Introduction
Pancreatic cancer is the fourth leading cause of
cancer-related mortality in Western countries. The medial survival
rate for patients with pancreatic cancer is approximately five
months and the five-year survival rate is approximately 5% for all
stages of the disease (1).
Chemotherapy is the main treatment regimen for pancreatic cancer.
Gemcitabine monotherapy is currently the first-line therapy
recommended by the National Comprehensive Cancer Network (NCCN)
panel (2). However, the majority of
patients are resistant to gemcitabine (3). One of the potential mechanisms
involved is insensitivity to gemcitabine-induced apoptosis
(4,5). Nuclear factor-κB (NF-κB) and X-linked
inhibitor of apoptosis protein (XIAP) are two important factors in
the apoptotic pathway (6), which
render them promising targets in reversing the chemoresistance of
pancreatic cancer cells.
Mammalian NF-κB is a family of ubiquitous
transcription factors formed by homo- or heterodimers of five NF-κB
members: Rel A (p65), c-Rel (Rel), Rel B, NF-κB1 (p50) and NF-κB2
(p52) (7). The heterodimer p65/p50
is the most abundant in many types of cells (8). In resting cells, NF-κB is sequestered
in the cytoplasm with inhibitory proteins termed IκBs; upon
multiple stimuli, such as cytokines, bacterial pathogens or
ionizing radiation, the IκB kinase (IKK) complex phosphorylates the
IκB molecules at conserved serine residues, leading to the
ubiquitination and degradation of IκB by the 26S proteasome. NF-κB
is subsequently released from IκB and translocates to the nucleus
to promote the transcription of various target genes (9). NF-κB most commonly suppresses
apoptosis by activating the transcription of anti-apoptotic genes,
such as XIAP and Bcl-2 family genes (10).
XIAP belongs to the inhibitor of apoptosis proteins
(IAPs) including eight family members defined by the presence of
the baculovirus IAP repeat (BIR) domain (11). IAPs function mainly by regulating
caspases involved in apoptosis. Of all IAPs, mammalian XIAP is the
only one that directly inhibits caspases. During apopotosis, XIAP
inhibits caspase-9, -3 and -7 to protect cell against death
(12).
It has been reported that several NF-κB subunits,
particularly the p65 subunit, are overexpressed in pancreatic
cancer cells and that patients with pancreatic cancer with a high
expression of p65 have a poor prognosis (13,14).
There are encouraging results which suggest that NF-κB is an
excellent therapeutic target for pancreatic cancer. Arlt et
al(15) demonstrated that the
inhibition of NF-κB activation sensitized pancreatic cancer
chemoresistant cells towards gemcitabine treatment. Kong et
al(16) demonstrated the
inhibition of NF-κB protein expression by the transfection of p65
small interfering RNA (siRNA) synergized with gemcitabine to induce
apoptosis. However, Pan et al(17) did not completely agree with these
conclusions. In their study, they concluded that silencing NF-κB
p65 led to gemcitabine-induced apoptosis only in chemosensitive
pancrea- tic cancer cells but not in resistant ones. Therefore, the
issue that remains is that the inhibition of NF-κB alone may not be
as effective in increasing the chemosensitivity of pancreatic
cancer cells as demonstrated previously.
On the basis of its ability to inhibit caspase
activity, XIAP has been described as a chemoresistance factor in
ammalian cancer (18). Elevated
XIAP expression has been reported in pancreatic cancer patients and
has been found to be associated with chemoresistance and decreased
patient survival (19). The
silencing of XIAP can enhance the chemosensitivity of pancreatic
cancer cells (19,20).
As NF-κB is a vital transcription factor regulating
XIAP and a possible chemoresistant factor, we hypothesized that
NF-κB in conjunction with XIAP may confer the chemoresistance of
pancreatic cancer cells; simultaneously targeting NF-κB and XIAP by
RNAi may enhance chemosusceptibility to gemcitabine.
Materials and methods
Cell lines and reagents
The human pancreatic cancer cell lines, Mia PaCa-2
and PANC-1, were stored in liquid nitrogen in the Cell Bank of the
State Key Laboratory of Medical Genetics, China. When used, they
were taken out and revivified. The cells were routinely cultured in
Dulbecco's modified Eagle's medium (DMEM) plus 10% fetal bovine
serum, streptomycin (100 mg/ml) and penicillin (100 U/ml) at 37°C
in a humidified incubator containing 5% CO2. Gemcitabine
(Lilly France, Fegersheim, France) was a gift from Dr Zhidong Wang,
Gerontism Hospital of Hunan Province.
Transfection of siRNA targeting NF-κB p65
subunit or XIAP
p65 siRNA (sense, 5′-CCAUCAACUAUGAUGAGUUdTdT-3′ and
antisense, 3′-dGdTGGUAGUUGAUACUACUCAA-5′) (17) was designed to target the NF-κB p65
subunit. XIAP siRNA (sense, 5′-GGUGAAGGUGAUAAAGUAA-3′ and
antisense, 3′-CCACUUCCACUAUUUCAUU-5′) (21) was designed to target XIAP. A
non-specific siRNA control (sense, 5′-UUCUCC GAACGUGUCACGU-3′ and
antisense, 3′-AAGAGGCUUGC ACAGUGCA-5′) was also designed. The
siRNAs were produced by GenePharma Co., Ltd., Shanghai, China. Mia
PaCa and PANC-1 cells (40,000 cells from each cell line) were grown
in six-well plates until 70% confluence. The cells were then
transfected with p65 siRNA or XIAP siRNA or control siRNA using
Lipofectamine™ 2000 (Invitrogen) in medium according to the
manufacturer's instructions. After 72 h, the protein was extracted
and used for western blot analysis to evaluate the effect of gene
silencing.
Electrophoretic mobility shift assay
(EMSA)
Nuclear extracts were prepared from cells using the
NucBuster™ Protein Exaction kit (Merck, Germany) according to the
manufacturer's instructions. Biotin-labeled NF-κB oligonucleotides
(sequence, 5′-AGTTGAGGGGACTTTCCCAGGC-3′ and
3′-TCAACTCCCCTGAAAGGGTCCG-5′) were used for gel retardation assay.
The obtained nuclear extracts (3 μg protein) were incubated with
the biotin-labeled NF-κB oligonucleotides at room temperature for
20 min and subjected to electrophoresis and chemiluminescent
reaction. Competition was performed by adding specific unlabeled
double-stranded oligonucleotide to the reaction mixture in 100-fold
molar excess. The gels were dried and visualized with a Cool Imager
imaging system (IMGR002).
Western blot analysis
Cells were washed in PBS, pH 7.4, lysed and
homogenized in RIPA buffer containing 50 mM Tris-HCl, pH 7.4, 150
mM NaCl, 1 mM EDTA, 1% NP-40, 0.1% SDS and 0.5% sodium deoxycholate
supplemented with protease inhibitor cocktail set (Pierce,
Rockford, IL, USA). The lysed material was collected and
centrifuged at 4°C for 10 min at 9,000 rpm. The total protein
concentration of the supernatant was measured using the BCA assay
kit (Sigma, Inc.). Proteins were run on 10% polyacrylamide SDS gels
and transferred onto nitrocellulose membranes. The blots were
blocked for 2 h at room temperature with 5% non-fat milk power. The
blots were then incubated with primary antibodies and subsequently
with secondary antibodies. Equal loading and transfer were
confirmed using an anti-GAPDH antibody (Santa Cruz Biotechnology,
Inc.). Primary and secondary antibodies used were: i) mouse
anti-human XIAP polyclonal antibody (Santa Cruz Biotechnology,
Inc.), 1:80,000; secondary antibody 1:10,000; ii) rabbit anti-human
NF-κB p65 polyclonal antibody (Abcam, Inc.), 1:40,000; secondary
antibody 1:10,000.
Detection of apoptosis
Apoptosis was assayed by Annexin V- FITC binding to
the externalized phosphatidylserine. Apoptosis was monitored with
the assay kit and protocol provided by the supplier (Beyotime
Institute of Biotechnology, China). The apoptosis rate was analyzed
by fluorescence-activated cell sorting analysis.
Detection of cell growth inhibition
rate
Analysis of the cell growth inhibition rate was used
to determine the sensitivity of the cell lines to gemcitabine. Mia
PaCa-2 and PANC-1 cells were seeded in 100 μl medium in 96-well
plates and various concentrations of gemcitabine were added to the
plates. After incubation for 72 h, 10 μl water-soluble tetrazolium
(WST-8) were added to each well and incubated for 1 h. The
OD450 nm was measured. The cell growth inhibition rate
was calculated using the following formula: percentage cell growth
inhibition rate = 100% - [(detected cell OD value - blanket control
OD value)/(control cell OD value - blanket control OD value)].
Statistical analysis
All data are expressed as the means ± SD. Analysis
was performed using analysis of variance (ANOVA). A value of
P<0.05 was considered to indicate a statistically significant
difference.
Results
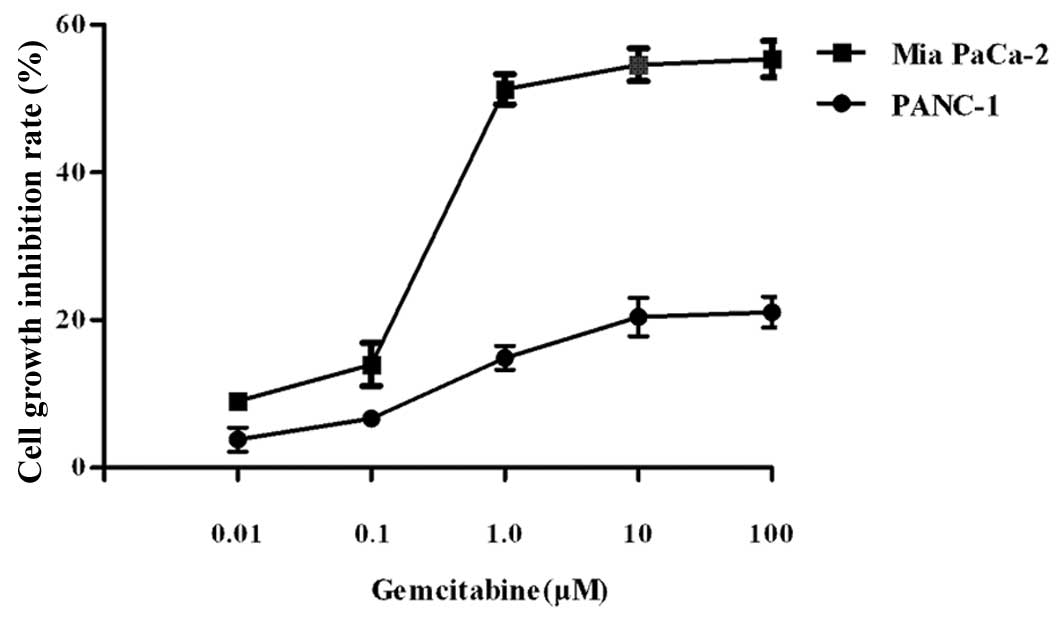
Gemcitabine causes greater cell growth
inhibition in Mia PaCa-2 compared to PANC-1 cells
Mia PaCa-2 and PANC-1 cells were treated with
various concentrations of gemcitabine for 72 h. Cell viability was
determined by the CCK-8 assay. The LD50 of gemcitabine
was approximately 1 μM for Mia PaCa-2 and >50 μM for PANC-1
cells (Fig. 1). Mia PaCa-2 cells
showed greater sensitivity to gemcitabine compared to PANC-1 cells.
We classified Mia PaCa-2 as gemcitabine-sensitive and PANC-1 as
gemcitabine-resistant based on their different LD50
values.
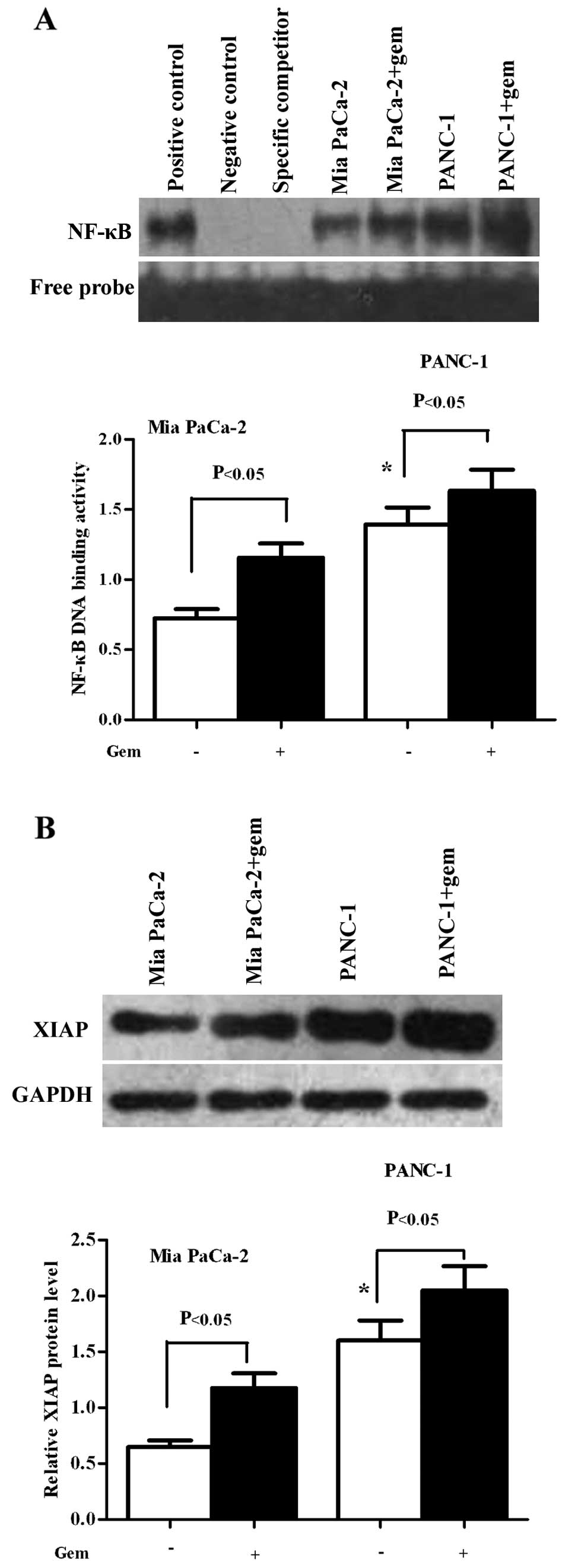
Mia PaCa-2 cells have a much lower basal
level of NF-κB and XIAP compared to PANC-1 cells
Gemcitabine induced an increase in NF-κB and XIAP
levels in the Mia PaCa-2 and PANC-1 cell lines. To determine
whether the chemoresitance of pancreatic cancer cells correlates
with NF-κB and/or XIAP, we detected their basal and induced levels
in Mia PaCa-2 and PANC-1 cells. The cells were treated with or
without gemcitabine for 6 h. Nuclear extracts were prepared and
EMSAs were performed for NF-κB DNA binding activity. As shown in
Fig. 2A, the Mia PaCa-2 and PANC-1
cells showed a high basal level NF-κB activity, although the Mia
PaCa-2 cells showed a relatively lower level (P<0.05);
gemcitabine induced an increase in NF-κB activity in both cell
lines. Homogenates of the treated cells were used to detect XIAP
protein expression by western blot analysis. We observed that the
Mia PaCa-2 and PANC-1 cells had high levels XIAP protein, although
the Mia PaCa-2 cells had a relatively lower level (P<0.05);
gemcitabine induced an increase in XIAP protein expression in both
cell lines (P<0.05) (Fig.
2B).
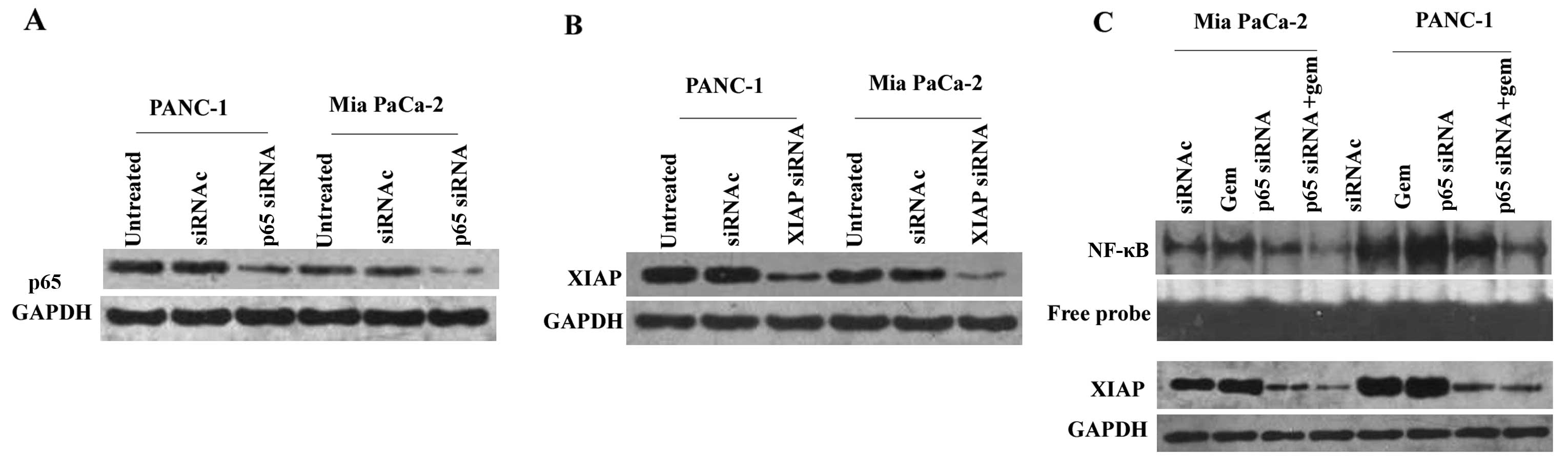
p65 siRNA effectively inhibits NF-κB
activity accompanied by the downregulation of XIAP protein
siRNA targeting NF-κB p65 was used to knockdown p65.
Mia PaCa-2 and PANC-1 cells were separately divided into three
groups and treated as follows: untreated; control siRNA or p65
siRNA. Subsequently, western blot analysis was conducted to detect
NF-κB p65 protein expression (Fig.
3A). In Mia PaCa-2 cells, p65 expression was reduced by
70.85±0.25% following treatment with p65 siRNA compared with
control siRNA (P<0.05). There was no difference between the
control siRNA and the untreated groups (P>0.05). p65 expression
was reduced by 54.19±0.67% following treatment with p65 siRNA
compared with control siRNA (P<0.05) in PANC-1 cells, and no
difference was observed between the control siRNA and the untreated
groups (P>0.05). The cells were then treated with control siRNA,
gemcitabine, p65 siRNA or gemcitabine + p65 siRNA. EMSAs were
performed. As shown in Fig. 3C, p65
siRNA downregulated basal (P<0.05, compared with control) and
gemcitabine-induced (P<0.05, compared with gemcitabine) NF-κB
DNA binding activity in the Mia PaCa-2 and PANC-1 cells (Fig. 3C, upper two lanes). To determined
whether the inhibition of NF-κB would affect XIAP expression,
western blot analysis was performed. In both cell lines, XIAP
protein (Fig. 3C, lower two panels)
was also downregulated by p65 siRNA compared with control
(P<0.05) or gemcitabine + p65 siRNA compared with gemcitabine
(P<0.05).
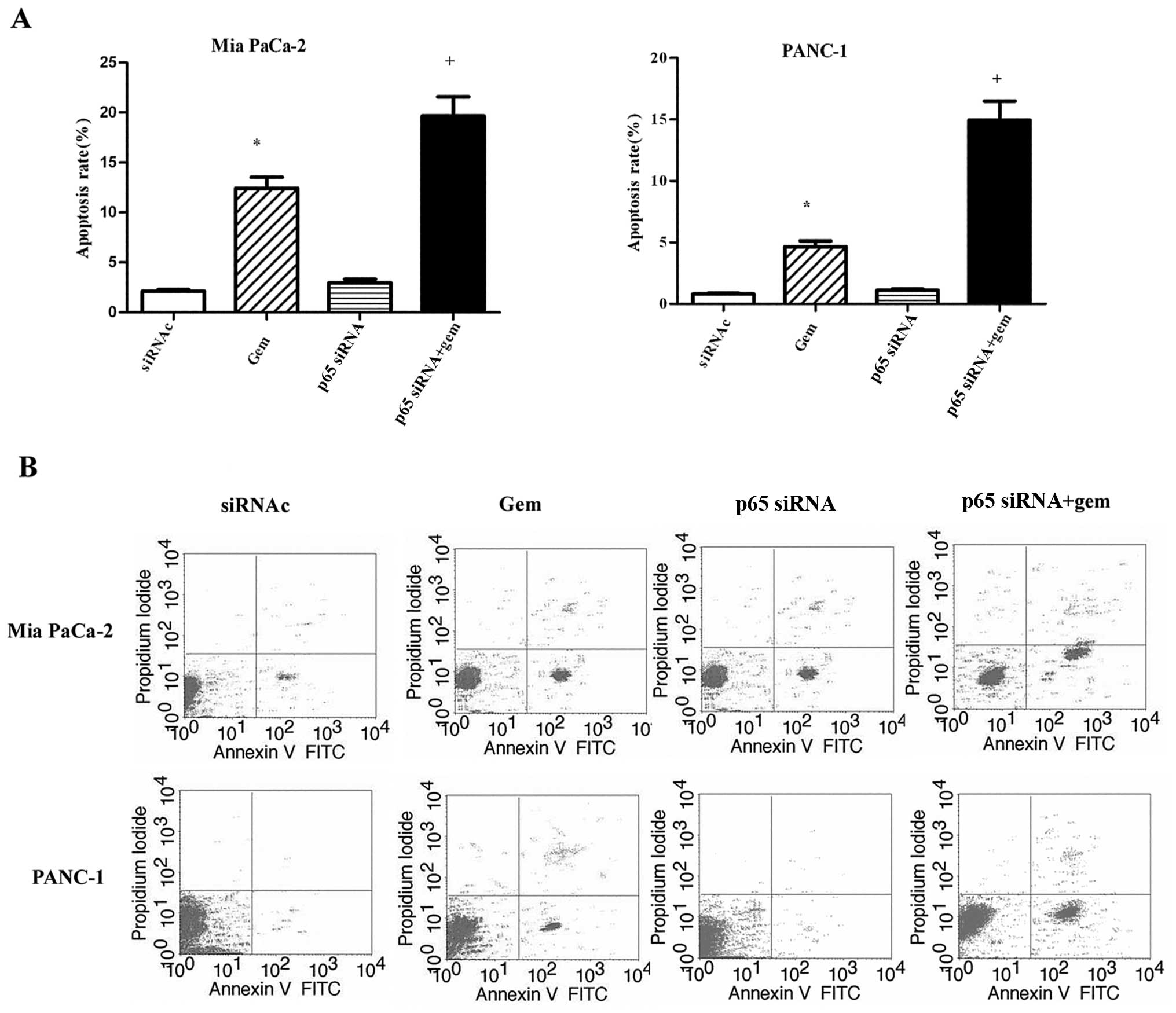
p65 siRNA enhance the sensitivity of
pancreatic cancer cells to gemcitabine; however, this was not
sufficient
Mia PaCa-2 and PANC-1 cells were treated with
control siRNA, gemcitabine, p65 siRNA or the combination of
gemcitabine and p65 siRNA for 72 h, then stained with Annexin V/PI,
and subjected to flow cytometry to measure the rate of apoptosis.
As shown in Fig. 4A, gemcitabine
alone increased the apoptotic rate compared with the control in
both cell lines (P<0.05); p65 siRNA alone did not alter the
apoptotic rate compared with the control siRNA (P>0.05) in Mia
PaCa-2 and PANC-1 cells; the combination of gemcitabine with p65
siRNA increased the apoptotic rate compared with the control siRNA
or gemcatabine or p65 siRNA in Mia PaCa-2 and PANC-1 cells
(P<0.05). The representative histograms of the flow cytometry
results shown in Fig. 4B indicate
that the apoptotic rates of the control, gemcitabine, p65 siRNA or
the combination of gemcitabine and p65 siRNA-treated groups in Mia
PaCa-2 cells were 2.1, 12.3, 3.0 and 19.6%, respectively; in the
PANC-1 cells, the rates were 0.8, 4.7, 1.1 and 14.9%, respectively.
Although gemcitabine in conjunction with p65 siRNA improved the
apoptotic rates of the Mia PaCa-2 and PANC-1 cells, this
improvement was not sufficient.
XIAP siRNA effectively downregulates XIAP
protein expression
siRNA targeting XIAP was used to knockdown XIAP.
Western blot analysis was performed to detect the expression of
XIAP protein. As demonstrated in Fig.
3B, XIAP protein expression was reduced by 79.86±0.37% in Mia
PaCa-2 and by 65.87±0.23% in PANC-1 cells.
XIAP siRNA in conjunction with p65 siRNA
downregulate XIAP protein expression and NF-κB DNA binding
activity
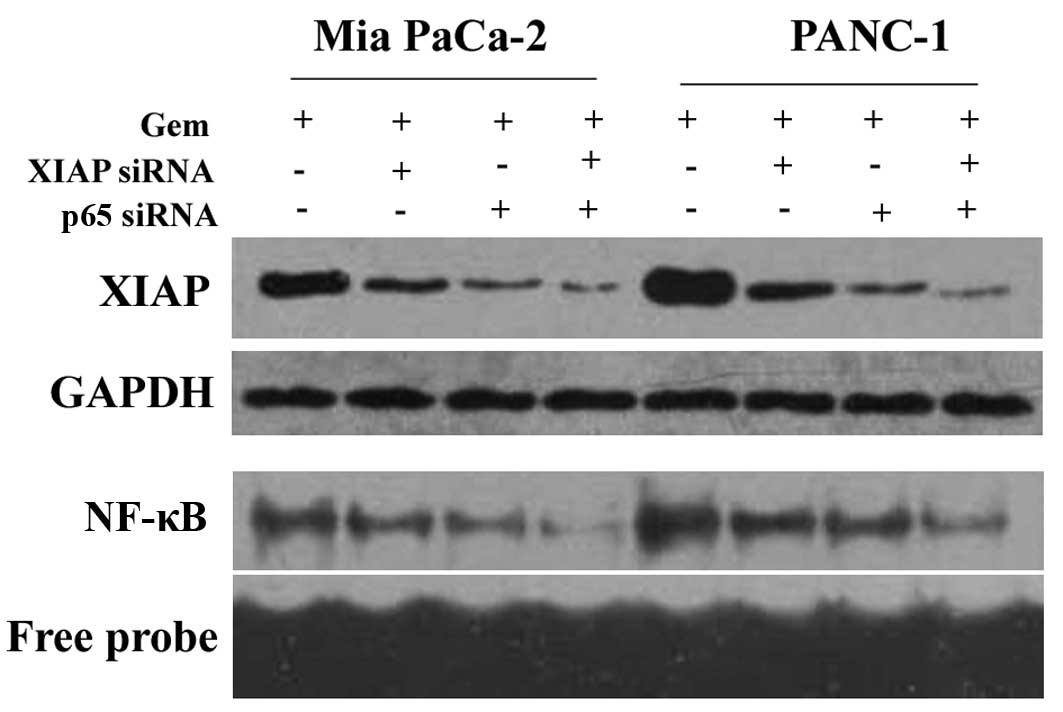
Mia PaCa-2 and PANC-1 cells were treated with
gemcitabine (control), gemcitabine + XIAP siRNA, gemcitabine + p65
siRNA or gemcitabine + XIAP siRNA + p65 siRNA, and subsequently
XIAP protein expression and NF-κB DNA binding activity were
detected. In the Mia PaCa-2 and PANC-1 cells, as shown by western
blot analysis (Fig. 5, top two
panels), gemcitabine + XIAP siRNA or gemcitabine + p65 siRNA caused
a greater reduction in XIAP protein expression compared with the
control (P<0.05). There was no difference between the groups
treated with gemcitabine + XIAP siRNA and gemcitabine + p65 siRNA
(P>0.05). Treatment with XIAP siRNA + p65 siRNA + gemcitabine
not only caused a greater reduction in XIAP protein expression
compared with the control (P<0.05), but also compared with XIAP
siRNA + gemcitabine or p65 siRNA + gemcitabine treatment. The
results of NF-κB DNA binding activity detected by EMSA (Fig. 5, bottom two panels) were the same as
those obtained from western blot analysis.
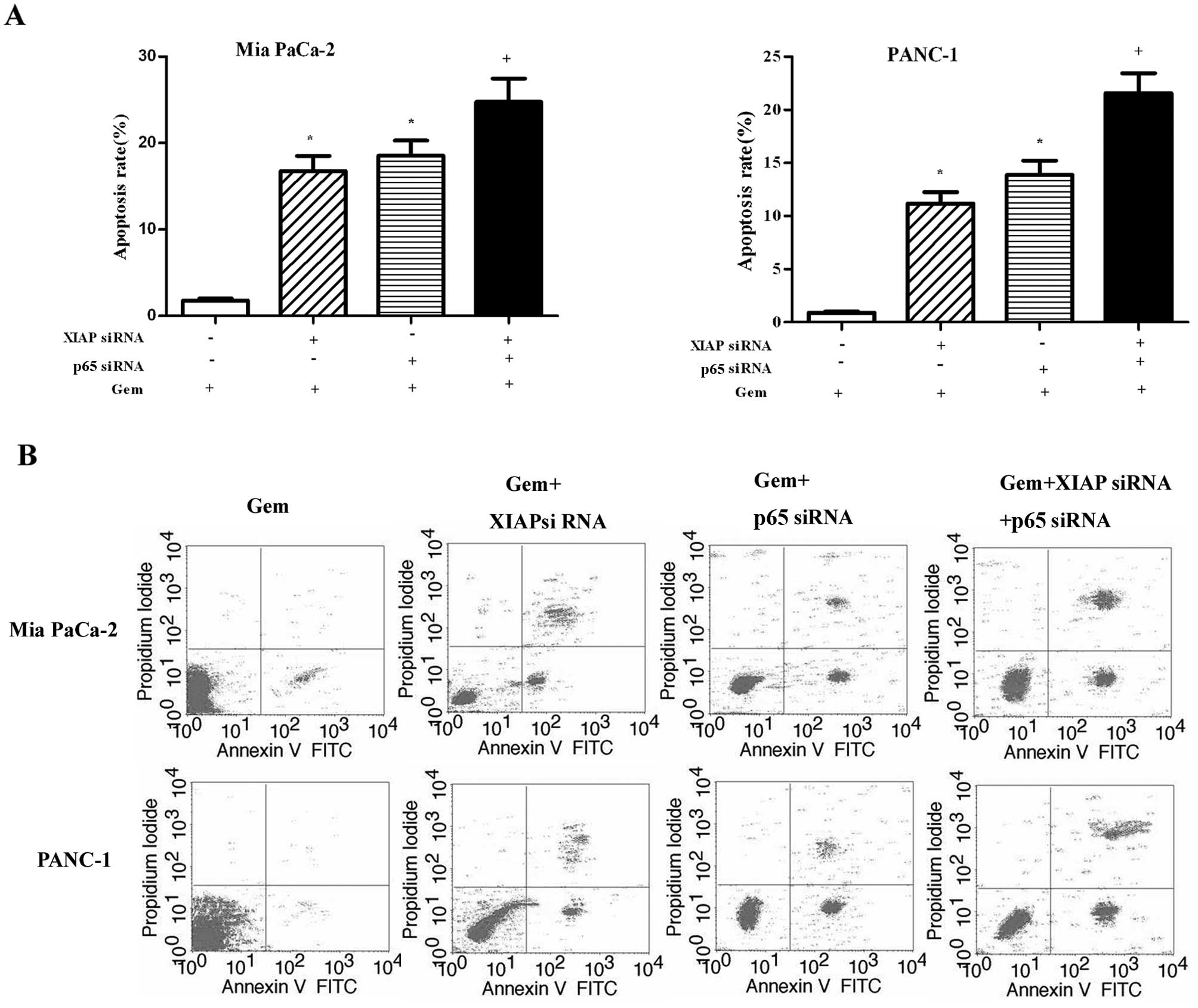
XIAP siRNA and p65 siRNA enhance the
chemosensitivity of pancreatic cancer cells to gemcitabine
Mia PaCa-2 and PANC-1 cells were treated as above
for 72 h, then stained with Annexin V/PI, and subjected to flow
cytometry to measure the rate of apoptosis. As shown in Fig. 6A, in the two types of pancreatic
cancer cells, the combination treatment of gemcitabine and XIAP
siRNA or gemcitabine and p65 siRNA increased the apoptotic rate
compared with the control (P<0.05). There was no difference
between the groups treated with gemcitabine + XIAP siRNA and
gemcitabine + p65 siRNA (P>0.05). The combination treatment of
gemcitabine + XIAP siRNA + p65 siRNA increased the apoptotic rate
compared with the control (P<0.05) or gemcitabine + XIAP siRNA
(P<0.05) or gemcitabine + p65 siRNA treatment (P<0.05).
Representative histograms of the flow cytometry results are shown
in Fig. 6B. The apoptotic rates of
Mia PaCa-2 cells in the control, gemcitabine + XIAP siRNA,
gemcitabine + p65 siRNA, and gemcitabine + XIAP siRNA + p65
siRNA-treated groups were 1.8, 16.8, 18.5 and 43.7%, respectively.
The corresponding apoptotic rates of the PANC-1 cells were 0.9,
11.1, 13.9 and 39.2%, respectively.
Discussion
The majority patients with pancreatic cancer show
great resistance to chemotherapy. The mechanisms involved remain
unknown. One of the potential mechanisms is the insensitivity to
drug-induced apoptosis (4). NF-κB
and XIAP play important roles in drug resistance in patients with
pancreatic cancer due to their involvement in the apoptotic pathway
(11,22). NF-κB is constitutively activated in
pancreatic cancer specimens as well as in pancreatic cancer cells
and contributes to anti-apoptosis (23). The same occurs with XIAP (24). Arlt et al(15) examined six types of pancreatic
cancer cells and found that chemoresistant cells had much higher
basal levels of NF-κB than chemosensitive ones and concluded that
the basal level of NF-κB predicted chemoresistance. In the present
study, we discovered that PANC-1 cells showed a relatively greater
resistance to gemcitabine compared to Mia PaCa-2 cells, as the
PANC-1 cells had a higher LD50 of gemcitabine. We
classified PANC-1 as gemcitabine-resistant and Mia PaCa-2 as
gemcitabine-sensitive. PANC-1 cells had a much higher basal level
of XIAP protein and NF-κB DNA binding activity compared to Mia
PaCa-2 cells. Therefore, XIAP and NF-κB are two potential factors
predicting chemoresistance. To detect the functional association of
NF-κB and XIAP with gemcitabine resistance, we downregulated NF-κB
and/or XIAP to determined the effects.
The inhibition of NF-κB activation by interference
with the IKK complex or blocking proteasome activity has been shown
to increase apoptosis by suppressing NF-κB target genes, including
XIAP and Bcl-2 (8). IL-32r
(25) or some natural products,
such as curcumin (26), morin
(27) and gossypol (28) have been shown to induce apoptosis or
inhibit cancer growth through the suppression of NF-κB and its
downstream genes (XIAP and Bcl-2) in many cancer cells. Neither of
these methods are specific for the inhibition of NF-κB, while RNAi
can inhibit the target gene directly (29). In the current study, we designed
siRNAs (p65 siRNA and XIAP siRNA) to downregulate the target genes.
Gemcitabine induced an increase in NF-κB DNA binding activity and
XIAP protein expression in Mia PaCa-2 and PANC-1 cells, rendering
them more chemoresisitant. p65 siRNA effectively downregulated
basal and gemcitabine-induced NF-κB DNA binding activity in the
chemoresistant and chemosensitive cells accompanied by the
downregulation of XIAP protein expression (which may lead to higher
apoptotic rates). As expected, the apoptotic rates of Mia PaCa-2
and PANC-1 cells treated with p65 siRNA combined with gemcitabine
were much higher than those of the control, or those treated with
gemcitabine or p65 siRNA alone. Therefore, p65 siRNA enhances
chemosensitivity in both chemoresistant and chemosensitive cells
through the downregulation of XIAP. These results were consistent
with those from a previous study (15). However, unlike the results of a
previous study (16), the apoptotic
rate in our study was not that high. XIAP may be another factor
affecting chemoresistance.
XIAP is a downstream target regulated by NF-κB and
was downregulated when NF-κB was inhibited by p65 siRNA. However,
there remained a possibility that it was not inhibited sufficiently
and exerted an influence on the apoptotic rate by the combined
treatment of p65 siRNA and gemcitabine. Thus, we designed XIAP
siRNA to knockdown XIAP. Bilim et al(30) demonstrated that the inhibition of
the XIAP and Bcl-2 axis retrieved the sensitivity of renal cancer
cells to apoptosis. We aimed to investigate whether the inhibition
of NF-κB and XIAP would increase the sensitivity of pancreatic
cancer cells to gemcitabine. As expected, XIAP siRNA and p65 siRNA
downregulated XIAP expression and NF-κB activity more effectively
compared to XIAP siRNA or p65 siRNA alone. The combination
treatment of gemcitabine with XIAP siRNA and p65 siRNA increased
apoptosis more efficiently than the combination of gemcitabine with
XIAP siRNA or gemcitabine with p65 siRNA in both chemoresistant and
chemosensitive pancreatic cancer cells. Thus far, there are few
studies on the multitargeted therapy of cancer treatment. Kunze
et al(31) demonstrated that
the targeted inhibition of anti-apoptotic genes (XIAP, Bcl-2 and
Bcl-xL) through siRNA combined with cisplatin increased apoptosis
in bladder cancer cells. Ruckert et al(32) found that the simultaneous gene
silencing of Bcl-2, XIAP and survivin without chemotherapy
increased the apoptosis of pancreatic cancer cells. In our study,
we first demonstrated that the double inhibition of NF-κB and XIAP
through RNAi enhanced the sensitivity of pancreatic cancer cells to
gemcitabine. IAPs, particularly XIAP are much more important to
cell apoptosis than Bcl-2 (33).
XIAP is a better target in cancer therapy. However, Seeger et
al(34) demonstrated that XIAP
overexpression alone had little effect on chemorisistance to
chemotherapeutic agents; only elevated XIAP together with the
downregulation of second mitochondria-derived activator of caspases
(SMAC), which control XIAP function, conferred chemoresistance in
cancer cells. NF-κB is an upstream factor regulating XIAP. In our
study, we demonstrated that the downregulation of XIAP alone by
RNAi was less effective in gemcitabine-induced apoptosis compared
to the double inhibition of XIAP and NF-κB. These data show that
NF-κB and XIAP together confer chemoresistance and that the
inhibition of XIAP together with the inhibition of its upstream
regulating factor, NF-κB, reverse the insensitivity of pancreatic
cancer cells to gemcitabine. This method may be used as a novel
strategy for the treatment of pancreatic cancer.
In conclusion, XIAP and NF-κB are two important
anti-apoptotic factors in pancreatic cancer cells. They are
overexpressed in either chemoresistant or chemosensitive cells and
can predict chemoresistance. The double inhibition of XIAP and
NF-κB via RNAi can enhance the chemosensitivity of pancreatic
cancer cells to gemcitabine.
Acknowledgements
This study was supported by a grant from the
National Natural Science Foundation of China (no. 30872492) and a
grant from the PhD Innovation Program of Hunan Province (no.
CX2011B064).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar
|
|
2
|
Tempero MA, Arnoletti JP, Behrman SW, et
al: Pancreatic Adenocarcinoma, version 2.2012: featured updates to
the NCCN Guidelines. J Natl Compr Cancer Netw. 10:703–713.
2012.PubMed/NCBI
|
|
3
|
Cunningham D, Chau I, Stocken DD, et al:
Phase III randomized comparison of gemcitabine versus gemcitabine
plus capecitabine in patients with advanced pancreatic cancer. J
Clin Oncol. 27:5513–5518. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gottesman MM: Mechanisms of cancer drug
resistance. Annu Rev Med. 53:615–627. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Raguz S and Yague E: Resistance to
chemotherapy: new treatments and novel insights into an old
problem. Br J Cancer. 99:387–391. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Arlt A, Muerkoster SS and Schafer H:
Targeting apoptosis pathways in pancreatic cancer. Cancer Lett.
November 13–2010. View Article : Google Scholar
|
|
7
|
Wan F and Lenardo MJ: The nuclear
signaling of NF-kappaB: current knowledge, new insights, and future
perspectives. Cell Res. 20:24–33. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sebens S, Arlt A and Schafer H: NF-kappaB
as a molecular target in the therapy of pancreatic carcinoma.
Recent Results Cancer Res. 177:151–164. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kanarek N, London N, Schueler-Furman O and
Ben-Neriah Y: Ubiquitination and degradation of the inhibitors of
NF-kappaB. Cold Spring Harb Perspect Biol. 2:a0001662010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Biswas DK, Martin KJ, McAlister C, et al:
Apoptosis caused by chemotherapeutic inhibition of nuclear
factor-kappaB activation. Cancer Res. 63:290–295. 2003.PubMed/NCBI
|
|
11
|
Gyrd-Hansen M and Meier P: IAPs: from
caspase inhibitors to modulators of NF-kappaB, inflammation and
cancer. Nat Rev Cancer. 10:561–574. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Eckelman BP, Salvesen GS and Scott FL:
Human inhibitor of apoptosis proteins: why XIAP is the black sheep
of the family. EMBO Rep. 7:988–994. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chandler NM, Canete JJ and Callery MP:
Increased expression of NF-kappa B subunits in human pancreatic
cancer cells. J Surg Res. 118:9–14. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Weichert W, Boehm M, Gekeler V, et al:
High expression of RelA/p65 is associated with activation of
nuclear factor-kappaB-dependent signaling in pancreatic cancer and
marks a patient population with poor prognosis. Br J Cancer.
97:523–530. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Arlt A, Gehrz A, Muerkoster S, et al: Role
of NF-kappaB and Akt/PI3K in the resistance of pancreatic carcinoma
cell lines against gemcitabine-induced cell death. Oncogene.
22:3243–3251. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kong R, Sun B, Jiang H, et al:
Downregulation of nuclear factor-kappaB p65 subunit by small
interfering RNA synergizes with gemcitabine to inhibit the growth
of pancreatic cancer. Cancer Lett. 291:90–98. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pan X, Arumugam T, Yamamoto T, et al:
Nuclear factor-kappaB p65/relA silencing induces apoptosis and
increases gemcitabine effectiveness in a subset of pancreatic
cancer cells. Clin Cancer Res. 14:8143–8151. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kashkar H: X-linked inhibitor of
apoptosis: a chemoresistance factor or a hollow promise. Clin
Cancer Res. 16:4496–4502. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shrikhande SV, Kleeff J, Kayed H, et al:
Silencing of X-linked inhibitor of apoptosis (XIAP) decreases
gemcitabine resistance of pancreatic cancer cells. Anticancer Res.
26:3265–3273. 2006.PubMed/NCBI
|
|
20
|
Lopes RB, Gangeswaran R, McNeish IA, Wang
Y and Lemoine NR: Expression of the IAP protein family is
dysregulated in pancreatic cancer cells and is important for
resistance to chemotherapy. Int J Cancer. 120:2344–2352. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li Y, Jian Z, Xia K, et al: XIAP is
related to the chemoresistance and inhibited its expression by RNA
interference sensitize pancreatic carcinoma cells to
chemotherapeutics. Pancreas. 32:288–296. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fan Y, Dutta J, Gupta N, Fan G and Gelinas
C: Regulation of programmed cell death by NF-kappaB and its role in
tumorigenesis and therapy. Adv Exp Med Biol. 615:223–250. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liptay S, Weber CK, Ludwig L, Wagner M,
Adler G and Schmid RM: Mitogenic and antiapoptotic role of
constitutive NF-kappaB/Rel activity in pancreatic cancer. Int J
Cancer. 105:735–746. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Trauzold A, Schmiedel S, Roder C, et al:
Multiple and synergistic deregulations of apoptosis-controlling
genes in pancreatic carcinoma cells. Br J Cancer. 89:1714–1721.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Oh JH, Cho MC, Kim JH, et al: IL-32γ
inhibits cancer cell growth through inactivation of NF-κB and STAT3
signals. Oncogene. 30:3345–3359. 2011.
|
|
26
|
Aggarwal S, Ichikawa H, Takada Y, Sandur
SK, Shishodia S and Aggarwal BB: Curcumin (diferuloylmethane)
down-regulates expression of cell proliferation and antiapoptotic
and metastatic gene products through suppression of IkappaBalpha
kinase and Akt activation. Mol Pharmacol. 69:195–206.
2006.PubMed/NCBI
|
|
27
|
Manna SK, Aggarwal RS, Sethi G, Aggarwal
BB and Ramesh GT: Morin (3,5,7,2′,4′-Pentahydroxyflavone) abolishes
nuclear factor-kappaB activation induced by various carcinogens and
inflammatory stimuli, leading to suppression of nuclear
factor-kappaB-regulated gene expression and up-regulation of
apoptosis. Clin Cancer Res. 13:2290–2297. 2007.
|
|
28
|
Moon DO, Kim MO, Lee JD and Kim GY:
Gossypol suppresses NF-kappaB activity and NF-kappaB-related gene
expression in human leukemia U937 cells. Cancer Lett. 264:192–200.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hannon GJ: RNA interference. Nature.
418:244–251. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bilim V, Yuuki K, Itoi T, et al: Double
inhibition of XIAP and Bcl-2 axis is beneficial for retrieving
sensitivity of renal cell cancer to apoptosis. Br J Cancer.
98:941–949. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kunze D, Wuttig D, Fuessel S, et al:
Multitarget siRNA inhibition of antiapoptotic genes (XIAP, BCL2,
BCL-X(L)) in bladder cancer cells. Anticancer Res. 28:2259–2263.
2008.PubMed/NCBI
|
|
32
|
Ruckert F, Samm N, Lehner AK, Saeger HD,
Grutzmann R and Pilarsky C: Simultaneous gene silencing of Bcl-2,
XIAP and Survivin re-sensitizes pancreatic cancer cells towards
apoptosis. BMC Cancer. 10:3792010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liston P, Fong WG and Korneluk RG: The
inhibitors of apoptosis: there is more to life than Bcl2. Oncogene.
22:8568–8580. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Seeger JM, Brinkmann K, Yazdanpanah B, et
al: Elevated XIAP expression alone does not confer chemoresistance.
Br J Cancer. 102:1717–1723. 2010. View Article : Google Scholar : PubMed/NCBI
|