Introduction
Cervical cancer is the third most common cancer
among females worldwide. Approximately 530,232 new cases of
cervical cancer were diagnosed and an estimated 275,008 deaths were
reported in 2008 (1). Although the
overall mortality of patients with cervical cancer has decreased
over the past few years due to the widespread availability of
effective screening programs, cervical cancer is a major cause of
morbidity and mortality in women. Overall, the 5-year survival rate
is 73%, yet the prognosis for advanced cervical cancer or recurrent
cervical cancer still remains poor (2).
Most women with early stage cervical cancer (stage
Ib–IIA) are treated with surgery, radiation or chemoradiation
therapy. Since patients with locally metastatic or advanced lesions
are at significant risk for recurrence, they require concurrent
chemoradiation therapy (3). To
eradicate micrometastases and sensitize radiation, concurrent
chemotherapy has been added to pelvic radiation, with an apparent
improvement in survival rates compared to radiation therapy alone
(4–8). Patients with distant metastases are
rarely curable, and most of the patients are treated either with
chemotherapy or supportive care.
Cisplatin is the most active agent against cervical
cancer, with a response rate of 17–21% (9). The most common non-platinum-based
agent for cervical cancer is paclitaxel; its response rate is 17%
(10). Single-agent chemotherapy
plays a limited role in improving survival rates among patients
with distant metastasis. Therefore, combination chemotherapy with
existing agents is necessary to improve response rates and patient
survival. Combination chemotherapy includes drugs that have
demonstrated single agent activity (antitumor effects), different
toxicity spectra, and synergistic activity with no increase in
toxicity to improve response rates and survival of patients. A
classic example of a combined regimen is cisplatin-based
chemotherapy (11). Cisplatin-based
combination chemotherapy with paclitaxel in stage IVb, recurrent,
or persistent cervical cancer has a 36% response rate but does not
improve the median survival when compared with the use of cisplatin
alone (12). A recent phase III
trial (GOG 204) evaluated the toxicity and efficacy of four
cisplatin-based doublet combinations (ciaplatin/paclitaxel,
cisplatin/vinorelbine, cisplatin/gemcitabine, and
cisplatin/topotecan) among patients with advanced and recurrent
cervical carcinoma (13). The
response rates for cisplatin/paclitaxel, cisplatin/vinorelbine,
cisplatin/gemcitabine and cisplatin/topotecan were 29.1, 25.9, 22.3
and 23.4%, respectively. That study was closed early since
cisplatin/vinorelbine, cisplatin/gemcitabine and
cisplatin/topotecan did not exhibit superior efficacy to
cisplatin/paclitaxel. Therefore, other regimens are needed to
improve survival in patients with advanced and recurrent cervical
cancer.
Arsenic compounds have been used to treat leukemia,
particularly chronic myeloid leukemia and Hodgkin’s lymphoma, since
1865 (14). Tetraarsenic oxide
(As4O6; TAO) is a new arsenic compound. TAO
is more effective at inhibiting human cervical cancer cell (SiHa
cells) growth than arsenic trioxide (As2O3)
(15). TAO may exert potential
anticancer activity via vascular shutdown in vivo(16). TAO exhibits a synergistic effect
with paclitaxel in gastric, cervix and head and neck cancer cell
lines by inducing caspase-3 and poly(ADP-ribose)
polymerase-dependent apoptosis (17).
The present study was conducted to investigate the
antitumor effect of TAO compared with cisplatin and paclitaxel,
which are conventional chemotherapy agents. The effects of a
combination of TAO and conventional chemotherapeutic agents were
evaluated and analyzed in vivo and in vitro using a
cervical cancer cell line.
Materials and methods
Cell lines and chemical reagents
HPV 16, an immortalized human cervical carcinoma
cell line, and CaSki cells (CRL-1550; American Type Culture
Collection, Manassas, VA, USA), were cultured in RPMI-1640 medium
(Gibco, Gaithersburg, MD, USA) supplemented with 10% fetal bovine
serum (Gibco-BRL, Grand Island, NY, USA), 100 U/ml penicillin and
streptomycin (Gibco-BRL) at 37°C in a humidified 5%
CO2-95% air incubator under standard conditions.
Paclitaxel (Genexol®, Samyang Co., Seoul,
Korea) and cisplatin (Unistin®, Korea United
Pharmaceutical, Seoul, Korea) were purchased and used, and TAO
(Tetras®) was provided from Chunjisan (Seoul,
Korea).
Cell viability
To evaluate the inhibition of tumor cell viability,
a water-soluble tetrazolium salt (WST)-1 assay (EZ-CyTox Enhanced
Cell Viability Assay kit; DaeiLab Service, Seoul, Korea) was used
according to the manufacturer’s instructions. Briefly,
5×103 cells were treated with various concentrations of
cisplatin (10–500 μM) and/or paclitaxel (0.001–10 μM) and/or TAO
(0.5–25 μM) for 72 h, and the WST-1 solution was added. After 4 h,
cell viability was measured at an absorbance of 480 nm using
BIO-TEL™ (EL-800). The experiment was repeated three times.
Apoptosis
Apoptosis in the CaSki cell line was measured using
the Annexin V-FITC Apoptosis Detection kit (BD Bioscience, San
Jose, CA, USA). After a 24-h incubation in 6-well plates at a
density of 2×105 cells/well, the cells were treated with
a single agent or with a combination of agents at the
IC50 concentration for each agent. Cells were washed
twice with cold PBS, resuspended in 100 μl binding buffer, and
incubated with 3 μl Annexin V-FITC (BD Bioscience) and 10 μl
propidium iodide (PI; BD Bioscience) at room temperature for 15
min. Fluorescent intensities were determined by flow cytometry
(Becton-Dickinson, San Jose, CA, USA). The experiment was repeated
three times.
Western blot analysis
CaSki cells were plated in a 6-cm dish at
1×106 cells/dish and incubated for 24 h. After a 24-h
incubation with the drugs, the CaSki cells were washed with PBS and
lysed with a mammalian tissue lysis/extraction reagent including a
protease inhibitor. After the protein was quantified with a BCA
protein assay kit, the proteins were separated using 10% sodium
dodecyl sulfate-polyacrylamide gel electrophoresis and
immunoblotted with anti-caspase-3, anti-cleaved caspase-3 and
anti-α-tubulin at 4°C overnight. Goat anti-rabbit or
anti-mouse-conjugated alkaline phosphatase secondary antibodies
were applied for 1 h at room temperature, and the membrane was
developed using an AP-Conjugated Development kit (Bio-Rad
Laboratories). The developed protein bands were quantified using
the Multi Gauge V2.2 program.
Treatment of human cervical cancer
xenografts with cisplatin, paclitaxel and TAO
BALB/c nu/nu female mice (age, 6 weeks; weight,
20–25 g) were obtained from Orient Bio Inc. (Seongnam, Korea). All
animal procedures and care were performed under the guidelines
approved by the Animal Ethics Committee of the College of Medicine
at Inje University. CaSki cells (2×106) were injected
subcutaneously into the backs of mice anesthetized with a mixture
of ketamine (90 mg/kg) and xylazine (10 mg/kg).
After 24 days, the mice were randomized into the
following 7 treatment groups with 6 mice in each group and treated
for 35 days. Each agent was administered by intraperitoneal
injection: i) control (0.9% sodium chloride injected once per
week), ii) cisplatin (4 mg/kg body weight per injection, once per
week), iii) paclitaxel (20 mg/kg body weight per injection, twice
per week), iv) TAO (8 mg/kg body weight per injection, once per
week), and v) cisplatin and paclitaxel and vi) TAO and cisplatin
and vii) TAO and paclitaxel at the same doses and schedule.
Tumor sizes were assessed twice per week. Tumor size
was calculated using the formula: Tumor size = length × width.
Histological examination
Tumor tissue was removed from each animal at 24, 48
and 72 h following the administration of each agent, and a terminal
deoxynucleotidyl transferase-mediated dUTP nick end labeling
(TUNEL) assay was carried out.
Assessment of cell death was carried out via the
TUNEL method using an In Situ Cell Death Detection kit conjugated
with horseradish peroxidase (Roche Applied Science, Indianapolis,
IN, USA), according to the manufacturer’s instructions. Five
equal-sized tissue section fields were randomly chosen and analyzed
under a Leica DMI microscope (Leica, Wetzlar, Germany).
Statistical analysis
Statistical analyses were performed using the
MedCalc version 10.0 program (Frank Schoonjans, University of Gent,
Belgium). The Mann-Whitney U-test, analysis of variance, and the
Kruskal-Wallis tests were used. P-value <0.05 was considered to
indicate a statistically significant result.
Results
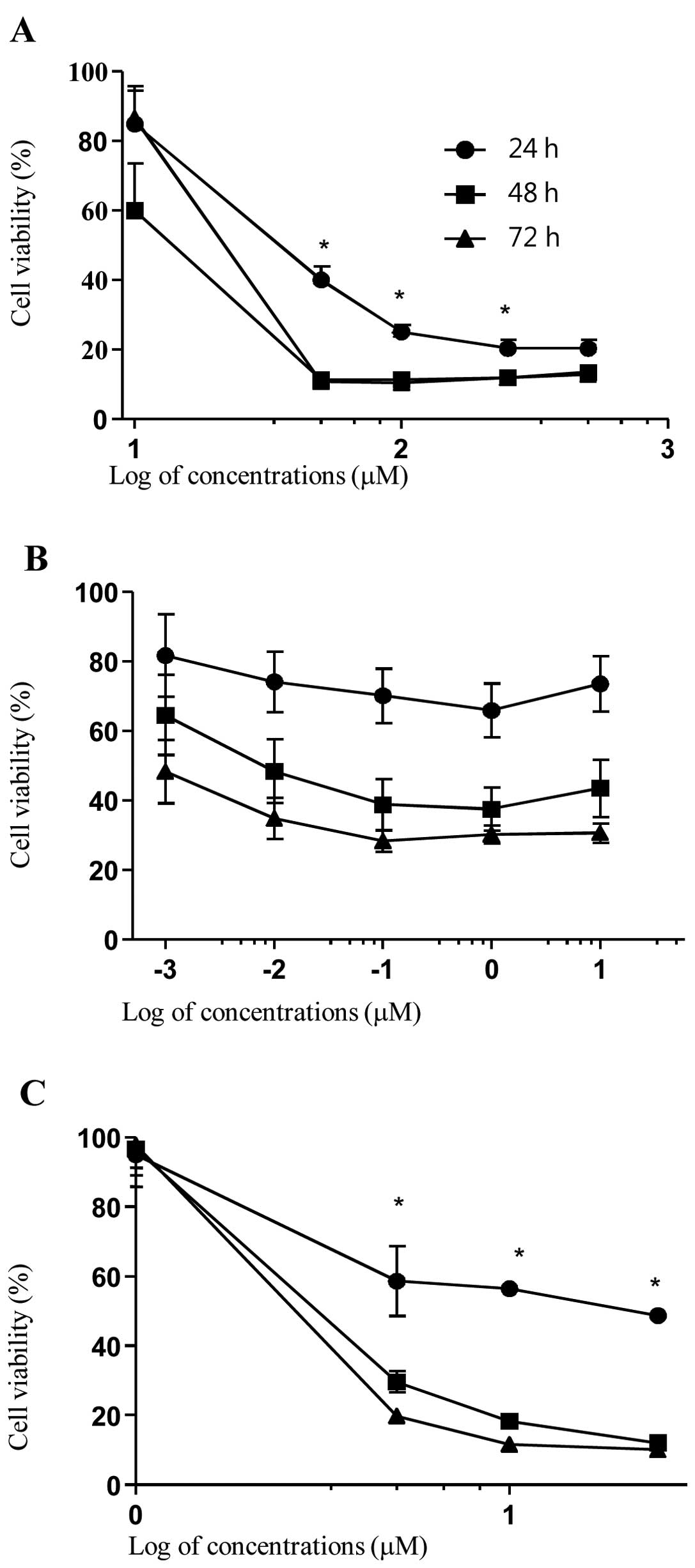
TAO induces time- and dose-dependent
growth inhibition of CaSki cells
o investigate the effect of cisplatin, paclitaxel,
and TAO on CaSki cells, proliferation of CaSki cells was assessed
via WST assay using various concentrations of each agent applied
over 24–72 h. Cisplatin decreased cell growth slowly at 10 μM but
decreased cell growth rapidly at 50 μM (at 24, 48 and 72 h;
P<0.001, P=0.001 and P<0.001, respectively). Paclitaxel also
tended to decrease cell growth at 0.001 μM, but cell growth did not
differ significantly from the control. TAO decreased cell growth at
1 μM and decreased cell growth rapidly at 5 μM (24, 48 and 72 h;
P<0.001, P<0.001 and P<0.001, respectively). Each agent
decreased cell growth over time. As shown in Fig. 1, time- and concentration-dependent
inhibition of cell growth was observed upon incubation with each
agent.
The IC50 values of cisplatin, paclitaxel,
and TAO at 48 h were 29.74, 0.009 and 3.72 μM, respectively.
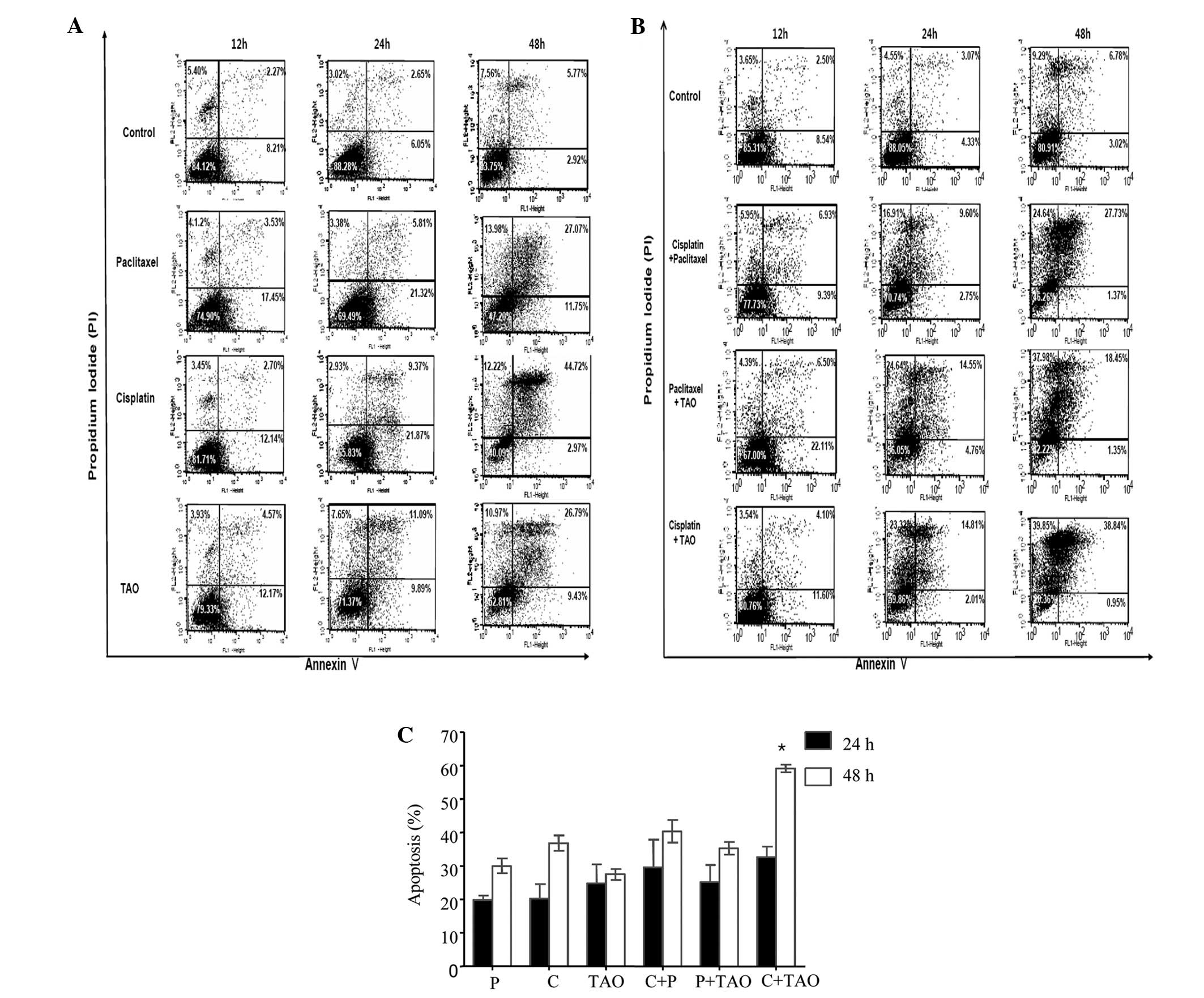
TAO induces apoptosis and interacts
synergistically with cisplatin
To determine whether the inhibited cell growth was a
result of apoptosis induced by each agent, an Annexin V/PI double
staining-based flow cytometric analysis was performed in the CaSki
cells after 12, 24 and 48 h of treatment using the IC50
concentrations of each agent alone and in combination. Early
apoptotic cell populations were regarded as Annexin V(+)/PI(−) and
late apoptotic cell populations were regarded as Annexin V(+)/PI(+)
(Fig. 2).
Apoptosis was observed following 12 h of treatment
with the IC50 concentrations of cisplatin, paclitaxel,
and TAO, and early and late apoptosis was increased compared to
that in the control group after 24 and 48 h (Fig. 2A). Cisplatin at 29.74 μM induced
apoptosis in 20.3 and 36.8% of the cells after 24 and 48 h,
respectively (Fig. 2A). Paclitaxel
at 0.009 μM induced apoptosis in 19.8 and 30.0% of cells after 24
and 48 h, respectively (Fig. 2A).
TAO at 3.72 μM induced apoptosis in 24.8 and 27.5% of cells after
24 and 48 h, respectively (Fig.
2A).
The combinations of cisplatin and paclitaxel,
cisplatin and TAO, or paclitaxel and TAO induced apoptosis after 12
h of treatment, and apoptosis was increased to a greater extent in
a time-dependent pattern compared to that in the control group
after 24 and 48 h (Fig. 2B). The
combination of cisplatin and paclitaxel increased the percentages
of apoptotic cells to 33.7 and 37.8% after 24 and 48 h of
treatment. The combination of paclitaxel and TAO increased the
percentages of apoptotic cells to 22.8 and 37.0% after 24 and 48 h
of treatment, respectively (Fig.
2B). The combination of cisplatin and TAO increased apoptotic
cells to 34.2 and 60.3% after 24 and 48 h of treatment,
respectively (Fig. 2B).
The combination of cisplatin and TAO significantly
increased apoptosis in the CaSki cell line compared to that of TAO
or cisplatin used alone after 24 and 48 h (TAO vs. cisplatin + TAO
after 48 h, P=0.0286; cisplatin vs. cisplatin + TAO after 24 and 48
h, P=0.0286 and 0.0286, respectively). The combination of cisplatin
and paclitaxel or paclitaxel and TAO tended to increase apoptosis
but the difference was not significant when compared to the
apoptosis rate with TAO or cisplatin used alone after 24 and 48 h.
Apoptosis in CaSki cells treated with a combination of cisplatin
and TAO after 48 h was increased to a greater exent when compared
with that in the other two-agent combinations (cisplatin +
paclitaxel or paclitaxel + TAO; P=0.0231). TAO exhibited more
synergism together with cisplatin in inducing apoptosis in CaSki
cells when compared with that in combination with paclitaxel
(Fig. 2C).
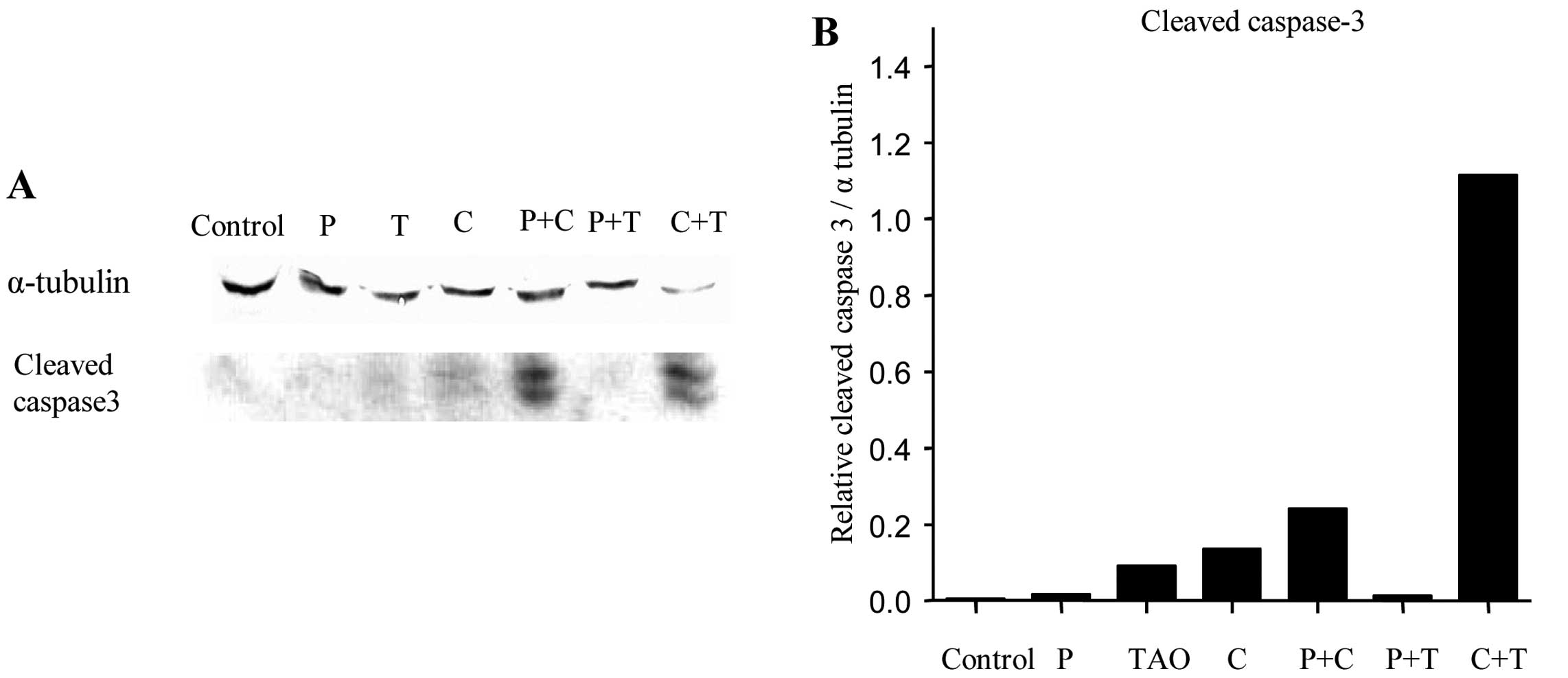
Caspase-3 is highly activated by TAO and
cisplatin
Western blot analysis was performed after treatment
with each agent alone and in combination to evaluate the expression
of apoptosis-related proteins (Fig.
3A). The cisplatin + TAO treatment resulted in prominent
caspase-3 activation compared to that in the other treatment groups
(Fig. 3B).
TAO in combination with other
chemotherapeutic agents inhibits tumor growth in CaSki cell
xenografts
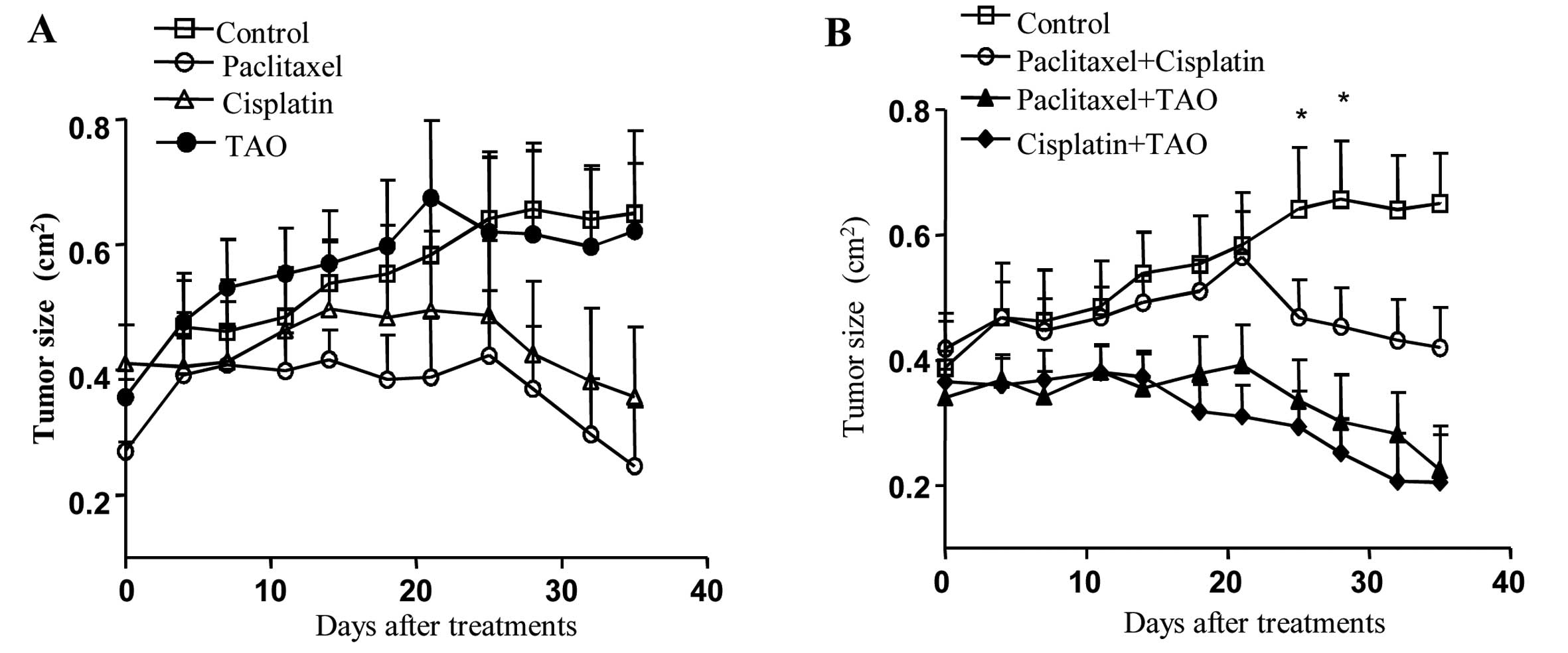
Mice bearing CaSki cell tumors were treated with
TAO, cisplatin and paclitaxel alone and in combination to test
whether TAO synergizes with conventional chemotherapeutic agents
(Fig. 5). Tumor size was decreased
consistently in the paclitaxel-treated group. Tumor size was
decreased significantly on days 32 and 35 after administration of
paclitaxel when compared with that in the control group (P=0.00152,
0.0087, respectively). While treatment with cisplatin alone
significantly suppressed tumor growth on day 35 of treatment
compared with that in the control group (P=0.0411), TAO alone did
not inhibit tumor growth (Fig.
4A).
Although the paclitaxel and cisplatin groups
exhibited significant differences in tumor size compared with the
control group, no significant differences were observed among the
paclitaxel, cisplatin and TAO treatments.
The tumor size was markedly decreased following
treatment with the cisplatin and TAO combination (Fig. 4B). Tumor size was significantly
decreased following treatment with the combination of paclitaxel
and cisplatin, cisplatin and TAO, or paclitaxel and TAO when
compared with that in the control group. Tumor size was decreased
significantly in the paclitaxel and cisplatin combination group on
day 35 of treatment (P=0.0411). Tumor size also decreased
significantly from days 21 to 35 after treatment in the TAO and
cisplatin combination group (P<0.05). Tumor size was
significantly decreased from days 14 to 35 of treatment in the TAO
and paclitaxel combination group (P<0.05).
Tumor size in the group treated with a combination
of TAO and cisplatin was significantly decreased when compared to
that in the group treated with a combination of cisplatin and
paclitaxel on days 18–21 of treatment (P=0.0380, 0.050,
respectively).
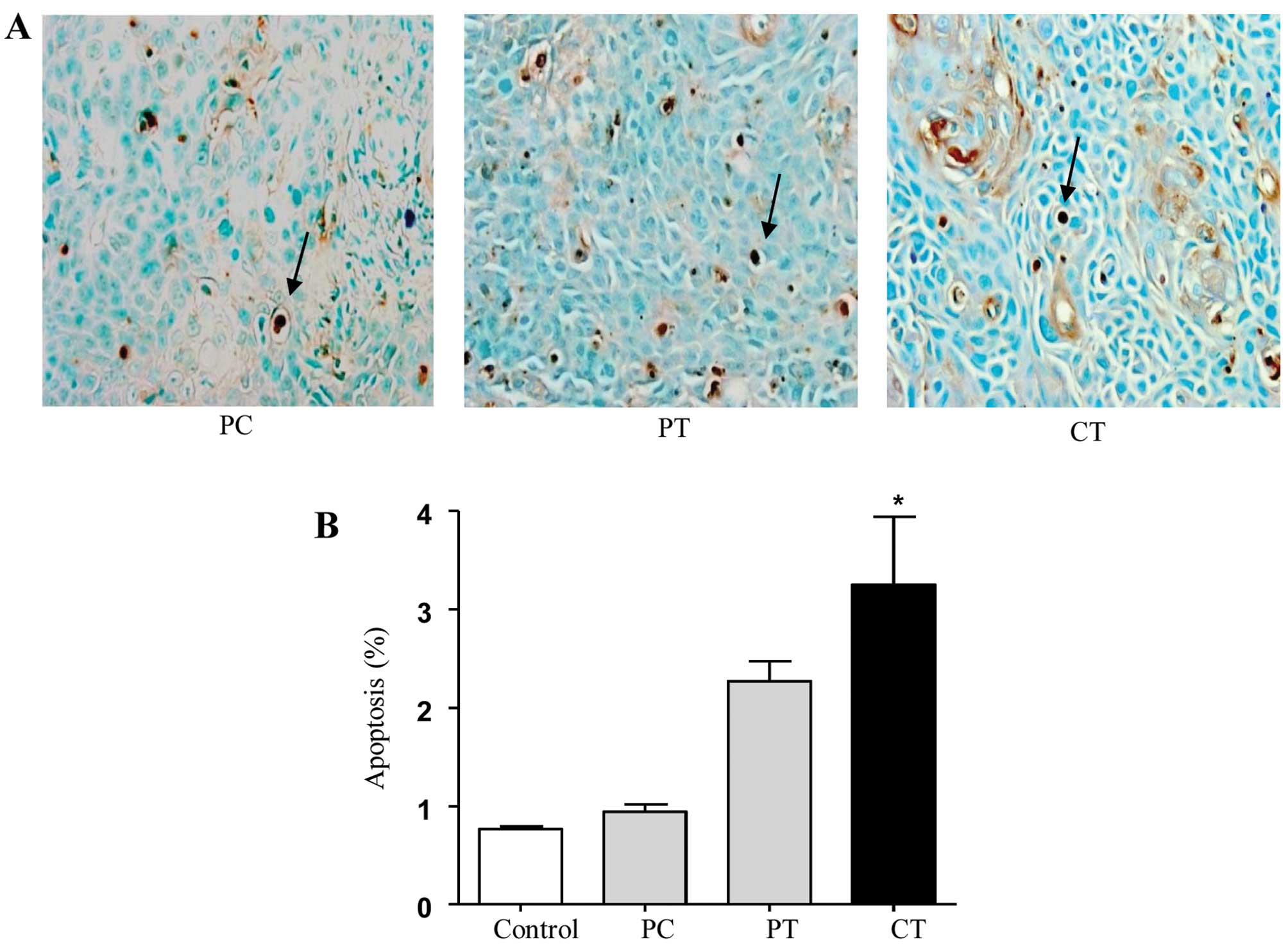
TAO and cisplatin combination induces
apoptosis in vivo
A TUNEL assay was conducted to determine whether the
drug-induced inhibition of tumor growth was caused by apoptosis.
Histochemical staining of TUNEL-positive cells (apoptotic cells)
(Fig. 5A) revealed that the number
of apoptotic cells increased significantly in the paclitaxel and
TAO and cisplatin and TAO combination groups compared with that in
the paclitaxel and cisplatin combination group, respectively
(P=0.0037, 0.024 respectively). The combination of paclitaxel and
TAO tended to increase apoptosis more than the combination of
paclitaxel and cisplatin, but the difference was not significant in
comparison to the apoptosis noted in the three groups (Fig. 5B). The TUNEL analysis in the three
groups revealed a significant increase in apoptosis induced by the
combination of cisplatin and TAO when compared with the combination
of paclitaxel and cisplatin (P=0.022).
Discussion
The recurrence rate of cervical cancer is 10–20% for
FIGO stages Ib–IIa and 50–70% for stages IIb–IVa (18). Recurrent and advanced cervical
cancers are associated with high mortality rates and a lack of
effective treatment options. To date, management of advanced cases
of cervical cancer has included radiotherapy and chemotherapy
(19). The efficacy of treatments
in patients with recurrent or metastatic disease is palliative, and
treatment for recurrent cervical cancer has not improved
significantly despite the progress in modern chemotherapy.
Cisplatin binds to and causes cross-linking of DNA
and induces DNA damage, which leads either to cell cycle arrest or
immediate activation of apoptosis and killing of cancer cells
(19). Cisplatin has been the
standard cytotoxic agent for treating advanced cervical cancer
(20) and has been combined with
other chemotherapeutic agents, including 5-fluorouracil (21), bleomycin (22), ifosfamide (23), gemcitabine (24), vinorelbine (25), paclitaxel (26–28)
and topotecan (29).
Zhang et al(30) reported a synergistic effect of
arsenic trioxide in combination with cisplatin in human ovarian
cancer cells. Arsenic compounds have been used as a treatment for
various hematologic diseases, and arsenic trioxide induces
apoptosis in numerous cancer cell lines in vitro(14). TAO has the same antitumor effects as
arsenic trioxide for inhibiting cell growth, inhibiting
angiogenesis, and inducing apoptosis in cancer cells by arresting
cells in the G1 or G2/M phases of the cell cycle (31).
In the present study, the antitumor effect of TAO
was investigated in the CaSki human cervical cancer cell line, and
in nude mice bearing CaSki xenografts. The antitumor activity of
TAO was then compared with cisplatin and paclitaxel and the
interactions among these agents were tested.
Cisplatin, paclitaxel and TAO induced similar rates
of apoptosis in vitro. Apoptosis in CaSki cells was also
induced by the combinations of the agents; in particular, the
combination of cisplatin and TAO after 48 h increased apoptosis
more than that of the other two-agent combinations (cisplatin +
paclitaxel and paclitaxel + TAO) (P=0.0231).
Western blot analysis revealed higher expression of
apoptosis-related proteins in cells treated with a combination of
TAO and cisplatin when compared with that in the other
combinations.
TAO alone demonstrated less of an antitumor effect
than cisplatin or paclitaxel, conventional chemotherapeutic agents.
However, TAO had a stronger synergistic antitumor effect in
combination with the other chemotherapeutic agents. When combined
with cisplatin, the effect of TAO was enhanced to a greater extent
than that with paclitaxel on days 18–21 in vivo (P=0.038,
0.050). The cytotoxic effect of TAO in vivo was similar to
that reported in vitro. Although we did not investigate the
mechanism of the synergistic interaction between TAO and cisplatin
in this study, we hypothesize that TAO may improve the apoptotic
effect of cisplatin. The TUNEL analysis confirmed that TAO combined
with paclitaxel or cisplatin induced cell death, and that TAO
enhanced apoptosis in combination with cisplatin more than in
combination with paclitaxel in vivo. The mechanism of action
of cisplatin is DNA damage, which induces apoptosis. Together,
these results suggest that TAO enhanced the apoptotic signaling of
cisplatin. Although the combination of TAO and paclitaxel induced
tumor shrinkage, the effect was not as strong as that of TAO +
cisplatin.
Cisplatin is considered the most effective agent for
treating cervical cancer and has been placed among the most active
drugs in combination with paclitaxel (32). Rowinsky et al(33)suggested a possible synergistic
interaction between cisplatin and paclitaxel. We demonstrated that
the antitumor effect of TAO and cisplatin was superior to that of
the cisplatin and paclitaxel combination in vitro and in
vivo. Furthermore, cell growth was inhibited more when TAO was
combined with cisplatin than when it was combined with
paclitaxel.
Various combination regimens have become a major
strategy for overcoming drug resistance and improving response and
cure rates (34–36), and these should be able to decrease
the adverse effects of the drugs. Therefore, drug interactions may
be fully recognized when TAO + cisplatin combination chemotherapy
is administered to patients with advanced cervical cancer.
In conclusion, TAO and cisplatin may be a key
regimen for cervical cancer chemotherapy. Therefore, a
comprehensive examination of the interaction of TAO and cisplatin
and the nature of the biochemical mechanisms of the synergistic
effect between them is warranted. The combination of TAO and
cisplatin merits further investigation.
Acknowledgements
This study was supported by a 2012 Inje University
research grant.
References
|
1
|
Ferlay J, Shin H, Bray F, Forman D,
Mathers C and Parkin D: GLOBOCAN 2008 v2.0. Cancer Incidence and
Mortality Worldwide: IARC CancerBase No. 10. International Agency
for Research on Cancer; Lyon: 2010, Available from: http://globocan.iarc.fr.
|
|
2
|
Jemal A, Siegel R, Ward E, Murray T, Xu J
and Thun MJ: Cancer statistics, 2007. CA Cancer J Clin. 57:43–66.
2007. View Article : Google Scholar
|
|
3
|
Im SS and Monk BJ: New developments in the
treatment of invasive cervical cancer. Obstet Gynecol Clin North
Am. 29:659–672. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Peters WA III, Liu P, Barrett RJ II, et
al: Concurrent chemotherapy and pelvic radiation therapy compared
with pelvic radiation therapy alone as adjuvant therapy after
radical surgery in high-risk early-stage cancer of the cervix. J
Clin Oncol. 18:1606–1613. 2000.
|
|
5
|
Morris M, Eifel PJ, Lu J, et al: Pelvic
radiation with concurrent chemotherapy compared with pelvic and
para-aortic radiation for high-risk cervical cancer. N Engl J Med.
340:1137–1143. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rose PG, Bundy BN, Watkins EB, et al:
Concurrent cisplatin-based radiotherapy and chemotherapy for
locally advanced cervical cancer. N Engl J Med. 340:1144–1153.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Whitney CW, Sause W, Bundy BN, et al:
Randomized comparison of fluorouracil plus cisplatin versus
hydroxyurea as an adjunct to radiation therapy in stage IIB–IVA
carcinoma of the cervix with negative para-aortic lymph nodes: a
Gynecologic Oncology Group and Southwest Oncology Group study. J
Clin Oncol. 17:1339–1348. 1999.PubMed/NCBI
|
|
8
|
Keys HM, Bundy BN, Stehman FB, et al:
Cisplatin, radiation, and adjuvant hysterectomy compared with
radiation and adjuvant hysterectomy for bulky stage IB cervical
carcinoma. N Engl J Med. 340:1154–1161. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bonomi P, Blessing JA, Stehman FB, DiSaia
PJ, Walton L and Major F: Randomized trial of three cisplatin dose
schedules in squamous-cell carcinoma of the cervix: a Gynecologic
Oncology Group study. J Clin Oncol. 3:1079–1085. 1985.PubMed/NCBI
|
|
10
|
McGuire WP, Blessing JA, Moore D, Lentz SS
and Photopulos G: Paclitaxel has moderate activity in squamous
cervix cancer. A Gynecologic Oncology Group study. J Clin Oncol.
14:792–795. 1996.PubMed/NCBI
|
|
11
|
Pectasides D, Kamposioras K, Papaxoinis G
and Pectasides E: Chemotherapy for recurrent cervical cancer.
Cancer Treat Rev. 34:603–613. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Moore DH, Blessing JA, McQuellon RP, et
al: Phase III study of cisplatin with or without paclitaxel in
stage IVB, recurrent, or persistent squamous cell carcinoma of the
cervix: a Gynecologic Oncology Group Study. J Clin Oncol.
22:3113–3119. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Monk BJ, Sill MW, McMeekin DS, et al:
Phase III trial of four cisplatin-containing doublet combinations
in stage IVB, recurrent, or persistent cervical carcinoma: a
Gynecologic Oncology Group study. J Clin Oncol. 27:4649–4655. 2009.
View Article : Google Scholar
|
|
14
|
Wintrobe MM: Clinical Hematology. 3rd
edition. Henry Kimpton; London: 1951
|
|
15
|
Kim J, Bae SM, Lim DS, et al: Tetraarsenic
oxide-mediated apoptosis in a cervical cancer cell line, SiHa.
Cancer Res Treat. 37:307–312. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Park SG, Jung JJ, Won HJ, et al:
Tetra-arsenic oxide (Tetras) enhances radiation sensitivity of
solid tumors by anti-vascular effect. Cancer Lett. 277:212–217.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chung WH, Sung BH, Kim SS, Rhim H and Kuh
HJ: Synergistic interaction between tetra-arsenic oxide and
paclitaxel in human cancer cells in vitro. Int J Oncol.
34:1669–1679. 2009.PubMed/NCBI
|
|
18
|
Benedet JL, Odicino F, Maisonneuve P, et
al: Carcinoma of the cervix uteri. Int J Gynaecol Obstet. 83(Suppl
1): 41–78. 2003. View Article : Google Scholar
|
|
19
|
Mandic A, Hansson J, Linder S and Shoshan
MC: Cisplatin induces endoplasmic reticulum stress and
nucleus-independent apoptotic signaling. J Biol Chem.
278:9100–9106. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Robati M, Holtz D and Dunton CJ: A review
of topotecan in combination chemotherapy for advanced cervical
cancer. Ther Clin Risk Manag. 4:213–218. 2008.PubMed/NCBI
|
|
21
|
Bonomi P, Blessing J, Ball H, Hanjani P
and DiSaia PJ: A phase II evaluation of cisplatin and
5-fluorouracil in patients with advanced squamous cell carcinoma of
the cervix: a Gynecologic Oncology Group study. Gynecol Oncol.
34:357–359. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Daghestani AN, Hakes TB, Lynch G and Lewis
JL: Cervix carcinoma: treatment with combination cisplatin and
bleomycin. Gynecol Oncol. 16:334–339. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cervellino JC, Araujo CE, Sánchez O, Miles
H and Nishihama A: Cisplatin and ifosfamide in patients with
advanced squamous cell carcinoma of the uterine cervix. A phase II
trial. Acta Oncol. 34:257–259. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Duenas-Gonzalez A, Lopez-Graniel C,
Gonzalez A, et al: Phase II study of gemcitabine and cisplatin
combination as induction chemotherapy for untreated locally
advanced cervical carcinoma. Ann Oncol. 12:541–547. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pignata S, Silvestro G, Ferrari E, et al:
Phase II study of cisplatin and vinorelbine as first-line
chemotherapy in patients with carcinoma of the uterine cervix. J
Clin Oncol. 17:756–760. 1999.PubMed/NCBI
|
|
26
|
Rose PG, Blessing JA, Gershenson DM and
McGehee R: Paclitaxel and cisplatin as first-line therapy in
recurrent or advanced squamous cell carcinoma of the cervix: a
Gynecologic Oncology Group study. J Clin Oncol. 17:2676–2680.
1999.
|
|
27
|
Papadimitriou CA, Sarris K, Moulopoulos
LA, et al: Phase II trial of paclitaxel and cisplatin in metastatic
and recurrent carcinoma of the uterine cervix. J Clin Oncol.
17:761–766. 1999.PubMed/NCBI
|
|
28
|
Piver MS, Ghamande SA, Eltabbakh GH and
O’Neill-Coppola C: First-line chemotherapy with paclitaxel and
platinum for advanced and recurrent cancer of the cervix: a phase
II study. Gynecol Oncol. 75:334–337. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fiorica J, Holloway R, Ndubisi B, et al:
Phase II trial of topotecan and cisplatin in persistent or
recurrent squamous and non-squamous carcinomas of the cervix.
Gynecol Oncol. 85:89–94. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang N, Wu ZM, McGowan E, et al: Arsenic
trioxide and cisplatin synergism increase cytotoxicity in human
ovarian cancer cells: therapeutic potential for ovarian cancer.
Cancer Sci. 100:2459–2464. 2009. View Article : Google Scholar
|
|
31
|
Woo SH, Park MJ, An S, et al: Diarsenic
and tetraarsenic oxide inhibit cell cycle progression and bFGF-and
VEGF-induced proliferation of human endothelial cells. J Cell
Biochem. 95:120–130. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Thigpen T, Vance R, Khansur T and Malamud
F: The role of paclitaxel in the management of patients with
carcinoma of the cervix. Semin Oncol. 24(1 Suppl 2): S2-41–S2-46.
1997.PubMed/NCBI
|
|
33
|
Rowinsky EK, Gilbert M, McGuire W, et al:
Sequences of taxol and cisplatin: a phase I and pharmacologic
study. J Clin Oncol. 9:1692–1703. 1991.PubMed/NCBI
|
|
34
|
Delaloge S, Laadem A, Taamma A, et al:
Pilot study of the paclitaxel, oxaliplatin, and cisplatin
combination in patients with advanced/recurrent ovarian cancer. Am
J Clin Oncol. 23:569–574. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gordinier M, Kudelka A, Kavanagh J,
Wharton J and Freedman R: Thiotepa in combination with cisplatin
for primary epithelial ovarian cancer: a phase II study. Int J
Gynecol Cancer. 12:710–714. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Serova M, Calvo F, Lokiec F, et al:
Characterizations of irofulven cytotoxicity in combination with
cisplatin and oxaliplatin in human colon, breast, and ovarian
cancer cells. Cancer Chemother Pharmacol. 57:491–499. 2006.
View Article : Google Scholar : PubMed/NCBI
|