Introduction
Hepatocellular carcinoma (HCC) is one of the most
frequent causes of death from solid tumors in the world,
particularly in China (1,2). Currently, surgical intervention,
including hepatic resection and liver transplantation, is the only
therapy considered to provide a cure. However, only a few patients
are diagnosed early enough to take advantage of that option.
Therefore, the prognosis of HCC patients remains extremely poor,
with a 5-year survival rate of only 5% (3). Chemotherapy is often used in
conjunction with surgery and radiation (4). However, HCC is a type of cancer with
high resistance to conventional anticancer agents. Paclitaxel is
one of the most effective anticancer drugs discovered in the past
few decades. For a number of years it has been clinically used in
the treatment of various cancers, including HCC (5). However, chemoresistance due to
paclitaxel-induced nuclear factor κB (NF-κB) activation is an
important cause of suboptimal therapeutic effect (6). Therefore, the search for new effective
chemopreventive and chemotherapeutic agents with the ability of
directly inhibiting HCC cell growth or enhancing the anticancer
activity of conventional chemotherapeutic agents is urgent.
Accumulating data suggest that a number of signaling
elements, such as NF-κB (7), TLR-4
(8), Ras (9) and Akt (10), not only promote cancer progression
but also confer chemoresistance. Among these signaling elements,
NF-κB is one of the most investigated transcription factors, the
activation of which has been found to control multiple cellular
processes in cancer. Currently, many researchers have reported that
NF-κB activation plays a facilitating role in cell cycle
progression and apoptotic resistance. Additionally,
chemotherapy-induced activation of NF-κB may blunt the ability of
chemotherapy itself (11,12). Therefore, the inhibition of NF-κB
may be an effective treatment strategy for inhibiting tumor growth
and progression as well as in restoring the sensitivity of cancer
cells to cytotoxic drugs (13).
With the above findings in mind, we speculated that
new strategies with NF-κB suppression activity, which directly
inhibits cancer cell growth and restores the sensitivity of cancer
cells to the number of cytotoxic drugs (such as paclitaxel), may be
an effective method for treating HCC. Swainsonine, a new class of
compounds that display a wide range of pharmacological effects, has
been under extensive research to examine its potential anticancer
and therapeutic biological activities. A number of
swainsonine-related studies confirmed that swainsonine may inhibit
growth and induce apoptosis in tumor cells (14–16).
However, the detailed mechanism underlying the use of swainsonine
as an anticancer drug, especially through inhibition of NK-κB
activation, remains unknown. Therefore, an in-depth study is
required. Nevertheless, research reports regarding anti-HCC
activity and related mechanisms remain scarce. To determine the
direct anti-HCC effect of swainsonine, the underlying molecular
mechanisms and whether swainsonine sensitizes HCC cells to
paclitaxel, we evaluated the effectiveness of swainsonine alone or
in combination with paclitaxel in vitro and in vivo.
To the best of our knowledge, our results provide for the first
time evidence that swainsonine is a reliable candidate for
chemotherapeutic treatment of HCC that should be further
investigated.
Materials and methods
Sample preparation
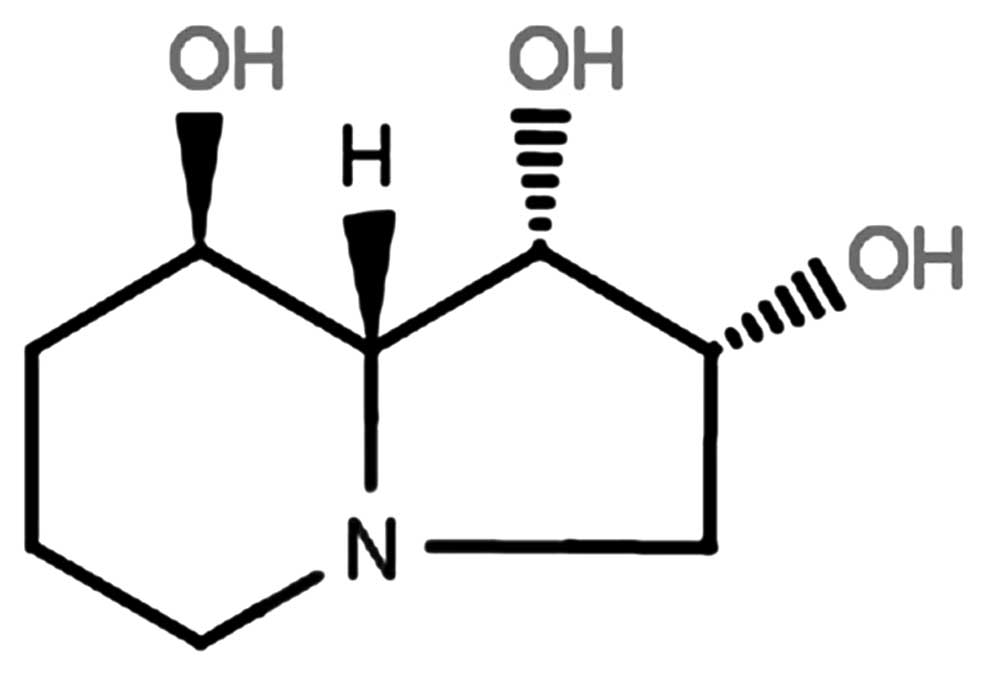
Endotoxin-free synthetic swainsonine (purity over
99%) was supplied by Sigma (St. Louis, MO, USA). The structure of
this compound is shown in Fig. 1.
Swainsonine was prepared in Ca2+-free and
Mg2+-free phosphate-buffered saline (PBS), sterilized by
ultrafiltration and stored at −20°C until diluted to the final
concentration in fresh medium before each experiment.
Cell lines and cell culture
Human hepatoma HepG2, SMCC7721, Huh7 and MHCC97-H
cells and human hepatocyte HL-7702 cells (maintained in our
laboratory, originally obtained from the Cell Bank of the Type
Culture Collection of Chinese Academy of Sciences) were cultured in
Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10%
fetal bovine serum (FBS) (Invitrogen, Carlsbad, CA, USA),
penicillin (100 IU/ml), streptomycin (100 μg/ml) and trypsin (400
IU/l). These were cultured at 37°C in 5% CO2 and 95%
humidified air. The medium was changed every 2 days. After reaching
~60–70% confluence, the cells were treated with different
concentrations of swainsonine as indicated in Results.
MTT assay for cytotoxicity
A
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay was performed to determine the number of viable cells. The
cells were cultured in 96-well plates at a density of
4×103 cells/well with fresh medium containing
swainsonine at the indicated concentrations (0.06, 0.12, 0.24, 0.48
and 0.96 μg/ml). After treatment for 24, 48 or 72 h, MTT solution
was added to each well (5 mg/ml) and incubated at 37°C for 4 h. The
MTT-formazan product dissolved in dimethyl sulfoxide (DMSO) was
measured at a wavelength of 490 nm with a microplate reader
(Bio-Rad Laboratories, Hercules, CA, USA). The reduction in the
viability of the swainsonine-treated cells was expressed as the
percentage compared with swainsonine-free control cells.
Ki-67 expression analysis for the
cellular growth
Flow cytometric analysis was performed for Ki-67
studies as previously described (17). Briefly, following incubation with
0.00, 0.09, 0.17 and 0.33 μg/ml of swainsonine for 24 h, MHCC97-H
cells treated with or without swainsonine were harvested, fixed in
70% (v/v) ethanol, washed and incubated for 20 min with 1% bovine
serum albumin (BSA). Then, the cells were incubated with 1:200
diluted anti-Ki-67 (FITC-conjugated; Bioscience, Beijing, China).
Data were obtained and analyzed by flow cytometry
(Becton-Dickinson, San Jose, CA, USA).
Effects of swainsonine on cell cycle
progression and apoptosis
To explore whether the growth inhibitory effect of
swainsonine was caused by cell cycle arrest and apoptosis, cell
cycle distribution and Annexin V analyses were utilized.
Cell cycle distribution analysis
Following incubation with 0.00, 0.09, 0.17 and 0.33
μg/ml of swainsonine for 24 h, MHCC97-H cells treated with or
without swainsonine were harvested by trypsinization and fixed with
ice-cold 70% ethanol for 48 h at 4°C. After being rinsed twice with
PBS, the cells were treated with RNase A at 1 mg/ml for 30 min at
37°C. After staining with 40 μl of 0.1 mg/l propidium iodide (PI),
stained cells were subjected to flow cytometric analysis.
Western blot analysis for cell cycle
regulatory proteins
The cells were processed for protein extraction and
western blot analysis as previously described (17). The primary antibodies were
anti-cyclin D1 (diluted 1:500, rabbit polyclonal), anti-cyclin E
(diluted 1:300, mouse monoclonal), anti-cyclin-dependent kinase
(Cdk) 2 (diluted 1:300, mouse monoclonal), anti-Cdk 4 (diluted
1:300, mouse monoclonal), anti-p21 (diluted 1:200, mouse
monoclonal), anti-p27 (diluted 1:200, mouse monoclonal) (all from
Santa Cruz Biotechnology, Santa Cruz, CA, USA) and
anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (diluted
1:400, rabbit monoclonal C-2; Santa Cruz Biotechnology).
Apoptotic cell determination
An Annexin V-FITC/PI apoptosis detection kit
(Annexin V-FITC/PI staining kit; Immunotech Co., Marseille, France)
was used for the detection of cell apoptosis. In brief,
1.5×105 cells were plated in 24-well plates and treated
with 0.00, 0.09, 0.17 and 0.33 μg/ml of swainsonine for 24 h. The
cells treated with or without swainsonine were collected, washed in
cold PBS, incubated for 15 min with fluorescein-conjugated Annexin
V and PI according to the manufacturer’s instructions and analyzed
by flow cytometry.
Morphological evaluation of apoptotic
MHCC97-H cells
The cells were treated with swainsonine for 24 h at
concentrations of 0.00 (PBS, vehicle as control) and 0.33 μg/ml.
Morphological changes were observed with an inverted microscope
(Olympus Corp., Tokyo, Japan). The Hoechst 33342 staining technique
was used to analyze the alterations in the nuclear morphology of
cells. Different interfered cells were washed in PBS and fixed in
70% ethanol for 2 h at 4°C. Cell nuclei were stained with 5 μg/ml
Hoechst 33342 (Sigma). After staining with Hoechst 33342, the
changes in nuclear morphology were visualized by fluorescence
microscopy (Olympus Corp.). The ultrastructure morphological change
was evaluated by transmission electron microscopy (TEM) as
described below. The cells were collected and fixed in 2.5%
glutaraldehyde, precooled at 4°C and stored at 4°C overnight. Then
the cells were dehydrated, embedded, cut into ultrathin sections
and stained routinely. The stained sections were scanned with an
electron microscope (JEM-2000EX, JEOL Ltd., Tokyo, Japan) for
ultrastructure observations.
Western blot analysis of apoptosis
regulatory proteins
Apoptosis-related gene fusion proteins were
identified by western blot analysis. The specific primary
antibodies were anti-Bax (diluted 1:300, mouse monoclonal),
anti-Bcl-2 (diluted 1:300, mouse monoclonal), anti-Fas (diluted
1:300, mouse monoclonal) and anti-Fas-L (diluted 1:300, rabbit
polyclonal) (all from Santa Cruz Biotechnology).
Western blot analysis for the effects of
swainsonine on the activation of NF-κB in MHCC97-H cells
The preparation of cytoplasmic and nuclear extracts
was performed using the Nuclear Extract kit (HyClone-Pierce, South
Logan, UT, USA) according to the manufacturer’s instructions. To
measure nuclear NF-κB/p65 and cytoplasmic NF-κB/p65 expression,
western blot analysis was performed. Related fusion proteins were
identified using anti-NF-κB/p65 (diluted 1:500, rabbit monoclonal;
Cell Signaling Technology, Beverly, MA, USA) primary antibody.
Effects of swainsonine on
chemosensitization of HCC for paclitaxel toxicity
To confirm the sensitization to paclitaxel toxicity
from swainsonine in vitro, MHCC97-H cells were treated with
swainsonine (0.03 μg/ml), paclitaxel (10 ng/ml; Beijing Sihuan
Pharmaceutical Co., Ltd., Beijing, China) or swainsonine (0.03
μg/ml) plus paclitaxel (10 ng/ml) for 24 h and the effect on growth
inhibition was examined using the cell viability assay described
above. In vivo, female athymic nude mice (6–8 weeks old)
were maintained in pathogen-limited conditions at the animal
resources center at the Fourth Military Medical University. The
Local Animal Care and Use Committee of the Fourth Military Medical
University approved all animal experimental procedures. MHCC97-H
cells (1×107 in 0.1 ml of serum-free growth medium) were
inoculated s.c. on the left side of the armpit. The injection
procedure took 2–3 min; all mice recovered in <5 min without
showing signs of stress or casualty. All mice were monitored for 5
min after cell administration and were returned to their cages. Two
weeks after tumor cell implantation, 40 tumor-bearing mice were
randomly divided into four groups and received one of the following
treatments by a single i.p. injection into the lower right quadrant
of the peritoneum. One group of mice (n=10) was treated with
swainsonine (1 mg/kg) thrice weekly plus paclitaxel (5 mg/kg)
weekly for 4 weeks. A second group of mice (n=10) was treated with
swainsonine alone (1 mg/kg) thrice weekly for 4 weeks. A third
group (n=10) was treated with paclitaxel alone (5 mg/kg) weekly for
4 weeks. The control group (n=10) received normal saline. Tumor
size was measured twice weekly. Tumor volume (V) was calculated
using the following formula: V = (a × b × c)/2, are a and b were
the shorter and longer diameters of each tumor, respectively and c
was the thickness. At the end of the experiment, mice underwent
euthanasia with CO2. The tumors of each group were
harvested to profile the gene expression associated with growth
(Ki-67; Santa Cruz Biotecnhology) and apoptosis (Bax and Bcl-2;
Santa Cruz Biotecnhology) by western blot analysis. To identify
whether swainsonine chemosensitizes HCC to paclitaxel-induced
cytotoxicity via attenuating the constitutive activation of NF-κB,
we also examined its cellular localization in paclitaxel (alone or
in combination with swainsonine)-treated MHCC97-H cells.
Statistical analysis
The significance of differences between the groups
was determined with one-way ANOVA (SPSS10.0 statistical software).
The results with a P-value ≤0.05 were considered statistically
significant. Data are expressed as the mean ± standard error of the
mean (SEM) of separate experiments (n ≥3, where n represents the
number of independent experiments).
Results
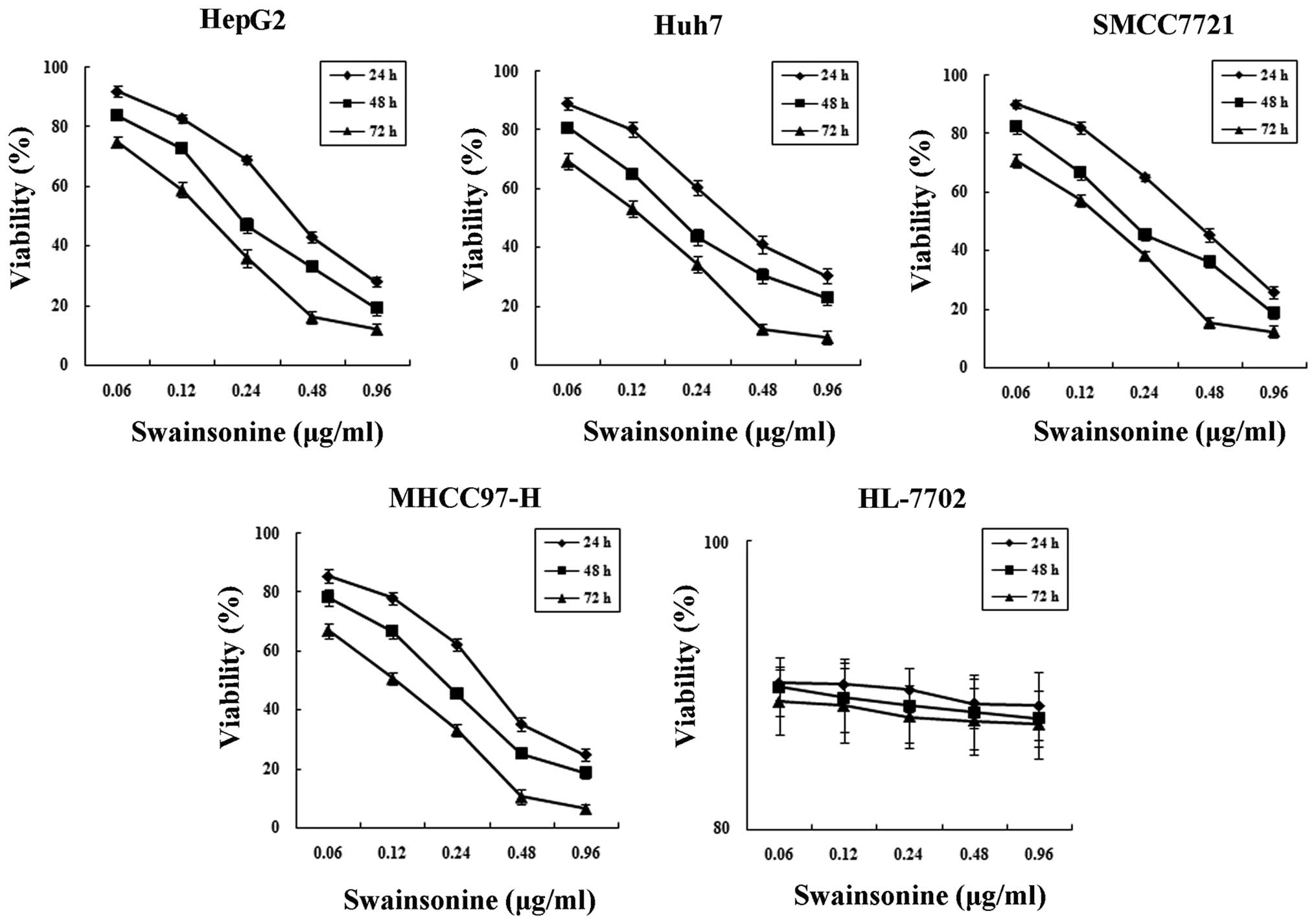
Cytotoxicity of swainsonine in both human
hepatoma and HL-7702 cells
The MTT experiment confirmed that human hepatoma
cells were sensitive to swainsonine and that swainsonine inhibited
the growth of human hepatoma cells in a dose- and time-dependent
manner. As shown in Fig. 2,
exposure of hepatoma cells to different concentrations of
swainsonine (0.06–0.96 μg/ml) at different time points resulted in
a statistically significant change in cell viability (n=3,
P<0.05). The IC50 value at 24 h post-treatment with
swainsonine for HepG2, SMCC7721, Huh7 and MHCC97-H cells was 0.43,
0.41, 0.39 and 0.33 μg/ml, respectively. However, different
concentrations of swainsonine had no significant toxic effects on
hepatocytes for the different time periods (n=3, P>0.05).
Therefore, swainsonine reduced the viability of hepatoma cells but
was only slightly toxic to HL-7702 hepatocyte cells. Taking into
account the more aggressive and highly metastatic nature of HCC,
MHCC97-H cells were then selected as a model system with which to
conduct mechanistic studies in vitro and in vivo.
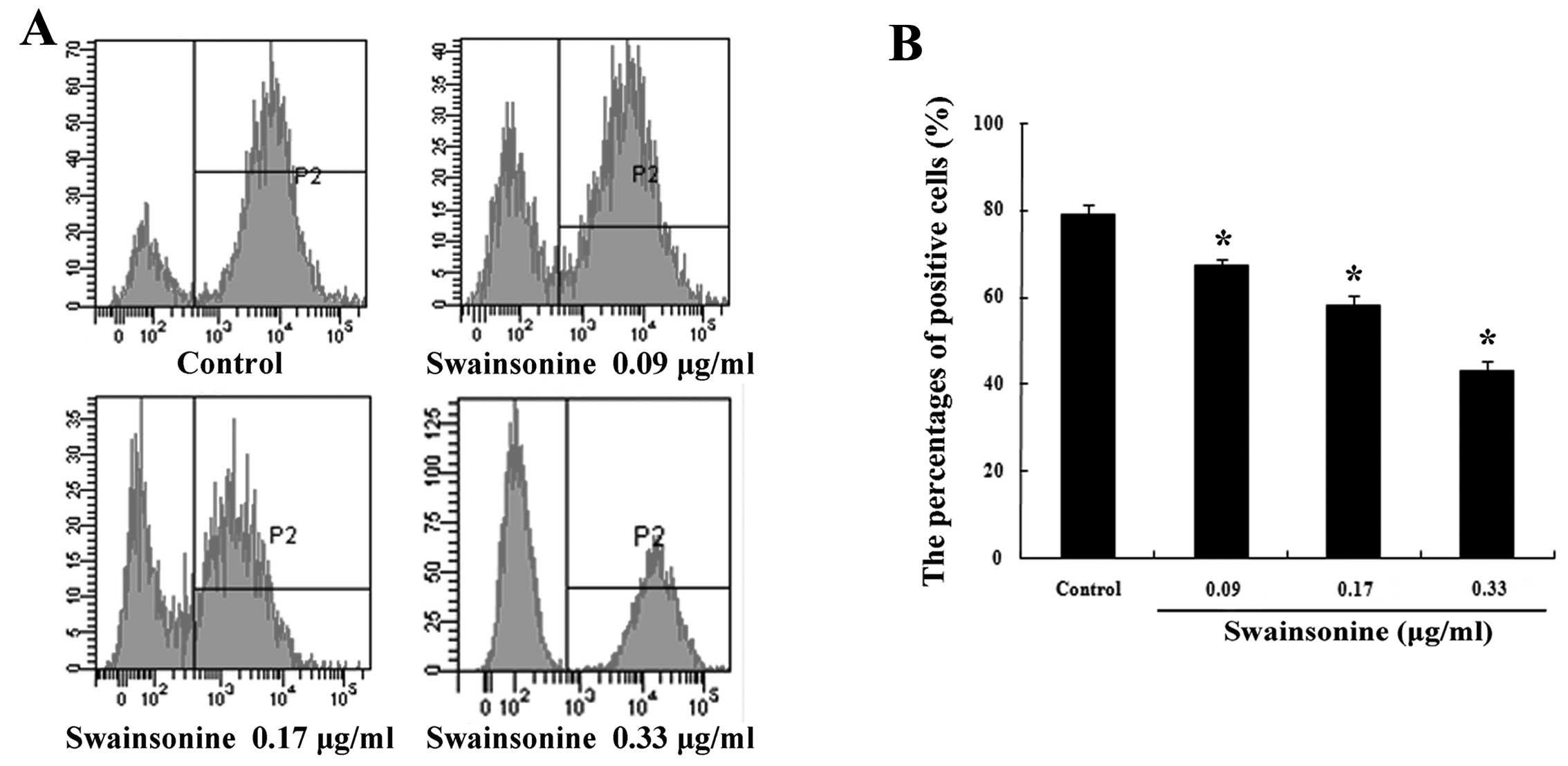
Swainsonine inhibits the growth of
MHCC97-H cells
The effects of swainsonine on the growth of MHCC97-H
cells were examined through the analysis of Ki-67 expression
(Fig. 3A and B). Our data
demonstrated that a considerable decrease in the level of
expression of Ki-67 occurred in a dose-dependent manner after 24 h
of swainsonine treatment. These outcomes suggest that treatment
with swainsonine results in the inhibition of cell growth of
MHCC97-H cells.
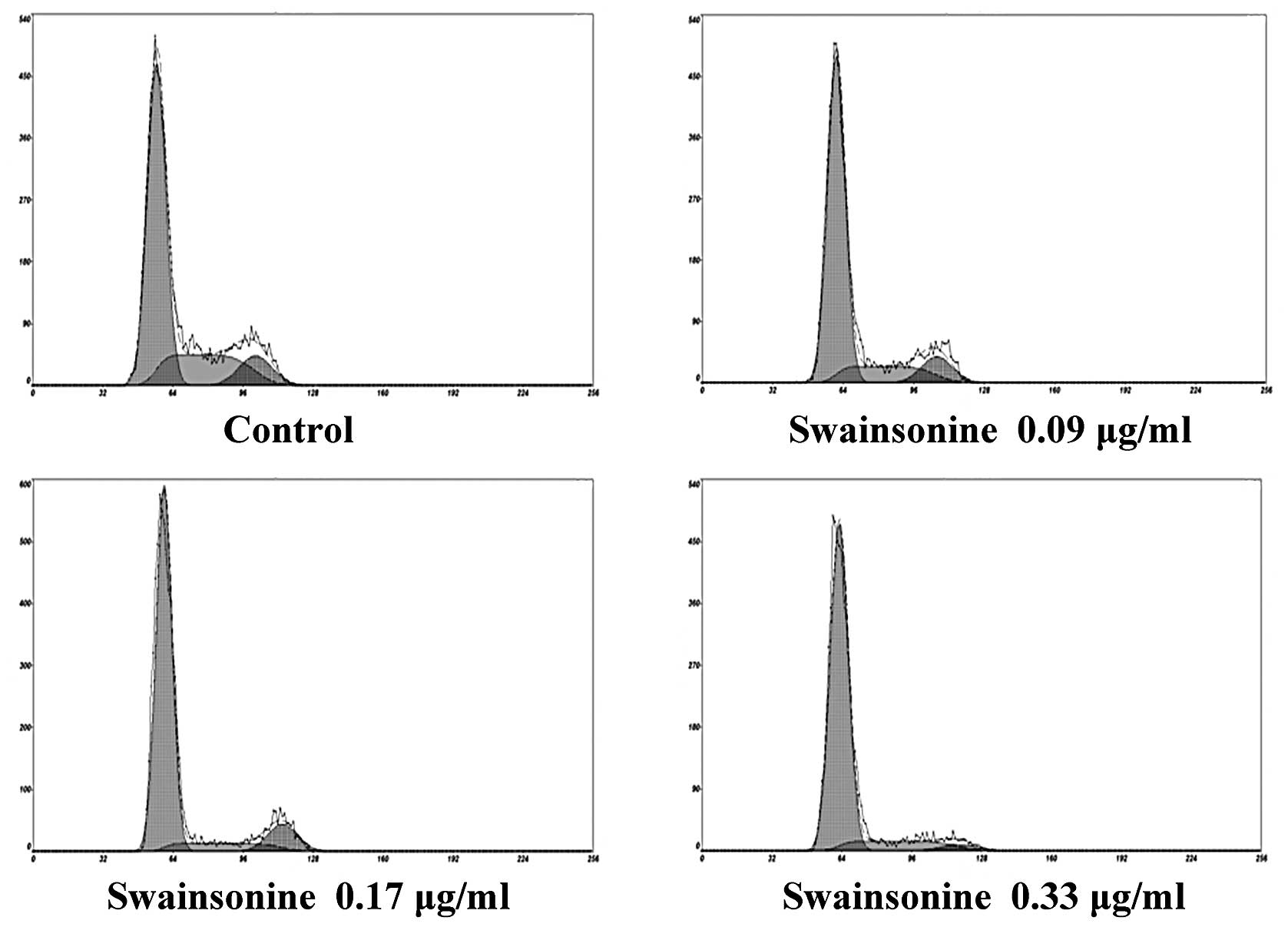
Effects of swainsonine on cell cycle
progression and apoptosis Swainsonine induces cell cycle arrest in
MHCC97-H cells
Dose-response analysis was performed, whereby
MHCC97-H cells were exposed to increased concentrations of
swainsonine for 24 h. As shown in Fig.
4 and Table I, following
incubation with different concentrations of swainsonine, the
proportion of cells in the G0/G1 phase increased substantially
compared to the control group; while the number of cells in the S
and G2/M phase were reduced to some extent within 24 h (n=3,
P<0.05), suggesting G0/G1 phase arrest. Our data suggest that
swainsonine inhibits MHCC97-H cell growth by blocking the G0/G1 to
S phase transition in the cell cycle in a dose-dependent
manner.
 | Table IEffects of swainsonine on cell cycle
distribution (%). |
Table I
Effects of swainsonine on cell cycle
distribution (%).
| G0/G1 | S | G2/M |
|---|
| Control | 62.77±2.06 | 25.68±2.12 | 11.55±1.83 |
| Swainsonine
(μg/ml) |
| 0.09 | 73.24±1.76a | 16.73±1.73a | 10.03±1.68 |
| 0.17 | 78.58±2.06a | 11.40±1.80a | 10.02±1.74 |
| 0.33 | 84.04±2.28a | 8.21±1.17a | 7.75±1.29a |
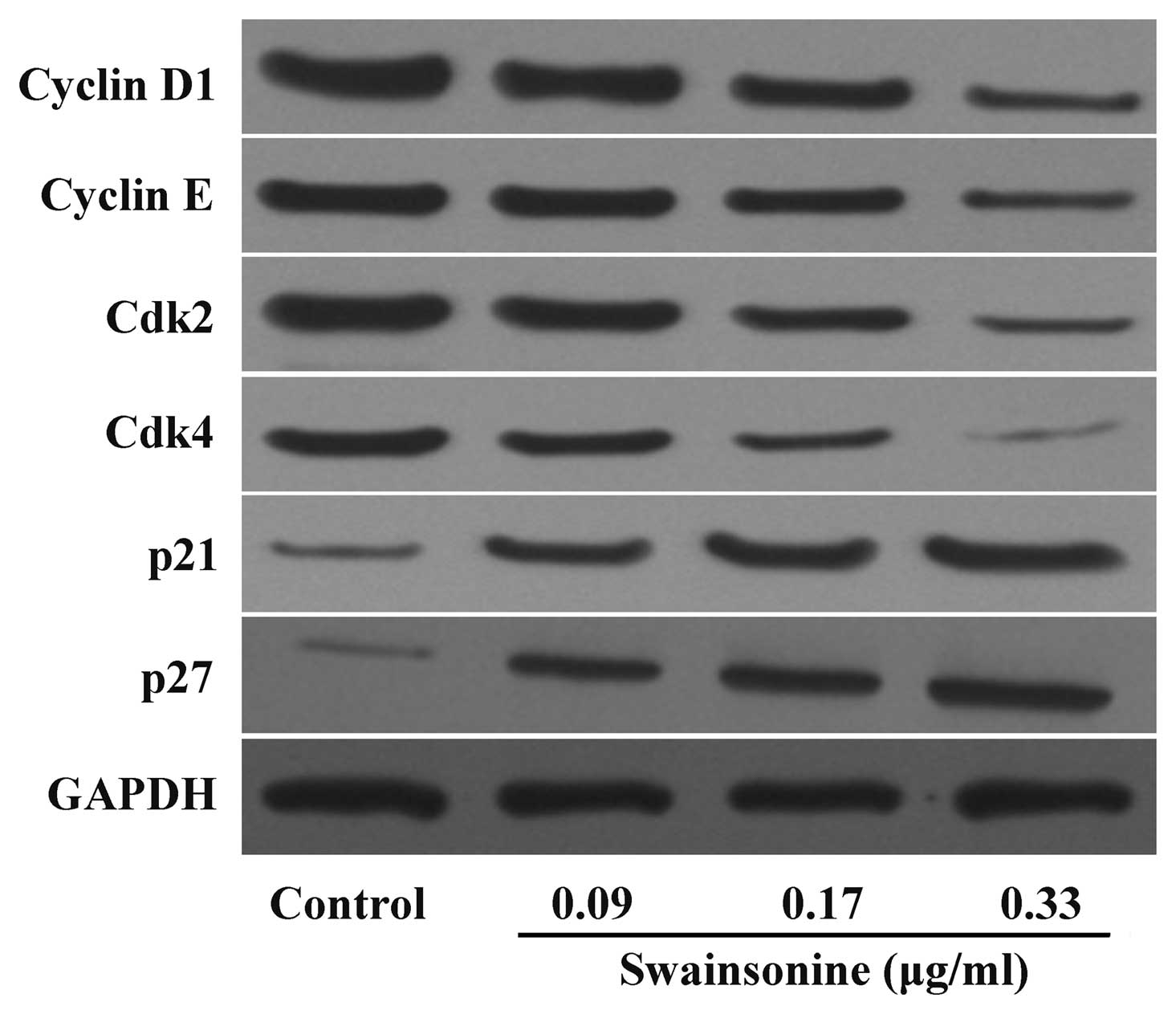
Swainsonine alters the expression of cell
cycle-associated proteins
Cyclins (D1 and E), Cdk2, Cdk4 and Cdk inhibitors,
p21 and p27 are important for cell growth and are key signaling
proteins in cell cycle progression. We investigated whether
swainsonine blocks the G0/G1 to S phase transition through altering
the expression of these proteins. As shown in Fig. 5, swainsonine induced a
dose-dependent decrease in cyclin D1, cyclin E, Cdk2 and Cdk4
protein levels, while an increased expression of p21 and p27 was
observed after swainsonine treatment. Taken together, these results
indicate that swainsonine decreased growth by blocking cell cycle
progression via the upregulation of p21 and p27 and/or the
downregulation of cyclin D1, cyclin E, Cdk2 and Cdk4
expression.
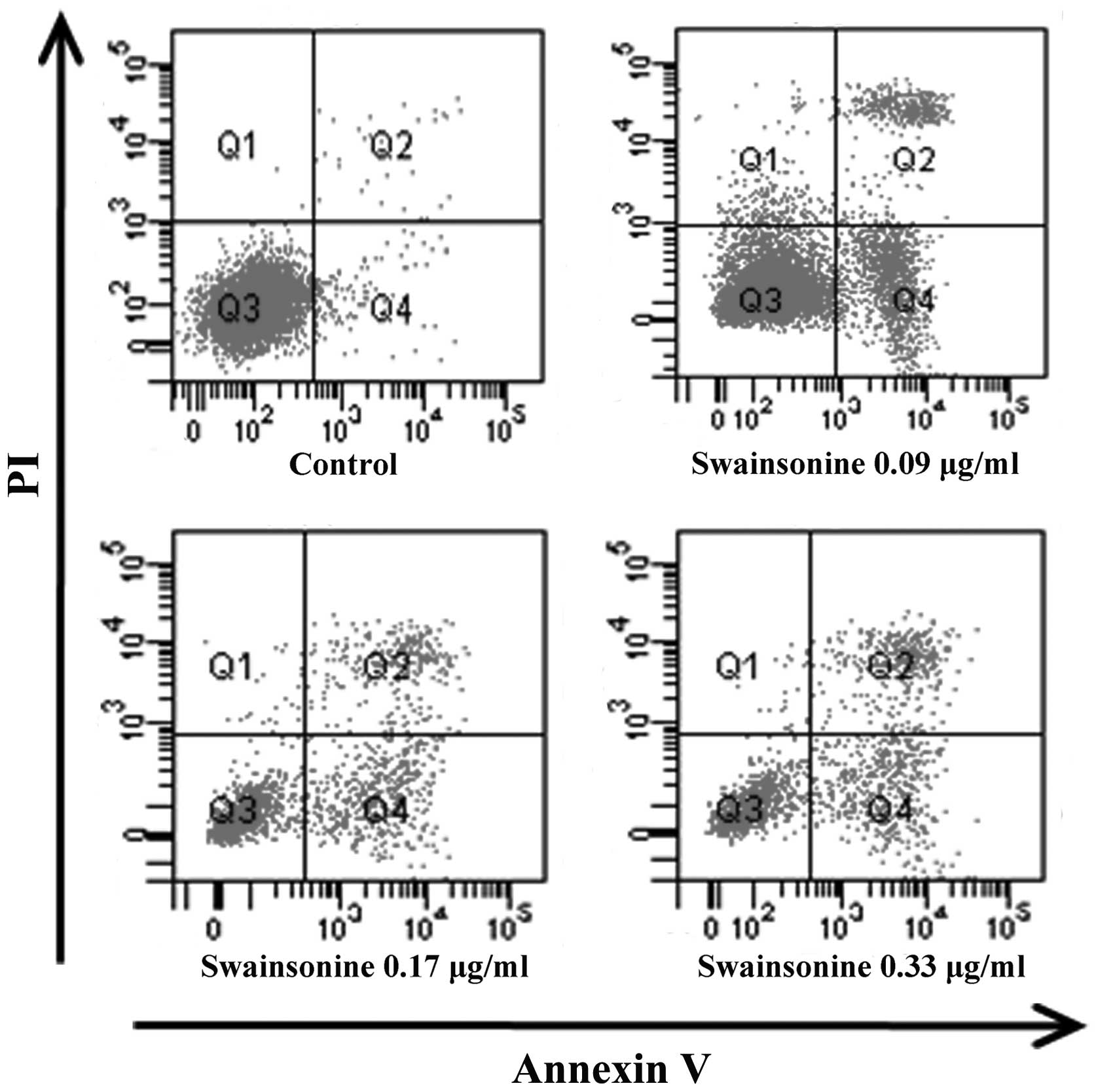
Swainsonine induces apoptosis in MHCC97-H
cells
We assessed the apoptotic rate of the cells treated
with different concentrations of swainsonine for 24 h using the
Annexin V/PI staining assay. The results (Fig. 6 and Table II) indicated that after swainsonine
treatment for 24 h, viable cells decreased clearly and the
proportion of early and late apoptotic cells increased (n=3,
P<0.05) in a dose-dependent manner. These results showed that
swainsonine significantly increased apoptosis.
 | Table IIEffects of swainsonine on cell
apoptosis (%). |
Table II
Effects of swainsonine on cell
apoptosis (%).
| Normal | Early
apoptosis | Late apoptosis | Necrosis |
|---|
| Control | 97.88±1.19 | 1.62±0.06 | 0.40±0.03 | 0.01±0.00 |
| Swainsonine
(μg/ml) |
| 0.09 | 79.36±2.03a | 13.30±1.57a | 4.74±0.29a | 2.60±0.11a |
| 0.17 | 64.88±1.77a | 21.19±1.00a | 12.72±0.51a | 1.21±0.06a |
| 0.33 | 50.35±1.56a | 27.61±1.03a | 20.96±0.97a | 1.08±0.02a |
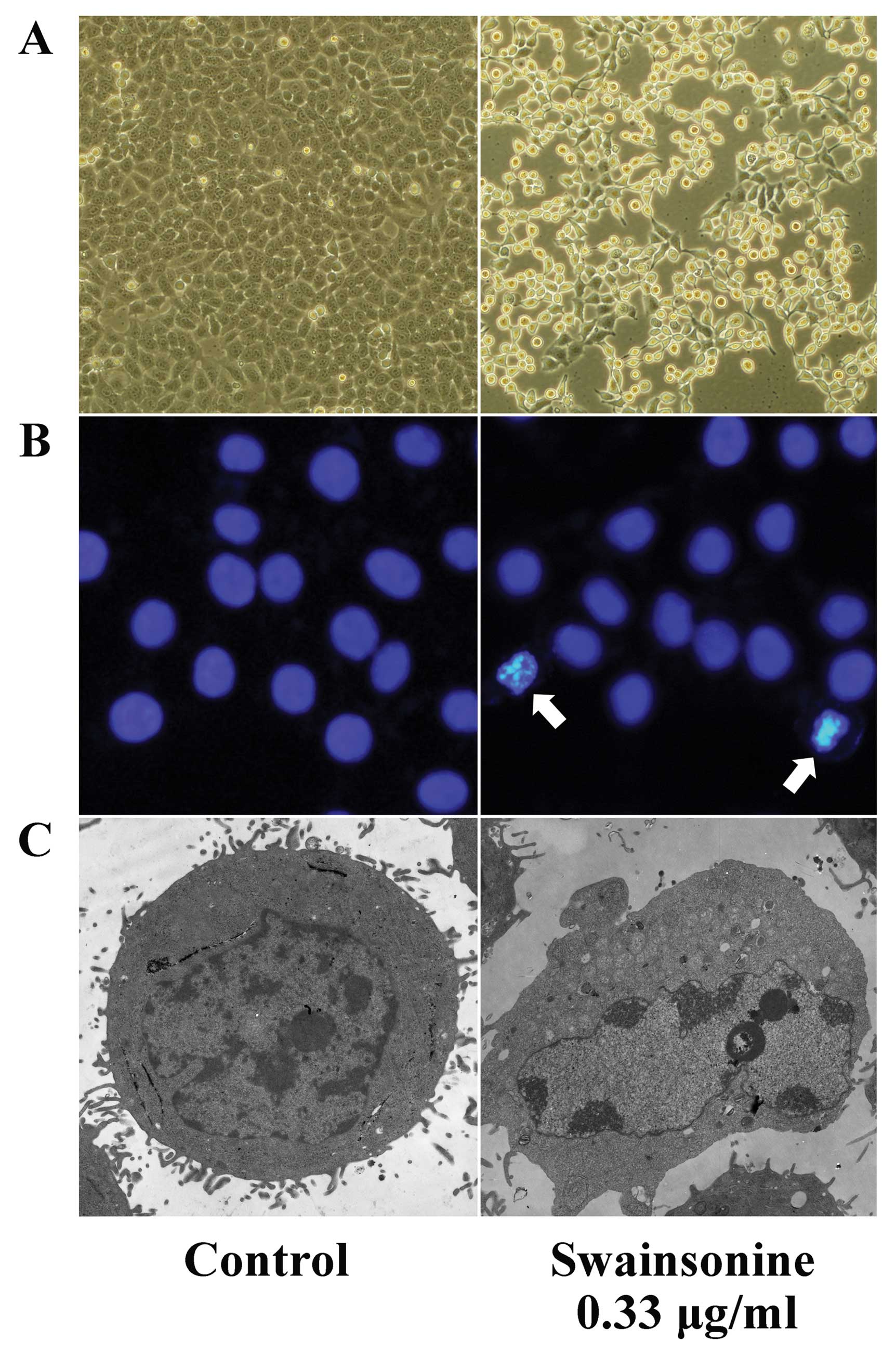
Swainsonine induces apoptotic
morphological changes in MHCC97-H cells
Morphological analyses showed that MHCC97-H cells
underwent marked morphological changes after incubation with
swainsonine. Bright field observations revealed that cells treated
with swainsonine (0.33 μg/ml) became round, shrunken and detached
from the surface of the flask (Fig.
7A). Subsequently, Hoechst 33342 staining also revealed
characteristic morphological features, for example, the cells
shrank, became circular, intensely fluorescent, fragmented and had
condensed nuclei when treated with swainsonine (Fig. 7B). The apoptotic features of cells
were further confirmed by electron microscopy. Cells were smaller
in size, lost microvilli, had irregular cell outlines, had
condensed, fractured and marginalized chromatin and had increased
numbers of lysosomes (Fig. 7C). The
morphological changes observed were consistent with changes
associated with apoptosis.
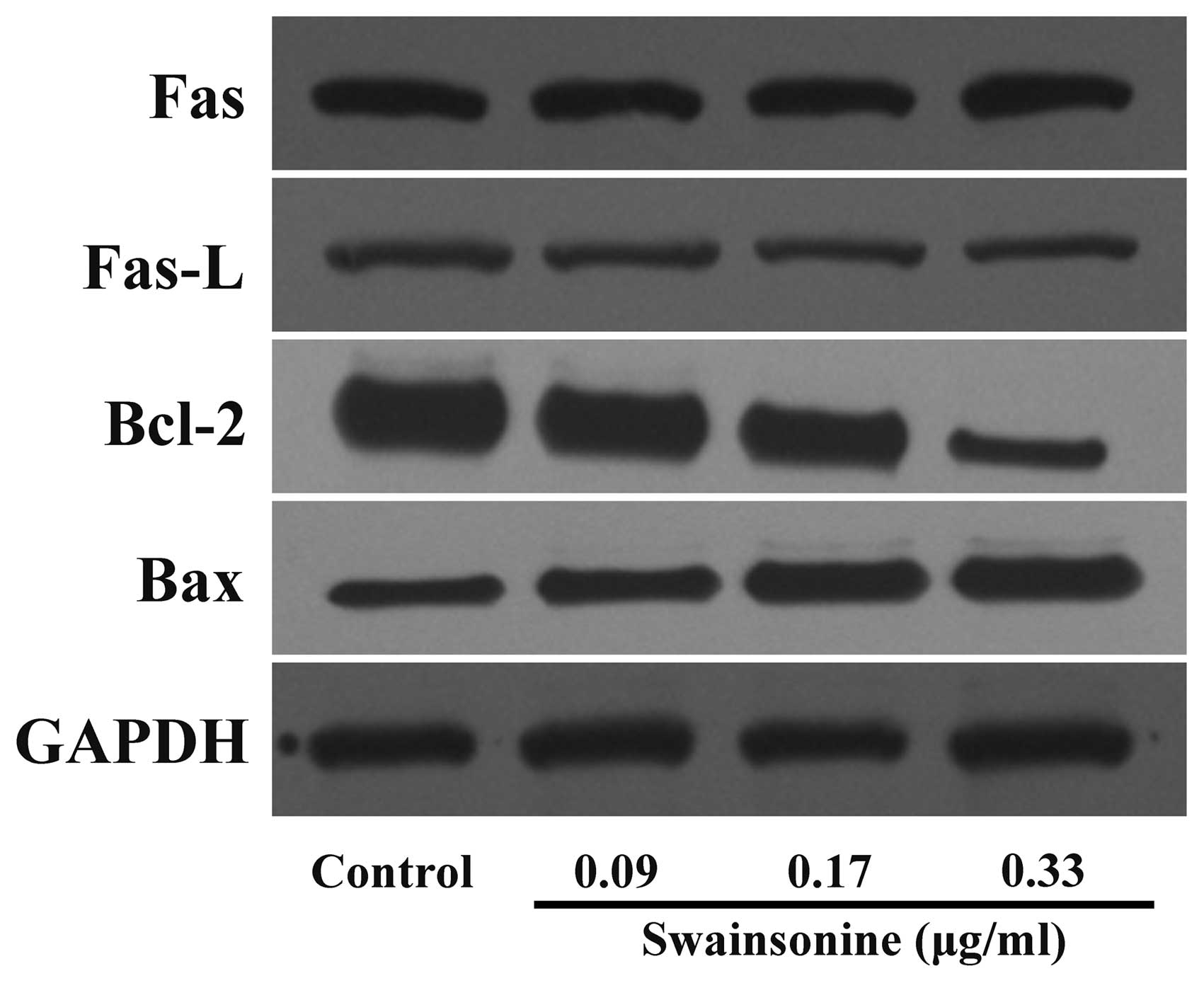
Swainsonine alters the expression of
apoptosis-related proteins
To further clarify the apoptotic mechanisms of human
hepatoma cells induced by swainsonine treatment, we analyzed the
proteins of MHCC97-H cells treated with different concentrations of
swainsonine using western blot assay. We found that the expression
levels of Fas and Fas-L were not changed significantly (n=3,
P>0.05). Furthermore, we observed a dose-dependent reduction in
the levels of the anti-apoptotic protein Bcl-2, whereas a
concomitant increase in the level of pro-apoptotic protein Bax was
observed (Fig. 8) (n=3, P<0.05).
The results indicate that swainsonine induces apoptosis in MHCC97-H
cells mainly through a mitochondria-mediated internal pathway.
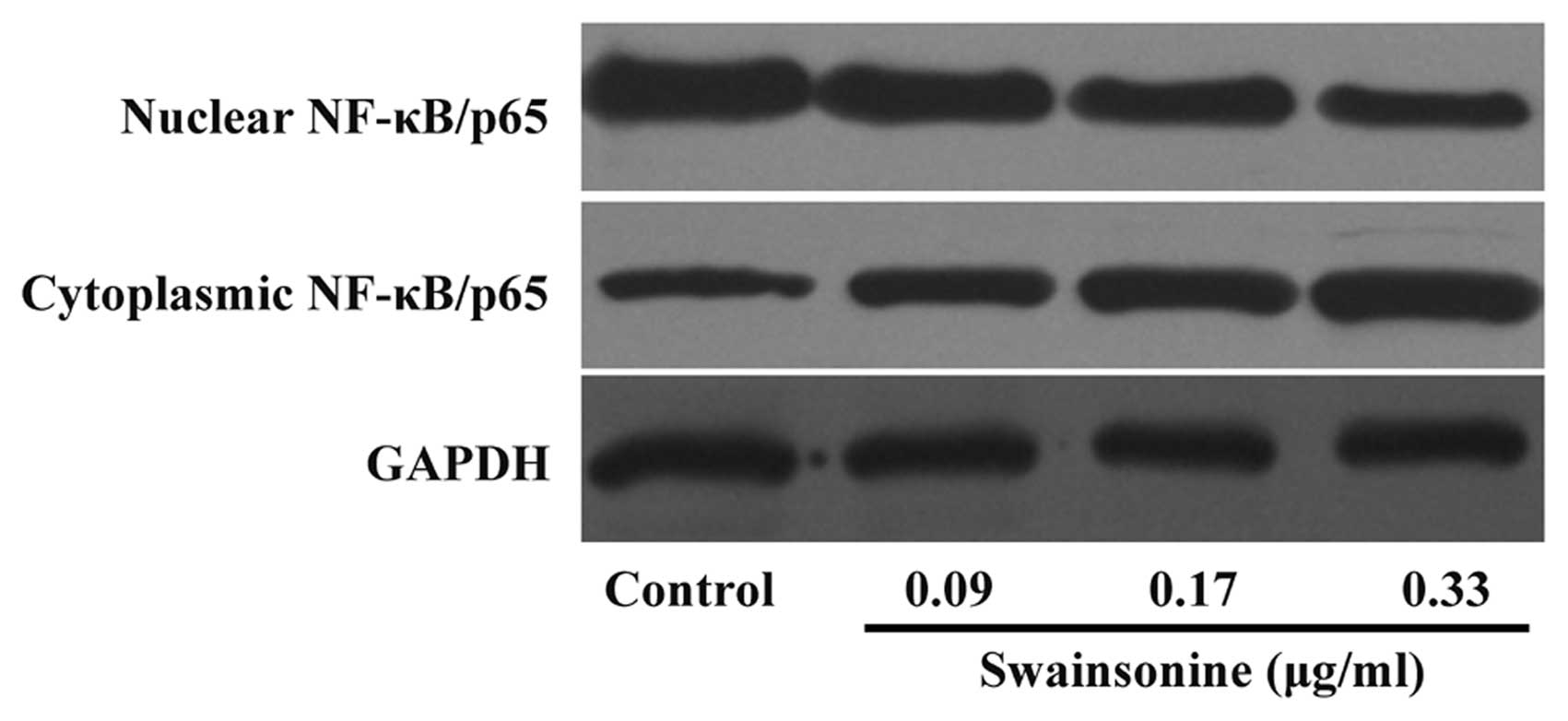
Swainsonine attenuates the constitutive
activation of NF-κB in MHCC97-H cells
Since NF-κB activation controls the expression of a
number of genes involved in cell growth and survival through direct
and indirect mechanisms, we investigated whether the treatment of
HCC cells with swainsonine has an impact on NF-κB activation in HCC
cells. We revealed that swainsonine treatment caused a marked and
dose-dependent increase in NF-κB levels in the cytoplasmic fraction
of MHCC97-H cells with a simultaneous decrease in the nuclear
fraction (Fig. 9). The results
indicate that swainsonine suppresses constitutive activation of
NF-κB in HCC cells.
Swainsonine chemosensitizes HCC to
paclitaxel-induced cytotoxicity
Since chemoresistance, due to paclitaxel-induced
NF-κB activation, is an important cause of a suboptimal therapeutic
effect we investigated whether swainsonine acts as a
chemosensitizer in HCC in vitro and in vivo. At a
concentration of 0.03 μg/ml, swainsonine did not significantly
inhibit the growth of the MHCC97-H cell line after 24 h of
treatment (Fig. 10A). We performed
further cell viability analysis in the MHCC97-H cells incubated
with paclitaxel alone or swainsonine plus paclitaxel. As noted in
Fig. 10A, paclitaxel treatment
alone inhibited the growth of MHCC97-H cells, while combined
treatment with swainsonine led to a significant enhancement in the
inhibitory effects of paclitaxel. This suggests that swainsonine at
a concentration of 0.03 μg/ml does not trigger apoptosis alone, but
is capable of sensitizing a cell response to paclitaxel.
Furthermore, the in vivo antitumor activity of the
paclitaxel/swainsonine combination was evaluated in nude mice
xenografts. The optimum doses of paclitaxel and swainsonine used in
this study were adopted according to our preliminary experiments
(data not shown). We found that paclitaxel alone inhibited the
growth of established tumors, while combined treatment of
paclitaxel plus swainsonine resulted in a more significant
suppression of established tumors compared to paclitaxel or
swainsonine alone (P<0.05). Swainsonine alone at low
concentrations was unable to significantly inhibit tumor growth in
the xenograft-bearing mice (Fig. 10B
and C). We further analyzed the growth- and apoptosis-related
molecules of tumors in each group. Importantly, we found that
tumors treated with paclitaxel plus swainsonine showed lower Ki-67
and Bcl-2, with higher Bax levels in comparison to tumors treated
alone with paclitaxel or swainsonine (Fig. 10D) (P<0.05). Our data
demonstrated that treatment with swainsonine increased the efficacy
of the paclitaxel-induced inhibition of tumor xenografts in mice.
In addition, we also determined the cellular localization of NF-κB
in paclitaxel (alone or in combination with swainsonine)-treated
MHCC97-H cells. Our data showed that paclitaxel induced an enhanced
accumulation of NF-κB in a nuclear compartment and a concomitant
decrease in cytoplasmic fraction in a dose-dependent manner
(Fig. 10E). However, swainsonine
was effective in inhibiting this paclitaxel-induced activation of
NF-κB in MHCC97-H cells (Fig.
10F). Our data indicate that swainsonine may be an important
chemotherapeutic agent for HCC and that it potentiates the
anticancer efficacy of paclitaxel by acting as a
chemosensitizer.
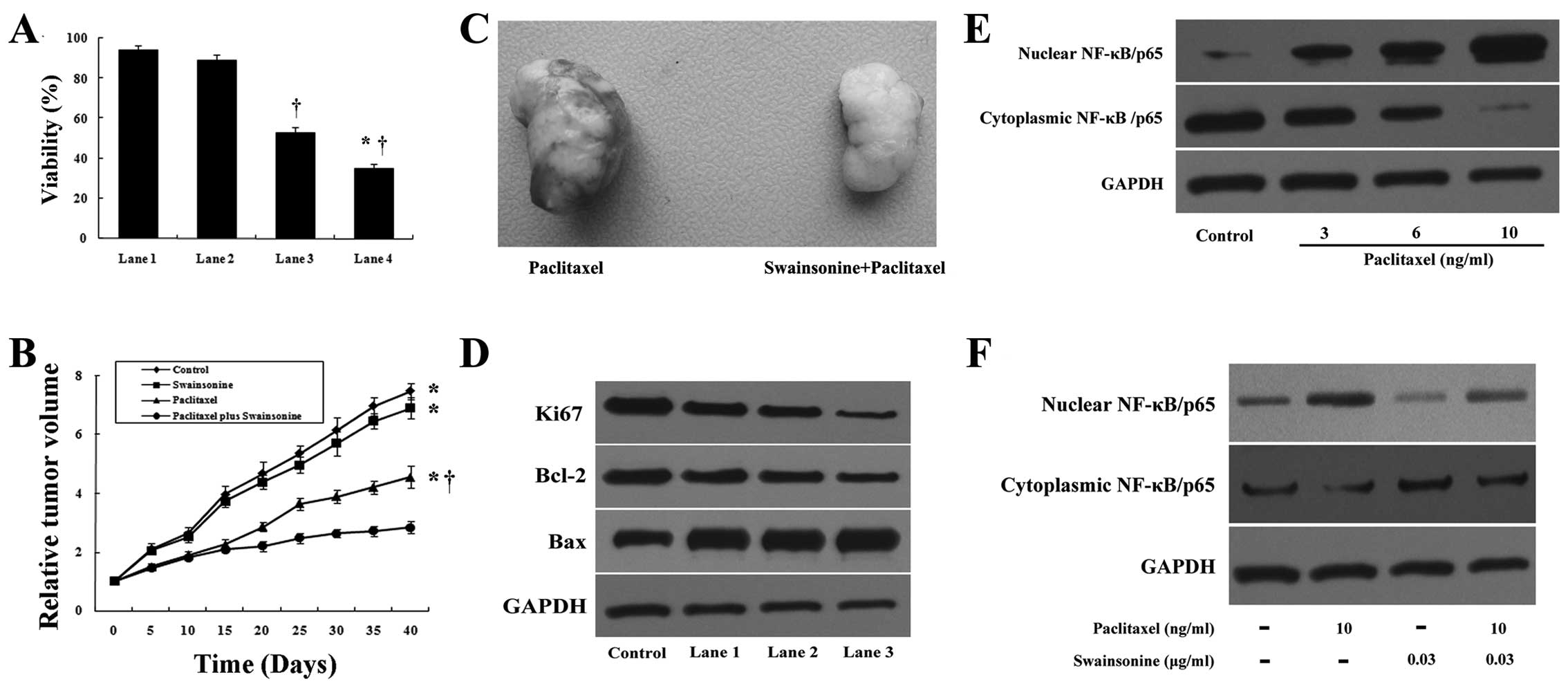 | Figure 10Swainsonine chemosensitizes MHCC97-H
cells to paclitaxel treatment in vitro and in vivo.
(A) MHCC97-H cells were treated with swainsonine (0.03 μg/ml),
paclitaxel (10 ng/ml) or swainsonine (0.03 μg/ml) plus paclitaxel
(10 ng/ml) for 24 h. Percent viability was measured by MTT assay.
Lane 1, control; lane 2, cells treated with swainsonine; lane 3,
cells treated with paclitaxel; lane 4, cells treated with
swainsonine plus paclitaxel. *P<0.05 compared to
paclitaxel alone. †P<0.05 compared to the control.
(B) In vivo anticancer activity assay. Drug treatment groups
received paclitaxel, swainsonine or paclitaxel and swainsonine. The
tumor volumes were measured on the indicated day.
*P<0.05 vs. paclitaxel plus swainsonine group.
†P<0.05 compared to the control. (C) At the end of
the experiment, representative tumors were collected. (D) The
expression levels of Bax, Bcl-2 and Ki-67 under in vivo
conditions. Lane 1, control; lane 2, mice treated with swainsonine;
lane 3, mice treated with paclitaxel; lane 4, mice treated with
swainsonine plus paclitaxel. (E) The cells were treated with
paclitaxel (0, 3, 6 and 10 ng/ml) for 24 h and the expression of
NF-κB/p65 in nuclear and cytoplasmic regions was determined by
western blot analysis. (F) Nuclear and cytoplasmic extracts were
prepared from cells treated with paclitaxel, swainsonine alone or
in combination for 24 h. Western blot analysis was used to
determine the expression of NF-κB/p65 in nuclear and cytoplasmic
regions. |
Discussion
Despite many years of intensive research, HCC
remains a devastating malignancy due to the lack of effective
therapy. Systemic chemotherapy is the only remaining option,
especially for patients with inoperable or metastatic disease.
Although systemic drugs have been tested for patients with HCC, the
prognosis of these patients is not yet favorable. Most patients
with advanced HCC are destined to develop resistance to
conventional anticancer agents. Therefore, the development of new
drugs with the ability to directly inhibit cancer cell growth or
potentiate the cytotoxic effect of other agents for the treatment
of HCC is required (18). Recently,
chemotherapy and other intervention strategies using naturally
occurring agents have emerged as a promising alternative option to
improve the quality of life for HCC patients (19).
Swainsonine, an extract from Astragalus
membranaceus, is a known potent and specific inhibitor of
lysosomal acid and Golgi α-mannosidase II (20). Golgi α-mannosidase II is an
important member of the N-glycosylation pathway, which is
associated with the progression, metastasis and clinical outcome of
a number of cancer types. Thus, the inhibition of Golgi
α-mannosidase II has shown clinical potential in cancer treatment
(21). Recently, numerous studies
have suggested through the expression changes of apoptosis-related
genes in vivo that swainsonine has potential for treating
glioma and gastric carcinoma through the mechanism of induced
apoptosis. Additionally, initial clinical research has confirmed a
clear curative effect against pate malignant tumor and chest and
abdominal lymphangioma (14–16).
Evidence indicates that swainsonine is a potent anticancer agent
and the anticancer action of swainsonine may result from the
co-operation of a number of pathways. However, a phase II clinical
trial of GD0039 (a hydrochloride salt of swainsonine) in 17
patients with renal carcinoma was discouraging (22). The reason for the disparity is
unknown; however, it may be due to the different types of cancer.
Despite the controversy surrounding its efficacy, swainsonine may
be considered a satisfactory candidate drug for cancer targeting
therapy. Further studies regarding this agent may possibly lead to
its clinical application.
Although numerous literature exists regarding
swainsonine acting on hepatoma cells, these studies applied
swainsonine on hepatoma cells for the related research of
oligosaccharides (23,24). Reports investigating the roles of
swainsonine in anti-HCC therapy, particularly regarding the
molecular mechanisms do not exist. Therefore in this study, we
focused on investigating the direct anti-HCC action of swainsonine
and the potential pathways involved. Furthermore, we investigated
whether swainsonine chemosensitizes HCC to paclitaxel-induced
cytotoxicity via attenuating the constitutive activation of
NF-κB.
Cancer is known as a disease for deregulating signal
transduction pathways that regulate fundamental processes such as
cell growth (25). In the last few
years, accumulating data also suggest that the regulation of
intracellular growth signaling events may be controlled by the
progression of the cell cycle and apoptosis (26). In addition, current cytotoxic
therapies such as chemotherapy, radiotherapy or immunotherapy
critically depend on inducing cell cycle arrest and cancer cell
apoptosis (27–29). Hence, a therapeutic strategy aimed
at the cell cycle and apoptosis is expected to provide an effective
treatment for cancer.
In this research, we demonstrated that the treatment
of HCC cells with swainsonine reduces the viability of hepatoma
cells, but induced little or no cytotoxicity to hepatocytes.
Furthermore, using flow cell cytometric analysis techniques, we
showed that swainsonine inhibited the growth of MHCC97-H cells
accompanied by increasing the number of cells in the G0/G1 phases
and decreasing the number of cells in the S and G2/M phases. Cell
cycle is regulated by the concerted actions of cyclins, Cdks and
Cdk inhibitors (30). The
physiological process of cell cycle from phase G1 to S is
controlled at several steps by cyclin D1, cyclin E, Cdk2 and Cdk4
(31). As cell cycle suppressor
molecules, the important function of p21 and p27 is to induce cell
cycle arrest by forming heterotrimeric complexes with G1-S Cdks and
cyclins to inhibit their activity (32). Our western blot results indicated
that cyclin D1, cyclin E, Cdk2 and Cdk4 were downregulated and that
p21 and p27 were upregulated by swainsonine treatment. However, our
results are not in total agreement with data obtained by Sun et
al (16), who reported that
swainsonine induced tumor cell arrest in S phase. This indicates
that swainsonine may induce different types of cell cycle arrest in
different cell types. Cell cycle regulation is complicated and
cell-specific. Further study is essential to disclose the mechanism
of swainsonine on cell cycle arrest.
There is growing evidence that apoptosis failure may
be involved in the pathogenesis of cancer. The ability of tumor
cells to evade the engagement of apoptosis may also play a
significant role in their resistance to conventional therapeutic
regimens (33). Therefore, a
therapeutic strategy aimed at specifically triggering apoptosis in
cancer cells may have a potential therapeutic effect. In our study,
we observed a significant induction of apoptosis in
swainsonine-treated MHCC97-H cells, indicating that swainsonine
potentiates the apoptotic machinery. There are two classic
apoptotic pathways in mammalian cells, namely, the
mitochondria-mediated apoptotic and the death receptor-mediated
apoptotic pathway (34). Both
apoptotic pathways are gene-regulated. We observed that the
treatment of MHCC97-H cells with swainsonine resulted in the
upregulation of Bax levels, along with the reduction in Bcl-2.
However, the expression levels of Fas and Fas-L were not changed
significantly. The results showed that swainsonine induced
apoptosis in MHCC97-H cells mainly through the
mitochondria-mediated pathway.
Constitutive activation of NF-κB has been described
in a number of solid tumors, including HCC. This activation is
involved in the regulation of transcription of various genes
involved in cell growth and apoptosis (35). Depending on the specific role of
NF-κB in the cell cycle and apoptosis, we investigated whether
NF-κB was involved in the inhibition of HCC cell growth by
swainsonine. Our results showed that swainsonine-induced inhibition
of cell growth, cell cycle arrest and apoptosis were accompanied by
a significant inactivation of NF-κB. Several other studies have
also shown that NF-κB induces the expression of cyclin D1, Bcl-2
and Bcl-xL (36). Our results
showed that the expression levels of cyclin D1 and Bcl-2 were
reduced when cells were treated with swainsonine and this occurred
at least partially via the inactivation of NF-κB signaling.
Therefore, it may be suggested that the inhibition of HCC cell
growth by swainsonine is possibly mediated through the inhibition
of NF-κB.
In addition, the activation of NF-κB plays an
important role in the development of drug resistance. Currently,
many researchers have focused their efforts on exploring
chemo-sensitizing drugs, with NF-κB suppression activity, used in
combination with other chemotherapeutic agents for effective
management of HCC (37). Paclitaxel
is a useful chemotherapeutic drug for the treatment of patients
with a variety of malignant tumors (38). However, chemoresistance due to
paclitaxel-induced NF-κB activation is an important cause of the
limits of paclitaxel efficacy (39). Several reports have shown the
advantage of combination treatments (40). In this study, we showed the
chemosensitizing effect of swainsonine on MHCC97-H cells to
paclitaxel toxicity in vitro and in vivo. Combination
treatment was significantly superior to paclitaxel or swainsonine
alone. In this study, we also revealed that combination treatment
of paclitaxel and swainsonine is associated with the inhibition of
NF-κB activity.
In conclusion, the present study demonstrated that
swainsonine significantly inhibits the growth of HCC cells by
causing cell cycle arrest and the induction of apoptosis.
Swainsonine causes G1 phase cell cycle arrest and the induction of
apoptosis by altering the cell cycle and expression of associated
survival proteins. Furthermore, our study suggests a role of
swainsonine in chemosensitizing HCC cells to the cytotoxic effects
of paclitaxel. Suppression of NF-κB activation may be one of the
mechanisms involved in the swainsonine-induced inhibition of growth
and chemosensitizing effects in HCC cells. Our study provides
important data with which to evaluate the possible clinical
application of swainsonine as a novel anti-HCC agent. Further
studies are in progress in our laboratory to provide additional
data for clinical application.
Acknowledgements
The authors would like to thank Juan Li and Jintao
Hu for their excellent technical assistance. The Chinese National
Natural Science Foundation grant no. 81170419 funded this
study.
Abbreviations:
|
BSA
|
bovine serum albumin
|
|
Cdk
|
cyclin-dependent kinases
|
|
DMEM
|
Dulbecco’s modified Eagle’s medium
|
|
DMSO
|
dimethyl sulfoxide
|
|
FBS
|
fetal bovine serum
|
|
GAPDH
|
glyceraldehyde-3-phosphate
dehydrogenase
|
|
HCC
|
hepatocellular carcinoma
|
|
MTT
|
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
|
|
NF-κB
|
nuclear factor κB
|
|
PBS
|
phosphate-buffered saline
|
|
PI
|
propidium iodide
|
|
TEM
|
transmission electron microscope
|
References
|
1
|
Lin H, van den Esschert J, Liu C and van
Gulik TM: Systematic review of hepatocellular adenoma in China and
other regions. J Gastroenterol Hepatol. 26:28–35. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Villanueva A and Llovet JM: Targeted
therapies for hepatocellular carcinoma. Gastroenterology.
140:1410–1426. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang JD and Roberts LR: Hepatocellular
carcinoma: a global view. Nat Rev Gastroenterol Hepatol. 7:448–458.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sanoff HK, Bernard S, Goldberg RM, et al:
Phase II study of capecitabine, oxaliplatin, and cetuximab for
advanced hepatocellular carcinoma. Gastrointest Cancer Res. 4:8–83.
2011.PubMed/NCBI
|
|
5
|
Okano J, Nagahara T, Matsumoto K and
Murawaki Y: The growth inhibition of liver cancer cells by
paclitaxel and the involvement of extracellular signal-regulated
kinase and apoptosis. Oncol Rep. 17:1195–1200. 2007.PubMed/NCBI
|
|
6
|
Caicedo-Granados EE, Wuertz BR, Marker PH,
Lee GS and Ondrey FG: The effect of indomethacin on paclitaxel
sensitivity and apoptosis in oral squamous carcinoma cells: the
role of nuclear factor-κB inhibition. Arch Otolaryngol Head Neck
Surg. 137:799–805. 2011.PubMed/NCBI
|
|
7
|
Sreekanth CN, Bava SV, Sreekumar E and
Anto RJ: Molecular evidences for the chemosensitizing efficacy of
liposomal curcumin in paclitaxel chemotherapy in mouse models of
cervical cancer. Oncogene. 30:3139–3152. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kelly MG, Alvero AB, Chen R, et al: TLR-4
signaling promotes tumor growth and paclitaxel chemoresistance in
ovarian cancer. Cancer Res. 66:3859–3868. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Baines AT, Xu D and Der CJ: Inhibition of
Ras for cancer treatment: the search continues. Future Med Chem.
3:1787–1808. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Stronach EA, Chen M, Maginn EN, Agarwal R,
Mills GB, Wasan H and Gabra H: DNA-PK mediates AKT activation and
apoptosis inhibition in clinically acquired platinum resistance.
Neoplasia. 13:1069–1080. 2011.PubMed/NCBI
|
|
11
|
Luedde T and Schwabe RF: NF-κB in the
liver - linking injury, fibrosis and hepatocellular carcinoma. Nat
Rev Gastroenterol Hepatol. 8:108–118. 2011.
|
|
12
|
Luqman S and Pezzuto JM: NFkappaB: a
promising target for natural products in cancer chemoprevention.
Phytother Res. 24:949–963. 2010.PubMed/NCBI
|
|
13
|
Nogueira L, Ruiz-Ontañon P,
Vazquez-Barquero A, Moris F and Fernandez-Luna JL: The NFκB
pathway: a therapeutic target in glioblastoma. Oncotarget.
2:646–653. 2011.
|
|
14
|
Goss PE, Baptiste J, Fernandes B, Baker M
and Dennis JW: A phase I study of swainsonine in patients with
advanced malignancies. Cancer Res. 54:1450–1457. 1994.PubMed/NCBI
|
|
15
|
Santos FM, Latorre AO, Hueza IM, Sanches
DS, Lippi LL, Gardner DR and Spinosa HS: Increased antitumor
efficacy by the combined administration of swainsonine and
cisplatin in vivo. Phytomedicine. 18:1096–1101. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sun JY, Yang H, Miao S, et al: Suppressive
effects of swainsonine on C6 glioma cell in vitro and in vivo.
Phytomedicine. 16:1070–1074. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
You N, Liu W, Zhong X, et al: Tg737
inhibition results in malignant transformation in fetal liver
stem/progenitor cells by promoting cell-cycle progression and
differentiation arrest. Mol Carcinog. 51:659–673. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Alves RC, Alves D, Guz B, et al: Advanced
hepatocellular carcinoma. Review of targeted molecular drugs. Ann
Hepatol. 10:21–27. 2011.PubMed/NCBI
|
|
19
|
Karikas GA: Anticancer and chemopreventing
natural products: some biochemical and therapeutic aspects. J BUON.
15:627–638. 2010.PubMed/NCBI
|
|
20
|
Tulsiani DR and Touster O: Swainsonine, a
potent mannosidase inhibitor, elevates rat liver and brain
lysosomal alpha-D-mannosidase, decreases Golgi alpha-D-mannosidase
II, and increases the plasma levels of several acid hydrolases.
Arch Biochem Biophys. 224:594–600. 1983. View Article : Google Scholar
|
|
21
|
van den Elsen JM, Kuntz DA and Rose DR:
Structure of Golgi alpha-mannosidase II: a target for inhibition of
growth and metastasis of cancer cells. EMBO J. 20:3008–3017.
2001.PubMed/NCBI
|
|
22
|
Shaheen PE, Stadler W, Elson P, Knox J,
Winquist E and Bukowski RM: Phase II study of the efficacy and
safety of oral GD0039 in patients with locally advanced or
metastatic renal cell carcinoma. Invest New Drugs. 23:577–581.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yanagida K, Natsuka S and Hase S:
Structural diversity of cytosolic free oligosaccharides in the
human hepatoma cell line, HepG2. Glycobiology. 16:294–304. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yeo TK, Yeo KT, Parent JB and Olden K:
Swainsonine treatment accelerates intracellular transport and
secretion of glycoproteins in human hepatoma cells. J Biol Chem.
260:2565–2569. 1985.PubMed/NCBI
|
|
25
|
Williams GH and Stoeber K: The cell cycle
and cancer. J Pathol. 226:352–364. 2012. View Article : Google Scholar
|
|
26
|
Fang J, Yu Z, Lian M, Ma H, Tai J, Zhang L
and Han D: Knockdown of zinc finger protein, X-linked (ZFX)
inhibits cell proliferation and induces apoptosis in human
laryngeal squamous cell carcinoma. Mol Cell Biochem. 360:301–307.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hu W, Shen T and Wang MH: Cell cycle
arrest and apoptosis induced by methyl 3,5-dicaffeoyl quinate in
human colon cancer cells: Involvement of the PI3K/Akt and MAP
kinase pathways. Chem Biol Interact. 194:48–57. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Palumbo C, Bei R, Procopio A and Modesti
A: Molecular targets and targeted therapies for malignant
mesothelioma. Curr Med Chem. 15:855–867. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rahbari NN, Mehrabi A, Mollberg NM, Müller
SA, Koch M, Büchler MW and Weitz J: Hepatocellular carcinoma:
current management and perspectives for the future. Ann Surg.
253:453–469. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Johansson M and Persson JL: Cancer
therapy: targeting cell cycle regulators. Anticancer Agents Med
Chem. 8:723–731. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chang HR, Lian JD, Lo CW, Chang YC, Yang
MY and Wang CJ: Induction of urothelial proliferation in rats by
aristolochic acid through cell cycle progression via activation of
cyclin D1/cdk4 and cyclin E/cdk2. Food Chem Toxicol. 44:28–35.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen K, Perez-Stable C, D’Ippolito G,
Schiller PC, Roos BA and Howard GA: Human bone marrow-derived stem
cell proliferation is inhibited by hepatocyte growth factor via
increasing the cell cycle inhibitors p53, p21 and p27. Bone.
49:1194–1204. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sayers TJ: Targeting the extrinsic
apoptosis signaling pathway for cancer therapy. Cancer Immunol
Immunother. 60:1173–1180. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chowdhury I, Tharakan B and Bhat GK:
Current concepts in apoptosis: the physiological suicide program
revisited. Cell Mol Biol Lett. 11:506–525. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
He G and Karin M: NF-κB and STAT3 - key
players in liver inflammation and cancer. Cell Res. 21:159–168.
2011.
|
|
36
|
Deeb D, Gao X, Dulchavsky SA and Gautam
SC: CDDO-Me inhibits proliferation, induces apoptosis,
down-regulates Akt, mTOR, NF-kappaB and NF-kappaB-regulated
antiapoptotic and proangiogenic proteins in TRAMP prostate cancer
cells. J Exp Ther Oncol. 7:31–39. 2008.
|
|
37
|
Ma Y, Wang J, Liu L, et al: Genistein
potentiates the effect of arsenic trioxide against human
hepatocellular carcinoma: role of Akt and nuclear factor-κB. Cancer
Lett. 301:75–84. 2011.PubMed/NCBI
|
|
38
|
Matsubara J, Shimada Y, Kato K, et al:
Phase II study of bolus 5-fluorouracil and leucovorin combined with
weekly paclitaxel as first-line therapy for advanced gastric
cancer. Oncology. 81:291–297. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fujiwara Y, Furukawa K, Shimada Y, et al:
Combination paclitaxel and inhibitor of nuclear factor κB
activation improves therapeutic outcome for model mice with
peritoneal dissemination of pancreatic cancer. Pancreas.
40:600–607. 2011.
|
|
40
|
Pasqualetti G, Ricciardi S, Mey V, Del
Tacca M and Danesi R: Synergistic cytotoxicity, inhibition of
signal transduction pathways and pharmacogenetics of sorafenib and
gemcitabine in human NSCLC cell lines. Lung Cancer. 74:197–205.
2011. View Article : Google Scholar : PubMed/NCBI
|