Introduction
Cancer cells grow more rapidly than tumoral blood
vessels (1). This characteristic of
cancer cells induces an intra-tumoral hypoxia (2) that in turn decreases the growth of
cancer cells. When cancer cell growth is decreased, so is the
cell’s sensitivity to oncologic therapies (3,4).
Mammalian cells produce energy primarily by
oxidative phosphorylation (OXPHOS) and to a lesser extent by
glycolysis. Cancer cells have increased glucose transporters,
glycolytic enzymes and the proteins that inhibit OXPHOS. As a
result, cancer cells have high levels of glycolysis and low levels
of OXPHOS. Therefore, cancer cells switch their primary pathway of
energy production from OXPHOS to glycolysis. This phenomenon was
first described by Warburg (5) and
is known as the Warburg effect. Mechanisms of the Warburg effect
are poorly defined and may involve the transcription factor
hypoxia-inducible factor-1 (HIF-1) (6).
HIF-1 comprises α and β subunits (7). Upon biosynthesis, HIF-1α is
hydroxylated in the presence of oxygen and is then degraded in
proteasomes (8). Thus, normal
mammalian cells have HIF-1β but not HIF-1α. When cells are
subjected to hypoxia, however, HIF-1α is stabilized and associated
with HIF-1β. Thus, intra-tumoral hypoxia appears to be causally
responsible for HIF-1α expression that is seen in different cancer
cells (6). In addition, cancer
cells may have an increased HIF-1α production (9). When cancer cells produce more HIF-1α
than they can degrade, HIF-1α is accumulated. HIF-1α expression
increases glucose transporters, glycolytic enzymes, and the
inhibitors of OXPHOS. Therefore, cancer-induced HIF-1α plays a key
role in the Warburg effect (10–13).
As the first step of glycolysis, glucose is
phosphorylated by hexokinase to produce glucose-6-phosphate
(G-6-P). Next, G-6-P is converted to fructose-6-phosphate by
phosphoglucose isomerase (PGI). In cancer cells, the predominant
form of hexokinase is hexokinase-2 (HK-II) that is attached to the
outer membrane of the mitochondria (12). Inhibition of HK-II not only
decreases energy production but also impairs the mitochondria in
cancer cells. Several HK-II inhibitors have been developed,
including 2-deoxy-D-glucose (2-DG) and 3-bromopyruvate (3-BrPA)
(14–25).
After 2-DG is transported in cancer cells by
overexpressed glucose transporters, it is phosphorylated by HK-II
to produce 2-DG-6-phosphate (2-DG-6-P). However, this product
cannot be metabolized further, so it accumulates in cancer cells
and inhibits HK-II by allosteric feedback. Meanwhile,
non-phosphorylated 2-DG competes with glucose for binding to HK-II,
and the substrate competition inhibits HK-II as well. Furthermore,
2-DG-6-P competes with G-6-P for binding to PGI, and the second
substrate competition inhibits PGI (14). Unlike 2-DG, 3-BrPA is an alkylating
reagent that inactivates HK-II and dissociates the enzyme from the
mitochondria (21). When 2-DG or
3-BrPA is used alone or in combination with other anticancer drugs,
cancer cells are inhibited (15–25).
However, it is unclear whether 2-DG and 3-BrPA have enhanced
anticancer effects when they are used together. Here, we addressed
this question in MiaPaCa2 and Panc-1 pancreatic cancer cells.
When cells are subjected to hypoxia, they undergo
biological changes to adapt themselves to the hypoxic conditions.
Whilst many of the hypoxia-induced changes are mediated by HIF-1α,
some hypoxia-induced changes may be independent of HIF-1α (26). In the present study, we studied
wild-type (wt) MiaPaCa2 and Panc-1 cells when they were incubated
under hypoxic (HIF-1α-positive) or normoxic (HIF-1α-negative)
conditions and we also studied MiaPaCa2 and Panc-1 variants that
had HIF-1α-specific small interfering RNA (siRNA) and were devoid
of HIF-1α in both normoxia and hypoxia. By comparing data from the
different cells, we differentiated the hypoxia-induced changes that
were dependent on HIF-1α from those that were independent of
HIF-1α. In different experiments, we measured cell population,
determined intracellular ATP and fumarate, and assessed the
expression of HIF-1α, HK-II, Cu/Zn-superoxide dismutase (SOD1), and
poly(ADP-ribose) polymerase (PARP).
Materials and methods
Pancreatic cancer cells
Wt MiaPaCa2 and Panc-1 cells were obtained from the
American Type Culture Collection (Rockville, MD, USA). The MiaPaCa2
and Panc-1 cells that had HIF-1α-specific siRNA (namely si-MiaPaCa2
and si-Panc-1) were prepared as we previously described (10). In different assays, cells were
plated in different vessels and were cultured at 37°C in 5%
CO2 and 95% air (normoxia), using Dulbecco’s modified
Eagle’s medium with 10% fetal bovine serum. When cells were 80%
confluent, they were subjected to experimental incubation in which
they were exposed to 2-DG (#D3179; Sigma) and 3-BrPA (#16490;
Sigma) in normoxia or hypoxia. Hypoxic incubation (1%
O2, 5% CO2 and 94% N2) was set up
as previously described (10).
Western blotting
Whole-cell proteins were extracted using RIPA lysis
and extraction buffer (Thermo Scientific). In some experiments,
nuclear proteins were extracted (10). Proteins were separated on 8% SDS gel
and transferred to PVDF membranes. HIF-1α was determined either in
nuclear proteins using a monoclonal anti-HIF-1α antiserum (#610958;
BD Biosciences) or in whole-cell proteins using a polyclonal
antiserum (#100-449; Novus). HK-II, SOD1 and PARP were determined
in whole-cell proteins, using antisera from Santa Cruz
Biotechnology Inc., (#6521), StressMarq Biosciences Inc.,
(SPC-115C/D) and Cell Signaling Technology (#9532). Topo1, β-actin
and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were
determined as loading controls, using antisera from TopoGEN, Inc.,
(#2012-2) and Abcam (#8227 and #9483). Secondary antisera were
produced by Amersham (#NA931 and #NA934) and Chemicom (#AP106P).
Each protein was assayed at least 3 times.
MTT viability assay
Cells in 96-well plates were exposed to: i) 2-DG
alone (0.3–18 mM), ii) 3-BrPA alone (50–200 μM), iii) both 3-BrPA
(50–200 μM) and 2-DG (1 mM), or iv) neither 2-DG nor 3-BrPA
(control) for 22 h in normoxic or hypoxic conditions. After a
solution of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT, 5 mg/ml) was added (10 μl/well), all cells were
incubated in normoxia for 2 h. The cells were dissolved in
isopropanol with 4 mM HCl, and MTT values were read in a
spectrophotometer using a wavelength of 570 nm.
Other assays
MiaPaCa2 cells were plated either in 24-well plates
for ATP assay or in Petri dishes for fumarate assay. During
experimental incubation, cells were exposed for 4 h to 2-DG alone
(4 mM), 3-BrPA alone (25 μM), both 2-DG (1 mM) and 3-BrPA (25 μM),
or neither 2-DG nor 3-BrPA. ATP and fumarate were determined using
BioVision kits #354 and #633 (Mountain View, CA, USA). Proteins
were determined using a BCA assay kit.
Statistical analysis
Data are the means ± SEM. When three or more groups
were involved, results were analyzed using analysis of variance
followed by the Student’s t-test. When two groups were involved,
the Student’s t-test was used. P<0.05 was considered to indicate
statistically significant differences.
Results
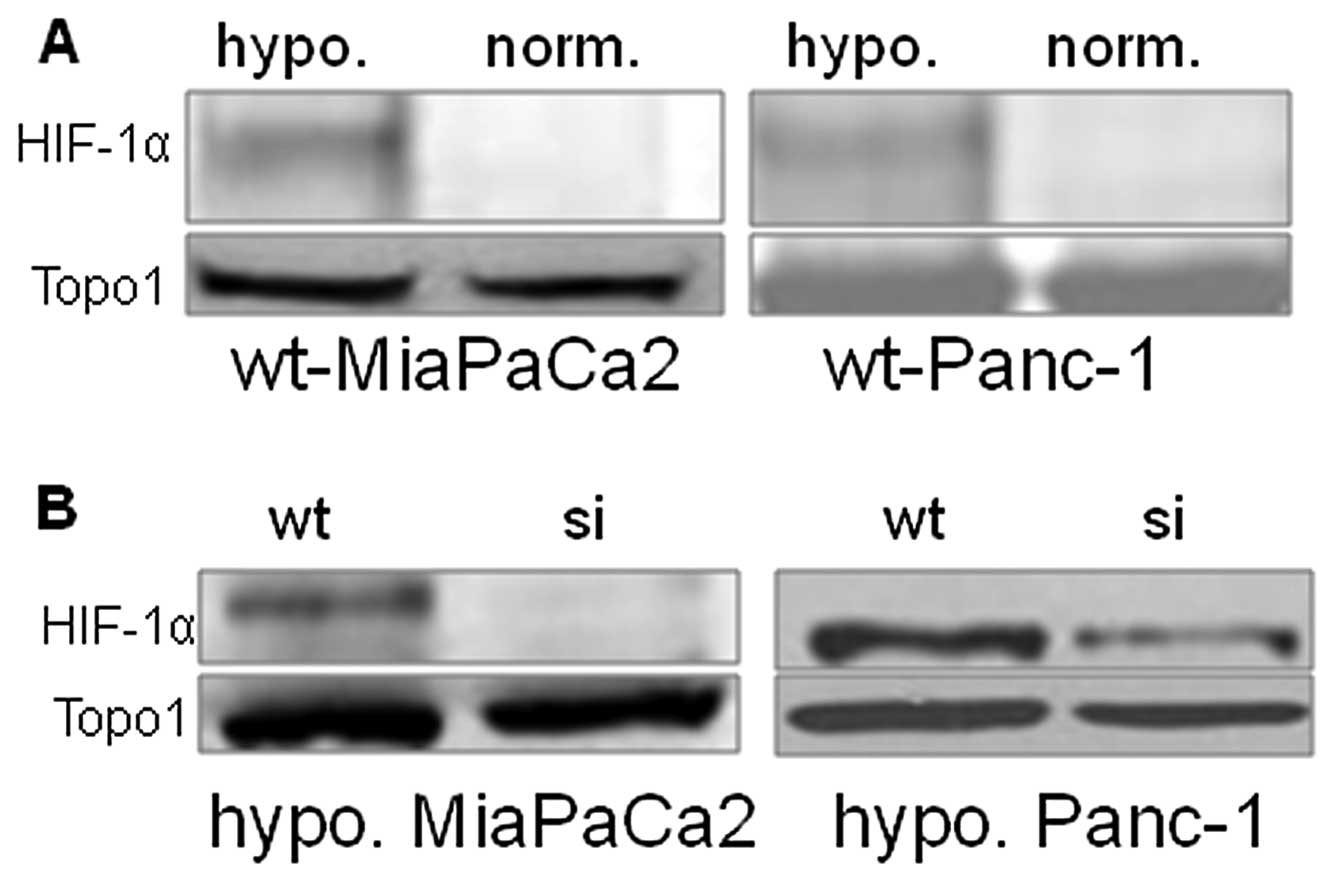
HIF-1α expression in studied cells
Wt-MiaPaCa2 and wt-Panc-1 cells expressed HIF-1α in
hypoxia but not normoxia (Fig. 1A).
As a result of RNA interference, HIF-1α expression was completely
inhibited in hypoxic si-MiaPaCa2 cells and largely inhibited in
hypoxic si-Panc-1 cells (Fig. 1B).
Neither si-MiaPaCa2 cells nor si-Panc-1 cells had HIF-1α in
normoxia (data not shown).
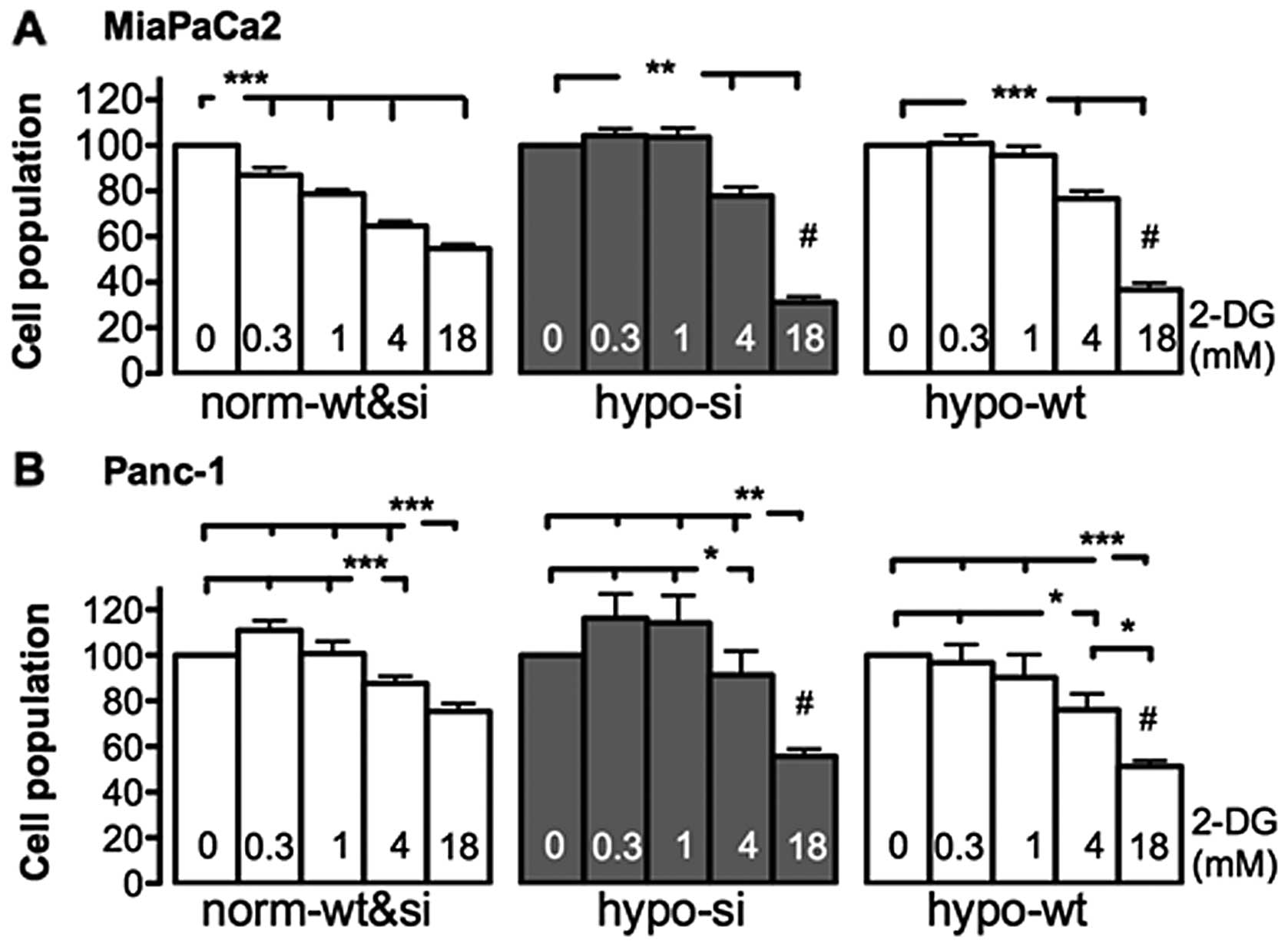
Effects of 2-DG and 3-BrPA on cell
viability
Given that neither wt-MiaPaCa2 nor si-MiaPaCA2 cells
had HIF-1α in normoxia, these normoxic cells were essentially
identical in terms of HIF-1α expression. Thus, although these
normoxic cells (i.e., wt and si cells) were incubated separately
during MTT assay, data from them were pooled and are shown in the
same groups. As shown in the left panel in Fig. 2A, normoxic MiaPaCa2 cells were
decreased by each of the four 2-DG concentrations tested (0.3-, 1-,
4- and 18-mM). In hypoxia, however, significant decreases were seen
when si-MiaPaCa2 and wt-MiaPaCa2 cells were exposed to the two
highest 2-DG concentrations but not the lower ones (Fig. 2A). Thus, the lowest effective dose
of 2-DG for hypoxic MiaPaCa2 cells (4 mM) was higher than that for
normoxic cells (0.3 mM), which suggests that hypoxia decreased 2-DG
sensitivity. When cell-inhibiting effects of 2-DG were compared
across different cell types, 18 mM 2-DG induced greater cell
decrease in hypoxic MiaPaCa2 cells, than in normoxic ones (Fig. 2A). When different types of Panc-1
cells underwent the same 2-DG treatment, they were all decreased by
the two highest concentrations of 2-DG (4 and 18 mM). Thus,
normoxic Panc-1 cells were less sensitive to 2-DG than normoxic
MiaPaCa2 cells, as reported in a previous study (15). In addition, 18 mM 2-DG decreased
Panc-1 cells more in hypoxia, than in normoxia (Fig. 2B).
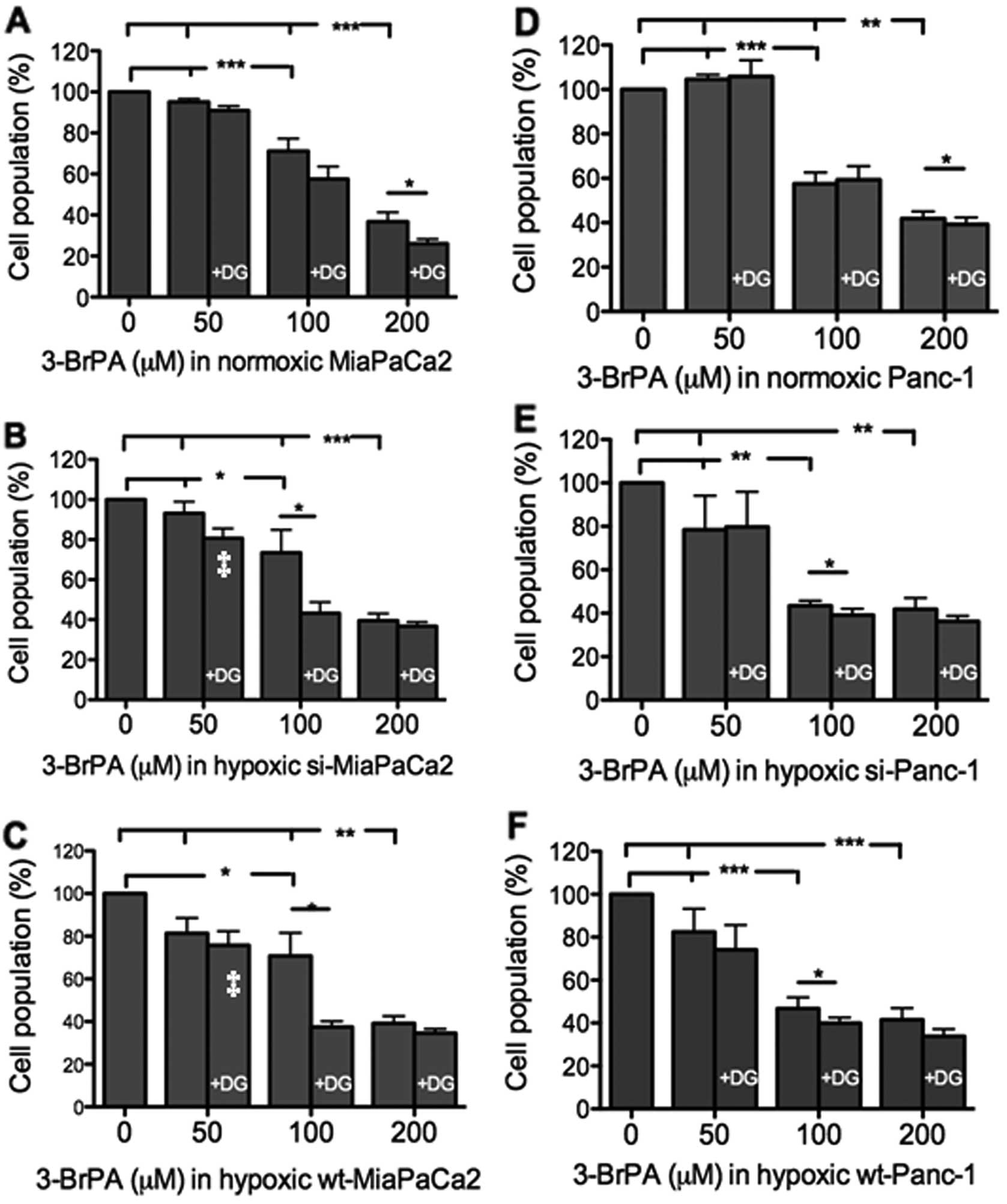
When used alone, 50 μM 3-BrPA did not decrease any
MiaPaCa2 cells (Fig. 3A-C). When 50
μM 3-BrPA was combined with 1 mM 2-DG, however, significant
decreases were seen in hypoxic wt- and si-MiaPaCa2 cells (Fig. 3B and C). When 3-BrPA was increased
to 100–200 μM, all MiaPaCa2 cells were decreased, and the cell
decrease was further augmented by additional 2-DG (Fig. 3A-C). When Panc-1 cells were
subjected to the same assay, results were similar to those seen in
MiaPaCa2 cells (Fig. 3D-F).
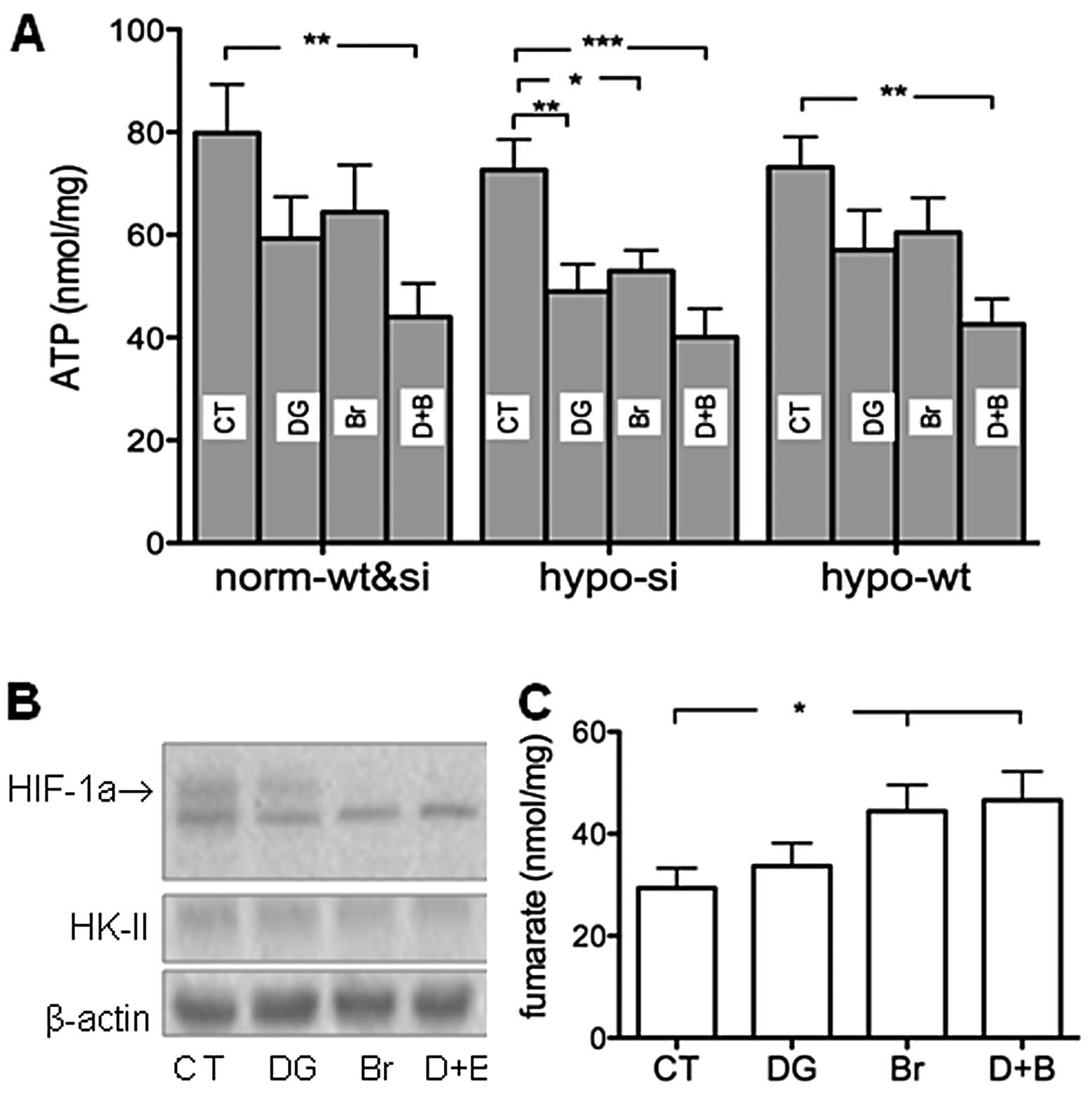
Mechanisms underlying the cell decrease
induced by 2-DG and 3-BrPA
Separate use of 2-DG and 3-BrPA significantly
decreased ATP in hypoxic si-MiaPaCa2 cells but not in any
wt-MiaPaCa2 cells (Fig. 4A). When
3-BrPA and 2-DG were combined, significant decreases in ATP were
seen in all cell types (Fig. 4A).
When HIF-1α and HK-II were determined in hypoxic wt-MiaPaCa2 cells,
we found that 2-DG decreased HIF-1α in a moderate manner but had no
effects on HK-II (Fig. 4B). By
contrast, 3-BrPA decreased HIF-1α profoundly in both the absence
and presence of 2-DG, and also decreased HK-II in the same cells
(Fig. 4B). When fumarate was
determined in hypoxic wt-MiaPaCa2 cells, we found that 3-BrPA
increased this mitochondrial metabolite in both the absence and
presence of 2-DG (Fig. 4C).
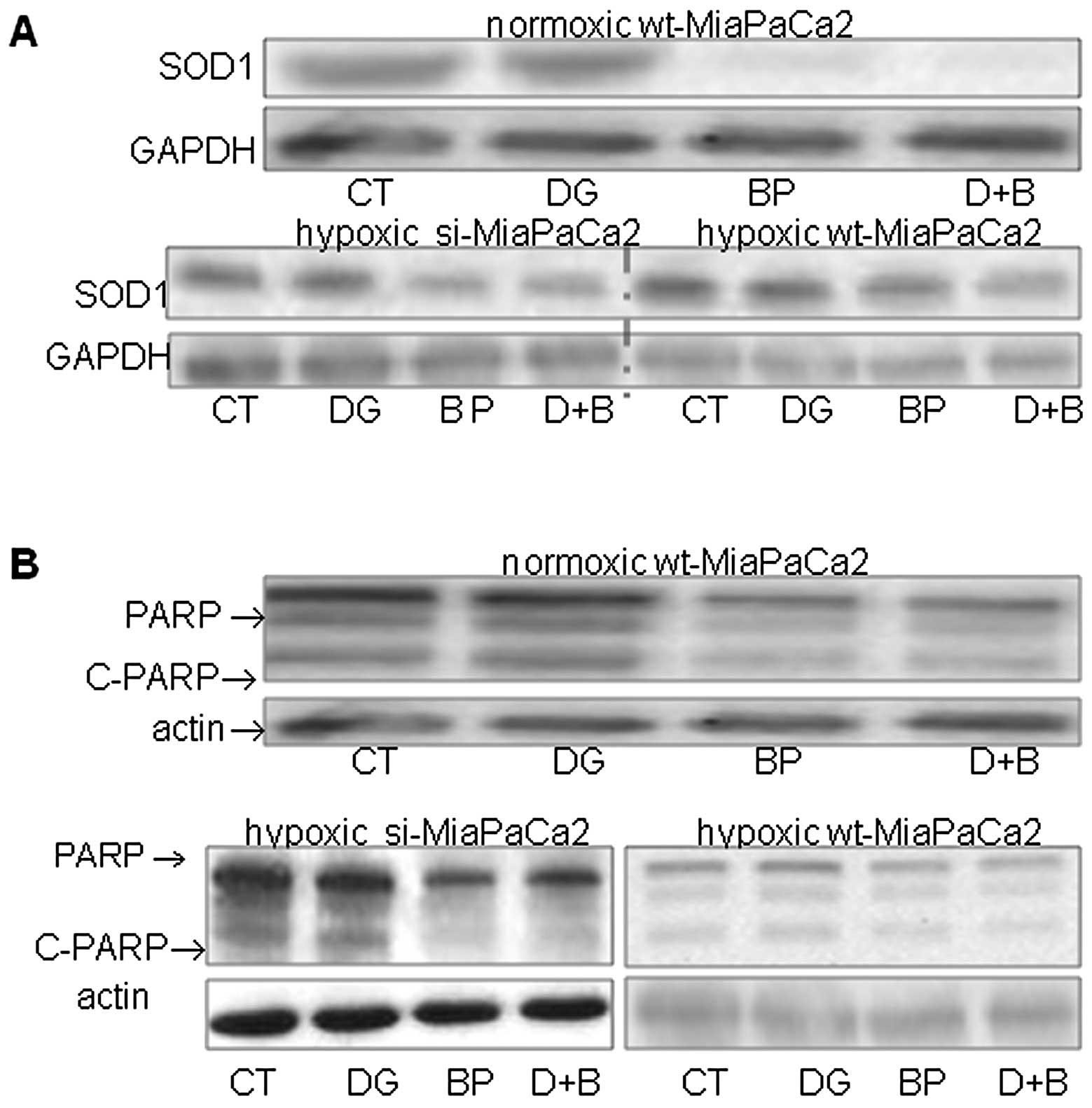
2-DG did not change SOD1 in any cells tested,
whereas 3-BrPA decreased this antioxidant enzyme in both the
absence and presence of 2-DG (Fig.
5A). Furthermore, the SOD1 decrease was more pronounced in two
HIF-1α-negative cell types, than in hypoxic wt-MiaPaCa2 cells
(Fig. 5A). When PARP was
determined, we used an antiserum that recognized both full-length
PARP (116 kDa) and a cleaved PARP molecule (c-PARP, 89 kDa). 2-DG
did not change PARP contents, whereas 3-BrPA decreased PARP and
c-PARP in both the absence and presence of 2-DG (Fig. 5B).
Discussion
When different cancer cells were exposed to 2-DG or
3-BrPA in previous studies, exposure times ranged from 6 to 96 h,
with 24 h being the usual choice (15–18,
20–24). In the MTT assay, we exposed cells to
2-DG and 3-BrPA for 24 h. The 2-DG and 3-BrPA concentrations we
used were comparable to those used by others (15–18,
20–24).
When cancer cells are stressed by hypoxia, they
increase glucose transporters and glycolytic enzymes to augment
energy production by glycolysis. Thus, hypoxia may decrease the
sensitivity of cancer cells to the reagents that inhibit glycolysis
by substrate competition and/or by allosteric feedback. This may
explain why hypoxic MiaPaCa2 cells were less sensitive to 2-DG than
their normoxic counterparts. Markedly, the hypoxia-induced decrease
in 2-DG sensitivity was seen in both wt- and si-MiaPaCa2 cells,
which suggests that the decrease in 2-DG sensitivity was
independent of HIF-1α.
In a previous study, hypoxic conditions
downregulated more than 100 genes in mouse fibroblasts, with many
of the genes being related to cell viability (26). This suggests that hypoxia has
negative effects on cell viability. Normally, these negative
effects may be masked by hypoxia-induced beneficial effects on cell
biology, such as increased glycolysis and energy production. When
antiglycolytic reagents completely inhibit glycolysis, however, the
hypoxia-induced effects on cell viability are revealed. This
explains why 18 mM 2-DG decreased cells to greater extents in
hypoxia, than it did in normoxia.
When 3-BrPA was used alone in 50 μM, it did not
affect the tested cells. However, when 50 μM 3-BrPA was combined
with 1 mM 2-DG, hypoxic MiaPaCa2 cells were decreased. Of note, 1
mM 2-DG did not decrease the hypoxic cells when it was used
independently. Thus, concurrent use of 2-DG (1 mM) and 3-BrPA (50
μM) decreased the lowest effective doses of both reagents. When
3-BrPA was used in higher concentrations (100–200 μM), its
anticancer effects were also augmented by the additional 2-DG.
Since 2-DG and 3-BrPA inhibit glycolysis by different molecular
mechanisms (14), these mechanisms
might cooperate when the two antiglycolytic reagents are used
simultaneously.
When stressed by hypoxia, HIF-1α-negative
si-MiaPaCa2 cells were unable to increase glycolysis adequately.
This may explain why separate use of 2-DG and 3-BrPA only decreased
ATP in the HIF-1α-negative cells. When glycolysis was completely
inhibited by concurrent use of 2-DG and 3-BrPA, however,
significant decreases in ATP were seen in all cell types. Thus, the
present ATP data suggest that HIF-1α helped cells maintain ATP
contents.
HIF-1α in hypoxic wt-MiaPaCa2 cells was moderately
inhibited by 2-DG and profoundly inhibited by 3-BrPA. The
mechanisms underlying the HIF-1α inhibition are unclear. However,
the data suggest that 2-DG and 3-BrPA compromised cancer cells not
only by inhibiting glycolysis but also by decreasing HIF-1α
expression. In 3-BrPA-treated cells, the HIF-1α decrease may be
responsible for the concomitant decrease in HK-II expression.
In cancer cells, defects in the mitochondria may
increase mitochondrial metabolites such as fumarate and succinate
(29). As a result, these
metabolites may exit mitochondria and accumulate in the cytosol.
Since fumarate and succinate inhibit HIF-1α degradation, their
accumulation in the cytosol may be associated with an increased
HIF-1α expression (29). In the
present study, however, 3-BrPA was seen to both increase fumarate
and decrease HIF-1α. Thus, it appears that fumarate did not play a
key role in HIF-1α expression by MiaPaCa2 cells. The increase in
fumarate may be a result of impairments in the mitochondria of the
3-BrPA-treated cells. In a recent study, 3-BrPA was shown to
inhibit mitochondrial respiration in hepatocellular carcinoma cells
(26).
Whole-cell SOD1 levels are an index of mitochondrial
integrity (30). In the present
study, SOD1 was decreased in 3-BrPA-treated cells, which suggests
that 3-BrPA impaired the mitochondria (27). Furthermore, 3-BrPA-induced SOD1
decreases were more pronounced in two HIF-1α-negative MiaPaCa2 cell
types, than in hypoxic wt-MiaPaCa2 cells. This suggests that HIF-1α
protected mitochondria from 3-BrPA-induced damages. Apoptosis and
necrosis are two cell-death modalities. When cancer cells undergo
apoptosis, they show decreased PARP and increased c-PARP (28). When cancer cells undergo necrosis,
their PARP contents are normal or decreased, and their c-PARP
contents are not increased (22,31).
In this light, the present PARP data suggest that 3-BrPA killed
MiaPaCa2 cells by necrosis. In a previous study, 3-BrPA was found
to induce necrosis in melanoma cells (32).
In summary, the present study shows that concurrent
use of 2-DG and 3-BrPA increases anticancer effects of these
antiglycolytic reagents; it also demonstrates that hypoxia and
HIF-1α regulate 2-DG and 3-BrPA effects. Addition of an HIF-1α
inhibitor to combined 2-DG and 3-BrPA may further increase the
anticancer effects of these antiglycolytic reagents.
Acknowledgements
The authors are grateful for the financial support
from the Tianjin Qian-Ren program.
References
|
1
|
Folkman J: Tumor angiogenesis: therapeutic
implications. N Engl J Med. 285:1182–1186. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Koong AC, Mehta VK, Le QT, et al:
Pancreatic tumors show high levels of hypoxia. Int J Radiat Oncol
Biol Phys. 48:919–922. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Erler JT, Cawthorne CJ, Williams KJ, et
al: Hypoxia-mediated down-regulation of Bid and Bax in tumors
occurs via hypoxia-inducible factor 1-dependent and -independent
mechanisms and contributes to drug resistance. Mol Cell Biol.
24:2875–2889. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brown JM, Diehn M and Loo BW Jr:
Stereotactic ablative radiotherapy should be combined with a
hypoxic cell radiosensitizer. Int J Radiat Oncol Biol Phys.
78:323–327. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Warburg O: On the origin of cancer cells.
Science. 123:309–314. 1956. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhong H, De Marzo AM, Laughner E, et al:
Overexpression of hypoxia-inducible factor 1alpha in common human
cancers and their metastases. Cancer Res. 59:5830–5835.
1999.PubMed/NCBI
|
|
7
|
Semenza GL and Wang GL: A nuclear factor
induced by hypoxia via de novo protein synthesis binds to the human
erythropoietin gene enhancer at a site required for transcriptional
activation. Mol Cell Biol. 12:5447–5454. 1992.
|
|
8
|
Salceda S and Caro J: Hypoxia-inducible
factor 1alpha (HIF-1alpha) protein is rapidly degraded by the
ubiquitin-proteasome system under normoxic conditions. Its
stabilization by hypoxia depends on redox-induced changes. J Biol
Chem. 272:22642–22647. 1997. View Article : Google Scholar
|
|
9
|
Fukuda R, Hirota K, Fan F, et al:
Insulin-like growth factor 1 induces hypoxia-inducible factor
1-mediated vascular endothelial growth factor expression, which is
dependent on MAP kinase and phosphatidylinositol 3-kinase signaling
in colon cancer cells. J Biol Chem. 277:38205–38211. 2002.
View Article : Google Scholar
|
|
10
|
Wang F, Li SS, Segersvärd R, et al:
Hypoxia inducible factor-1 mediates effects of insulin on
pancreatic cancer cells and disturbs host energy homeostasis. Am J
Pathol. 170:469–477. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Natsuizaka M, Ozasa M, Darmanin S, et al:
Synergistic up-regulation of Hexokinase-2, glucose transporters and
angiogenic factors in pancreatic cancer cells by glucose
deprivation and hypoxia. Exp Cell Res. 313:3337–3348. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mathupala SP, Ko YH and Pedersen PL:
Hexokinase-2 bound to mitochondria: cancer’s stygian link to the
‘Warburg Effect’ and a pivotal target for effective therapy. Semin
Cancer Biol. 19:17–24. 2009.
|
|
13
|
Kirito K, Hu Y and Komatsu N: HIF-1
prevents the overproduction of mitochondrial ROS after cytokine
stimulation through induction of PDK-1. Cell Cycle. 8:2844–2849.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kurtoglu M, Maher JC and Lampidis TJ:
Different toxic mechanisms of 2-deoxy-D-glucose versus
2-fluorodeoxy-D-glucose in hypoxic and normoxic tumor cells.
Antioxid Redox Signal. 9:1383–1390. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Maher JC, Savaraj N, Priebe W, et al:
Differential sensitivity to 2-deoxy-D-glucose between two
pancreatic cell lines correlates with GLUT-1 expression. Pancreas.
30:e34–e39. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wangpaichitr M, Savaraj N, Maher J, et al:
Intrinsically lower AKT, mTOR and HIF activity correlates with
increased sensitivity to 2-deoxy-D-glucose under hypoxia in lung
cancer cell lines. Mol Cancer Ther. 7:1506–1513. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Maher JC, Wangpaichitr M, Savaraj N, et
al: Hypoxia-inducible factor-1 confers resistance to the glycolytic
inhibitor 2-deoxy-D-glucose. Mol Cancer Ther. 6:732–741. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hernlund E, Ihrlund LS, Khan O, et al:
Potentiation of chemotherapeutic drugs by energy metabolism
inhibitors 2-deoxyglucose and etomoxir. Int J Cancer. 123:476–483.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Maschek G, Savaraj N, Priebe W, et al:
2-Deoxy-D-glucose increases the efficacy of adriamycin and
paclitaxel in human osteosarcoma and non-small cell lung cancers in
vivo. Cancer Res. 64:31–34. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ko YH, Pedersen PL and Geschwind JF:
Glucose catabolism in the rabbit VX2 tumor model for liver cancer:
characterization and targeting hexokinase. Cancer Lett. 173:83–91.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen Z, Zhang H, Lu W and Huang P: Role of
mitochondria-associated hexokinase II in cancer cell death induced
by 3-bromopyruvate. Biochim Biophys Acta. 1787:553–560. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim JS, Ahn KJ, Kim JA, et al: Role of
reactive oxygen species-mediated mitochondrial dysregulation in
3-bromopyruvate induced cell death in hepatoma cells: ROS-mediated
cell death by 3-BrPA. J Bioenerg Biomembr. 40:607–618. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hulleman E, Kazemier KM, Holleman A, et
al: Inhibition of glycolysis modulates prednisolone resistance in
acute lymphoblastic leukemia cells. Blood. 113:2014–2021. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ko YH, Smith BL, Wang Y, et al: Advanced
cancers: eradication in all cases using 3-bromopyruvate therapy to
deplete ATP. Biochem Biophys Res Commun. 324:269–275. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pereira da Silva AP, El-Bacha T, Kyaw N,
et al: Inhibition of energy-producing pathways of HepG2 cells by
3-bromopyruvate. Biochem J. 417:717–726. 2009.PubMed/NCBI
|
|
26
|
Greijer AE, van der Groep P, Kemming D, et
al: Up-regulation of gene expression by hypoxia is mediated
predominantly by hypoxia-inducible factor 1 (HIF-1). J Pathol.
206:291–304. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Indran IR, Tufo G, Pervaiz S and Brenner
C: Recent advances in apoptosis, mitochondria and drug resistance
in cancer cells. Biochim Biophys Acta. 1807:735–745. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mangerich A and Bürkle A: How to kill
tumor cells with inhibitors of poly(ADP-ribosyl)ation. Int J
Cancer. 128:251–265. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Koivunen P, Hirsilä M, Remes AM, et al:
Inhibition of hypoxia-inducible factor (HIF) hydroxylases by citric
acid cycle intermediates: possible links between cell metabolism
and stabilization of HIF. J Biol Chem. 282:4524–4532. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liang HL, Sedlic F, Bosnjak Z and
Nilakantan V: SOD1 and MitoTEMPO partially prevent mitochondrial
permeability transition pore opening, necrosis, and mitochondrial
apoptosis after ATP depletion recovery. Free Radic Biol Med.
49:1550–1560. 2010. View Article : Google Scholar
|
|
31
|
Ha HC and Snyder SH: Poly(ADP-ribose)
polymerase is a mediator of necrotic cell death by ATP depletion.
Proc Natl Acad Sci USA. 96:13978–13982. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Qin JZ, Xin H and Nickoloff BJ:
3-Bromopyruvate induces necrotic cell death in sensitive melanoma
cell lines. Biochem Biophys Res Commun. 396:495–500. 2010.
View Article : Google Scholar : PubMed/NCBI
|