Introduction
Osteosarcoma is the most common primary malignant
bone tumor in children and adolescents. Standard treatment consists
of multi-agent neoadjuvant chemotherapy, radical excision of the
tumor and adjuvant chemotherapy (1,2).
However, many patients still succumb to the disease as a result of
tumor metastasis and relapse (3,4). The
chemoresistance of tumor cells is one of the most prevalent causes
of therapeutic failure (5,6). Although patients with chemoresistant
cells require palliative treatment such as radiotherapy,
osteosarcomas are considered to be radioresistant tumors (7,8),
necessitating the combination of chemotherapy and radiotherapy for
these patients (8,9).
In general, tumor cells are the most radiosensitive
during G2/M phase of the cell cycle and the most
radioresistant in S phase (10,11).
Radiotherapy has been shown to activate the mitogen-activated
protein kinase (MAPK) and phosphatidylinositol-3 kinase (PI3K)/Akt
pathways, which regulate cell proliferation and apoptosis.
Inhibition of these pathways has been reported to enhance
radiosensitivity of cells (12,13).
New osteosarcoma treatment regimens have been
investigated, including many clinical trials of novel agents, among
which is sulforaphane (SFN) (14),
a naturally occurring member of the isothiocyanate family produced
by cruciferous vegetables such as broccoli (15). SFN has been shown to suppress the
growth of T-cell leukemia, colon, breast and prostate cancer cells
in vitro by inhibiting cell cycle progression (16–20)
and/or causing apoptosis (17,18).
We previously reported that SFN inhibited the proliferation of
cultured murine osteosarcoma LM8 cells i) by inducing
G2/M phase arrest, as shown by the appearance of cells
with sub-G1 DNA content; and ii) by inducing apoptosis,
as shown by the cleavage and activation of caspase-3 (21). In addition, SFN inhibited the
activation of the PI3K/Akt and MAPK pathways in pancreatic and
prostate cancer cells (22,23).
Our findings that SFN induced G2/M-phase arrest and
inhibited the PI3K/Akt and MAPK pathways, suggest that SFN may
enhance the radiosensitivity of LM8 cells. Moreover, the
combination of SFN and radiotherapy may further inhibit cell
growth, thereby allowing a decrease in the doses of both drug and
irradiation to safer levels than when used alone, ensuring a lower
incidence and grade of side effects. Although SFN has been found to
promote the radiosensitization of cancer cells (24,25),
the combined effects of SFN and radiation in osteosarcoma cells
have not been studied. We, therefore, analyzed the effects of SFN
and radiation on LM8 cells, including their effects on cell cycle,
the inhibition of the MAPK and PI3K/Akt pathways and the induction
of apoptosis.
Materials and methods
Reagents
Sulforaphane (SFN) was purchased from LKT
Laboratories, Inc. (St. Paul, MN, USA), and was dissolved in
dimethyl sulfoxide (DMSO); equivalent volumes of DMSO were used as
controls. The maximum percentage of DMSO in the assays was
0.1%.
X-ray irradiation
Cultured cells were irradiated with 2 Gy X-rays
using Softex M-150WE (Softex Co., Ltd., Tokyo, Japan). The cells
were placed 1 cm from the focus and the irradiation rate was 0.5
Gy/min in air.
Cell culture
The LM8 murine osteosarcoma cell line was
established from the murine Dunn osteosarcoma cell line and exhibit
high metastatic potential to the lungs (27). LM8 cells were cultured in Dulbecco’s
modified Eagle’s medium (DMEM) with 10% fetal calf serum (FCS)
containing antibiotics (100 U/ml penicillin G, 100 mg/ml
streptomycin) and incubated at 37°C in a humidified atmosphere of
5% CO2.
Concurrent exposure to SFN and
radiation
Effects on cell growth
LM8 cells were cultivated in 6-well plates at
2×104 cells/well in 2 ml medium for 24 h, followed by
incubation with various concentrations of SFN for 24 h and/or
X-irradiation at 2 Gy. After 24 or 48 h, the number of viable cells
was counted using a trypan blue dye exclusion test. The data are
presented as the means ± standard deviation (SD) of at least three
independent experiments.
Analysis of cell cycle
progression
To assess the effects of SFN alone, radiation alone
or the two treatments together on the cell cycle, LM8 cells were
cultivated in 6-well plates at 2×104 cells/well and
exposed to various doses of SFN and/or irradiation for 24 h. After
48 h, the cells were stained with propidium iodide (Sigma Aldrich,
St. Louis, MO, USA), and the stained nuclei were analyzed by flow
cytometry (FACSCalibur, Becton-Dickinson, Franklin Lakes, NJ, USA).
DNA histograms were created using CellQuest software for Apple
Macintosh (Becton-Dickinson). For all assays 10,000 events were
counted, with each assay performed in triplicate.
Western blot analysis
LM8 cells were plated in 6-well culture plates at
2.0×104 cells/well and incubated for 24 h, followed by
incubation with 20 μM SFN for 24 h and/or radiation at 2 Gy. After
1 and 48 h, the cells were washed twice with PBS and lysed with
RIPA buffer [20 mM Tris-HCl (pH 7.4), 150 mM NaCl, 0.1% SDS, 1%
Nonidet P-40, 0.5% sodium deoxycholate, 40 mM NaF, and protease
inhibitor cocktail (Sigma Aldrich)]. The lysates were centrifuged
at 15,000 rpm for 20 min; the supernatant lysate was incubated in
sample buffer [0.0625 M Tris-HCl (pH 6.8), 2% SDS, 5% glycerol, 5%
2-ME] at 95°C for 5 min; and the samples were separated by sodium
dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE),
followed by electroblotting onto nitrocellulose membranes (Amersham
Biosciences, Tokyo, Japan). The membranes were incubated in 5%
(wt/vol) non-fat dry milk in Tris-buffered saline with Tween 20
(TBST) [25 mM Tris HCl (pH 7.8), 140 mM NaCl, 0.1% (vol/vol) Tween
20] and incubated overnight with the following antibodies (each
from Cell Signaling Technology, Beverly, MA, USA, and diluted
1:1,000 in TBST): extracellular signal-regulated kinase (ERK1/2),
phosphorylated ERK1/2 (pERK1/2), Akt, phosphorylated Akt (p-Akt),
caspase-3, cleaved caspase-3 and GAPDH. The membranes were washed
thoroughly with TBST, incubated for 1 h with horseradish
peroxidase-conjugated anti-mouse or -rabbit IgG (Santa Cruz
Biotechnology, Santa Cruz, CA, USA), diluted 1:5,000 in TBST, and
developed with enhanced chemiluminescence kits (Amersham
Biosciences).
Analysis of nuclear morphology
LM8 cells treated with SFN and/or radiation under
appropriate conditions were cultured for 48 h, fixed with 2%
paraformaldehyde in PBS for 10 min, and stained with DAPI
(4′,6-diamidino-2-phenylindole dihydrochloride) (Nacalai Tesque,
Inc., Kyoto, Japan) at 4°C in the dark. For fluorescence
microscopy, cells were cytospun onto slides and examined using a
fluorescence microscope Eclipse 1000 (Nikon, Tokyo, Japan) with UV
illumination. Apoptotic cells were identified on the basis of
characteristic changes, including nuclear condensation,
fragmentation and apoptotic bodies.
Statistical analysis
All data are represented as the means ± SD.
Statistical significance was determined using Student’s t-tests.
P<0.05 was considered to indicate a statistically significant
result.
Results
Growth inhibitory effects of combination
therapy in murine osteosarcoma LM8 cells
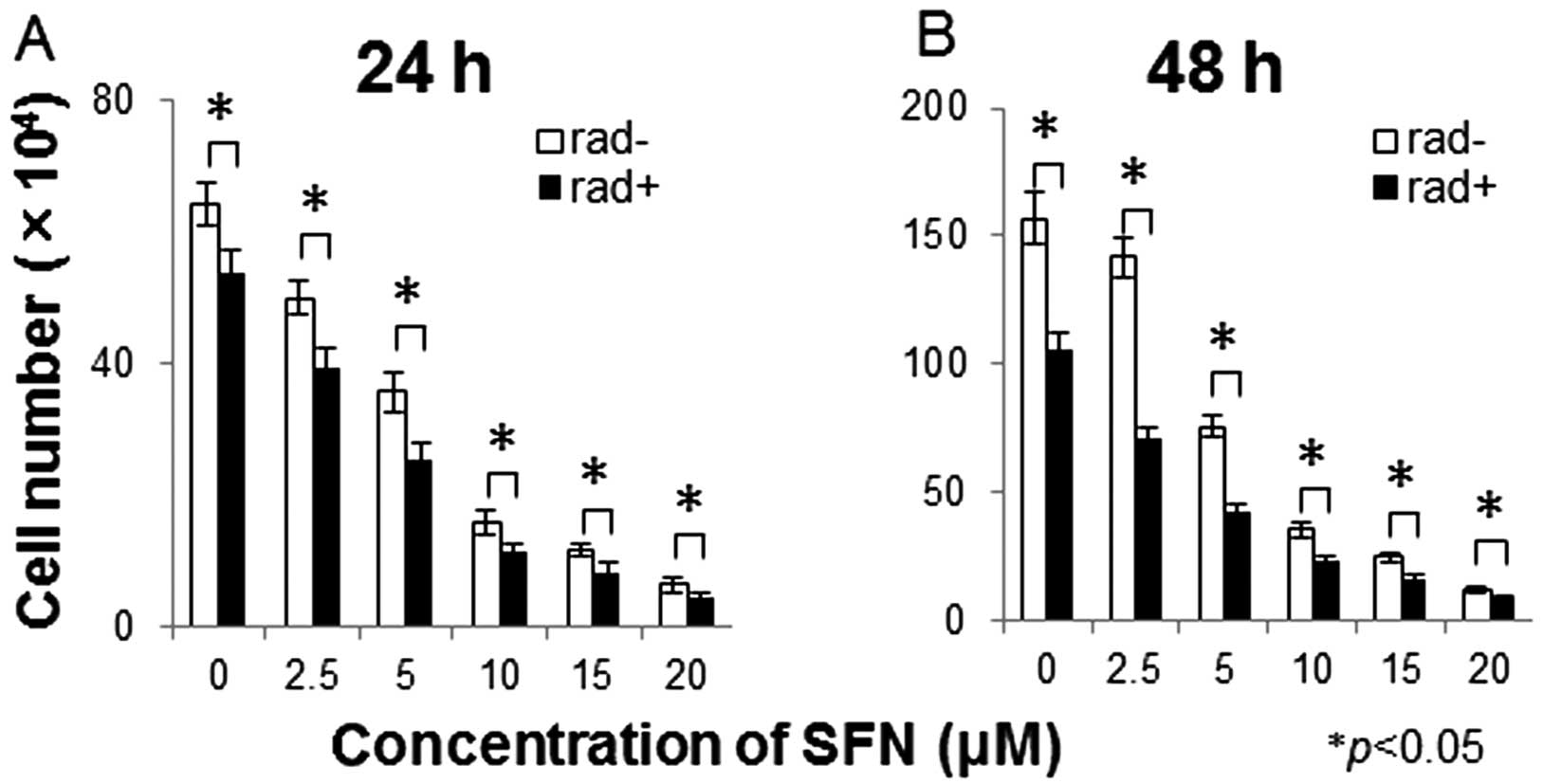
The combination of SFN and radiation treatment
produced significantly greater antitumor effects on the LM8
osteosarcoma cells than either treatment alone (Fig. 1). Stronger combined effects were
observed 48 h after treatment of SFN and radiation than effects
obtained after 24 h.
Combined effects of SFN and radiation on
the distribution of the cell cycle
To determine the effects of SFN and radiation on
cell cycle progression in LM8 cells, the DNA content of their
nuclei was assessed by flow cytometry. Exposure to SFN for 72 h
dose-dependently increased the population of cells in the
G2/M phase (Fig. 2A).
Following exposure to SFN plus 2 Gy radiation, the numbers of cells
in the G2/M phase (Fig.
2A) and in sub-G1 (Fig.
2B) were greater than these values after exposure to SFN
alone.
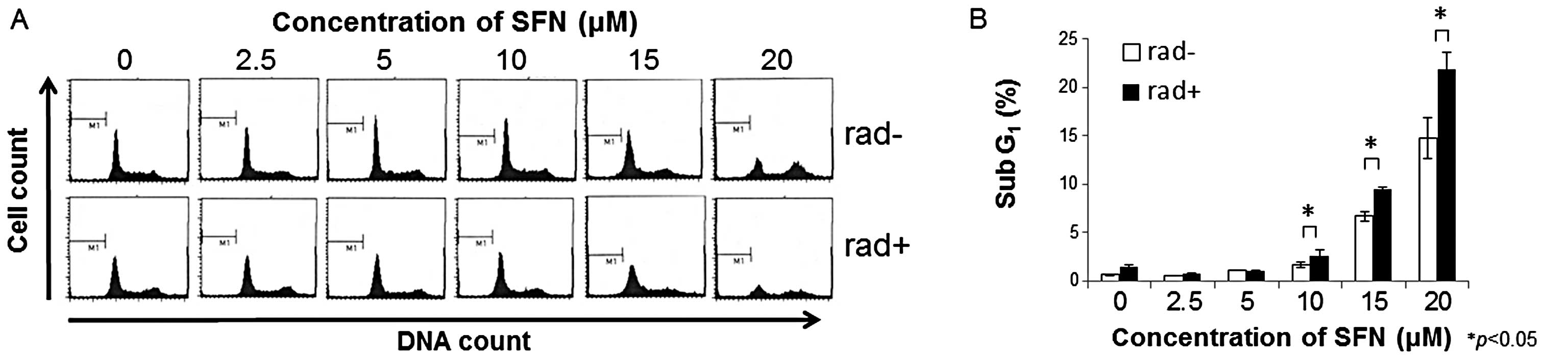 | Figure 2Effects of SFN plus radiation on the
cell cycle and the proportion of cells in sub-G1. (A)
Cell cycle analysis following combined treatment with SFN plus
radiation. Twenty-four hours after seeding, LM8 cells were treated
with 0, 2.5, 5, 10, 15 and 20 μM SFN for 24 h, followed by
treatment with (rad+) or without (rad−) 2 Gy X-irradiation. After
48 h, the DNA content of propidium iodide-stained nuclei was
analyzed by FACSCalibur flow cytometry, as described in Materials
and methods. (B) Percentage of cells in Sub-G1. LM8
cells were treated with the indicated concentrations of SFN in the
presence (black bars, rad+) or absence (white bars, rad−) of 2 Gy
X-irradiation, and the cells were analyzed by FACSCalibur flow
cytometry. Data are shown as means (bars, SD) (n=3).
*P<0.05. |
Combined effects of SFN and radiation on
apoptosis of LM8 cells
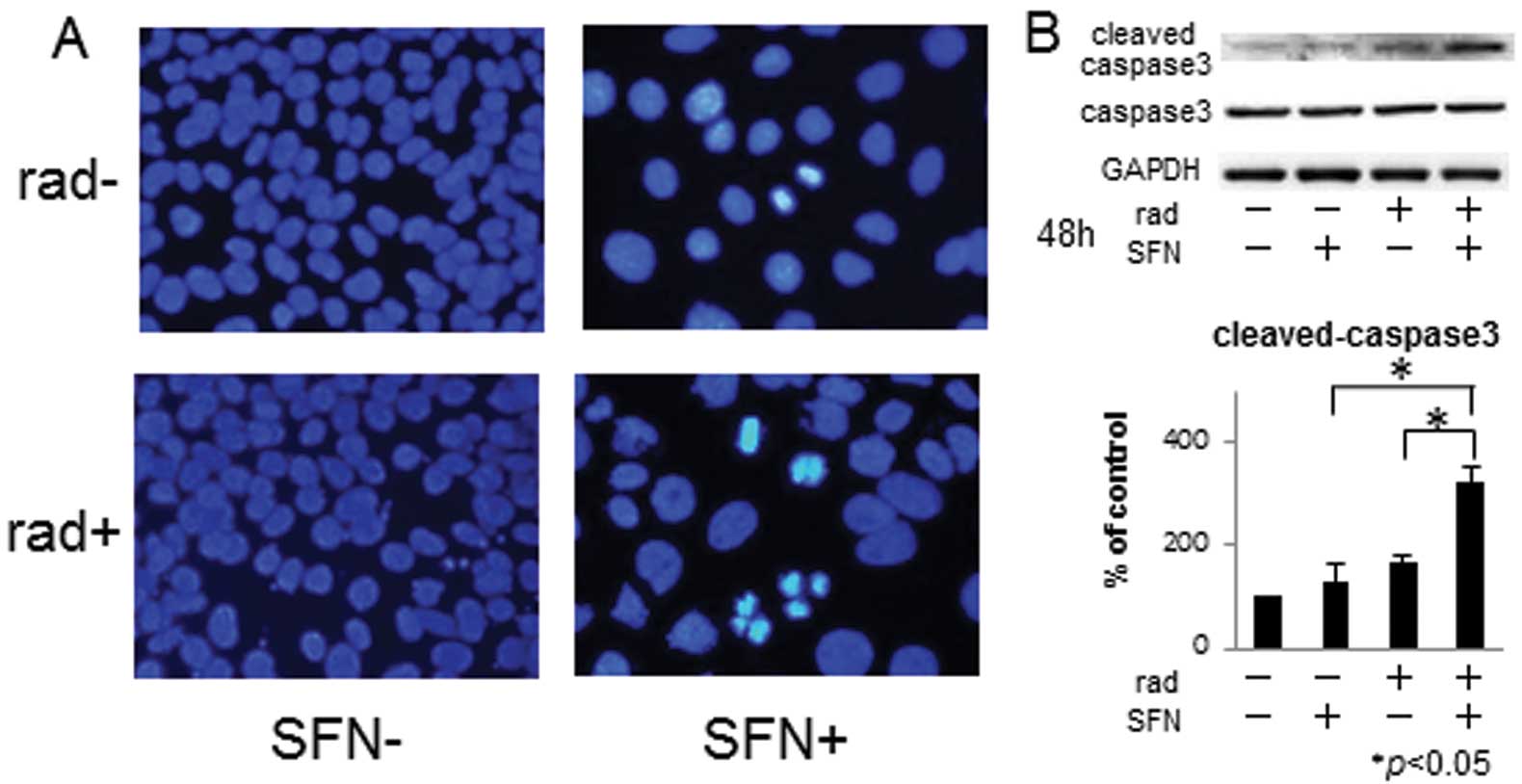
Nuclear fragmentation and apoptotic bodies
characteristic of apoptosis were observed with DAPI staining in LM8
cells treated with 20 μM SFN for 48 h plus 2 Gy X-irradiation for
48 h (Fig. 3A), and were more
frequently observed than in cells treated with SFN alone. In
addition, western blotting showed an increase in the amount of
activated caspase-3 in cells treated with SFN plus irradiation when
compared with that in cells treated with SFN alone (Fig. 3B).
Combined effects of SFN and radiation on
the phosphorylation of ERK and Akt
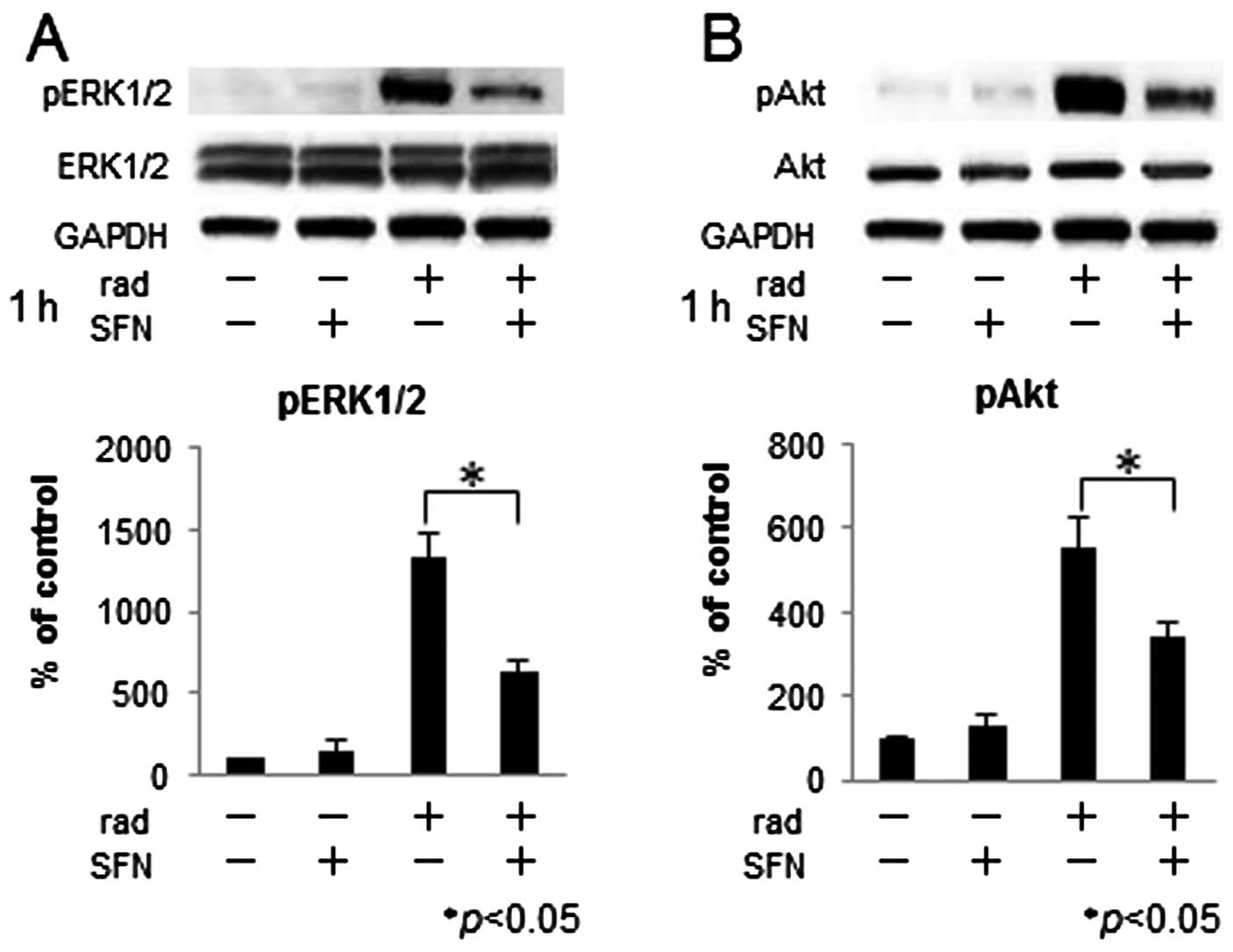
To assess the effect of SFN and radiation on the
phosphorylation of ERK and Akt, LM8 cells were treated with 20 μM
SFN and 2 Gy X-irradiation for 1 h, and the expression levels of
ERK, phosphorylated ERK, Akt and phosphorylated Akt protein were
evaluated by western blotting (Fig.
4). We found that X-irradiation alone increased the expression
of phosphorylated ERK and Akt proteins, whereas the levels of
phosphorylation were lower in cells treated with both SFN and
X-irradiation than in cells treated with X-irradiation alone.
Discussion
SFN, first identified in broccoli sprouts in 1992
(15), is a cancer chemopreventive
agent that suppresses the growth of osteosarcoma cells and other
malignant tumors. It is already being assessed in clinical trials,
including a phase II trial in patients with prostate cancer. We
previously reported that intraperitoneal administration of SFN
significantly inhibited the growth of LM8 xenografts to less than
30% of the controls in a murine tumor model, without causing any
toxicity (21).
Cell cycle arrest and apoptosis are considered to be
most important among the suggested mechanisms of action of SFN. SFN
has been reported to induce G2/M arrest and apoptosis in
human osteosarcoma U2-OS cells (28), as well as to induce growth arrest
and upregulate the expression of p21WAF1/CIP1 protein in
a p53-independent manner in human osteosarcoma MG63 cells (14). Moreover, SFN inhibited the growth of
LM8 cells i) by causing G2/M-phase arrest, as shown by
the appearance of cells with sub-G1 DNA content; and ii)
by inducing apoptosis, as shown by the cleavage and activation of
caspase-3 (21). In addition, SFN
was found to inhibit the phosphorylation of Akt and ERK and to
regulate apoptosis and cell proliferation. In pancreatic cancer
cells, SFN was shown to induce apoptosis through the inhibition of
both the PI3K/Akt and MEK/ERK pathways (22).
Following oral administration of the effective dose
of SFN to rats, its maximum plasma concentration was 20 μM
(28). However, it was found that
in humans the maximum plasma concentrations were only 2 μM after
oral intake of SFN-rich broccoli sprouts (29). Therefore, we studied whether or not
the effects of SFN can be enhanced when combined with
X-irradiation.
Radiotherapy has long been used to treat malignant
tumors. In the treatment of osteosarcoma, however, standard
treatment consists of neoadjuvant chemotherapy, surgical excision
and adjuvant chemotherapy. The use of radiotherapy has been limited
to patients in poor general condition and those with unresectable
tumors (30). In general, cells are
most radiosensitive during the G2/M phase and most
radioresistant during the S phase (10,11).
Agents that induce cell cycle arrest in the G2/M phase
have thus exhibited potent radiosensitivity in vitro and
in vivo(31–34). Inhibition of WEE1 kinase has been
reported to abrogate G2 arrest and may sensitize OS
cells to irradiation-induced cell death (34). In contrast, radioresistance may be
due to radiation-induced activation of ERK and Akt, resulting in
the dynamic and rapid adaptation of tumor cells to maintain growth
and viability (12). Thus,
inhibition of ERK and Akt activation may enhance the
radiosensitivity of tumors (13,35).
SFN has been reported to enhance the
radiosensitivity of HeLa human cervical carcinoma cells in
vitro and in vivo by inhibiting the repair of DNA
double-strand breaks (DSB), through the inhibition of the
DNA-dependent protein kinase catalytic subunit (DNA-PKcs) and RAD51
(24). Moreover, a combination of
SFN and radiation was found to decrease clonogenic survival in 4
human cancer cell lines derived from head and neck squamous cell
carcinomas, in which apoptosis is not regulated through Akt or the
Mcl-1 protein (25).
We found that either SFN alone or radiation alone
significantly and dose-dependently inhibited the growth of LM8
cells, whereas the combination of SFN and irradiation further
enhanced the growth inhibitory effects. However, the precise
synergistic mechanism of action of SFN and radiation is currently
unknown. We, therefore, investigated the mechanisms involved when
SFN and radiation were combined. Incubation of LM8 cells with SFN
alone dose-dependently increased the number of cells in the
G2/M phase and in sub-G1, as previously
described (21,27). Although radiation alone had no
effect on the cell cycle, the combination of SFN and irradiation
significantly increased the number of cells in sub-G1.
These findings suggest that combination treatment may induce
apoptosis more efficiently. Indeed, we found that combination
treatment increased the number of cells showing nuclear
fragmentation and apoptotic bodies and the expression of activated
caspase-3. Thus, the SFN-induced death of LM8 cells is considered
to be apoptotic.
We also studied whether the combination of SFN and
radiation activates the pathway of ERK and Akt. It turned out that
SFN inhibited the radiation-induced phosphorylation of ERK and Akt,
suggesting that SFN enhanced the radiosensitivity of LM8 cells.
These results were similar to previous findings, although the
induction of apoptosis by SFN and radiation was regulated through
Akt in head and neck squamous cell carcinoma cell lines (25). It is known that squamous cell
carcinomas are considered radioreactive, whereas osteosarcomas are
not. It could thus be argued that tumor cell-intrinsic properties
in terms of radiosensitivity may predispose to the difference
between our results and those by Kotowski et al(25).
In conclusion, we found that SFN enhanced the
radiosensitivity of murine osteosarcoma LM8 cells by inducing
apoptosis through G2/M-phase arrest and inhibiting ERK and Akt
activation. Thus, combined treatment with SFN and radiotherapy may
be useful in enhancing the antitumor effects of SFN alone. We would
propose a novel therapeutic regimen for patients with osteosarcoma
in which SFN and radiation are combined.
Acknowledgements
This study was supported by KAKENHI (Grant-in-Aid
for Scientific Research C: 22591668 to Y.T. and H.M.).
References
|
1
|
Unni KK and Inwards CY: Dahlin’s Bone
Tumors, General Aspects and Data on 10,165 Cases. 6th edition.
Lippincott Williams & Wilkins; Philadelphia, PA: pp. 122–168.
2010
|
|
2
|
Ferrari S, Palmerini E, Staals EL, et al:
The treatment of non-metastatic high grade osteosarcoma of the
extremity: review of the Italian Rizzoli experience. Impact on the
future. Cancer Treat Res. 152:275–287. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gelderblom H, Jinks RC, Sydes M, et al:
Survival after recurrent osteosarcoma: data from 3 European
Osteosarcoma Intergroup (EOI) randomized controlled trials. Eur J
Cancer. 47:895–902. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zalupski MM, Rankin C, Ryan JR, et al:
Adjuvant therapy of osteosarcoma - A phase II trial: Southwest
Oncology Group study 9139. Cancer. 100:818–825. 2004.PubMed/NCBI
|
|
5
|
Takeshita H, Gebhardt MC, Springfield DS,
Kusuzaki K and Mankin HJ: Experimental models for the study of drug
resistance in osteosarcoma: P-glycoprotein-positive, murine
osteosarcoma cell lines. J Bone Joint Surg Am. 78:366–375.
1996.PubMed/NCBI
|
|
6
|
Hirata M, Kusuzaki K, Takeshita H,
Hashiguchi S, Hirasawa Y and Ashihara T: Drug resistance
modification using pulsing electromagnetic field stimulation for
multidrug resistant mouse osteosarcoma cell line. Anticancer Res.
21:317–320. 2001.PubMed/NCBI
|
|
7
|
Sack H: Radiation therapy and chemotherapy
of primary malignant tumors of the bone. Rontgenblatter.
29:424–429. 1976.(In German).
|
|
8
|
Schwarz R, Bruland O, Cassoni A, Schomberg
P and Bielack S: The role of radiotherapy in osteosarcoma. Cancer
Treat Res. 152:147–164. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ryu K, Murata H, Koto K, et al: Combined
effects of bisphosphonate and radiation on osteosarcoma cells.
Anticancer Res. 30:2713–2720. 2010.PubMed/NCBI
|
|
10
|
Quiet CA, Weichselbaum RR and Grdina DJ:
Variation in radiation sensitivity during the cell cycle of two
human squamous cell carcinomas. Int J Radiat Oncol Biol Phys.
20:733–738. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tell R, Heiden T, Granath F, Borg AL, Skog
S and Lewensohn R: Comparison between radiation-induced cell cycle
delay in lymphocytes and radiotherapy response in head and neck
cancer. Br J Cancer. 77:643–649. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yacoub A, Miller A, Caron RW, et al:
Radiotherapy-induced signal transduction. Endocr Relat Cancer.
13:S99–S114. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Marampon F, Gravina GL, Di Rocco A, et al:
MEK/ERK inhibitor U0126 increases the radiosensitivity of
rhabdomyosarcoma cells in vitro and in vivo by downregulating
growth and DNA repair signals. Mol Cancer Ther. 10:159–168. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Matsui TA, Sowa Y, Yoshida T, et al:
Sulforaphane enhances TRAIL-induced apoptosis through the induction
of DR5 expression in human osteosarcoma cells. Carcinogenesis.
27:1768–1777. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang Y, Talalay P, Cho CG and Posner GH:
A major inducer of anticarcinogenic protective enzymes from
broccoli: isolation and elucidation of structure. Proc Natl Acad
Sci USA. 89:2399–2403. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jackson SJT and Singletary KW:
Sulforaphane: a naturally occurring mammary carcinoma mitotic
inhibitor, which disrupts tubulin polymerization. Carcinogenesis.
25:219–227. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gamet-Payrastre L, Li P, Lumeau S, et al:
Sulforaphane, a naturally occurring isothiocyanate, induces cell
cycle arrest and apoptosis in HT29 human colon cancer cells. Cancer
Res. 60:1426–1433. 2000.PubMed/NCBI
|
|
18
|
Fimognari C, Nusse M, Cesari R, Iori R,
Cantelli-Forti G and Hrelia P: Growth inhibition, cell-cycle arrest
and apoptosis in human T-cell leukemia by the isothiocyanate
sulforaphane. Carcinogenesis. 23:581–586. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Parnaud G, Li P, Cassar G, et al:
Mechanism of sulforaphane-induced cell cycle arrest and apoptosis
in human colon cancer cells. Nutr Cancer. 48:198–206. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Singh SV, Herman-Antosiewicz A, Singh AV,
et al: Sulforaphane-induced G2/M phase cell cycle arrest involves
checkpoint kinase 2-mediated phosphorylation of cell division cycle
25C. J Biol Chem. 279:25813–25822. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Matsui TA, Murata H, Sakabe T, et al:
Sulforaphane induces cell cycle arrest and apoptosis in murine
osteosarcoma cells in vitro and inhibits tumor growth in
vivo. Oncol Rep. 18:1263–1268. 2007.PubMed/NCBI
|
|
22
|
Roy SK, Srivastava RK and Shankar S:
Inhibition of PI3K/AKT and MAPK/ERK pathways causes activation of
FOXO transcription factor, leading to cell cycle arrest and
apoptosis in pancreatic cancer. J Mol Signal. 5:102010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shankar S, Ganapathy S and Srivastava RK:
Sulforaphane enhances the therapeutic potential of TRAIL in
prostate cancer orthotopic model through regulation of apoptosis,
metastasis, and angiogenesis. Clin Cancer Res. 14:6855–6866. 2008.
View Article : Google Scholar
|
|
24
|
Yu D, Sekine-Suzuki E, Xue L, Fujimori A,
Kubota N and Okayasu R: Chemopreventive agent sulforaphane enhances
radiosensitivity in human tumor cells. Int J Cancer. 125:1205–1211.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kotowski U, Heiduschka G, Brunner M, et
al: Radiosensitization of head and neck cancer cells by the
phytochemical agent sulforaphane. Strahlenther Onkol. 187:575–580.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Asai T, Ueda T, Itoh K, et al:
Establishment and characterization of a murine osteosarcoma cell
line (LM8) with high metastatic potential to the lung. Int J
Cancer. 76:418–422. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim MR, Zhou L, Park BH and Kim JR:
Induction of G2/M arrest and apoptosis by sulforaphane
in human osteosarcoma U2-OS cells. Mol Med Rep. 4:929–934.
2011.PubMed/NCBI
|
|
28
|
Hu R, Hebbar V, Kim BR, et al: In vivo
pharmacokinetics and regulation of gene expression profiles by
isothiocyanate sulforaphane in the rat. J Pharmacol Exp Ther.
310:263–271. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ye L, Dinkova-Kostova AT, Wade KL, Zhang
Y, Shapiro TA and Talalay P: Quantitative determination of
dithiocarbamates in human plasma, serum, erythrocytes and urine:
pharmacokinetics of broccoli sprout isothiocyanates in humans. Clin
Chim Acta. 316:43–53. 2002. View Article : Google Scholar
|
|
30
|
Sheplan LJ and Juliano JJ: Use of
radiation therapy for patients with soft-tissue and bone sarcomas.
Cleve Clin J Med. 77:S27–S29. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhao Y, Jiang W, Li B, et al: Artesunate
enhances radiosensitivity of human non-small cell lung cancer A549
cells via increasing NO production to induce cell cycle arrest at
G2/M phase. Int Immunopharmacol. 11:2039–2046. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yu J, Liu F, Sun M, Sun Z and Sun S:
Enhancement of radiosensitivity and the potential mechanism on
human esophageal carcinoma cells by tetrandrine. Cancer Biother
Radiopharm. 26:437–442. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Forde JC, Maginn EN, McNamara G, et al:
Microtubule-targeting-compound PBOX-15 radiosensitizes cancer cells
in vitro. Cancer Biol Ther. 11:421–428. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
PosthumaDeBoer J, Würdinger T, Graat HCA,
et al: WEE1 inhibition sensitizes osteosarcoma to radiotherapy. BMC
Cancer. 11:1562011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kim IA, Bae SS, Fernandes A, et al:
Selective inhibition of Ras, phosphoinositide 3 kinase, and Akt
isoforms increases the radiosensitivity of human carcinoma cell
lines. Cancer Res. 65:7902–7910. 2005.PubMed/NCBI
|