Introduction
Ewing sarcoma is an aggressive cancer of bone.
Successful therapy requires a multimodal strategy combining
systemic multiagent chemotherapy with local treatment (1). Current 3-year event-free survival
ranges between 70% in patients with localized disease and good
response to chemotherapy and only 25–30% in patients with skeletal
metastases. Novel therapies are needed to eliminate residual
disease after conventional treatment and prevent disease
recurrence. One example of a conceptually novel non-cytotoxic drug
is the bisphosphonate compound zoledronic acid (ZA).
Bisphosphonates are a class of drugs that inhibit osteoclast
activity and bone resorption and are widely used in patients with
osteolytic skeletal disorders (2).
Based on the rationale that ZA may interfere with stimulatory
interactions between the bone microenvironment and tumor cells and
on observations of direct antiproliferative effects against tumor
cells (3–5), the drug was also evaluated for the
treatment of primary and metastatic bone tumors. As expected, ZA
demonstrated clinical antitumor activity in multiple myeloma
(6) and osteosarcoma (7) and against (micro)metastatic bone
and/or bone marrow disease in solid tumors (8,9). In
preclinical studies in Ewing sarcoma, ZA was found to inhibit in
vivo tumor growth both alone and in synergism with
chemotherapies (4,5), and initial clinical reports suggest
that ZA combined with chemotherapy may be effective in refractory
disease (10). The drug has now
entered randomized clinical evaluation to improve overall survival
as an add-on therapy in localized disease (4).
In a parallel development, cellular therapies have
emerged as promising novel modalities for cancer treatment
(11). Among pediatric solid
tumors, Ewing sarcoma cells were found to be particularly sensitive
to lysis by activated natural killer (NK) cells in
vitro(12). NK cells are innate
effector lymphocytes that provide a first-line defense against
viral infection and tumor cells. Thus, NK cell therapies may have
therapeutic benefit in Ewing sarcoma without additive toxicity.
Modulatory or synergistic interactions between novel drugs and
immunotherapies have only begun to be investigated. Antiapoptotic
proteins, kinase inhibitors and epigenetic agents can sensitize
cancer cells to antigen-specific immunotherapies and reverse immune
escape (13–17). On the other hand, various
non-cytotoxic anticancer drugs were found to negatively interfere
with critical lymphocyte and antigen-presenting functions and
thereby conflict with rational combinations of the two strategies
(18,19).
Apart from their antitumor and antiresorptive
activities, ZA and other bisphosphonates have potent
immunomodulating effects. At clinically relevant concentrations,
these agents were found to induce specific activation and
IL-2-dependent proliferation of a peripheral blood lymphocyte
subset, γδ T cells (20). Moreover,
ZA can inhibit the activation of monocyte-derived dendritic cells
(21). The immune effects of ZA are
further documented by the clinical inflammatory syndrome that
occurs in many patients upon first doses and is associated with a
transient decrease in the number of lymphocytes circulating in
peripheral blood (22,23). Through indirect mechanisms, ZA was
found to enhance NK cell effector functions (24), supporting the combination of the two
strategies. In the present study, we explored the direct in
vitro effects of ZA on expansion, phenotype and cytolytic
activity of human NK cells in Ewing sarcoma.
Materials and methods
Cell lines
The identity of all cancer cell lines was confirmed
by short tandem repeat (STR) profiling. The Ewing sarcoma cell
lines TC-71 and CADO-ES1 were obtained from DSMZ (Braunschweig,
Germany). VH-64 and WE-68 cells were gifts from Frans van Valen’s
Laboratory at the Institute of Experimental Orthopedics of the
University of Muenster, Germany. These cell lines were
characterized by the EuroBoNeT consortium (25). Tumor cells were cultured in
collagen-coated 25-cm2 tissue culture flasks (VH-64,
WE-68, CADO-ES1) or in uncoated flasks (TC-71) in RPMI-1640 medium
(Invitrogen, Darmstadt, Germany), supplemented with 10%
heat-inactivated fetal calf serum (FCS) (Thermo Fisher, Bonn,
Germany) and 2 mM L-glutamine and maintained at 37°C in 5%
CO2. K562 (ATCC) is a human erythroleukemia cell line
that is sensitive to lysis by NK cells. Generation of the
K562-mb15–41BBL stimulator cells was previously described (26). The human myeloid ML-2 cell line
(ATCC) was used as a control target.
In vitro expansion of human NK cells
Approval for using peripheral blood samples of both
healthy donors and pediatric sarcoma patients was obtained from the
University of Muenster Board of Ethics. Peripheral blood
mononuclear cells (PBMCs) were purified by density gradient
centrifugation and resuspended in RPMI-1640 medium, supplemented
with 10% FCS and 2 mM L-glutamine (RPMI culture medium). The cells
were seeded at 1×106/well in a 24-well tissue-culture
plate in the presence of 40 IU/ml recombinant human IL-2 (rhIL-2)
(Proleukin; Chiron, Emeryville, CA, USA) in RPMI-1640 and 10% FCS
and stimulated once with 0.75×106 irradiated (120 Gy)
K562-mb15–41BBL stimulator cells, as described by Imai et
al(26). TCR γδ-expressing T
cells were depleted by magnetic cell sorting using the anti-TCR γδ
MicroBead kit (Miltenyi Biotec, Bergisch Gladbach, Germany).
Flow cytometry
For immunophenotyping, lymphocytes were stained with
fluorescence-labeled anti-human antibodies against the following
surface molecules: CD3, CD4, CD56, CD16, CD137 (4-1BB), NKG2D,
NKG2A, NKG2C, CD94, CD57, 2B4 (CD244), DNAM-1 (CD226), NKp30,
NKp44, NKp46, TCRγδ for 30 min at 4°C. Anti-NKG2A and anti-NKG2C
antibodies were from R&D Systems (Minneapolis, MN, USA). All
others were from BD Biosciences (Heidelberg, Germany). Cells were
analyzed using a FACSCanto cytometer (BD Biosciences).
CD107a assay
Degranulation responses were assessed by flow
cytometric analysis of CD107a expression after a 4-h co-incubation
with target cells. Co-incubations were performed in the presence of
PE-labeled anti-human CD107a antibody (BD Biosciences) and 2 μM
monensin (Sigma, Munich, Germany). The NK cells were washed and
stained with FITC-labeled anti-CD56 and PerCP-labeled anti-CD3
antibody, followed by analysis of cells within the
CD56+CD3− gate.
Statistics
The Student’s t-test was used to test whether the
means in each set of values differed significantly, assuming two
possible tails as well as unequal variance. A P-value <0.05 was
defined as indicative of statistical significance. Values depict
the means ± standard deviation unless otherwise stated.
Results
Ewing sarcoma cells effectively induce NK
cell degranulation responses
First, we assessed the capacity of individual Ewing
sarcoma cell lines to functionally interact with in vitro
activated and expanded human NK cells. CD107a upregulation
corresponds to NK cell degranulation (27) and was used as a functional marker
for cytolytic activity. PBMCs from three healthy donors were
stimulated with irradiated K562 cells that had been gene-modified
to express membrane-bound IL-15 and 41BB ligand, as described by
Imai et al(26), and
expanded in the presence of low-dose rhIL-2 for 12 days. This
resulted in a mean 34.5±8.3-fold increase in
CD56+CD3− NK cells among the stimulated bulk
populations on day 12 of culture (range, 27.8–43.7) and a mean
purity of 66.8±11.9% (range, 53.1–74.2%). Expanded NK cells from
all donors were highly responsive to the NK cell-sensitive target
cell line K562, while the NK cell-resistant leukemia ML2 cell line
failed to induce degranulation responses above the background of
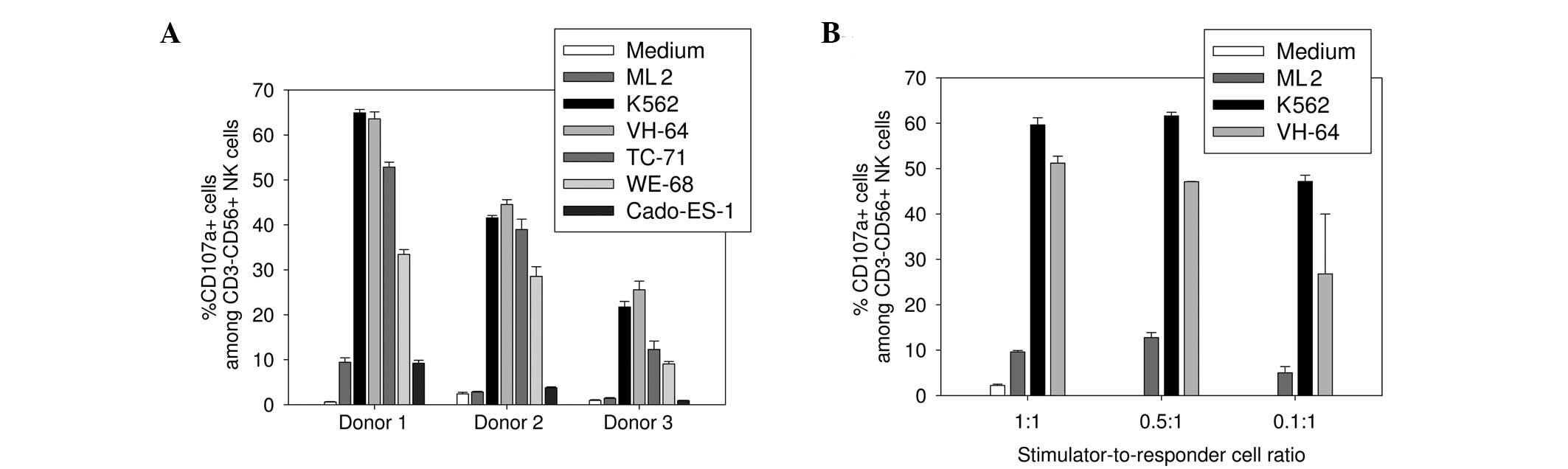
the medium alone (Fig. 1A). At a
1:1 stimulator-to-responder cell ratio, VH-64, WE-68 and TC-71
Ewing sarcoma cells effectively induced CD107a upregulation
responses in all three donors, with 44.6±16.5% (range, 23.3–65.2%),
23.7±11.2% (range, 8.7–34.5%) and 34.7±17.9% (range, 16.2–53.9%)
CD107a-expressing cells within the CD56+CD3−
NK cell gate, respectively. While degranulation responses to VH-64
cells were comparable to K562 cells in all donors, TC-71 and WE-68
cells induced variable responses among the three donors. Cado-ES-1
Ewing sarcoma cells failed to induce NK cell activation above the
medium control in all donors (4.6±3.7%; range, 0.8–9.9%). Specific
degranulation responses to VH-64 cells were maintained with reduced
stimulator-to-responder cell ratios of 0.5:1 and even 0.1:1
(Fig. 1B). These results confirm
that Ewing sarcoma cells can be highly efficient activators of
allogeneic NK cells. Subsequent experiments were performed at the
lowest ratio of 0.1:1 unless otherwise stated, and the NK
cell-sensitive cell lines VH-64 and WE-68 were used as targets.
Zoledronic acid impairs the in vitro
expansion of NK cells from healthy donors whereas the cytolytic
responses to tumor cells are essentially maintained
To investigate the effects of ZA on the in
vitro expansion and functionality of human NK cells, NK cells
were in vitro activated and expanded from PBMCs of five
healthy donors as described above, in the absence or presence of
increasing concentrations of ZA. These concentrations correspond to
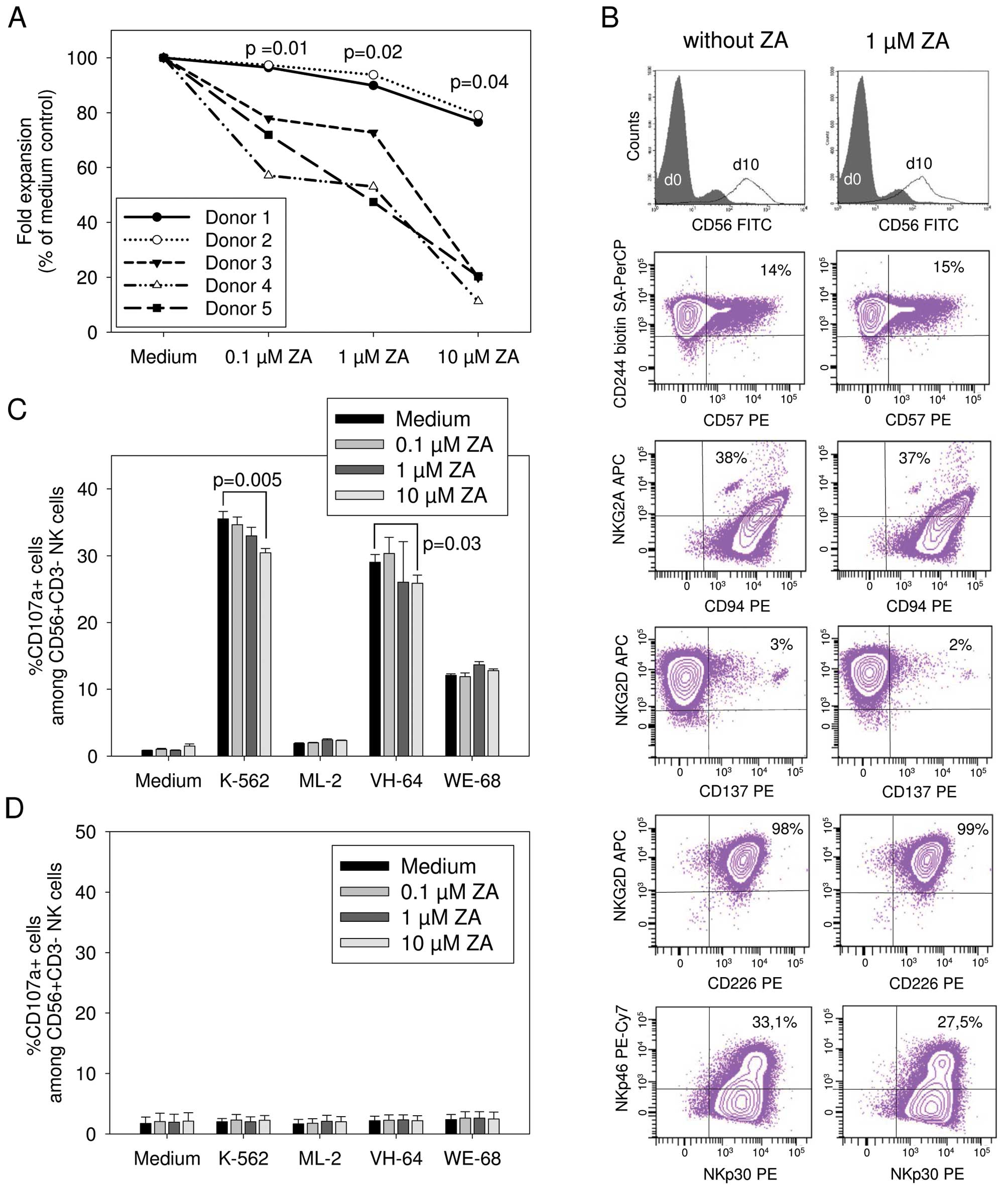
plasma levels obtained in patients after ZA infusion (28). NK cell expansion was significantly
diminished in the presence of ZA in a dose-dependent manner
compared to the ZA-free medium control (Fig. 2A). Substantial inter-donor
variability was observed; whereas addition of 10 μM ZA to the
culture medium almost completely prevented NK cell expansion in
three donors, expansion rates of 76.6 and 79.2% of controls,
respectively, were obtained under the same conditions in the two
other donors.
Next, we analyzed the phenotypes and NK cell
receptor expression of NK cells expanded from three healthy donors
in the presence or absence of ZA by gating on
CD56+CD3− lymphocytes and flow cytometryic
analysis. Our in vitro stimulation method preferentially
expanded NK cells with a CD56bright phenotype (Fig. 2B). Under the two culture conditions,
equal proportions of NK cells expressed CD57, a marker of highly
mature and differentiated NK cells (29). Over 95% of expanded NK cells
co-expressed the activating receptors NKG2D and DNAM-1 (CD226),
which have both been implicated in the recognition of target cells
via specific ligands, as well as the (co)stimulatory receptor 2B4
(CD244) and the activating receptors NKp30 and NKp46, as well as
NKp44 and CD94/NKG2C (data not shown), regardless of the presence
or absence of ZA (Fig. 2B).
CD94/NKG2A heterodimers, which have inhibitory function, were also
equally expressed under all culture conditions. Median fluorescence
intensities of expression of all of these receptors did not
significantly vary between the two populations (Table I).
 | Table ISurface expression density of cell
receptors on NK cells expanded in the presence or absence of
aminobisphosphonate zoledronic acid. |
Table I
Surface expression density of cell
receptors on NK cells expanded in the presence or absence of
aminobisphosphonate zoledronic acid.
| Without ZA | With 1 μM ZA | |
|---|
|
|
| |
|---|
| Marker | MFI (means ±
SD) | Range | MFI (means ±
SD) | Range | P-value |
|---|
| CD56 | 3210±2133 | 1875–5670 | 2764±409 | 2292–3022 | ns |
| CD57 | 160±47 | 130–214 | 138±24 | 113–161 | ns |
| CD244 (2B4) | 2007±546 | 1599–2627 | 1998±13 | 1568–2487 | ns |
| NKG2D | 5376±885 | 4378–6065 | 6187±2623 | 3213–8172 | ns |
| CD137 (4-1BBL) | 105±18 | 85–118 | 105±13 | 90–109 | ns |
| CD226 (DNAM-1) | 3841±461 | 3524–4370 | 3906±363 | 3641–4319 | ns |
| NKp46 | 380±198 | 179–574 | 257±80 | 165–312 | ns |
| NKp44 | 128±40 | 97–174 | 143±75 | 60–205 | ns |
| NKp30 | 4025±668 | 3510–4780 | 3923±1643 | 2049–5119 | ns |
To address the effect of ZA on the in vitro
functionality of NK cells, we compared CD107a upregulation by NK
cells expanded either in the presence or absence of ZA in response
to stimulation with K562 or tumor cells. Zoledronic acid is a
strong activator of γδ T cells (20). Therefore, to avoid confounding
effects by co-expanded γδ T cells, these cells were depleted prior
to expansion by magnetic cell sorting. In the presence of 0.1 and 1
μM ZA, potent degranulation responses to K562 and VH-64 cells and
intermediate responses to WE-68 cells were maintained (Fig. 2C). In two of the five donors,
responses to K562 and VH-64 cells by NK cells were reduced when the
highest concentration (10 μM) of ZA was added to the 4-h
co-incubation.
We further investigated the effects of ZA on the
functionality of native NK cells obtained by isolation from
peripheral blood of three healthy donors without prior stimulation.
NK cells were positively selected by magnetic cell selection, then
co-incubated for 4 h with the various target cells at an 0.1:1
stimulator-to-responder cell ratio in the presence or absence of
ZA. CD107a expression of non-activated NK cells from all donors did
not exceed background expression even upon co-incubation with K562
targets (Fig. 2D). These results
were unaffected by the presence of ZA.
Thus, whereas in vitro expansion of NK cells
from healthy donors was significantly impaired in the presence of
ZA, their phenotype and activating receptor expression were
unaffected by the drug, and degranulation responses of activated NK
cells to tumor targets were maintained at ZA concentrations
reflecting pharmaceutical serum levels.
Zoledronic acid impairs both in vitro
expansion and degranulation responses of NK cells from patients
with Ewing sarcoma
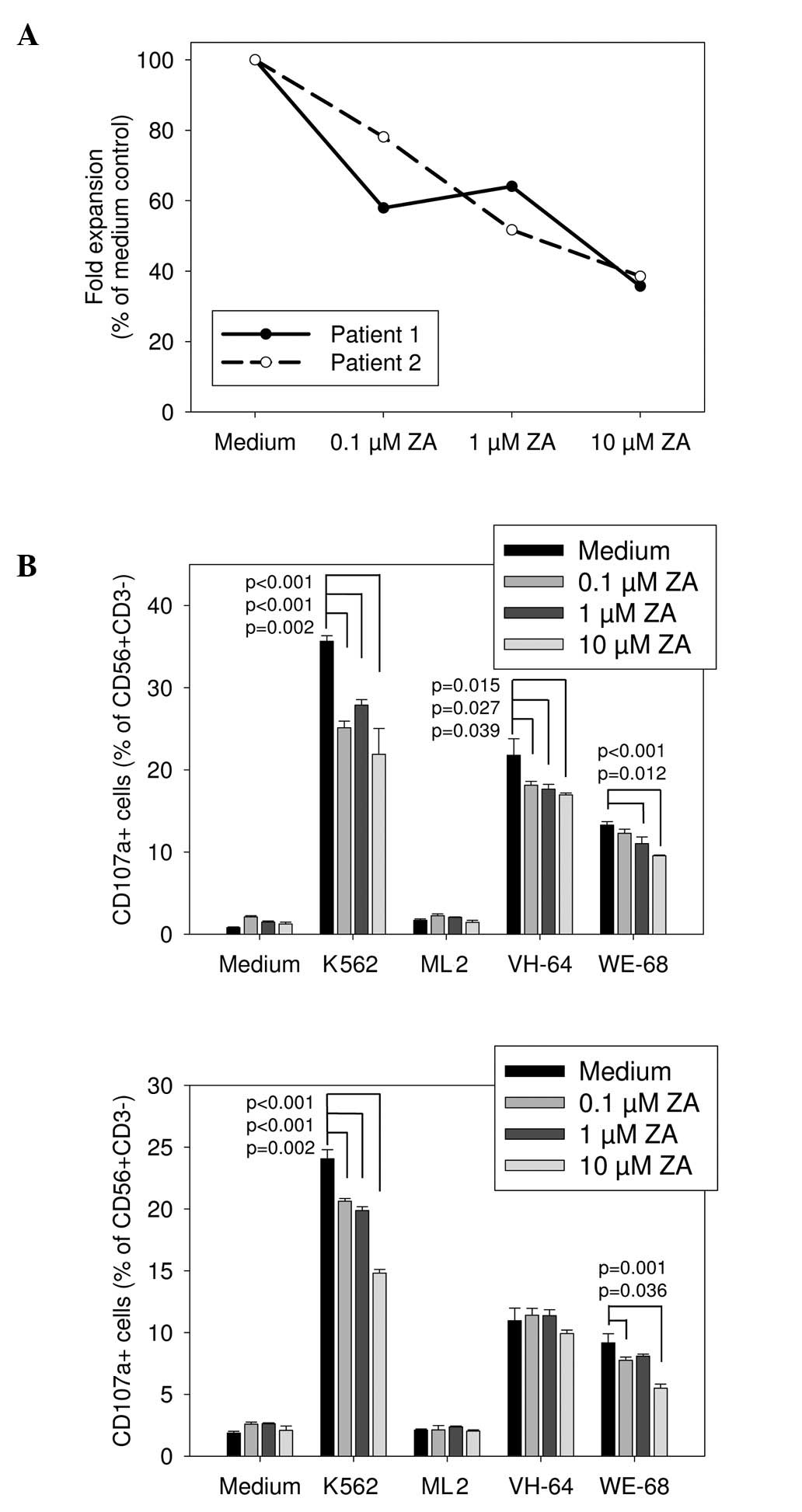
To determine the effects of ZA on the functionality
of NK cells from Ewing sarcoma patients, PBMCs were obtained from
two patients with relapsed refractory disease. NK cells from both
patients were in vitro stimulated and expanded using the
above-described technique, with 0.1 to 10 μM of ZA added during
expansion. NK cells were effectively expanded after in vitro
activation from both donors with a median 28.7-fold increase in
CD56+CD3− NK cells (range, 27.56–29.8) and a
mean purity of 88.1% (range, 80.5–95.6%). Similar to the healthy
donors, expansion of NK cells from both patients was significantly
diminished in the presence of 10, 1 and even 0.1 μM ZA (Fig. 3A). Moreover, in contrast to healthy
donors, NK cells expanded from Ewing sarcoma patients in the
presence of even low concentrations of 1 and 0.1 μM ZA had
significantly impaired degranulation responses to both K562 cells
and several Ewing sarcoma targets (Fig.
3B). Thus, ZA negatively affects both in vitro expansion
and cytolytic antitumor activity of activated NK cells from Ewing
sarcoma patients.
Discussion
ZA has attracted recent interest as a non-cytotoxic
anticancer drug and is under clinical investigation for use in
various diseases, including primary bone tumors (3,4,7–9).
Promising features of ZA include the observed anticancer synergies
between bisphosphonates and cytotoxic chemotherapies (4,30), the
favorable toxicity profile, and the proposed effect on the
tumor-promoting bone/bone marrow microenvironment to prevent
dissemination. However, drug therapy alone is unlikely to
completely eliminate residual disease in patients with high-risk
disseminated tumors. Instead, the combination of anticancer drugs
and immune-based treatments is an attractive strategy to overcome
drug resistance and clonal escape. Rational synergistic
combinations may exploit the capacity of individual drugs to
facilitate immune recognition of tumors by manipulation of
immunogenicity and immunosuppressive effects of the
microenvironment (13,14,16).
Potential combination partners must also be explored for their
effects on the antitumor effector functions of therapeutic immune
cells.
In the present study, we demonstrated that ZA
significantly impeded in vitro NK cell expansion. Moreover,
cytolytic NK cell responses to Ewing sarcoma cells were
substantially impaired in the presence of ZA. Both effects were
even more apparent with NK cells from Ewing sarcoma patients in
disease-refractory situations in which such alternative combination
therapies may be considered.
One of the most studied effects of ZA is the
selective activation of γδ T cells. ZA and other
aminobisphosphonates stimulate γδ T cells through indirect
mechanisms, including inhibition of a critical enzyme of the
mevalonate metabolism. This results in accumulation of the upstream
metabolite isopentenyl pyrophosphate that directly activates γδ T
cells (31). By the same mechanism,
pretreatment of solid cancer cells with ZA sensitizes cancer cells
to γδ T cell-mediated killing (32). Apart from γδ T cells, ZA was
reported to have profound effects on alternative immune cells,
including dendritic cells (DCs) (21). Previous studies of ZA and NK cells
have shown activating interactions (24,33,34)
which appear to contradict our own observations. A major conceptual
difference was that these previous studies relied on the effects of
ZA on accessory cells. ZA-activated γδ T cells and monocytes,
respectively, were shown to induce NK cell cytolysis of tumor cells
by providing CD137L-induced co-stimulation to upregulate NKG2D
expression on NK cells (33) or by
IFN-γ-mediated upregulation of TNF-related apoptosis-inducing
ligand (TRAIL) (34). In another
study, activation of NK cells in the presence of ZA was found to be
a consequence of ZA-induced cytokine support by DC-like cells
(24). In the present study, we
investigated the effects of ZA on a population of in vitro
preactivated NK cells under consideration for the cell therapy of
cancer (12,26,35).
Preactivation relies on stimulation with the human leukemia cell
line K562 and co-stimulation by 4-1BBL along with cytokine support
by IL-15 and subsequent low-dose IL-2. This method results in an
enrichment of highly cytotoxic NK cells with strong potency against
tumor cells, including Ewing sarcoma (12,35).
Although we cannot exclude that residual non-NK cell lymphocyte
populations contributed to our observations, interactions with γδ T
cells were avoided by depleting this subset prior to expansion.
Whereas ZA significantly suppressed
activation-induced expansion of NK cells in most donors and in both
Ewing sarcoma patients, the phenotypes of the resulting cell
populations were comparable and correspond to the in vitro
activated phenotype that was previously reported for 4-1BBL-based
stimulation methods (26,36). A practical consequence is that PBMCs
or activated and expanded NK cell products should be cryopreserved
prior to ZA treatment to maximize the rates of ex vivo NK
cell expansion. More relevant from the translational perspective is
the negative effect on degranulation responses particularly in
Ewing sarcoma patients. Although potent responses were still found
even at high concentrations of ZA, clinical efficacy of NK cell
therapy may be more dependent on an optimal activity of therapeutic
cells, and even moderate inhibitory effects may interfere with
tumor cell clearance.
The mechanisms by which ZA affects NK cell expansion
and responses to target cells remain to be resolved. Cytolytic
responses of activated NK cells to Ewing sarcoma targets were shown
to involve interaction of the NK cell activation receptors NKG2D
and DNAM-1 with their respective ligands on tumor cells (12,37).
Expression levels of these receptors were unaffected by ZA in our
studies. Alternatively, at least the effect on degranulation
responses may be explained by effects of ZA on the Ewing sarcoma
target cells, e.g. by downregulation of activating receptor
ligands. The variability observed among donors may be explained by
the impact of allogeneic KIR (killer immunoglobulin-like receptor)
mismatches on the outcome of the interaction.
Whether autologous NK cells in Ewing sarcoma
patients have a role in the natural defense against this disease is
unclear. In the present study, non-activated NK cells were not
capable of functionally interacting with Ewing sarcoma targets, and
the results were unaffected by ZA.
Collectively, our data suggest that ZA maintenance
therapy in Ewing sarcoma is not an ideal platform for adoptive NK
cell transfer. Drugs that potentiate the therapeutic immune effects
of NK cells and the mechanisms underlying these interactions must
be identified to develop effective combinations of novel drugs and
cellular therapies.
Acknowledgements
This study was supported by a grant from the Dr
Mildred-Scheel Stiftung der Deutschen Krebshilfe (to C.R.) and an
institutional grant by IMF Muenster.
References
|
1
|
Potratz J, Dirksen U, Jurgens H and Craft
A: Ewing sarcoma: clinical state-of-the-art. Pediatr Hematol Oncol.
29:1–11. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Berenson JR, Lichtenstein A, Porter L,
Dimopoulos MA, Bordoni R, George S, Lipton A, Keller A, Ballester
O, Kovacs MJ, Blacklock HA, Bell R, et al: Efficacy of pamidronate
in reducing skeletal events in patients with advanced multiple
myeloma. Myeloma Aredia Study Group. N Engl J Med. 22:488–493.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tassone P, Tagliaferri P, Viscomi C,
Palmieri C, Caraglia M, D’Alessandro A, Galea E, Goel A, Abbruzzese
A, Boland CR and Venuta S: Zoledronic acid induces
antiproliferative and apoptotic effects in human pancreatic cancer
cells in vitro. Br J Cancer. 88:1971–1978. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Odri GA, Dumoucel S, Picarda G, Battaglia
S, Lamoureux F, Corradini N, Rousseau J, Tirode F, Laud K, Delattre
O, Gouin F, Heymann D, et al: Zoledronic acid as a new adjuvant
therapeutic strategy for Ewing’s sarcoma patients. Cancer Res.
70:7610–7619. 2010.PubMed/NCBI
|
|
5
|
Zhou Z, Guan H, Duan X and Kleinerman ES:
Zoledronic acid inhibits primary bone tumor growth in Ewing
sarcoma. Cancer. 104:1713–1720. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Morgan GJ, Davies FE, Gregory WM, Szubert
AJ, Bell SE, Drayson MT, Owen RG, Ashcroft AJ, Jackson GH and Child
JA: Effects of induction and maintenance plus long-term
bisphosphonates on bone disease in patients with multiple myeloma:
the Medical Research Council Myeloma IX Trial. Blood.
119:5374–5383. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Heymann D, Ory B, Blanchard F, Heymann MF,
Coipeau P, Charrier C, Couillaud S, Thiery JP, Gouin F and Redini
F: Enhanced tumor regression and tissue repair when zoledronic acid
is combined with ifosfamide in rat osteosarcoma. Bone. 37:74–86.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Aft R, Naughton M, Trinkaus K, Watson M,
Ylagan L, Chavez-MacGregor M, Zhai J, Kuo S, Shannon W, Diemer K,
Herrmann V, Dietz J, et al: Effect of zoledronic acid on
disseminated tumour cells in women with locally advanced breast
cancer: an open label, randomised, phase 2 trial. Lancet Oncol.
11:421–428. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zarogoulidis K, Boutsikou E, Zarogoulidis
P, Eleftheriadou E, Kontakiotis T, Lithoxopoulou H, Tzanakakis G,
Kanakis I and Karamanos NK: The impact of zoledronic acid therapy
in survival of lung cancer patients with bone metastasis. Int J
Cancer. 125:1705–1709. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Siddiqui T, Marsh RW, Allegra C, Whittaker
D, Scarborough M, Gibbs P, Zlotecki R, Reith JD and Drane W:
Effective salvage treatment of recurrent Ewing sarcoma utilizing
chemotherapy and zoledronic acid. Clin Adv Hematol Oncol.
8:499–504. 2010.PubMed/NCBI
|
|
11
|
Kalos M, Levine BL, Porter DL, Katz S,
Grupp SA, Bagg A and June CH: T cells with chimeric antigen
receptors have potent antitumor effects and can establish memory in
patients with advanced leukemia. Sci Transl Med. 3:95ra732011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cho D, Shook DR, Shimasaki N, Chang YH,
Fujisaki H and Campana D: Cytotoxicity of activated natural killer
cells against pediatric solid tumors. Clin Cancer Res.
16:3901–3909. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Begley J, Vo DD, Morris LF, Bruhn KW,
Prins RM, Mok S, Koya RC, Garban HJ, Comin-Anduix B, Craft N and
Ribas A: Immunosensitization with a Bcl-2 small molecule inhibitor.
Cancer Immunol Immunother. 58:699–708. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Boni A, Cogdill AP, Dang P, Udayakumar D,
Njauw CN, Sloss CM, Ferrone CR, Flaherty KT, Lawrence DP, Fisher
DE, Tsao H and Wargo JA: Selective BRAFV600E inhibition enhances
T-cell recognition of melanoma without affecting lymphocyte
function. Cancer Res. 70:5213–5219. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Finke JH, Rini B, Ireland J, Rayman P,
Richmond A, Golshayan A, Wood L, Elson P, Garcia J, Dreicer R and
Bukowski R: Sunitinib reverses type-1 immune suppression and
decreases T-regulatory cells in renal cell carcinoma patients. Clin
Cancer Res. 14:6674–6682. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Skov S, Pedersen MT, Andresen L, Straten
PT, Woetmann A and Odum N: Cancer cells become susceptible to
natural killer cell killing after exposure to histone deacetylase
inhibitors due to glycogen synthase kinase-3-dependent expression
of MHC class I-related chain A and B. Cancer Res. 65:11136–11145.
2005. View Article : Google Scholar
|
|
17
|
Berghuis D, Schilham MW, Vos HI, Santos
SJ, Kloess S, Buddingh EP, Egeler RM, Hogendoorn PC and Lankester
AC: Histone deacetylase inhibitors enhance expression of NKG2D
ligands in Ewing sarcoma and sensitize for natural killer
cell-mediated cytolysis. Clin Sarcoma Res. 2:82012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fraser CK, Blake SJ, Diener KR, Lyons AB,
Brown MP, Hughes TP and Hayball JD: Dasatinib inhibits recombinant
viral antigen-specific murine CD4+ and CD8+
T-cell responses and NK-cell cytolytic activity in vitro and in
vivo. Exp Hematol. 37:256–265. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rossi LE, Avila DE, Spallanzani RG, Ziblat
A, Fuertes MB, Lapyckyj L, Croci DO, Rabinovich GA, Domaica CI and
Zwirner NW: Histone deacetylase inhibitors impair NK cell viability
and effector functions through inhibition of activation and
receptor expression. J Leukoc Biol. 91:321–331. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mariani S, Muraro M, Pantaleoni F, Fiore
F, Nuschak B, Peola S, Foglietta M, Palumbo A, Coscia M, Castella
B, Bruno B, Bertieri R, et al: Effector gamma delta T cells and
tumor cells as immune targets of zoledronic acid in multiple
myeloma. Leukemia. 19:664–670. 2005.PubMed/NCBI
|
|
21
|
Bringmann A, Schmidt SM, Weck MM, Brauer
KM, von Schwarzenberg K, Werth D, Grünebach F and Brossart P:
Zoledronic acid inhibits the function of Toll-like receptor 4
ligand activated monocyte-derived dendritic cells. Leukemia.
21:732–738. 2007.PubMed/NCBI
|
|
22
|
Pecherstorfer M, Jilch R, Sauty A, Horn E,
Keck AV, Zimmer-Roth I and Thiebaud D: Effect of first treatment
with aminobisphosphonates pamidronate and ibandronate on
circulating lymphocyte subpopulations. J Bone Miner Res.
15:147–154. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liote F, Boval-Boizard B, Fritz P and
Kuntz D: Lymphocyte subsets in pamidronate-induced lymphopenia. Br
J Rheumatol. 34:993–995. 1995. View Article : Google Scholar
|
|
24
|
Nussbaumer O, Gruenbacher G, Gander H and
Thurnher M: DC-like cell-dependent activation of human natural
killer cells by the bisphosphonate zoledronic acid is regulated by
γδ T lymphocytes. Blood. 118:2743–2751. 2011.PubMed/NCBI
|
|
25
|
Ottaviano L, Schaefer KL, Gajewski M,
Huckenbeck W, Baldus S, Rogel U, Mackintosh C, de Alava E,
Myklebost O, Kresse SH, Meza-Zepeda LA, Serra M, et al: Molecular
characterization of commonly used cell lines for bone tumor
research: a trans-European EuroBoNet effort. Genes Chromosomes
Cancer. 49:40–51. 2010.PubMed/NCBI
|
|
26
|
Imai C, Iwamoto S and Campana D: Genetic
modification of primary natural killer cells overcomes inhibitory
signals and induces specific killing of leukemic cells. Blood.
106:376–383. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Alter G, Malenfant JM and Altfeld M:
CD107a as a functional marker for the identification of natural
killer cell activity. J Immunol Methods. 294:15–22. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen T, Berenson J, Vescio R, Swift R,
Gilchick A, Goodin S, LoRusso P, Ma P, Ravera C, Deckert F, Schran
H, Seaman J, et al: Pharmacokinetics and pharmacodynamics of
zoledronic acid in cancer patients with bone metastases. J Clin
Pharmacol. 42:1228–1236. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lopez-Verges S, Milush JM, Pandey S, York
VA, Arakawa-Hoyt J, Pircher H, Norris PJ, Nixon DF and Lanier LL:
CD57 defines a functionally distinct population of mature NK cells
in the human CD56dimCD16+ NK-cell subset.
Blood. 116:3865–3874. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Coleman RE, Winter MC, Cameron D, Bell R,
Dodwell D, Keane MM, Gil M, Ritchie D, Passos-Coelho JL, Wheatley
D, Burkinshaw R, Marshall SJ, et al: The effects of adding
zoledronic acid to neoadjuvant chemotherapy on tumour response:
exploratory evidence for direct anti-tumour activity in breast
cancer. Br J Cancer. 102:1099–1105. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang H, Sarikonda G, Puan KJ, Tanaka Y,
Feng J, Giner JL, Cao R, Monkkonen J, Oldfield E and Morita CT:
Indirect Stimulation of Human Vγ2Vδ2 T cells through alterations in
isoprenoid metabolism. J Immunol. 187:5099–5113. 2011.
|
|
32
|
Marcu-Malina V, Heijhuurs S, van Buuren M,
Hartkamp L, Strand S, Sebestyen Z, Scholten K, Martens A and Kuball
J: Redirecting αβ T cells against cancer cells by transfer of a
broadly tumor-reactive γδT-cell receptor. Blood. 118:50–59.
2011.
|
|
33
|
Maniar A, Zhang X, Lin W, Gastman BR,
Pauza CD, Strome SE and Chapoval AI: Human γδ T lymphocytes induce
robust NK cell-mediated antitumor cytotoxicity through CD137
engagement. Blood. 116:1726–1733. 2010.
|
|
34
|
Sarhan D, D’Arcy P, Wennerberg E, Liden M,
Hu J, Winqvist O, Rolny C and Lundqvist A: Activated monocytes
augment TRAIL-mediated cytotoxicity by human NK cells through
release of IFN-gamma. Eur J Immunol. Sep 19–2012.(Epub ahead of
print).
|
|
35
|
Fujisaki H, Kakuda H, Shimasaki N, Imai C,
Ma J, Lockey T, Eldridge P, Leung WH and Campana D: Expansion of
highly cytotoxic human natural killer cells for cancer cell
therapy. Cancer Res. 69:4010–4017. 2009. View Article : Google Scholar
|
|
36
|
Dowell AC, Oldham KA, Bhatt RI, Lee SP and
Searle PF: Long-term proliferation of functional human NK cells,
with conversion of CD56(dim) NK cells to a CD56 (bright) phenotype,
induced by carcinoma cells co-expressing 4–1BBL and IL-12. Cancer
Immunol Immunother. 61:615–628. 2012.PubMed/NCBI
|
|
37
|
Verhoeven DH, de Hooge AS, Mooiman EC,
Santos SJ, ten Dam MM, Gelderblom H, Melief CJ, Hogendoorn PC,
Egeler RM, van Tol MJ, Schilham MW and Lankester AC: NK cells
recognize and lyse Ewing sarcoma cells through NKG2D and DNAM-1
receptor dependent pathways. Mol Immunol. 45:3917–3925. 2008.
View Article : Google Scholar : PubMed/NCBI
|