Introduction
In general, apoptosis may be initiated by either an
extrinsic (death receptor-mediated) or an intrinsic
(mitochondrial-mediated) pathway. The extrinsic pathway functions
via death receptors on the cell surface that may directly activate
caspase-8. The intrinsic pathway regulates apoptotic cascades that
occur as a result of the convergence of the signaling at the
mitochondrion, which results in the alteration of the mitochondrial
membrane potentials (MMP, ΔΨm), the release of
cytochrome c into the cytosol and the activation of
caspase-9 (1). Mitochondria are the
major energy generators of adenosine triphosphate (ATP) by
oxidative phosphorylation, and mitochondrial-mediated apoptosis
occurs in response to a wide range of stimuli. Reactive oxygen
species (ROS), which are the byproducts of normal cellular
oxidative processes, are generated in and around mitochondria, and
it has been suggested that they are involved in regulating the
process involved in the initiation of apoptotic signaling (2–4).
Therefore, constitutively elevated levels of cellular oxidative
stress and the dependence on ROS signaling may represent a redox
vulnerability of malignancies, which may be targeted by
chemotherapeutic intervention using redox modulators; both anti-
and pro-oxidant agents have been shown to exert anticancer activity
(5,6). Consistent with the role of
mitochondria in the control of cell death, survival or apoptotic
factors such as Bcl-2 and Bax act on the organelle to prevent or
facilitate the release of apoptogenic factors, such as cytochrome
c (5–7).
Garlic (Allium sativum), used for both
culinary and medical purposes in Asia for many years, has come
under intensive study in the past few decades because of its
ability to impart a beneficial effect on several human disease
processes (8). Garlic derivatives
have various biological properties, such as anti-inflammatory
(9), antimicrobial (10), antithrombotic (11), antihypertensive (12), antihyperlipidemic (13), antihyperglycemic (8) and immune system enhancement (14). Moreover, recent studies have
indicated that garlic compounds inhibit the growth of cancer cells,
and that this inhibition of growth is associated with cell cycle
arrest and the stimulation of apoptosis (8,15–23).
However, the biochemical mechanisms underlying garlic clove
extract-induced apoptosis in cancer cells have not yet been
explored.
The primary purpose of this study was to evaluate
the role of mitochondria in apoptosis induced by hexane extracts of
garlic cloves (HEGCs), using a Hep3B human hepatocarcinoma cell
line. As such, we examined whether ROS were critical mediators of
HEGC-induced Hep3B cell death and determined the sequence of events
leading to the activation of downstream caspases and apoptosis. In
this report, we present evidence that HEGCs elicit ROS, which in
turn triggers a decrease in MMP, consequently leading to caspase
activation.
Materials and methods
Plant materials and preparation of hexane
extract
The garlic cloves were purchased directly from the
Danyang Food Company in Danyang, Korea, in January 2009. The fresh
garlic cloves (100 g) were macerated in a blender, extracted with
300 ml of 80% MeOH 3 times, and then filtered with Whatman no. 2
filter paper. The extracted garlic solution was successively
partitioned with 300 ml of hexane, chloroform and n-butanol 3
times. The upper layer suspension was filtered and evaporated under
reduced pressure at 45°C and then lyophilized. A yellow, oily
residue of the hexane extract (285 mg) was obtained. The remaining
aqueous layer was partitioned again, with chloroform and n-butanol
sequentially, to yield chloroform (155 mg) and n-butanol extract
fractions (2,223 mg). HEGCs were used in this study. Hexane,
chloroform, and methanol were purchased from Fisher Scientific,
Ltd. (Pittsburgh, PA, USA).
Cell culture and viability assay
Hep3B cells were obtained from the American Type
Culture Collection (Rockville, MD, USA). The cells were cultured in
RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS,
Gibco-BRL, Gaithersburg, MD, USA) and 1% penicillin-streptomycin at
37°C in a humid environment containing 5% CO2. For the
cell viability study, Hep3B cells were grown to 70% confluence and
treated with various concentrations of HEGCs. The control cells
were supplemented with complete media containing 0.1% DMSO (vehicle
control). Following treatment, cell viability was determined using
the MTT assay, which is based on the conversion of MTT to
MTT-formazan by mitochondrial enzymes. The effect of HEGCs on the
inhibition of cell growth was assessed as the percentage of cell
viability, where the vehicle-treated cells were considered 100%
viable.
Nuclear staining with DAPI
After treating the cells with HEGCs for 48 h, the
cells were harvested, washed in ice-cold phosphate-buffered saline
(PBS) and fixed with 3.7% paraformaldehyde (Sigma) in PBS for 10
min at room temperature. The fixed cells were washed with PBS and
stained with a 4,6-diamidino-2-phenylindole (DAPI, Sigma) solution
for 10 min at room temperature. The nuclear morphology of the cells
was examined using fluorescence microscopy (Carl Zeiss,
Germany).
DNA fragmentation assay
The cells were treated with different concentrations
of HEGCs for 48 h and lysed on ice in a buffer containing 10 mM
Tris-HCl (pH 7.4), 150 mM NaCl, 5 mM EDTA and 0.5% Triton X-100 for
30 min. The lysates were vortexed and cleared by centrifugation at
10,000 × g for 20 min. The fragmented DNA in the supernatant was
extracted using an equal volume of neutral
phenol:chloroform:isoamyl alcohol (25:24:1, v/v/v) and analyzed
electrophoretically on 1% agarose gel containing ethidium bromide
(EtBr, Sigma) (24).
Flow cytometric analysis for measurement
of sub-G1 phase
The cells were harvested and washed once with PBS,
fixed in ice-cold 70% ethanol and stored at 4°C. Prior to analysis,
the cells were washed once again with PBS, suspended in 1 ml of a
cold propidium iodide (PI, Sigma) solution containing 100 μg/ml
RNase A, 50 μg/ml PI, 0.1% (w/v) sodium citrate and 0.1% (v/v)
NP-40, and further incubated on ice for 30 min in the dark. Flow
cytometric analyses were carried out using a flow cytometer
(FACSCalibur, Becton-Dickinson, San Jose, CA, USA). CellQuest
software was used to determine the relative DNA content, based on
the presence of a red fluorescence.
Measurement of intracellular ROS and MMP
(ΔΨm)
ROS production was monitored using the stable
non-polar dye 2,7 dichlorofluorescein diacetate (DCFH-DA), which
readily diffuses into cells (25).
The cells were seeded in 24-well plates and incubated in the
absence or presence of HEGCs for different periods of time, after
which they were incubated with 10 μM DCFH-DA for 30 min. ROS
production in the cells was monitored using a flow cytometer with
CellQuest Software. To measure the MMP, the dual-emission
potential-sensitive probe 5,5V,6,6V-tetrachloro-1,1V,3,3
V-tetraethyl-imidacarbocyanine iodide (JC-1, Sigma), was used.
After treatment with HEGC, 5×105 cells were collected,
stained with 2 mg/l JC-1 at 37°C for 20 min and then analyzed with
a flow cytometer (26).
Protein extraction and western
blotting
The cells were harvested and lysed. The protein
concentrations were measured using a Bio-Rad protein assay (Bio-Rad
Laboratories, Hercules, CA, USA), according to the manufacturer’s
instructions. For western blot analysis, an equal amount of protein
was subjected to electrophoresis on SDS-polyacrylamide gel and
transferred by electroblotting onto a nitrocellulose membrane
(Schleicher & Schuell, Keene, NH, USA). The blots were probed
with the desired antibodies for 1 h, incubated with the diluted
enzyme-linked secondary antibody and visualized by enhanced
chemiluminescence (ECL), according to the manufacturer’s
instructions (Amersham Corp., Arlington Heights, IL, USA). The
primary antibodies were purchased from Santa Cruz Biotechnology
Inc. (Santa Cruz, CA, USA) and Cell Signaling Technology, Inc.
(Boston, MA, USA). The peroxidase-labeled donkey anti-rabbit
immunoglobulin and peroxidase-labeled sheep anti-mouse
immunoglobulin were purchased from Amersham Corp. (27).
In vitro caspase activity assay
Caspase activity was determined by a colorimetric
assay using a caspase-3, -8 and -9 activation kit, according to the
manufacturer’s instructions (R&D Systems, Minneapolis, MN,
USA). Briefly, the cells were lysed in a lysis buffer for 30 min in
an ice bath. The supernatants were collected and incubated at 37°C
with the reaction buffer supplied, which contained dithiothreitol
and substrates, Asp-Glu-Val-Asp (DEVD)-p-nitroaniline (pNA) for
caspase-3, Ile-Glu-Thr-Asp (IETD)-pNA for caspase-8 and
Leu-Glu-His-Asp (LEHD)-pNA for caspase-9. The optical density of
the reaction mixture was quantified spectrophotometrically at a
wavelength of 405 nm (28).
Statistical analysis
The data are expressed as the means ± SD. A
statistical comparison was performed using one-way ANOVA, followed
by a Fisher’s test. The significant differences between the groups
were determined using an unpaired Student’s t-test. A P-value
<0.05 was considered to indicate a statistically significant
difference.
Results
HEGCs induce apoptosis in Hep3B
cells
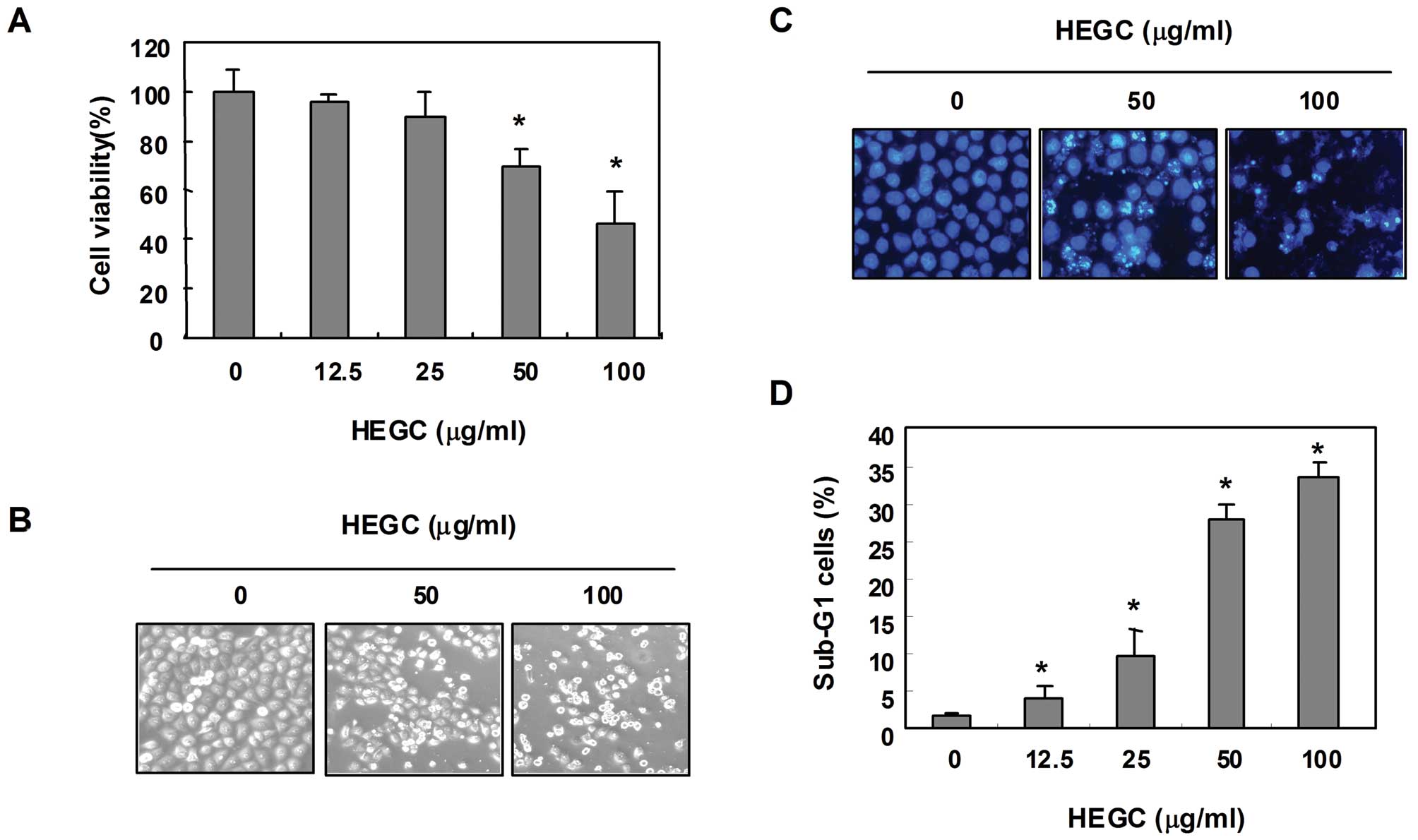
In order to evaluate the ability of HEGCs to inhibit
the growth of Hep3B cells, the cells were exposed to different
concentrations of HEGCs, after which MTT assays were performed.
After being cultured in the presence of 50 and 100 μg/ml HEGC for
48 h, cell viability decreased by ~70 and 58%, respectively, when
compared to that of the controls (Fig.
1A), which was associated with characteristic features of cell
shrinking, rounding and detachment (Fig. 1B). Further experiments were then
conducted to determine whether HEGCs inhibit the proliferation of
Hep3B cells through the induction of apoptosis. As shown in
Fig. 1C, DAPI staining revealed
that the number of nuclei showing chromatin condensation and the
formation of apoptotic bodies increased in cells cultured with
HEGCs in a concentration-dependent manner. Therefore, the degree of
apoptosis was determined by analyzing the amount of sub-G1 DNA that
was in the cells treated with HEGCs, using a flow cytometer. As
shown in Fig. 1D, the addition of
HEGCs resulted in the increased accumulation of cells in the sub-G1
phase, which was similar to the results observed in the
HEGC-induced loss of cell viability and formation of apoptotic
bodies. These results indicated that HEGCs inhibit the
proliferation of Hep3B cells through the induction of
apoptosis.
Modulation of Bcl-2 family proteins and
loss of MMP by HEGCs in Hep3B cells
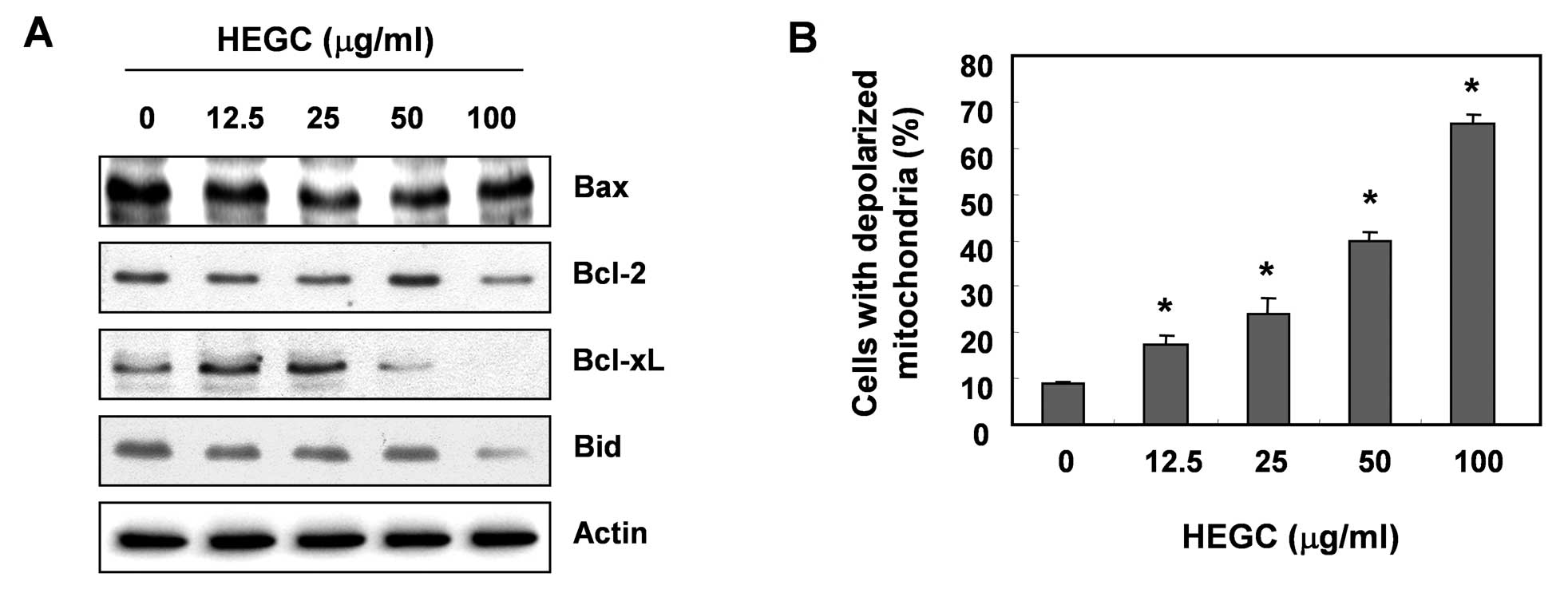
The role of the mitochondria in HEGC-induced
apoptosis of Hep3B cells was investigated by examining the effect
of HEGCs on the levels of the Bcl-2 family proteins, as well as the
MMP values. As shown in Fig. 2A,
the protein levels of anti-apoptotic Bcl-2 and Bcl-xL were
decreased in a concentration-dependent manner in response to HEGC
treatment; however, the protein levels of pro-apoptotic Bax
remained unchanged. Under these conditions, the levels of total
pro-apoptotic protein Bid, a BH3-only pro-apoptotic member of the
Bcl-2 family, also decreased in response to HEGC treatment, in a
concentration-dependent manner. To examine the role of mitochondria
in apoptosis induced by HEGCs, we analyzed the profiles of the MMP
values via a flow cytometer, using the mitochondrial-specific probe
JC-1. As depicted in Fig. 2B, a
loss of MMP was observed with an increased concentration of HEGCs,
indicating that HEGCs induced mitochondrial dysfunction through the
disruption of the outer mitochondrial membrane.
Activation of caspases by HEGCs in Hep3B
cells
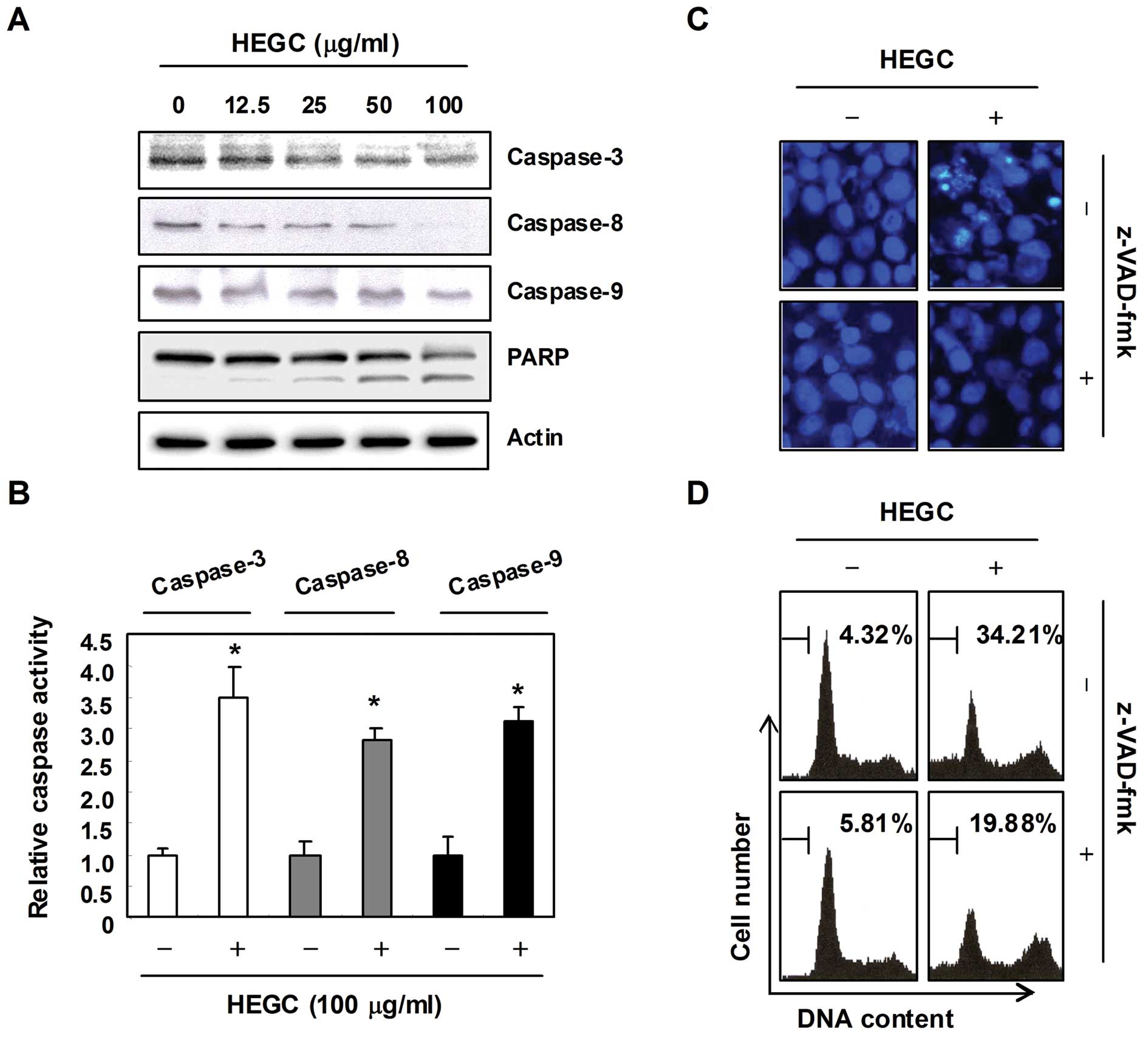
In order to determine if HEGC-induced apoptosis is
associated with the activation of caspases, the expression and
activity of caspases such as capases-3, -8 and -9 in the
HEGC-treated cells were examined using western blot analysis and an
in vitro activity assay. As shown in Fig. 3A and B, the HEGC treatment decreased
the expression levels of pro-caspase-3, -8 and -9 in a
concentration-dependent manner, and increased the in vitro
activity of those caspases and concomitant degradation of
poly(ADP-ribose) polymerase (PARP), which is a substrate protein of
caspase-3. In order to demonstrate that the activation of caspases
is a key step in the apoptotic pathway induced by HEGCs, Hep3B
cells were pretreated for 1 h with z-VAD-fmk, a cell-permeable
pan-caspase inhibitor, followed by treatment with HEGCs. As shown
in Fig. 3C and D, pretreatment of
the cells with z-VAD-fmk significantly blocked the chromatin
condensation and an increase in the sub-G1 population induced by
HEGCs, indicating that HEGC-induced apoptosis is
caspase-dependent.
HEGC-induced mitochondrial dysfunction is
associated with the generation of intracellular ROS
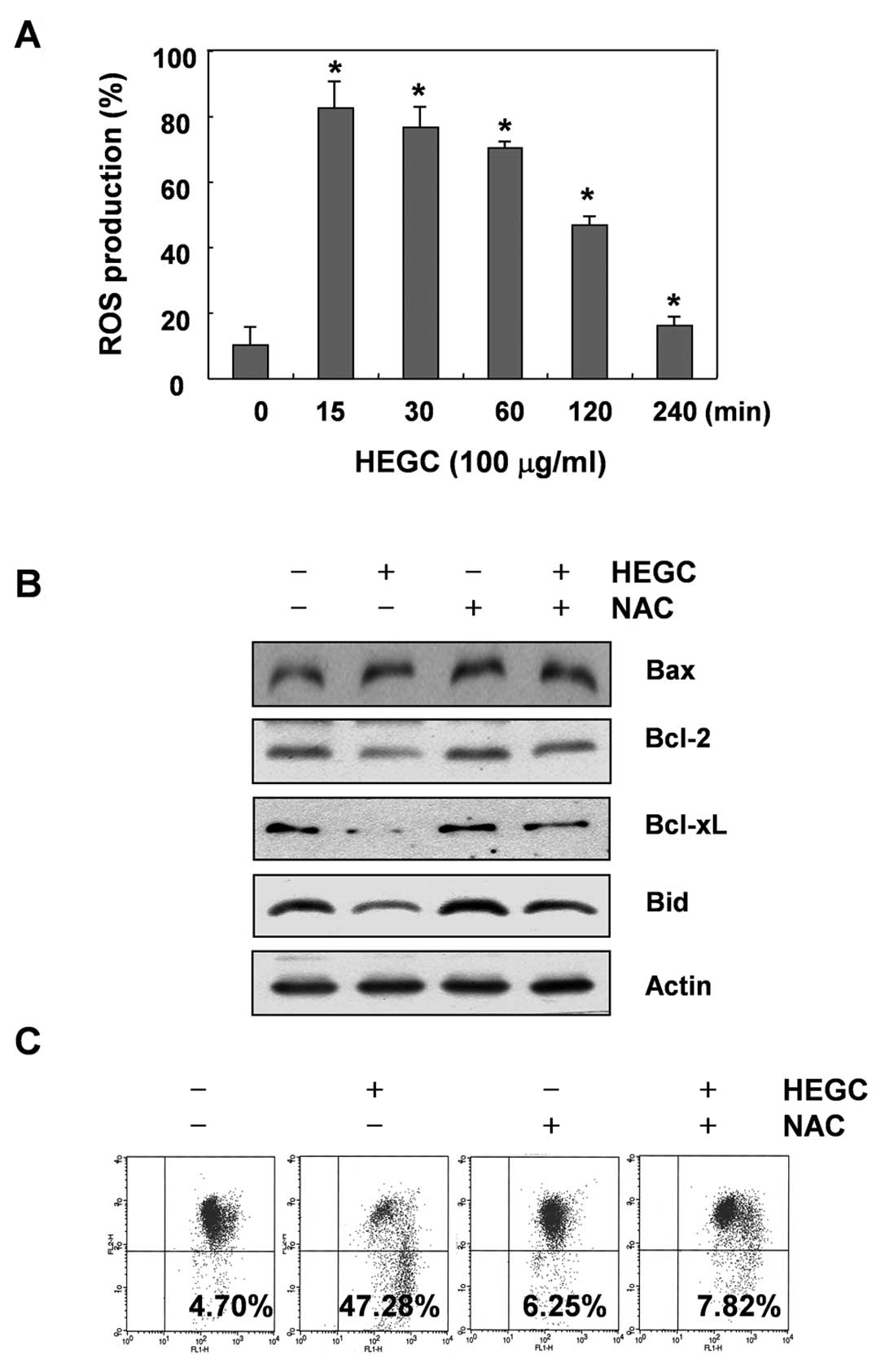
Since generation and acumination of ROS in cancer
cells may be related to mitochondrial dysfunction and cell
apoptosis, we attempted to characterize the correlation between ROS
production and changes in the MMP. For this investigation, we
performed kinetic studies to evaluate HEGC-stimulated intracellular
ROS productions, which were measured by using the cell-permeant
oxidation-sensitive dye DCFH-DA. As shown in Fig. 4A, ROS generation increased
significantly, as early as 15 min and began to decrease afterwards,
eventually dropping below the untreated control level at 4 h. We
reasoned that if ROS were a crucial factor in the induction of
mitochondrial dysfunction by HEGCs, the inhibition of ROS
generation must abrogate the loss of MMP. Therefore, cells were
pretreated for 1 h with 10 mM N-acetyl-L-cysteine (NAC), a commonly
used reactive oxygen intermediate scavenger, and then treated with
HEGCs. As shown in Fig. 4C,
HEGC-induced loss of MMP in Hep3B cells that were co-cultured with
NAC was effectively blocked, indicating that rapidly and
transiently produced ROS are capable of triggering mitochondrial
dysfunction. In addition, blocking the generation of ROS by
pretreatment of the cells with NAC prevented the HEGC-induced
downregulation of Bcl-2 and Bcl-xL expression and a decrease in the
Bid protein (Fig. 4B).
HEGC-induced caspase activation is
associated with the generation of intracellular ROS
To determine whether ROS generation is involved in
HEGC-induced caspase activation, the cells were pretreated with NAC
for 1 h and then exposed to 100 μg/ml HEGC for 6 h to determine the
expression levels of caspase-3, -8 and -9, as well as their
activities. As shown in Fig. 5A and
B, blocking the generation of ROS by pretreatment of the cells
with NAC prevented the HEGC-induced caspase activation, as well as
the degradation of PARP protein. Furthermore, the presence of NAC
almost completely suppressed the HEGC-induced chromatin
condensation and apoptotic ratio (Fig.
5C and D). Thus, our data indicate that HEGCs may cause
apoptosis in Hep3B cells through a ROS-mediated pathway.
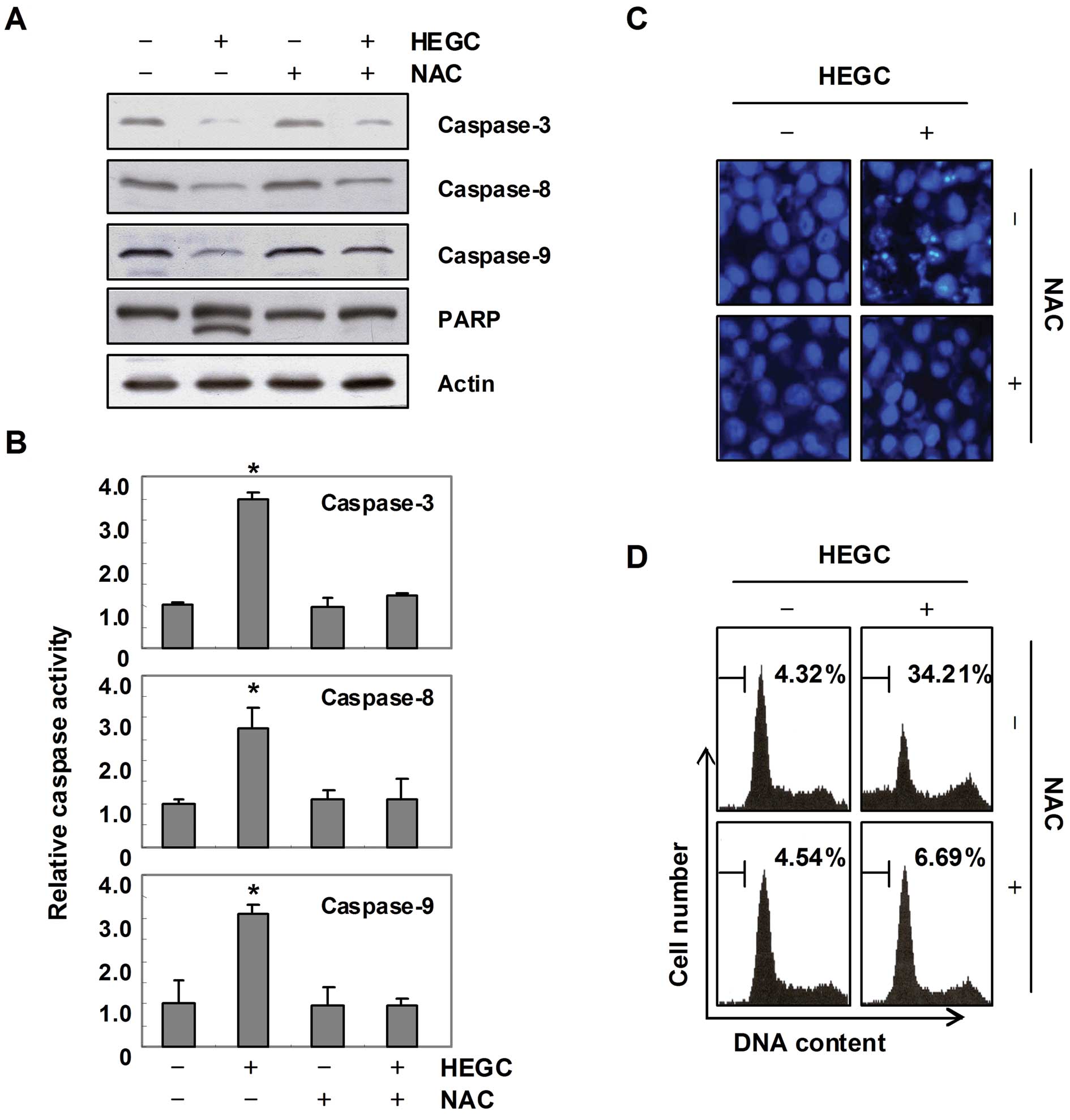 | Figure 5HEGC-induced activation of caspases
and apoptosis are associated with ROS generation in Hep3B cells.
(A) Cells were treated with or without NAC (10 mM) for 1 h before
treatment with 100 μg/ml of HEGCs for 48 h. The cellular proteins
were extracted, separated by SDS-polyacrylamide gels, and
transferred onto nitrocellulose membranes. The membranes were
probed with the indicated antibodies. Proteins were visualized
using an ECL detection system. Actin was used as the internal
control. (B) The cell lysates obtained from cells grown under the
same conditions as (A) those assayed for in vitro caspase-3,
-8 and -9 activity, using DEVD-pNA, IETD-pNA and LEHD-pNA,
respectively, as substrates. The relative concentrations of the
fluorescent products released were then measured. The results are
expressed as the means ± SD of 3 independent experiments. The
significance was determined by a Student’s t-test
(*P<0.05, compared with control). (C) The cells were
incubated with or without 100 μg/ml of HEGC for 48 h after 1 h
pretreatment with or without NAC (10 mM), and then stained with
DAPI for 10 min and images were captured with a fluorescence
microscope using a blue filter (×400). (D) The cells under the same
conditions as (C) were evaluated for sub-G1 DNA content, using a
flow cytometer. Results represent the means of 2 independent
experiments. |
Discussion
An increasing amount of data show that the potential
of antitumor activity of garlic compounds may be mediated through a
mitochondria-caspase-dependent pathway (16–18,20,21).
However, the signaling pathway associated with the induction of
apoptosis by extracts of garlic cloves is poorly defined. In the
course of our screening program of bioactive products from garlic,
we isolated HEGCs and demonstrated that HEGCs induced apoptosis in
Hep3B hepatocarcinoma cells through the generation of ROS.
Furthermore, we showed that HEGC-induced ROS generation was
accompanied by the disruption of the MMP, which led to the
activation of caspase-9, and eventually to cell death. In addition,
the quenching of ROS generation by NAC, a ROS scavenger, was shown
to prevent ROS generation and to confer almost complete protection
against HEGC-induced MMP disruption and apoptosis. Taken together,
these results suggest that ROS act upstream, signaling molecules to
initiate apoptosis.
Mitochondria are a rich source of ROS, which are
toxic byproducts of aerobic cells. ROS play an important role in
cell proliferation, inflammation and cancer development. However,
an excessive amount of ROS may lead to cell death by apoptosis or
necrosis (2–4). Recent investigations have suggested
that damaged mitochondria stimulate increased ROS production, which
subsequently activates the signaling pathways that control cancer
cell growth. However, the loss of MMP as a result of mitochondrial
depolarization in association with apoptosis appears to be more
common. This decrease in the MMP causes disruption of the outer
mitochondrial membrane and contributes to the release of cytochrome
c. Furthermore, the release of cytochrome c has been
reported to contribute to the activation of caspase-9, which in
turn causes activation of caspase-3 (5,6,29).
Many studies have demonstrated that the Bcl-2 family proteins
regulate apoptosis either as an activator (Bax) or inhibitor (Bcl-2
and Bcl-xL) and the Bax/Bcl-2 or Bcl-2 ratio is considered a key
factor in regulating the apoptotic process. Bcl-2 forms ion
channels in mitochondrial membranes, and its ion channel activity
may control apoptosis by influencing permeability in the
intracellular membranes (30,31).
In this study, we observed that the MMP levels in Hep3B cells
decreased after HEGC treatment (Fig.
2B), the activity of caspase-9 and -3 increased and the PARP
proteins, the substrate of caspase-3, were cleaved (Fig. 3). Our data also demonstrated that
HEGC-induced apoptosis is related to the downregulation of
anti-apoptotic Bcl-2 and Bcl-xL, without altering Bax levels,
indicating that HEGCs may increase the Bax/Bcl-2 or Bcl-2 ratio
(Fig. 2) and that induced
mitochondrial dysfunction leads to the apoptosis of Hep3B cells.
However, blocking caspase activity by pretreating the cells with a
pan-caspase inhibitor, z-VAD-fmk, prevented HEGC-induced chromatin
condensation and an increase in the sub-G1 population (Fig. 3), indicating that HEGCs induced
apoptosis in a caspase-dependent manner. Further experiments showed
that the HEGC treatment significantly increased the ROS-dependent
activation of caspase-9 and -3 and degradation of PARP, indicating
the existence of mitochondrial-mediated caspase-3 activity. The
results demonstrated that HEGCs are capable of inducing
mitochondrial dysfunction through ROS generation.
The present study also revealed that the activation
of caspase-8 in HEGC-treated cells is ROS-dependent (Fig. 5), which suggests that ROS may act
upstream of caspase-8 activation in Hep3B cells. Therefore, it is
reasonable to assume that the initial signal for the activation of
caspase-8 after treatment with HEGCs also derives from ROS.
Caspase-8 activation causes cleavage of Bid, which is a BH3-only
pro-apoptotic Bcl-2 family member exclusively localized in the
cytoplasm. The cleaved Bid, however, translocates to the
mitochondria and triggers cytochrome c release, which leads to the
activation of caspase-9 (5,7). In this study, we aimed to determine
whether or not HEGC-induced apoptosis is regulated by Bid. To
accomplish this, we examined Bid cleavage and found that treatment
of Hep3B cells with HEGCs resulted in the downregulation of the
total Bid expression that was perfectly blocked by NAC
pretreatment. Based on these observations, we conclude that the Bid
protein is involved in the regulation of HEGC-induced apoptosis of
Hep3B cells, and that this regulation also occurs in a
ROS-dependent manner.
In conclusion, the present study demonstrates that
Hep3B cells undergo apoptosis in response to treatment with HEGCs,
which occurs through a mitochondrial-mediated pathway that requires
ROS generation upstream to disrupt the MMP, leading to the
activation of caspase-9 and -8. Our data emphasize the key role of
ROS in apoptosis induced by HEGCs in hepatocarcinoma cells and
indicate that a positive correlation exists between ROS and
mitochondrial events leading to apoptosis.
Acknowledgements
This study was supported by a grant (code #7-19-42)
from the Rural Development Administration, Republic of Korea.
References
|
1
|
Chowdhury I, Tharakan B and Bhat GK:
Current concepts in apoptosis: the physiological suicide program
revisited. Cell Mol Biol Lett. 11:506–525. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Waldbaum S and Patel M: Mitochondria,
oxidative stress, and temporal lobe epilepsy. Epilepsy Res.
88:23–45. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stowe DF and Camara AK: Mitochondrial
reactive oxygen species production in excitable cells: modulators
of mitochondrial and cell function. Antioxid Redox Signal.
11:1373–1414. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Matés JM, Segura JA, Alonso FJ and Márquez
J: Intracellular redox status and oxidative stress: implications
for cell proliferation, apoptosis, and carcinogenesis. Arch
Toxicol. 82:273–299. 2008.PubMed/NCBI
|
|
5
|
Mohamad N, Gutierrez A, Nunez M, Cocca C,
Martin G, Cricco G, Medina V, Rivera E and Bergoc R: Mitochondrial
apoptotic pathways. Biocell. 29:149–161. 2005.
|
|
6
|
Orrenius S, Gogvadze V and Zhivotovsky B:
Mitochondrial oxidative stress: implications for cell death. Annu
Rev Pharmacol Toxicol. 47:143–183. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yin XM: Signal transduction mediated by
Bid, a pro-death Bcl-2 family proteins, connects the death receptor
and mitochondria apoptosis pathways. Cell Res. 10:161–167. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Alpers DH: Garlic and its potential for
prevention of colorectal cancer and other conditions. Curr Opin
Gastroenterol. 25:116–121. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hodge G, Hodge S and Han P: Allium
sativum (garlic) suppresses leukocyte inflammatory cytokine
production in vitro: potential therapeutic use in the treatment of
inflammatory bowel disease. Cytometry. 48:209–215. 2002. View Article : Google Scholar
|
|
10
|
Cellini L, Campli ED and Masulli M:
Inhibition of Helicobacter pylori by garlic extract
(Allium sativum). FEMS Immunol Med Microbiol. 13:273–277.
1996.
|
|
11
|
Bordia T, Mohammed N and Thomson M: An
evaluation of garlic and onion as antithrombotic agents.
Prostaglandins Leukot Essent Fatty Acids. 54:183–186. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
McMahon FG and Vargas R: Can garlic lower
blood pressure? A pilot study. Pharmacotherapy. 13:406–407.
1993.PubMed/NCBI
|
|
13
|
Yeh YY and Yeh SM: Garlic reduces plasma
lipids by inhibiting hepatic cholesterol and triacylglycerol
synthesis. Lipids. 29:189–193. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Patya M, Zahalka MA, Vanichkin A, Rabinkov
A, Miron T, Mirelman D, Wilchek M, Lander HM and Novogrodsky A:
Allicin stimulates lymphocytes and elicits an antitumor effect: a
possible role of p21ras. Int Immunol. 16:275–281. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Thomson M and Ali M: Garlic [Allium
sativum]: a review of its potential use as an anti-cancer
agent. Curr Cancer Drug Targets. 3:67–81. 2003.
|
|
16
|
Wu PP, Chung HW, Liu KC, Wu RS, Yang JS,
Tang NY, Lo C, Hsia TC, Yu CC, Chueh FS, et al: Diallyl sulfide
induces cell cycle arrest and apoptosis in HeLa human cervical
cancer cells through the p53, caspase- and mitochondria-dependent
pathways. Int J Oncol. 38:1605–1613. 2011.PubMed/NCBI
|
|
17
|
Nagaraj NS, Anilakumar KR and Singh OV:
Diallyl disulfide causes caspase-dependent apoptosis in human
cancer cells through a Bax-triggered mitochondrial pathway. J Nutr
Biochem. 21:405–412. 2010. View Article : Google Scholar
|
|
18
|
Karmakar S, Banik NL, Patel SJ and Ray SK:
Garlic compounds induced calpain and intrinsic caspase cascade for
apoptosis in human malignant neuroblastoma SH-SY5Y cells.
Apoptosis. 12:671–684. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Karmakar S, Choudhury SR, Banik NL and Ray
SK: Molecular mechanisms of anti-cancer action of garlic compounds
in neuroblastoma. Anticancer Agents Med Chem. 11:398–407. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Su CC, Chen GW, Tan TW, Lin JG and Chung
JG: Crude extract of garlic induced caspase-3 gene expression
leading to apoptosis in human colon cancer cells. In Vivo.
20:85–90. 2006.PubMed/NCBI
|
|
21
|
Xiao D, Pinto JT, Gundersen GG and
Weinstein IB: Effects of a series of organosulfur compounds on
mitotic arrest and induction of apoptosis in colon cancer cells.
Mol Cancer Ther. 4:1388–1398. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Herman-Antosiewicz A, Powolny AA and Singh
SV: Molecular targets of cancer chemoprevention by garlic-derived
organosulfides. Acta Pharmacol Sin. 28:1355–1364. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Herman-Antosiewicz A and Singh SV: Signal
transduction pathways leading to cell cycle arrest and apoptosis
induction in cancer cells by Allium vegetable-derived organosulfur
compounds: a review. Mutat Res. 555:121–131. 2004. View Article : Google Scholar
|
|
24
|
An SH, Kang JH, Kim DH and Lee MS: Vitamin
C increases the apoptosis via up-regulation p53 during cisplatin
treatment in human colon cancer cells. BMB Rep. 44:211–216. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cathcart R, Schwiers E and Ames BN:
Detection of picomole levels of lipid hydroperoxides using a
dichlorofluorescein fluorescent assay. Methods Enzymol.
105:352–358. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Choi JH, Choi AY, Yoon H, Choe W, Yoon KS,
Ha J, Yeo EJ and Kang I: Baicalein protects HT22 murine hippocampal
neuronal cells against endoplasmic reticulum stress-induced
apoptosis through inhibition of reactive oxygen species production
and CHOP induction. Exp Mol Med. 42:811–822. 2010. View Article : Google Scholar
|
|
27
|
Jeong JH, Ryu DS, Suk DH and Lee DS:
Anti-inflammatory effects of ethanol extract from Orostachys
japonicus on modulation of signal pathways in LPS-stimulated
RAW 264.7 cells. BMB Rep. 44:399–404. 2011.PubMed/NCBI
|
|
28
|
Cho SY, Lee JH, Bae HD, Jeong EM, Jang GY,
Kim CW, Shin DM, Jeon JH and Kim IG: Transglutaminase 2 inhibits
apoptosis induced by calcium-overload through down-regulation of
Bax. Exp Mol Med. 42:639–650. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Harada H and Grant S: Apoptosis
regulators. Rev Clin Exp Hematol. 7:117–138. 2003.
|
|
30
|
Antignani A and Youle RJ: How do Bax and
Bak lead to permeabilization of the outer mitochondrial membrane?
Curr Opin Cell Biol. 18:685–689. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Halestrap AP, McStay GP and Clarke SJ: The
permeability transition pore complex: another view. Biochimie.
84:153–166. 2002. View Article : Google Scholar : PubMed/NCBI
|