Introduction
Programmed cell death 5 (PDCD5) is a novel
apoptosis-related gene which was cloned from the human leukemia
cell line TF-1 and designated TFAR19 (TF1 cell apoptosis-related
gene 19) (1). It was able to induce
DNA damage-induced apoptosis through interacting with the histone
acetyltransferase Tip60 and phosphorylated in vitro or in
vivo by the multifunctional kinase CK2 (2,3). The
introduction of anti-TFAR19 antibody into HeLa cells could suppress
apoptosis (4). In addition, PDCD5
fragments could suppress the tumorigenesis by inhibiting the
Ras/Raf/MEK/ERK signaling pathway (5). Recent studies have also revealed that
decreased PDCD5 expression has been observed in multiple types of
human tumor cells and cancer including ovarian carcinomas (6), high-grade astrocytic gliomas (7), chondrosarcoma (8), colorectal cancer (9), myeloma (10), prostate cancer (11), acute myeloid leukemia (12), renal clear cell carcinoma (13), bladder carcinoma (14) and lung cancer (15). Decreased PDCD5 expression also
correlates with tumor progression and prognosis. It has been
demonstrated that PDCD5 expression in the gastric tumor tissues was
significantly lower than that of the normal tissues and it improved
the prognosis of patients compared with that of decreased PDCD5
expression (16). These findings
suggest that PDCD5 might represent a novel tumor suppressor
gene.
Gastrointestinal stromal tumors (GISTs) are
non-epithelial, mesenchymal tumors, 30% of which are malignant or
have a high potential for malignancy, although it comprises <1%
of all gastrointestinal tumors (17). The 5-year survival rate following
surgery of GISTs is approximately 50–65% (18). The overall survival of the patients
suffering from the disease has improved slightly, although the
treatment-resistant tumors have a well-recognized, well-understood,
and treatable tumor entity within only one decade (19). There have been marked achievements
in finding molecular targeted agents that cause the progression and
metastasis of GISTs (20). It is
now known that approximately 85% of GISTs arise from the mutations
in KIT or the homologous receptor tyrosine kinase platelet-derived
growth factor receptor alpha (PDGFRA) gene (19). Therefore, molecular biological
studies of the features of the tumor are key in understanding the
mechanisms underlying the pathogenesis of GISTs, and, in turn,
providing insights into therapeutics.
To our knowledge, there have been no studies on the
clinical significance of PDCD5 expression in GISTs. In the present
study, we investigated the PDCD5 expression levels by RT-PCR,
western blotting and immunohistochemistry (IHC) in GISTs. We
examined lower PDCD5 expression in the tumors compared with that of
adjacent normal gastrointestinal tissues. In addition, we found
that decreased PDCD5 expression was associated with the clinical
pathological characteristics of GISTs.
Materials and methods
Tumor samples
Sixty-six GIST specimens (28 frozen and 38
paraffin-embedded tissues) were obtained from patients aged between
40 and 70 years (median, 60 years), who underwent surgery at the
Department of General Surgery, Qilu Hospital and Shandong
Provincial Hospital, Shandong University, from 2006 to 2010. None
of the patients had received adjuvant immunosuppressive treatments
including chemotherapy or radiotherapy prior to the surgery in
order to eliminate their effects on gene expression. Tumor
specimens were immediately frozen in liquid nitrogen following
surgery. The normal non-tumor tissues were obtained from the
adjacent area of the primary tumor. The study was in compliance
with the ethics guidelines of the hospital, and all tumor samples
were obtained with the patients’ informed consent after the
surgery. The analysis of clinicopathological tumor characteristics
was performed according to the criteria of the National Institutes
of Health (NIH) (21).
RNA isolation and semi-quantitative
RT-PCR
Total-RNAs were extracted as previously described by
the modified TRIzol® one-step method and treated with
DNase (22,23). Total-RNAs (3 μg) were subjected to
reverse transcription to cDNA with the a kit. First-strand cDNA was
synthesized using the Reverse-Transcribe kit (Promega, Madison, WI,
USA) and PCR was performed using the gene-specific primers: sense
5′-CCATGG CGG ACG AGG AGC TTG-3′, and anti-sense 5′-TCAATA ATC GTC
ATC TTC ATC-3′ for 30 cycles at 94°C for 30 sec, 58°C for 30 sec
and 72°C for 30 sec followed by an extension cycle at 72°C for 7
min. Human β-actin primer was used as a positive control. Water
instead of the cDNA template was used in negative control
reactions. All PCR products were analyzed with 2% agarose gel
electrophoresis. RT-PCR was performed at least three times for each
sample.
SDS-PAGE and western blotting
The proteins were extracted from non-tumor and tumor
samples using a modified TRIzol one-step extraction method. The
concentration of the protein was determined by Bradford analysis
(Bio-Rad, Hercules, CA, USA). The protein extract was dissolved in
a loading buffer (1 mM Tris-Cl, 3% SDS, 60% glycerol and 75 mM DTT)
and each sample was separated and analyzed by SDS-PAGE on a 15% gel
and transferred onto PVDF membranes. The PVDF membrane was
incubated with anti-human PDCD5 or rabbit anti-human β-actin at 4°C
overnight. Immunoreactive bands were visualized using the enhanced
chemiluminescence method according to the manufacturer’s
instructions (ECL, Amersham Biosciences, Little Chalfont, UK).
Western blotting was performed at least three times for each
sample.
Immunohistochemistry
Formalin-fixed, paraffin-embedded tissue sections
from 28 fresh and 38 paraffin-embedded tissues were cut at 4–6 μm
and transferred to slides. Tumor tissues were deparaffinized in
xylene and rehydrated through alcohol gradient. The slides were
washed, blocked for endogenous peroxidase activity, pre-incubated
with goat serum, and were then stained with anti-PDCD5 polyclonal
antibody overnight at 4°C. Subsequently, the slides were incubated
with biotin-conjugated anti-rabbit IgG and developed with an
HRP-streptavidin-based ABC kit and a DAB Peroxidase Substrate kit
(Maixin Co., Fuzhou, China). The nuclei were counterstained with
hematoxylin. Negative controls for the specificity of IHC were
carried out by replacing the primary antibody with non-immune
rabbit IgG. The PDCD5 staining was divided into five (0–5) grades
according to staining intensity: − (score 0), + (score 1), ++
(score 2), +++ (score 3), ++++ (score 4) and +++++ (score 5). The
expression of PDCD5 was defined as follows: scores 0–3 were
classified as weak expression of PDCD5, whereas 4 and 5 were graded
as high expression of PDCD5. Slides were analyzed by two
independent pathologists blind to tumor sample origin. All staining
experiments were performed in duplicate for each sample.
Statistical analysis
The χ2 test was used to analyze the
correlation of expression of PDCD5 protein with clinicopathological
parameters. Pearson’s coefficient test was used to analyze the
correlation between PDCD5 expression labeling index. P<0.05 was
considered to indicate statistically significant differences. All
calculations were performed using the SPSS statistical software
package.
Results
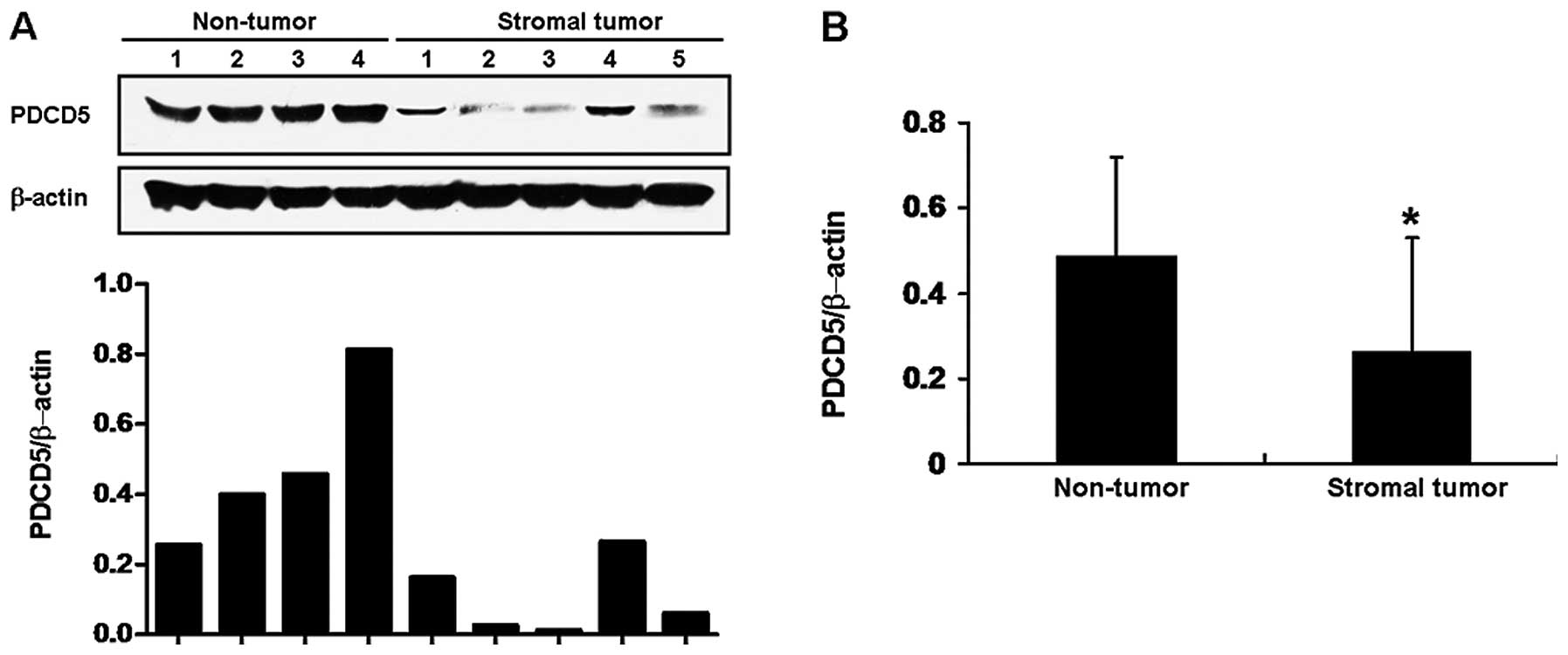
Expression of PDCD5 mRNA in GISTs
To explore the potential role of PDCD5 expression in
primary GISTs, we first detected the expression of PDCD5 mRNA by
semi-quantitative RT-PCR. As shown in Fig. 1A, non-tumorous tissues expressed
high levels of PDCD5 mRNA, whereas 16/28 (57%) of GIST samples
showed low levels of PDCD5 mRNA expression. These results suggested
that the expression of PDCD5 at the mRNA levels was decreased in
human GISTs compared with that in non-tumorous tissues (Fig. 1B).
Decreased expression of PDCD5 protein in
GISTs
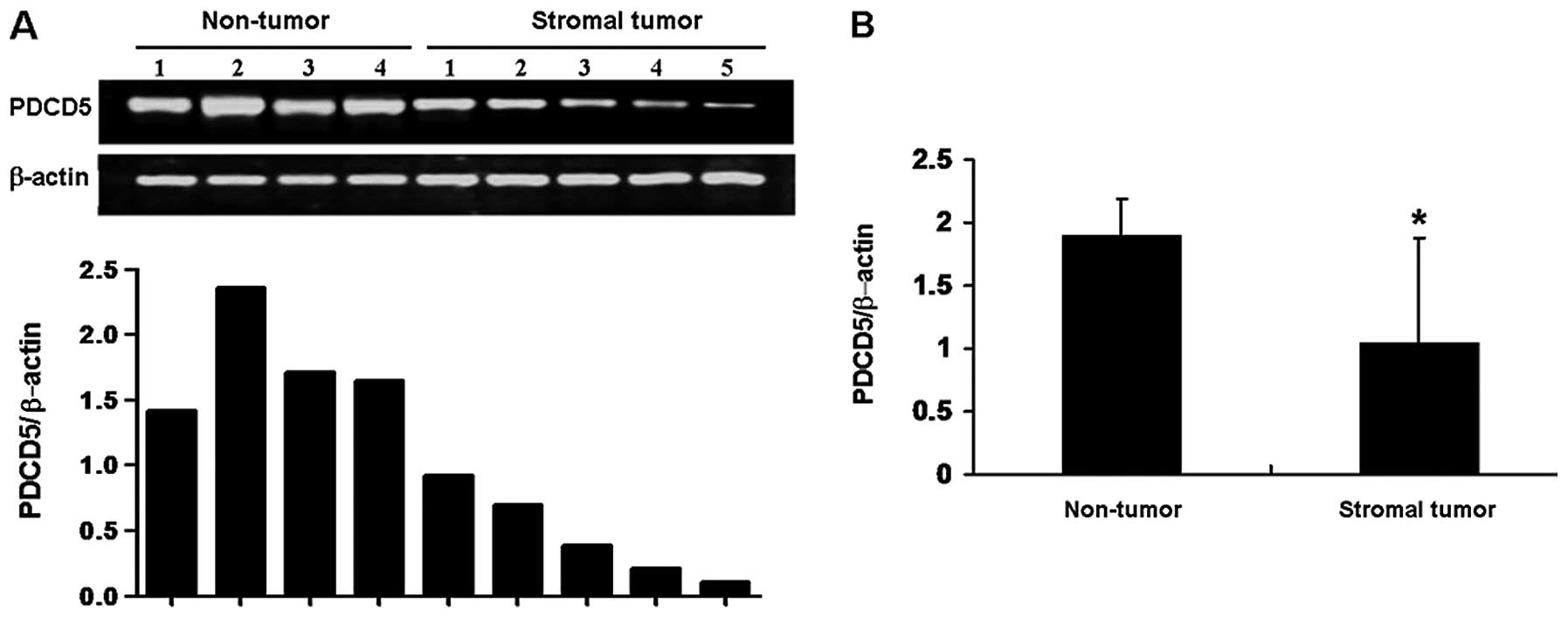
We further detected the expression of PDCD5 protein
by western blotting and IHC. Compared with normal tissues adjacent
to the tumor, the results of western blotting showed that PDCD5
protein expression in frozen GIST specimens was significantly
decreased (Fig. 2). Additionally,
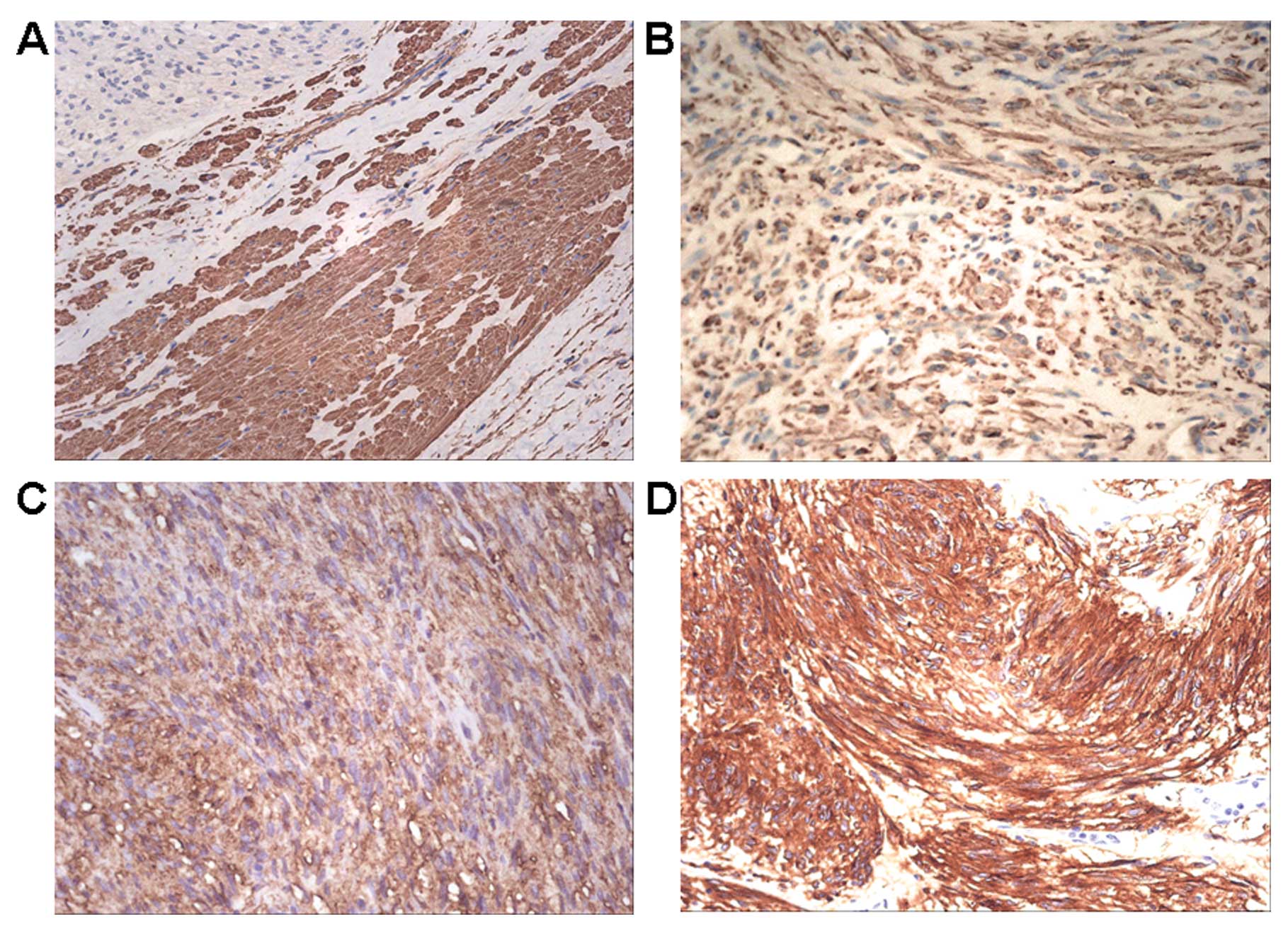
we examined the expression of PDCD5 proteins by IHC. In
non-tumorous tissues, there was a strong positive staining of PDCD5
(Fig. 3A). By contrast, PDCD5
expression of 28 frozen cases and 38 paraffin-embedded sample
tissues of GISTs exhibited weak or faint staining of PDCD5 protein
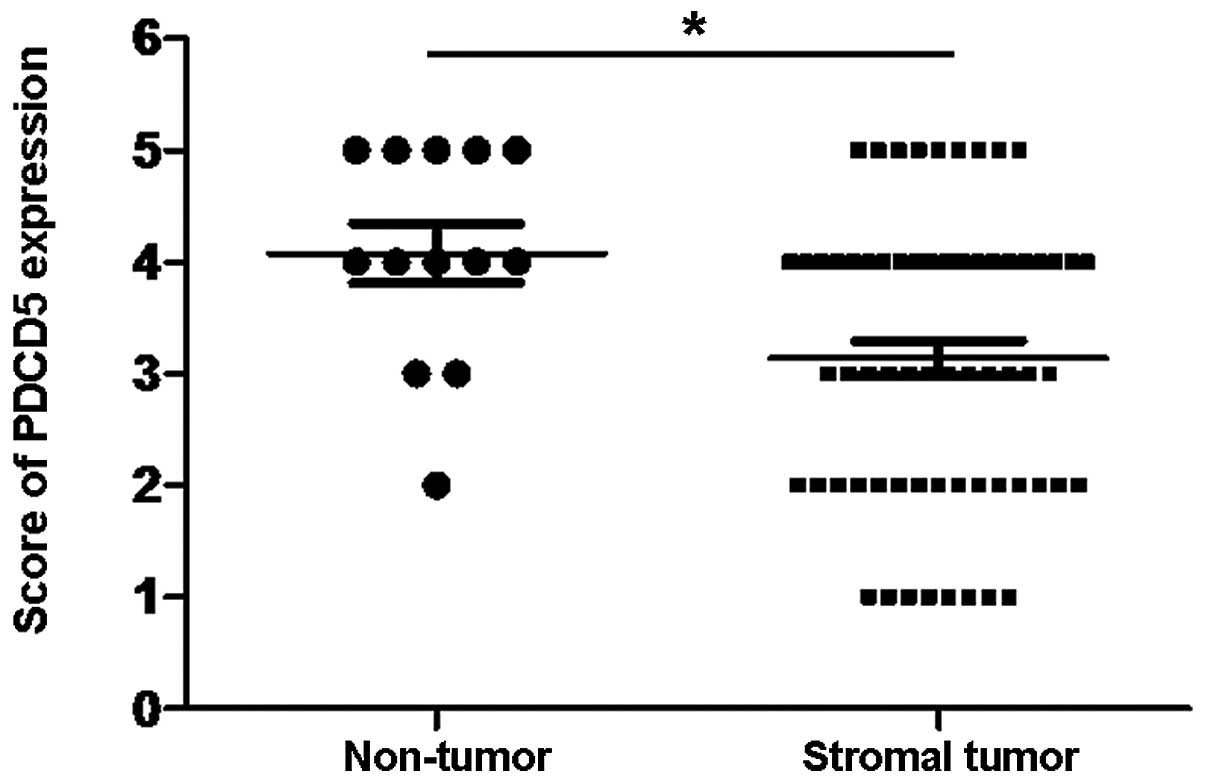
(Fig. 3B–D). Among them, 53%
(35/66) of tumor tissues expressed decreased expression of PDCD5
protein by IHC analysis, and the overall expression of PDCD5 in
GISTs was significantly lower compared with normal tissues adjacent
to tumor (Fig. 4).
Correlation of PDCD5 expression at the
protein level with the clinicopathological characteristics in
primary GISTs
To determine the clinical value of decreased PDCD5
expression in primary GISTs, we further examined the correlation of
PDCD5 expression with the clinicopathological parameters, such as
age, gender, tumor size, mitosis and risk group. The results showed
no significant relationship between PDCD5 expression and age,
gender and risk group. However, PDCD5 expression significantly
correlated with tumor size (P<0.05) and mitosis (P<0.05) of
patients (Table I), suggesting that
PDCD5 expression might be associated with the malignant progression
of GISTs.
 | Table IPDCD5 expression in GISTs. |
Table I
PDCD5 expression in GISTs.
| Parameters | PDCD5
Low expression | PDCD5
High expression | P-value |
|---|
| Total no. of
patients | 35 | 31 | |
| Gender |
| Male | 19 | 17 | 0.964 |
| Female | 16 | 14 | |
| Age (years) |
| ≥60 | 21 | 16 | 0.493 |
| <60 | 14 | 15 | |
| Risk group |
| High risk | 14 | 8 | 0.222 |
| Non-high risk | 21 | 23 | |
| Tumor size |
| ≤5 cm | 6 | 15 | 0.024a |
| 5–10 cm | 23 | 12 | |
| ≥10 cm | 6 | 4 | |
| Mitosis |
| ≤5/50 HPF | 17 | 23 | 0.043a |
| 5–10/50 HPF | 8 | 6 | |
| ≥10/50 HPF | 10 | 2 | |
Discussion
Previous studies have confirmed that PDCD5 is a
novel apoptosis-related gene, and research from mice and human
tumors has shown that PDCD5 might be a new tumor suppressor gene
that significantly affects tumor development and prognosis
(25,26). PDCD5 could inhibit the growth of
tumor cells through the induction of apoptosis and the inhibition
of the cell cycle (27,28). Previously, we had also found that
diminished PDCD5 expression existed in the high-grade astrocytic
gliomas and epithelial ovarian carcinomas (6,7).
However, the clinical role of PDCD5 in human GISTs, including the
relationship with tumor progression was, to date, unclear.
Gastrointestinal stromal tumors (GISTs) are the most
common primary mesenchymal tumors of the gastrointestinal tract
(24). It was distinguished from
other similar tumors in which the presence of a KIT protein and the
possibility of kit mutations was discovered. A large-scale clinical
trial indicated that 85% of GISTs had an active mutation in the kit
proto-oncogene, while only 3–5% mutation of PDGFRA existed in GISTs
(29). Although targeted agents,
such as imatinib, were studied for the treatment of the tumor,
40–50% of patients developed imatinib resistance within the first
two years of therapy. Most cases of primary resistance appeared kit
and PDGFRA wild-type (30). With
the advancement in understanding of the molecular biology, GIST
progresses together with improvement in immunohistochemical
staining. Many more target molecules are required to prevent the
high incidence of recurrence.
In the present study, we demonstrated for the first
time, that PDCD5 expression was decreased in human primary GISTs at
the mRNA or protein levels. Statistical analysis showed that the
overall expression level of PDCD5 in tumor samples was markedly
reduced compared with adjacent non-tumor normal tissues. To date,
little is known about the regulation mechanisms of abnormal PDCD5
expression. Downregulation of PDCD5 expression was found in human
breast epithelial cells after bisphenol A exposure accompanied by
DNA methylation. These suggested that the abnormal expression of
PDCD5 might be associated with DNA methylation and required further
investigations in order to be determined. Therefore, further
studies regarding the detailed molecular mechanisms of abnormal
expression of the gene in GISTs are required.
In addition, we also discovered that the decreased
expression of PDCD5 proteins was significantly associated with the
tumor size and mitosis of GISTs. It has been reported that the
reduced expression of PDCD5 correlates with short survival periods
of patients with gastric tumor tissues (16). At the same time, we also found that
lost or decreased PDCD5 expression in serous cystadenocarcinomas
was associated significantly with FIGO stage and poorer
disease-specific survival of patients (6). These suggested that reduced PDCD5
expression might contribute to the pathogenesis of human GISTs and
PDCD5 might be an important suppressor gene impacting the malignant
progression of the disease.
In conclusion, results of the present study show
that PDCD5 might serve as a novel therapeutic target for the
treatment of the tumor and the downregulation of PDCD5 gene
expression might be a key factor in the malignant progression of
GISTs. However, the detailed regulation mechanism of decreased
PDCD5 expression in GISTs requires further investigation.
Acknowledgements
This study was supported by the National 973 Basic
Research Program of China (no. 2012CB722406), the National Natural
Science Foundation of China (no. 81000126), the Natural Science
Foundation of Shandong Province (no. ZR2009CM021-2009ZRB01192) and
Grants from the Specialized Research Fund for the Doctoral Program
of Higher Education of China (no. 20090131120066).
References
|
1
|
Liu H, Wang Y, Zhang Y, et al: TFAR19, a
novel apoptosis-related gene cloned from human leukemia cell line
TF-1, could enhance apoptosis of some tumor cells induced by growth
factor withdrawal. Biochem Biophys Res Commun. 254:203–210. 1999.
View Article : Google Scholar
|
|
2
|
Xu L, Chen Y, Song Q, Xu D, Wang Y and Ma
D: PDCD5 interacts with Tip60 and functions as a cooperator in
acetyltransferase activity and DNA damage-induced apoptosis.
Neoplasia. 11:345–354. 2009.PubMed/NCBI
|
|
3
|
Salvi M, Xu D, Chen Y, Cabrelle A, Sarno S
and Pinna LA: Programmed cell death protein 5 (PDCD5) is
phosphorylated by CK2 in vitro and in 293T cells. Biochem Biophys
Res Commun. 387:606–610. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rui M, Chen Y, Zhang Y and Ma D: Transfer
of anti-TFAR19 monoclonal antibody into HeLa cells by in situ
electroporation can inhibit the apoptosis. Life Sci. 71:1771–1778.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Han XR, Sun Y and Bai XZ: The anti-tumor
role and mechanism of integrated and truncated PDCD5 proteins in
osteosarcoma cells. Cell Signal. 24:1713–1721. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang X, Wang X, Song X, et al: Clinical
and prognostic significance of lost or decreased PDCD5 expression
in human epithelial ovarian carcinomas. Oncol Rep. 25:353–358.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li H, Wang Q, Gao F, et al: Reduced
expression of PDCD5 is associated with high-grade astrocytic
gliomas. Oncol Rep. 20:573–579. 2008.PubMed/NCBI
|
|
8
|
Chen C, Zhou H, Xu L, et al: Prognostic
significance of downregulated expression of programmed cell death 5
in chondrosarcoma. J Surg Oncol. 102:838–843. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yin A, Jiang Y, Zhang X, Zhao J and Luo H:
Transfection of PDCD5 sensitizes colorectal cancer cells to
cisplatin-induced apoptosis in vitro and in vivo. Eur J Pharmacol.
649:120–126. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bao L, Ruan GR, Lu XJ, et al: Abnormal
expression of programmed cell death 5 gene in multiple myeloma
patients. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 18:634–637. 2010.(In
Chinese).
|
|
11
|
Du YJ, Xiong L, Lou Y, Tan WL and Zheng
SB: Reduced expression of programmed cell death 5 protein in tissue
of human prostate cancer. Chin Med Sci J. 24:241–245. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ruan GR, Chen SS, Ma X, et al: Abnormal
expression of PDCD5 in the bone marrow cells of adult acute myeloid
leukemia. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 15:462–465. 2007.(In
Chinese).
|
|
13
|
Tan WL, Xiong L, Zheng SB, et al:
Relationship between programmed cell death 5 protein expression and
prognosis of renal clear cell carcinoma. Nan Fang Yi Ke Da Xue Xue
Bao. 26:1316–1318. 2006.(In Chinese).
|
|
14
|
Xiong L, Tan WL, Yu ZC, et al: Expression
of TFAR19 (PDCD5) in normal human kidney, renal clear cell
carcinoma, normal human bladder and bladder carcinoma. Nan Fang Yi
Ke Da Xue Xue Bao. 26:805–809. 2006.(In Chinese).
|
|
15
|
Spinola M, Meyer P, Kammerer S, et al:
Association of the PDCD5 locus with lung cancer risk and prognosis
in smokers. J Clin Oncol. 24:1672–1678. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang YH, Zhao M, Li WM, et al: Expression
of programmed cell death 5 gene involves in regulation of apoptosis
in gastric tumor cells. Apoptosis. 11:993–1001. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Paral J, Slaninka I, Kalabova H and
Hadzi-Nikolov D: Gastrointestinal stromal tumors: review on
morphology, molecular pathology, diagnostics, prognosis and
treatment options. Acta Gastroenterol Belg. 73:349–359. 2010.
|
|
18
|
Lai Eric CH, Lau Stephanie HY and Lau WY:
Current management of gastrointestinal stromal tumors - a
comprehensive review. Int J Surg. 10:334–340. 2012.PubMed/NCBI
|
|
19
|
Bayraktar UD, Bayraktar S and Rocha-Lima
CM: Molecular basis and management of gastrointestinal stromal
tumors. World J Gastroenterol. 16:2726–2734. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liegl-Atzwanger B, Fletcher JA and
Fletcher CD: Gastrointestinal stromal tumors. Virchows Arch.
456:111–127. 2010. View Article : Google Scholar
|
|
21
|
Fletcher CD, Berman JJ, Corless C, et al:
Diagnosis of gastrointestinal stromal tumors: a consensus approach.
Int J Surg Pathol. 10:81–89. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chadderton T, Wilson C, Bewick M and Gluck
S: Evaluation of three rapid RNA extraction reagents: relevance for
use in RT-PCR’s and measurement of low level gene expression in
clinical samples. Cell Mol Biol. 43:1227–1234. 1997.PubMed/NCBI
|
|
23
|
Culley DE, Kovacik WP Jr, Brockman FJ and
Zhang W: Optimization of RNA isolation from the archaebacterium
Methanosarcina barkeri and validation for oligonucleotide
microarray analysis. J Microbiol Methods. 67:36–43. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Laurini JA and Carter JE: Gastrointestinal
stromal tumors: a review of the literature. Arch Pathol Lab Med.
134:134–141. 2010.
|
|
25
|
Xu HY, Chen ZW, Pan YM, et al:
Transfection of PDCD5 effect on the biological behavior of tumor
cells and sensitized gastric cancer cells to cisplatin-induced
apoptosis. Dig Dis Sci. 57:1847–1856. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang Y, Chu H, Zhou L, et al: Correlation
of PDCD5 and apoptosis in hair cells and spiral ganglion neurons of
different age of C57BL/6J mice. J Huazhong Univ Sci Technolog Med
Sci. 32:113–118. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ruan GR, Zhao HS, Chang Y, et al:
Adenovirus-mediated PDCD5 gene transfer sensitizes K562 cells to
apoptosis induced by idarubicin in vitro and in vivo. Apoptosis.
13:641–648. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang YF, Song QS, Zhang YM, Ma DL, Wang Y
and Ke XY: Sensitizing effect of recombinant human PDCD5 protein on
chemotherapy of acute monocytic leukemia cell line U937 and its
mechanism. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 18:277–281. 2010.(In
Chinese).
|
|
29
|
de Matteo RP, Ballman KV, Antonescu CR, et
al: Adjuvant imatinib mesylate after resection of localised,
primary gastrointestinal stromal tumour: a randomised,
double-blind, placebo-controlled trial. Lancet. 373:1097–1104.
2009.
|
|
30
|
Gramza AW, Corless CL and Heinrich MC:
Resistance to tyrosine kinase inhibitors in gastrointestinal
stromal tumors. Clin Cancer Res. 15:7510–7518. 2009. View Article : Google Scholar : PubMed/NCBI
|