statistical analysis
The results are presented as means ± standard
deviation (SD) and differences between groups were compared by
one-way ANOVA and considered significant at P<0.05. The
statistical analysis was carried out by using SSPS edition
16.0.
Results
Effects of Huaier extract on cell
morphology and viability of HUVECs
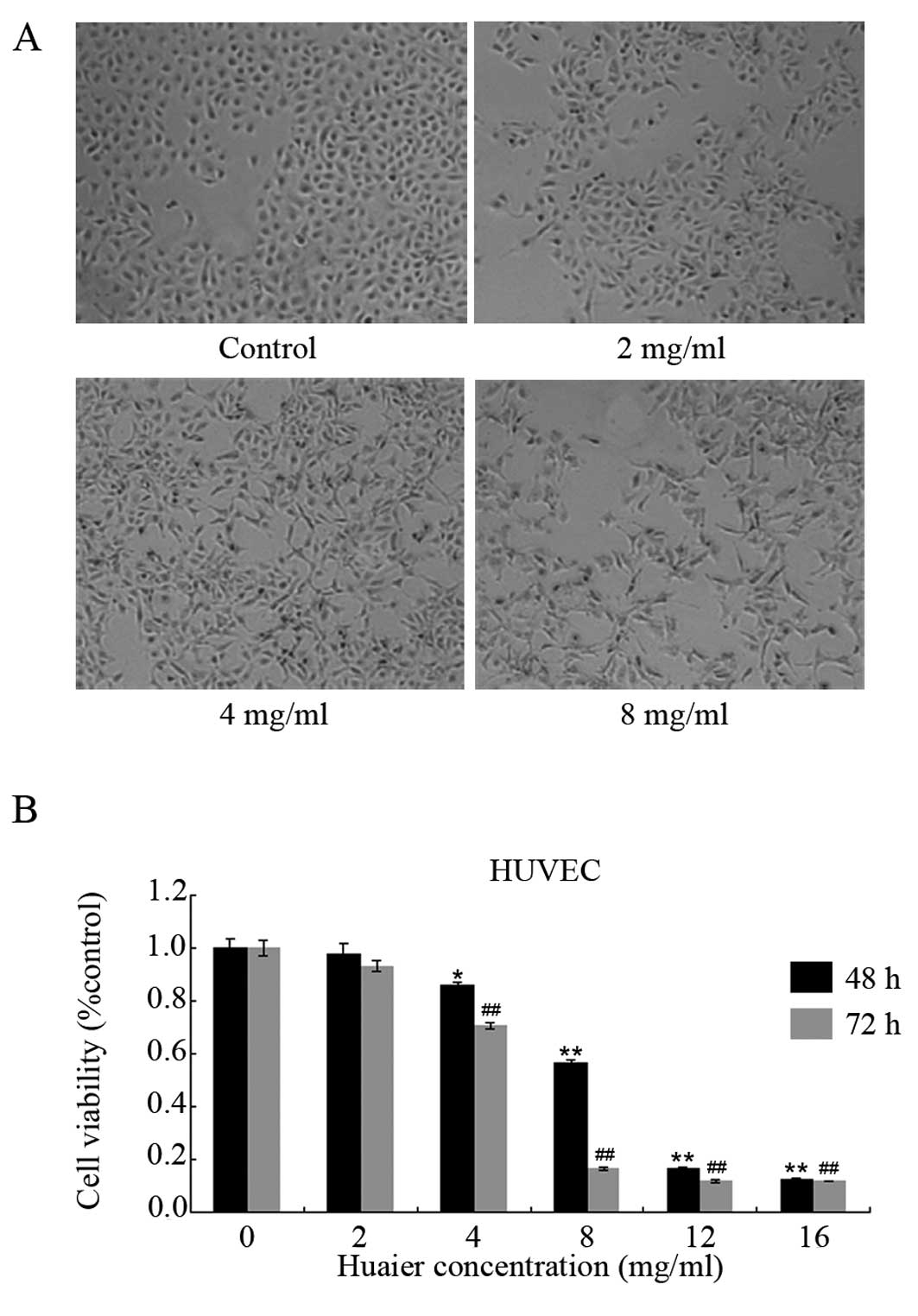
To investigate the effect of Huaier extract on
angiogenesis, we first observed the cell morphology of the HUVECs
after exposure to Huaier extract. Following 24 h of incubation with
various concentrations of Huaier extract, the morphological changes
in the HUVECs were observed (Fig.
1A). Obvious morphological changes were observed in the HUVECs.
Compared with the untreated cells, the majority of the cells in the
Huaier-treated groups became enlongated and star-shaped with sharp
outlines. These results suggest that Huaier extract causes cell
skeleton rearrangement in HUVECs.
We then examined the cell viability by using MTT
assay. As shown in Fig. 1B, Huaier
extract suppressed the proliferation of the HUVECs in a time- and
dose-dependent manner. The inhibitory rates of Huaier extract
varied from 2.3±3.7% to a maximum of 87.7±0.5% after 48-h
incubation, with an IC50 of 8.1±0.9 mg/ml. After
treatment with increasing concentrations of Huaier extract for 72
h, the viabilities of the HUVECs were suppressed by 6.9±2.2,
29.4±0.9, 83.7±0.5, 87.9±4.5 and 88.0±0.2%, respectively. A
significant reduction was firstly observed at 4 mg/ml
(P<0.05).
Suppressive effect on HUVEC proliferation
correlates with cell cycle arrest and apoptosis induction
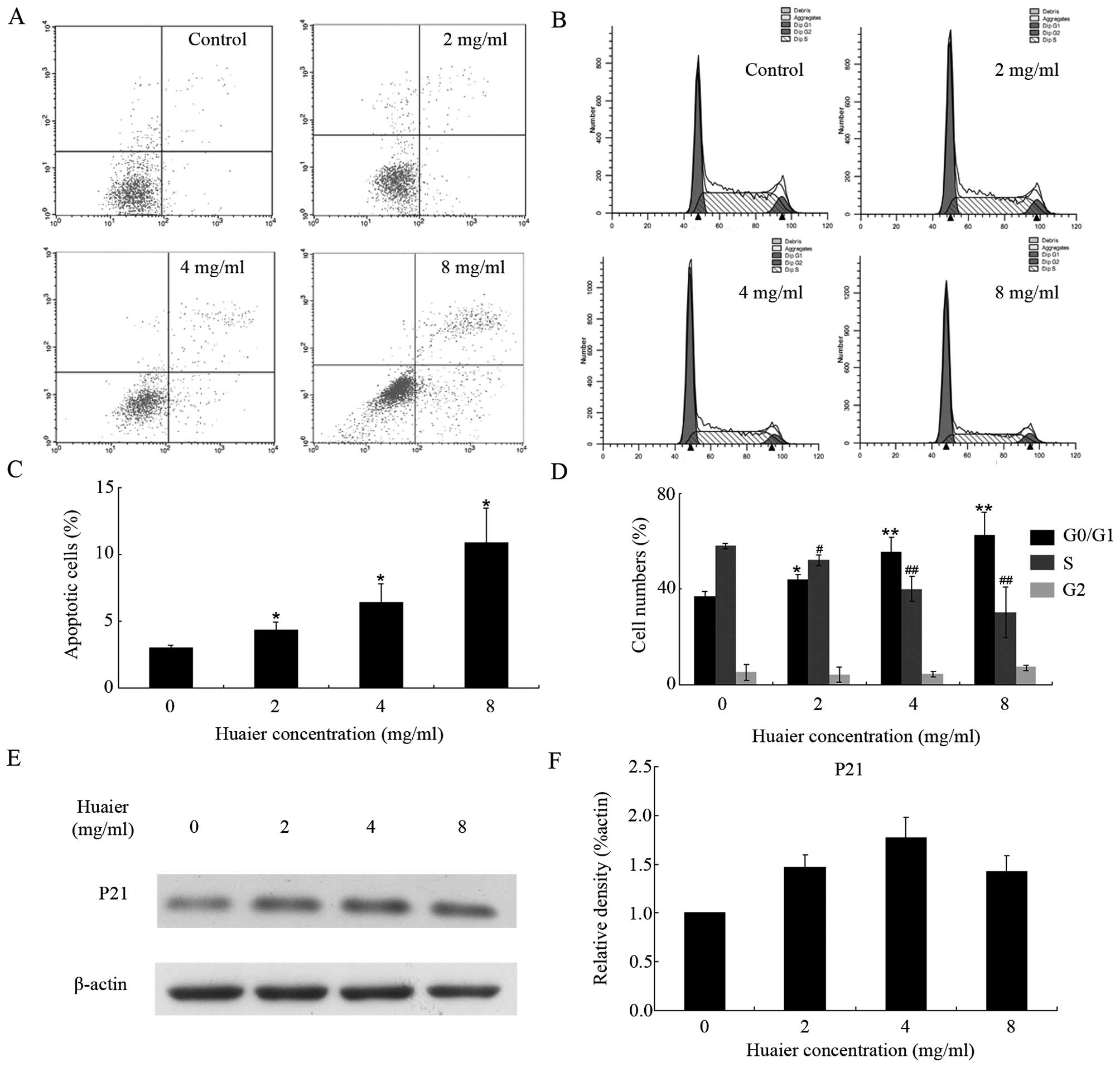
To further explore the underlying mechanism of the
antiproliferative effect of Huaier extract, we applied flow
cytometry to analyze the apoptotic rate and cell cycle distribution
after treatment with Huaier extract. The data demonstrated that the
ratios of apoptotic HUVECs were 4.4±0.6, 6.4±1.4 and 10.9±2.6% in
the presence of increasing concentrations of Huaier extract for 48
h, respectively (Fig. 2A and C). In
addition, as shown in Fig. 2B and
D, after 24 h of treatment, the proportion of cells at the
G0/G1 phase was dose-dependently increased (from 36.79±2.25% in
control group to 62.41±9.77% in 8 mg/ml Huaier group). Furthermore,
treatment with Huaier extract resulted in the accumulation of p21
with a maximum at 4 mg/ml (Fig. 2E and
F), and this partly contributed to the cell cycle arrest
mentioned above.
Effect of Huaier extract on motility of
HUVECs
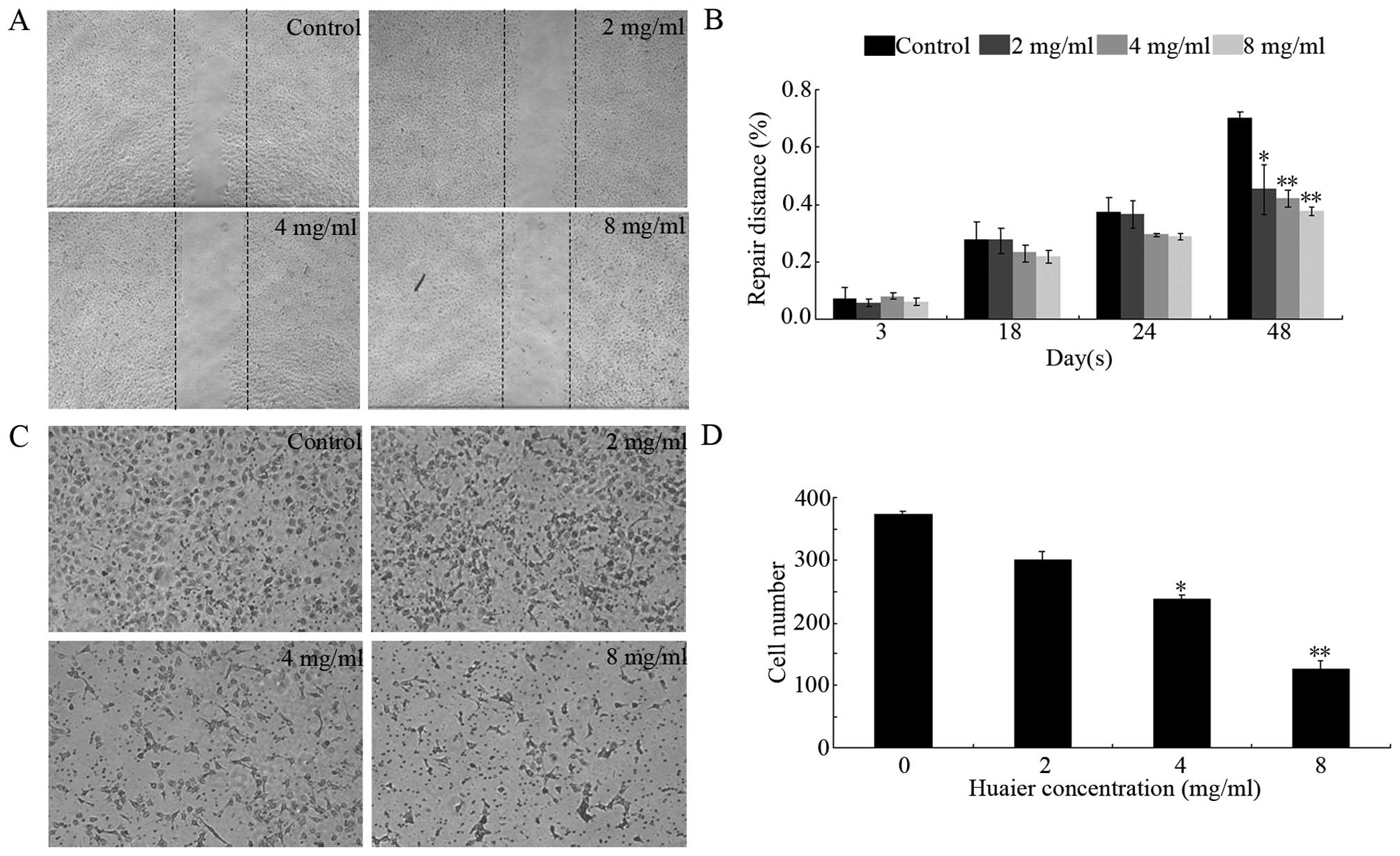
We then examined the influence of Huaier extract on
cell motility by using modified scratch assay (26) and cell migration assay (29). As shown in Fig. 3A and B, after treatment with Huaier
extract, the migration of the HUVECs was dose- and time-dependently
inhibited. This result was consistent with the results presented in
Fig. 3C and D. At 8 mg/ml, the
number of cells which had successfully migrated to the lower side
of the filter was reduced by 66.2±2.8% (P<0.01) after 24 h of
treatment with Huaier extract.
Effect of Huaier extract on angiogenesis
in vitro and ex vivo
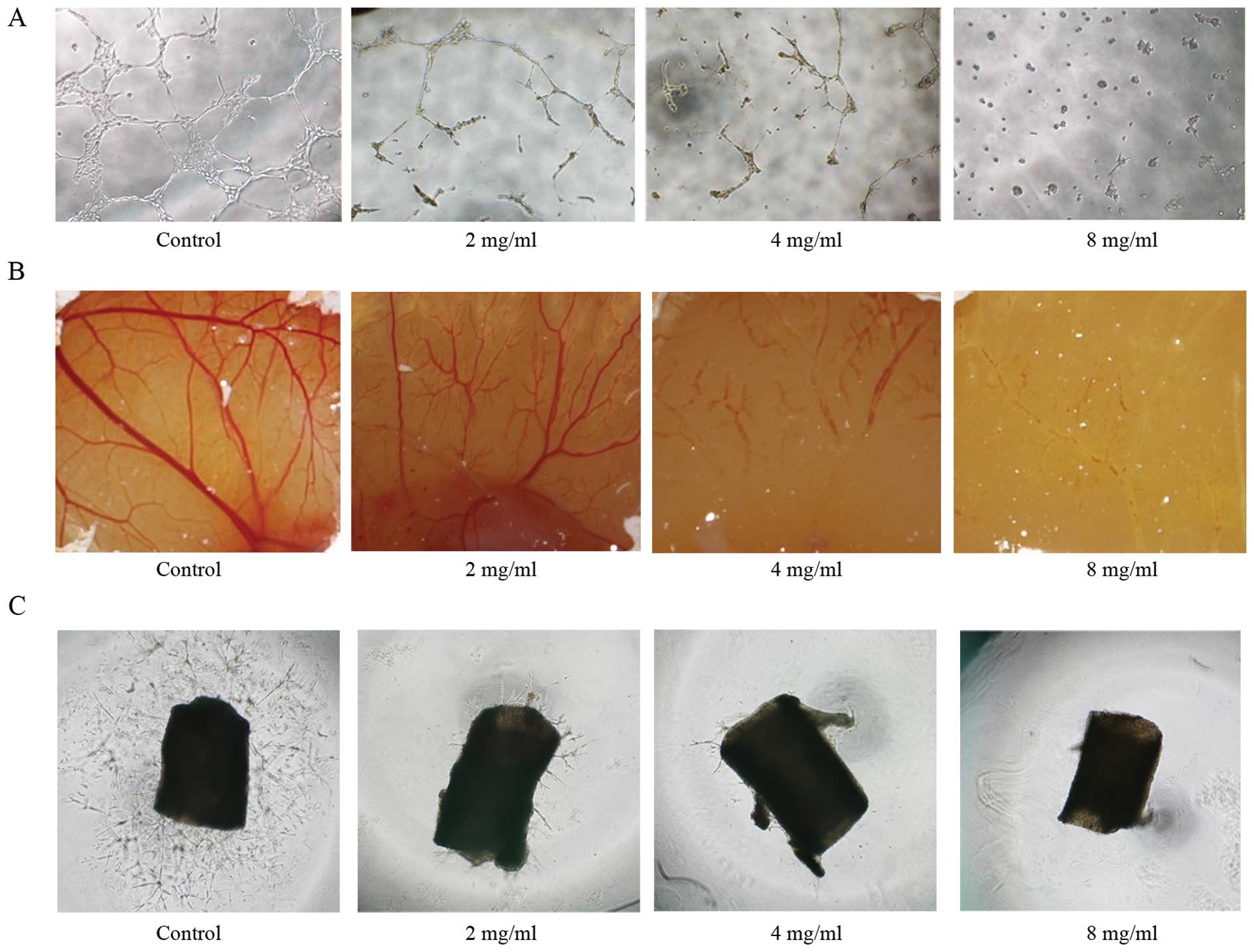
As an essential step for angiogenesis, the formation
of tube-like structures involves matrix degradation, rearrangement
and apoptosis of endothelial cells. As shown in Fig. 4A, untreated HUVECs formed organized
capillary tubes within 9 h. While in the presence of Huaier
extract, the HUVECs rounded up and rendered incomplete network
structures.
To verify the anti-angiogenic effect of Huaier
extract ex vivo, CAM assay and aortic ring assay were also
applied. On day 9 of embryo development, fertilized chick eggs were
treated with various concentrations of Huaier extract. After 24 h
of incubation, normal vascular pattern with numerous branchings was
observed in the control group. However, Huaier extract
significantly distorted the vasculature architecture on the
chorioallantoic membrane in a dose-dependent manner (Fig. 4B).
The results demonstrated that Huaier extract caused
a dramatic decrease in sprout length and density from the aortic
ring in a dose-dependent manner (Fig.
4C). In conclusion, Huaier exhibited anti-angiogenic activity
both in vitro and ex vivo.
Effect of Huaier extract on endothelial
signaling pathways
To identify whether Huaier extract can regulate
multiple molecules involved in angiogenesis, we used western blot
analysis to investigate the changes between the vehicle- and
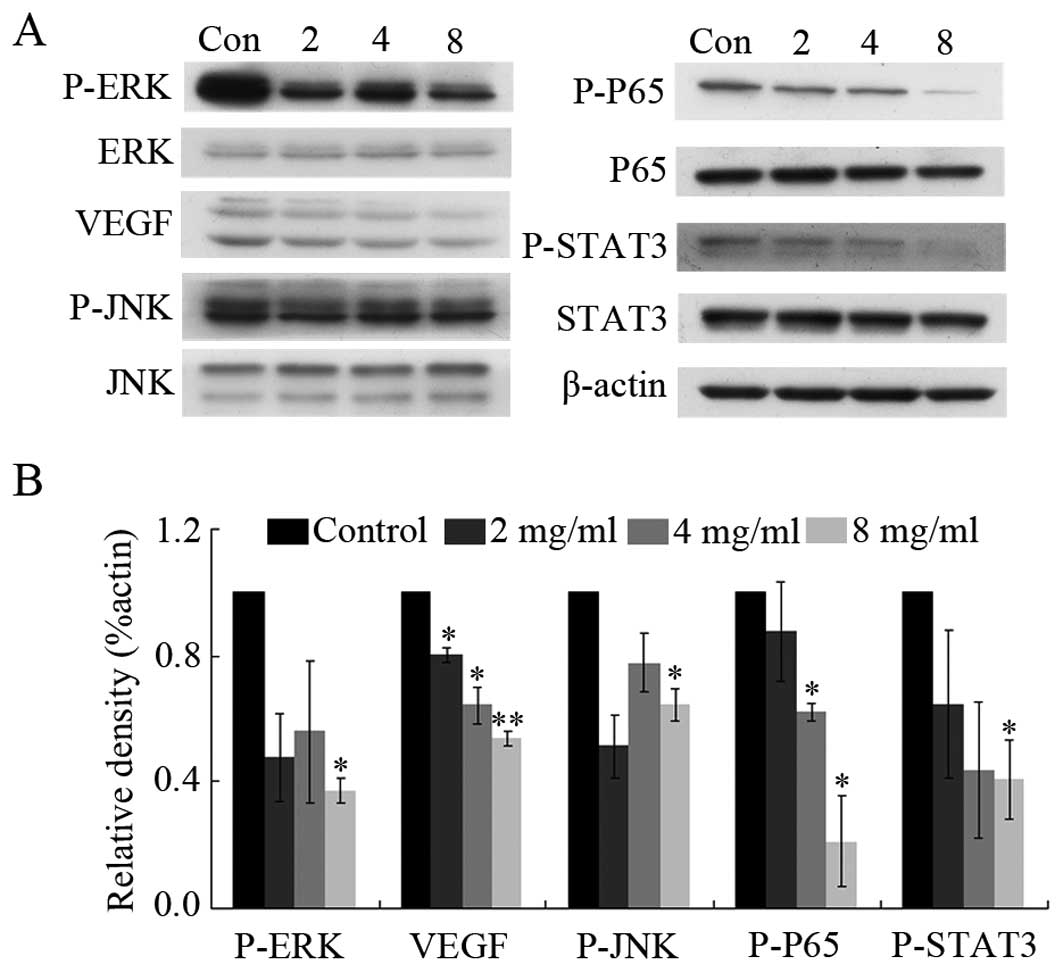
Huaier-treated groups. The results showed that Huaier extract
regulated the ERK pathway by down-regulating the phosphorylation of
ERK without affecting overall ERK expression levels (Fig. 5). In addition, the expression of
VEGF was significantly reduced by 46.4±2.1% after incubation with 8
mg/ml Huaier extract for 24 h (P<0.01). Similarly, Huaier
extract reduced the phosphorylation of JNK, STAT3 and p65. However,
no effect was observed on Akt signaling and Huaier extract was
unable to suppress the level of HIF (data not shown).
Huaier extract inhibits tumor growth in
vitro and in a xenograft model
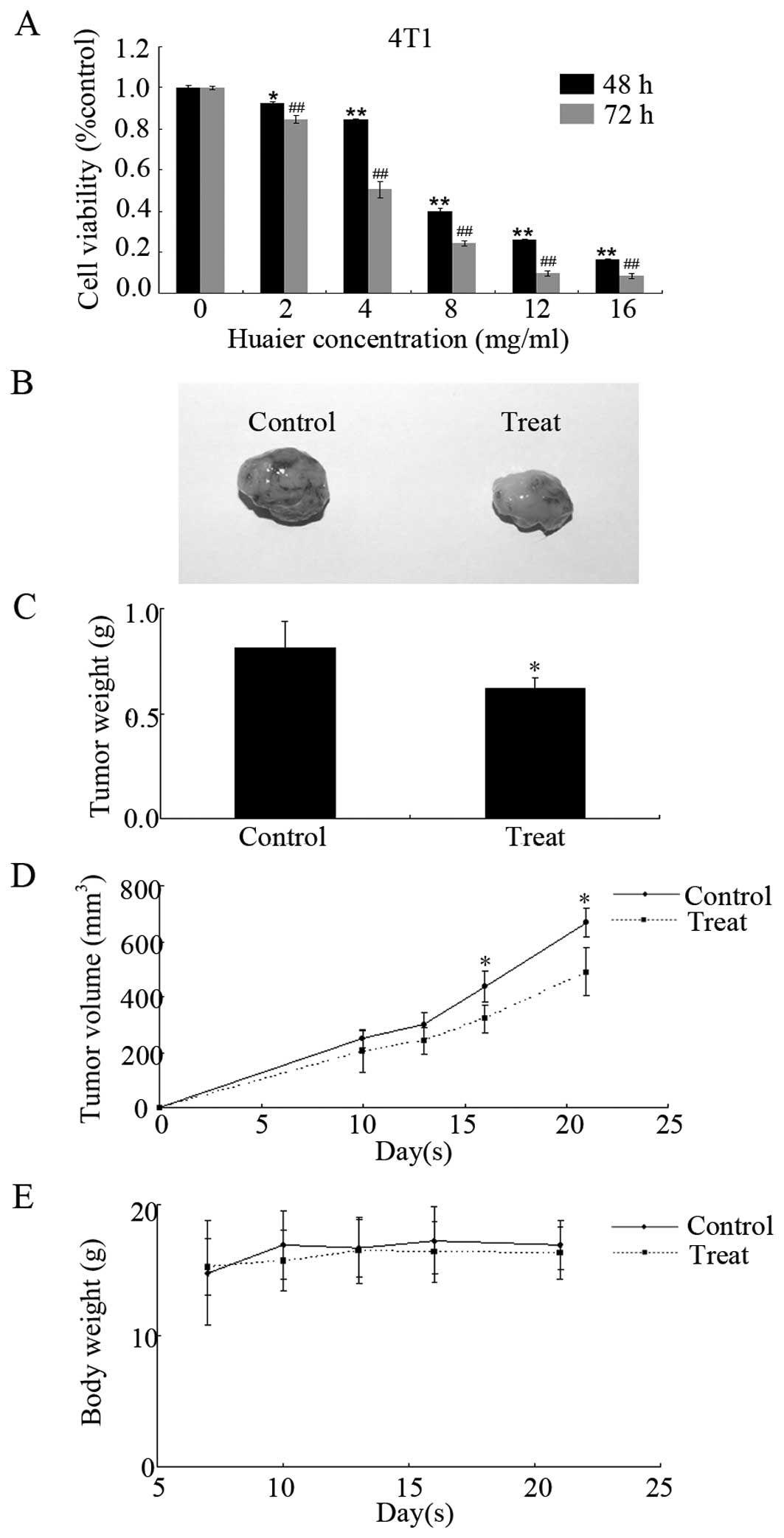
The antiproliferative effect of Huaier extract on
4T1 cells was examined by MTT assay. The results demonstrated that
Huaier extract significantly inhibited the proliferation of 4T1
cells in a time- and dose-dependent manner (Fig. 6A). With 2 mg/ml of Huaier extract, a
significant suppression on 4T1 cell proliferation was observed with
a 7.7±0.9% (at 48 h, P<0.05) and 15.2±1.8% (at 72 h, P<0.01)
reduction. The IC50 for 4T1 cells was 7.9±0.9 mg/ml (at
48 h) or 4.4±0.6 mg/ml (at 72 h). These data suggest that 4T1 cells
are more sensitive to Huaier extract than HUVECs.
Taking into account the anti-angiogenic and
antitumor effects of Huaier extract in vitro and ex
vivo, we then examined the antitumor effect of Huaier extract
in BALB/c mice. As shown in Fig. 6B and
C, the administration of Huaier extract by gavage delayed the
tumor volume at concentration of 2.5 g/kg per day. Compared with
the control group (667.0±52.6 mm3), tumor volumes in the
Huaier-treated group were significantly smaller (488.9±86.5
mm3, P<0.05) at day 21. The antitumor activity of
Huaier extract in vivo was confirmed by measuring the tumor
weights after the mice were sacrificed. The weights of tumors
isolated from the Huaier-treated groups were significantly
decreased by 23.6±5.1% (0.81±0.13 g in the control group, and
0.62±0.05 g in the treated group, P<0.05). However, we observed
no significant difference between the 2 groups in body weight,
which indicated no obvious toxicity to mice at the curative dose
(Fig. 6D). Our data prove the
antitumor effect of Huaier extract on 4T1 mouse mammary cancer
without causing marked toxicity in vivo.
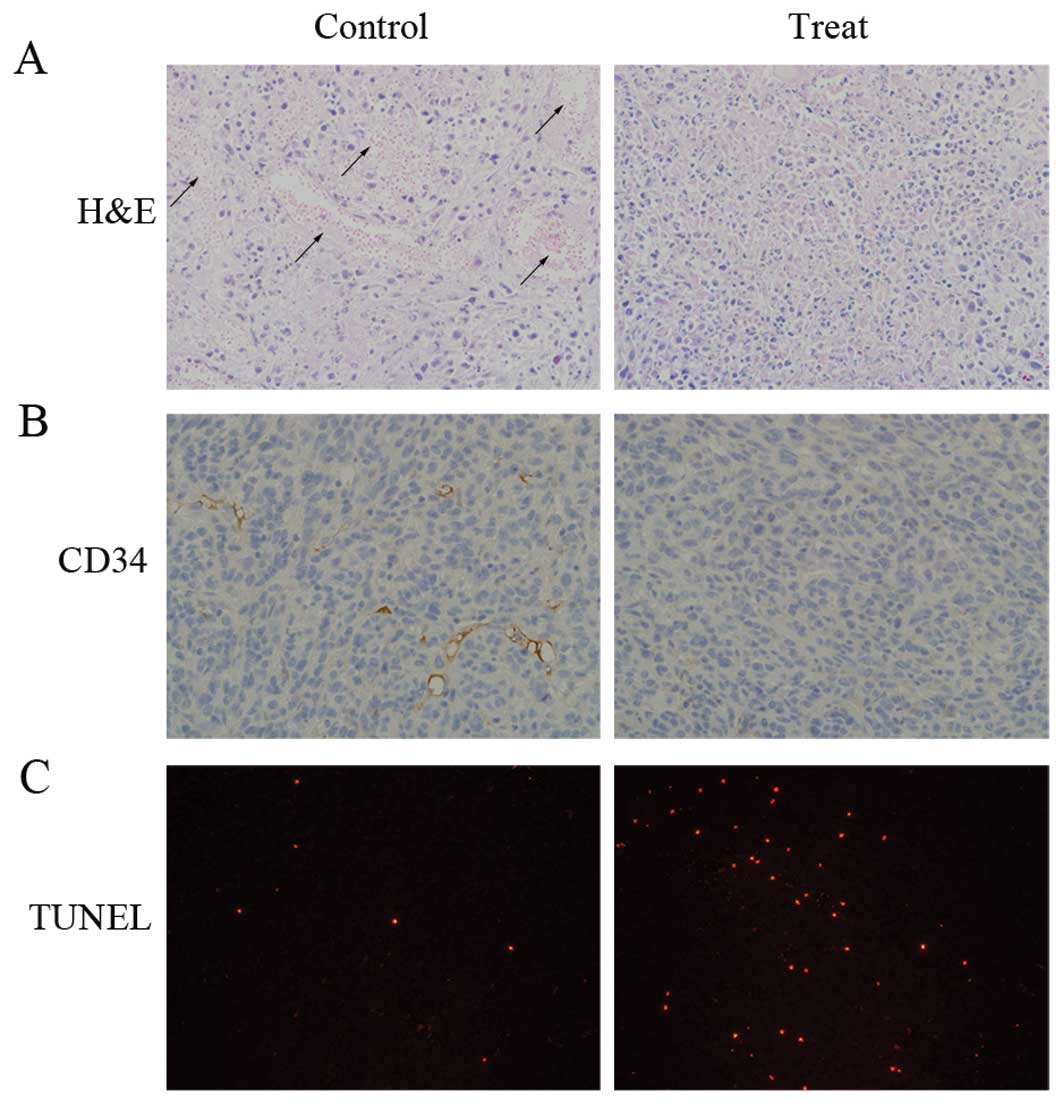
To elucidate the mechanisms behind the effect of
Huaier extract in vivo, the tumor tissues from the animal
models were stained with H&E, CD34 and TUNEL (Fig. 7). The H&E-stained sections
revealed large areas of necrosis occurring in both groups. The
necrotic part in the untreated tumors was hemorrhagic. However, an
ischemic type of necrosis with little or no blood was observed in
the Huaier-treated tumors. We then performed TUNEL staining to
explore the apoptotic effect induced by Huaier extract in
vivo. The increased ratio of TUNEL-positive cells clearly
demonstrated that the induction of apoptosis was involved in the
antitumor activity of Huaier extract. Furthermore, CD34 staining
was performed to evaluate the MVD in the tumor. Under microscopic
analysis, a marked decrease in the expression of CD34 was observed
in the treated group compared with the control group.
Discussion
Conventional treatments for cancer patients include
surgery, radiotherapy and chemotherapy. Recently, some alternative
treatments, such as gene therapy and targeted therapy have
attracted some attention. However, these therapies are usually
unaffordable for most patients and have limited efficiency and
serious side-effects. TCM has been used in China for thousands of
years. Along with its anticancer effect, TCM has been widely
applied to reduce toxic side-effects, improve quality of life,
enhance immune function as well as prevent recurrence and
metastasis for cancer patients (30). In recent years, TCM has been
increasingly accepted and studied worldwide. For example, ‘Chong
Lou Fu Fang’ was proven to improve the effect of chemotherapeutic
agents on gastric cancer cells (31). Treatment with Iscador, extracted
from mistletoe, resulted in a better survival among cancer patients
(32). Despite of the ever-growing
interest, rigorous and systematic pre-clinical evaluation is
required for the globalization of TCM.
As an indispensable step for metastasis,
angiogenesis is a promising target in anticancer therapy.
Anti-angiogenic agents exert their effect in 2 ways (33,34).
Direct inhibitors disrupt the proliferation, migration and
differentiation of endothelial cells. On the other hand, indirect
inhibitors interfere with the communication between tumor cells and
endothelial cells by suppressing the expression of pro-angiogenic
cytokines or blocking the binding of factors with their receptors.
Although anti-angiogenic agents exhibit obvious antitumor
activities, serious side-effects are often observed following
treatment, such as hypertension, impaired wound healing,
haemorrhaging and thrombosis (35).
Therefore, novel natural herbs, such as grape seed extract
(36) and dihydroartemisinin (DHA)
(37), which have been proven to be
safe for humans, are recognized as sources of effective antitumor
agents.
Huaier, one of the most popular medical fungi in
China, belongs to the Polyporaceae family and has been used as a
TCM for almost 1,600 years. In the present study, the
anti-angiogenic and antitumor effects of Huaier extract were
assessed using HUVECs and 4T1 cells as a model. The results of MTT
assay demonstrated that Huaier extract significantly attenuated the
proliferation of HUVECs and 4T1 cells in a time- and dose-dependent
manner (P<0.05). To our knowledge, this was the first study that
investigated and demonstrated Huaier extract inhibited the
proliferation of endothelial cells and mouse mammary tumor cells.
Importantly, Huaier extract was more cytotoxic for the tumor cells
with a lower IC50. In addition, the inhibition of the
proliferation of HUVECs was caused by cell cycle arrest and
pro-apoptotic activities. P21 is a well-studied cyclin-dependent
kinase (CDK) inhibitor. It inhibits the activity of the cyclin-CDK2
or -CDK1 complex, leading to G1/S cell cycle arrest (38). After incubation with Huaier extract
for 24 h, the protein level of p21 was increased. This suggested
that Huaier extract caused cell cycle arrest in the HUVECs partly
by promoting p21 accumulation.
In order to reveal the potential signaling pathways
underlying the potent anti-angiogenic activity of Huaier extract,
we investigated the expression of some angiogenic molecules. As one
of the key pro-angiogenic molecules, VEGF is a highly specific
mitogen for vascular endothelial cells and a potent vascular
permeability enhancer. During the process of angiogenesis, VEGF is
responsible for endothelial cell proliferation, migration, and
antiapoptosis (39). Certain
studies have demonstrated that the overexpression of VEGF in cancer
patients is associated with a poor prognosis and decreased survival
(40). As shown in Fig. 5, Huaier extract inhibited the
expression of VEGF in a concentration-dependent manner. Among the
upstream pathways that mediate VEGF expression, PI3K/AKT and
MEK/ERK play important roles (41).
As shown in Fig. 5, Huaier extract
dose-dependently suppressed the activation of ERK without exerting
any influence on AKT. Richard et al(42), as well as a previous study (43) demonstrated that active p42/p44 MAPK
increased HIF-1-dependent transcriptional activity, which finally
increased the expression of downstream proteins, including VEGF.
Recently, the addition of U0126, a known selective inhibitor of
MAPK/ERK kinase, was shown to inhibit the tube formation and induce
apoptosis in HUVECs (44).
Therefore, we hypothesized that Huaier extract could suppress the
activation of ERK, inhibit VEGF expression and eventually exhibit
anti-angiogenic activity. In addition, JNK, STAT3 and NF-κB are
important pathways that regulate cell migration (45–47).
In this study, we provide evidence that Huaier extract inhibits the
phosphorylation of JNK, STAT3 and p65 (the major component in NF-κB
complex). These data reveal the mechanisms underlying the
anti-angiogenic activity of Huaier extract.
In addition to the anti-angiogenic and antitumor
activities, the results from animal studies showed no significant
adverse effects of Huaier extract on the body weights of the
treated mice. As shown in Fig. 6E,
in the treated group, gavage with 50 mg of Huaier extract per day
did not cause body weight loss compared to the control group.
In conclusion, Huaier extract is a potent
anti-angiogenic and antitumor agent. A gavage dose of 2.5 g/kg per
day given to the mice was safe and effective against angiogenesis
and solid tumor growth. These results highlight the possible
application of Huaier extract in cancer chemoprevention and lay a
solid foundation for clinical use in humans. However, further
investigations are required to assess the detailed mechanisms, the
responsible component(s) and to ascertain its beneficial role in
the clinical setting.
Acknowledgements
This study was supported by grants from the Program
for New Century Excellent Talents in the University of China and
the National Natural Science Foundation of China to Professor
Qifeng Yang (No. 81072150 and 81172529).
References
|
1
|
WHO. Cancer. http://www.who.int/mediacentre/factsheets/fs297/en/index.html.
2012
|
|
2
|
DeSantis C, Siegel R, Bandi P and Jemal A:
Breast cancer statistics, 2011. CA Cancer J Clin. 61:409–418. 2011.
View Article : Google Scholar
|
|
3
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
4
|
IARC. Cancer Epidemiology Database.
GLOBOCAN. 2002
|
|
5
|
IARC. Cancer Epidemiology Database.
GLOBOCAN. 2008
|
|
6
|
Monsuez JJ, Charniot JC, Vignat N and
Artigou JY: Cardiac side-effects of cancer chemotherapy. Int J
Cardiol. 144:3–15. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bhat TA and Singh RP: Tumor angiogenesis -
a potential target in cancer chemoprevention. Food Chem Toxicol.
46:1334–1345. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sagar SM, Yance D and Wong R: Natural
health products that inhibit angiogenesis: a potential source for
investigational new agents to treat cancer - Part 1. Curr Oncol.
13:14–26. 2006.
|
|
9
|
Risau W: Mechanisms of angiogenesis.
Nature. 386:671–674. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Carmeliet P and Jain RK: Angiogenesis in
cancer and other diseases. Nature. 407:249–257. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Folkman J: Angiogenesis in cancer,
vascular, rheumatoid and other disease. Nat Med. 1:27–31. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hanahan D and Folkman J: Patterns and
emerging mechanisms of the angiogenic switch during tumorigenesis.
Cell. 86:353–364. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tassi E and Wellstein A: Tumor
angiogenesis: initiation and targeting - therapeutic targeting of
an FGF-binding protein, an angiogenic switch molecule, and
indicator of early stages of gastrointestinal adenocarcinomas.
Cancer Res Treat. 38:189–197. 2006. View Article : Google Scholar
|
|
14
|
Weidner N, Carroll P, Flax J, Blumenfeld W
and Folkman J: Tumor angiogenesis correlates with metastasis in
invasive prostate carcinoma. Am J Pathol. 143:401–409.
1993.PubMed/NCBI
|
|
15
|
Maeda K, Chung YS, Takatsuka S, et al:
Tumor angiogenesis as a predictor of recurrence in gastric
carcinoma. J Clin Oncol. 13:477–481. 1995.PubMed/NCBI
|
|
16
|
Choi HJ, Hyun MS, Jung GJ, Kim SS and Hong
SH: Tumor angiogenesis as a prognostic predictor in colorectal
carcinoma with special reference to mode of metastasis and
recurrence. Oncology. 55:575–581. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Frank RE, Saclarides TJ, Leurgans S,
Speziale NJ, Drab EA and Rubin DB: Tumor angiogenesis as a
predictor of recurrence and survival in patients with node-negative
colon cancer. Ann Surg. 222:695–699. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Folkman J and Cotran R: Relation of
vascular proliferation to tumor growth. Int Rev Exp Pathol.
16:207–248. 1976.PubMed/NCBI
|
|
19
|
Weidner N, Semple JP, Welch WR and Folkman
J: Tumor angiogenesis and metastasis - correlation in invasive
breast carcinoma. N Engl J Med. 324:1–8. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Keshavarz M, Mostafaie A, Mansouri K,
Bidmeshkipour A, Motlagh HR and Parvaneh S: In vitro and ex vivo
antiangiogenic activity of Salvia officinalis. Phytother
Res. 24:1526–1531. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lu J, Zhang K, Nam S, Anderson RA, Jove R
and Wen W: Novel angiogenesis inhibitory activity in cinnamon
extract blocks VEGFR2 kinase and downstream signaling.
Carcinogenesis. 31:481–488. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nassar ZD, Aisha AF, Ahamed MB, et al:
Antiangiogenic properties of Koetjapic acid, a natural triterpene
isolated from Sandoricum koetjaoe Merr. Cancer Cell Int.
11:122011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ren J, Zheng C, Feng G, et al: Inhibitory
effect of extract of fungi of Huaier on hepatocellular carcinoma
cells. J Huazhong Univ Sci Technolog Med Sci. 29:198–201. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu X, Wei Q, Wang K, et al: Anticancer
effects of Huaier are associated with down-regulation of P53. Asian
Pac J Cancer Prev. 12:2251–2254. 2011.PubMed/NCBI
|
|
25
|
Zhang N, Kong X, Yan S, Yuan C and Yang Q:
Huaier aqueous extract inhibits proliferation of breast cancer
cells by inducing apoptosis. Cancer Sci. 101:2375–2383. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liang CC, Park AY and Guan JL: In vitro
scratch assay: a convenient and inexpensive method for analysis of
cell migration in vitro. Nat Protoc. 2:329–333. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wu J, Lu Y, Gao M and Zhang W: The
advances of angiogenesis assay models. Chin Pharmacol Bull.
24:112008.
|
|
28
|
Kruger EA, Duray PH, Tsokos MG, et al:
Endostatin inhibits microvessel formation in the ex vivo rat aortic
ring angiogenesis assay. Biochem Biophys Res Commun. 268:183–191.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Patsouris D, Neels JG, Fan W, Li PP,
Nguyen MT and Olefsky JM: Glucocorticoids and thiazolidinediones
interfere with adipocyte-mediated macrophage chemotaxis and
recruitment. J Biol Chem. 284:31223–31235. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hu B, Du Q, Shen K and Xu L: Principles
and scientific basis of traditional Chinese medicine in cancer
treatment. J Bioanal Biomed S. 6:22012.
|
|
31
|
Liu Y, Ling Y, Hu W, et al: The herb
medicine formula ‘Chong Lou Fu Fang’ increases the cytotoxicity of
chemotherapeutic agents and down-regulates the expression of
chemotherapeutic agent resistance-related genes in human gastric
cancer cells in vitro. Evid Based Complement Alternat Med.
2011:8342312011.
|
|
32
|
Ostermann T, Raak C and Bussing A:
Survival of cancer patients treated with mistletoe extract
(Iscador): a systematic literature review. BMC Cancer. 9:4512009.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Abdollahi A, Lipson KE, Sckell A, et al:
Combined therapy with direct and indirect angiogenesis inhibition
results in enhanced antiangiogenic and antitumor effects. Cancer
Res. 63:8890–8898. 2003.PubMed/NCBI
|
|
34
|
Folkman J, Hahnfeldt P and Hlatky L: The
logic of anti-angiogenic gene therapy. Cold Spring Harbor Monograph
Archive. 36:527–543. 1999.
|
|
35
|
Kamba T and McDonald DM: Mechanisms of
adverse effects of anti-VEGF therapy for cancer. Br J Cancer.
96:1788–1795. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wen W, Lu J, Zhang K and Chen S: Grape
seed extract inhibits angiogenesis via suppression of the vascular
endothelial growth factor receptor signaling pathway. Cancer Prev
Res. 1:554–561. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang SJ, Sun B, Cheng ZX, et al:
Dihydroartemisinin inhibits angiogenesis in pancreatic cancer by
targeting the NF-kappaB pathway. Cancer Chemother Pharmacol.
68:1421–1430. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Brugarolas J, Moberg K, Boyd SD, Taya Y,
Jacks T and Lees JA: Inhibition of cyclin-dependent kinase 2 by p21
is necessary for retinoblastoma protein-mediated G1 arrest after
γ-irradiation. Proc Natl Acad Sci USA. 96:1002–1007.
1999.PubMed/NCBI
|
|
39
|
Neufeld G, Cohen T, Gengrinovitch S and
Poltorak Z: Vascular endothelial growth factor (VEGF) and its
receptors. FASEB J. 13:9–22. 1999.PubMed/NCBI
|
|
40
|
Paley PJ, Staskus KA, Gebhard K, et al:
Vascular endothelial growth factor expression in early stage
ovarian carcinoma. Cancer. 80:98–106. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yang XM, Wang YS, Zhang J, et al: Role of
PI3K/Akt and MEK/ERK in mediating hypoxia-induced expression of
HIF-1alpha and VEGF in laser-induced rat choroidal
neovascularization. Invest Ophthalmol Vis Sci. 50:1873–1879. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Richard DE, Berra E, Gothie E, Roux D and
Pouyssegur J: p42/p44 mitogen-activated protein kinases
phosphorylate hypoxia-inducible factor 1alpha (HIF-1alpha) and
enhance the transcriptional activity of HIF-1. J Biol Chem.
274:32631–32637. 1999. View Article : Google Scholar
|
|
43
|
Wang FS, Wang CJ, Chen YJ, et al: Ras
induction of superoxide activates ERK-dependent angiogenic
transcription factor HIF-1alpha and VEGF-A expression in shock
wave-stimulated osteoblasts. J Biol Chem. 279:10331–10337. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kunimasa K, Ahn MR, Kobayashi T, et al:
Brazilian propolis suppresses angiogenesis by inducing apoptosis in
tube-forming endothelial cells through inactivation of survival
signal ERK1/2. Evid Based Complement Alternat Med. 2011:8707532011.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Huang C, Rajfur Z, Borchers C, Schaller MD
and Jacobson K: JNK phosphorylates paxillin and regulates cell
migration. Nature. 424:219–223. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yahata Y, Shirakata Y, Tokumaru S, et al:
Nuclear translocation of phosphorylated STAT3 is essential for
vascular endothelial growth factor-induced human dermal
microvascular endothelial cell migration and tube formation. J Biol
Chem. 278:40026–40031. 2003. View Article : Google Scholar
|
|
47
|
Collins T, Read MA, Neish AS, Whitley MZ,
Thanos D and Maniatis T: Transcriptional regulation of endothelial
cell adhesion molecules: NF-kappa B and cytokine-inducible
enhancers. FASEB J. 9:899–909. 1995.PubMed/NCBI
|