Introduction
Urothelial carcinoma of the bladder (UCB) is the
fourth and tenth most common solid malignancy among US men and
women, respectively (1). Radical
cystectomy achieves a durable cure in most patients with
organ-confined, lymph node-negative muscle-invasive or high-risk
non-muscle invasive tumors, but a third of patients recur (2,3). Worse
oncologic outcomes are linked to surgical histopathology, including
higher pT stage, lymphovascular invasion (LVI), lymph node
metastasis and surgical margin involvement, as well as preoperative
characteristics including female gender, hydronephrosis, pelvic
radiation and lack of neoadjuvant chemotherapy (2–13). A
role for hormonal factors in bladder carcinogenesis has been
suggested by the male predominance (~3:1) of UCB diagnoses in
industrialized nations independent of tobacco usage or occupational
carcinogen exposure (3). Estrogen
plays a role in bladder development and homeostasis (14) and has been implicated in more
advanced stage bladder tumors and worse bladder cancer-specific
survival among females (5–13,15).
Epidemiologic support for a role of estrogen in bladder
carcinogenesis includes a 50–60% increase in UCB diagnoses among
females with early menopause, bilateral oophorectomy or absence of
combined estrogen-progesterone hormonal replacement therapy
(16–18). A 2-fold increase in UCB incidence is
also observed among nulliparous women, possibly related to
unopposed estrogen (16).
The estrogen receptor genes, ERα and ERβ, have
differential tissue expression patterns and functions (19). An important role for estrogen
signaling is becoming apparent in UCB (20–24).
ERβ protein is the predominant ER in bladder, expressed in up to
77% of clinical UCB, whereas ERα expression is infrequent (<5%)
(22,25,26).
ERβ expression is unrelated to UCB patient gender or age, but has
not been studied with regard to other clinical risk factors, such
as smoking or radiation. Furthermore, the relationship between ERβ
and tumor stage or patient outcomes is disputed (22,25,26),
and the relationship of ERβ to histological cancer features such as
perineural invasion, concomitant carcinoma in situ or LVI
has not yet been described. While selective estrogen receptor
modulators (SERM) have been shown to inhibit the proliferation of
some UCB cell lines and murine xenografts, which ER isoform(s) is
being targeted has not been addressed (20–24).
Research in preclinical models has generally not interrogated the
ERβ isoform specifically, with most studies evaluating a
non-specific ERα/ERβ agonist in UCB cell lines expressing both ERα
and ERβ.
As ERβ is the predominant estrogen receptor isoform
in normal and malignant bladder tissue, our study aimed to better
delineate the role of ERβ in bladder carcinogenesis. We quantified
ERβ expression in both malignant and benign tissues from UCB
patients undergoing cystectomy and tested its association with
tumor pathology, including for the first time histological features
such as lymphovascular, perineural invasion and concomitant
carcinoma in situ, in addition to survival outcomes. We also
provide the first investigation of ERβ in relation to known
clinical risk factors for UCB diagnosis and UCB-specific mortality
including smoking history, race, pelvic radiation, hydronephrosis
and neoadjuvant chemotherapy. Finally, we evaluated the effect of
selective ERβ activation on UCB cell line proliferation with and
without SERM treatment. Our findings indicate a crucial role for
ERβ in UCB, including a novel stage-independent association with
aggressive cancer histology and poor survival outcomes after
cystectomy, and suggest clinical utility of ERβ pharmacologic
targeting in this disease.
Materials and methods
Patients and tissue specimens
Institutional Review Board approval was acquired for
this study. Tissue samples (N=129), including urothelial cell
carcinomas (n=59) and benign urothelium (n=70), were obtained from
May 2002 to December 2007 at the Department of Pathology and
Laboratory Medicine, New York Presbyterian Hospital, Weill Cornell
Medical College as previously described (27). All patients had preoperatively
diagnosed UCB and underwent pelvic lymphadenectomy during
cystectomy. Thirteen patients with no detectable cancer (pT0) at
surgery had only benign urothelium acquired. All but two patients
without pT0 disease had both benign and malignant tissue samples
obtained; the two remaining patients had large primary tumors such
that only malignant tissue was available. Death certificates and
autopsy reports were reviewed to determine cause of death as
cancer-related or from other causes.
Immunohistochemistry
Immunohistochemical staining for human ERβ protein
(14C8 antibody, 1:300 dilution; Novus Biologicals, Littleton, CO,
USA) was performed using the Leica BOND-MAX Autostainer and the
Leica Microsystems Refine Detection kit as previously described
(27). Negative controls were
performed without the primary antibody and with normal mouse serum.
Benign and malignant prostate tissues were used as a positive
control. All stained tissue sections were evaluated by a urologic
pathologist (B.D.R.), and separate scores were assigned to each
sample based on: i) the percentage of tissue staining positive
(0–100%); and ii) intensity of positively staining cells (none, 0;
weak, 1; moderate, 2; and strong, 3) as previously described
(27). Only nuclear staining was
considered positive, and a minimum of 100 tumor nuclei was
evaluated per case.
Bladder cancer cell lines, culture
conditions, RNA extraction and qRT-PCR
The HTB-1 (J82), HTB-5 (TCCSUP) and HT1376 UCB cell
lines were derived from high-grade invasive urothelial carcinomas,
and the HTB-3 (SCaBER) cell line was derived from an invasive
squamous cell carcinoma of the bladder (ATCC, Rockville, MD, USA).
RNA was extracted and cDNA was synthesized as previously described
(27). Primers for ERα and ERβ were
as previously described (22) and
HPRT (5′-tgctcgagatgtgatgaagg-3′ and 5′-tcccctgttgactggtcatt-3′)
was used as a normalizing control. cDNA from the MCF-7 breast
cancer cell line was used as a positive control, and expression of
ERα and ERβ was normalized by HPRT and compared to the
levels detected in MCF-7 using the ΔΔCt qPCR method.
Effects of ER agonists and a selective ER
modulator on bladder cancer cell growth
The effects of 17β-estradiol (E2)(Sigma-Aldrich, St.
Louis, MO, USA) and selective ERβ agonist diarylpropionitrile (DPN)
(Tocris, Minneapolis, MN, USA), with and without tamoxifen (Sigma
Aldrich), were assessed on bladder cancer cell lines at a 10 nM
concentration as previously reported (22,28).
Cells (1×106/well) were treated with the drugs for 48 h
and the number of cells was counted using a Coulter Counter
(Beckman-Coulter, Brea, CA, USA). Each treatment condition
represents a minimum of 12 data points accrued over 4 separate
experiments.
Statistical analysis
Fisher’s exact test was used to test for statistical
association between ERβ immunostaining scores and
clinicopathological variables (Table
I). The staining percentage was assessed as a categorical
variable using a 4-tier scale (0–10%, 11–40%, 41–70% and 71–100%),
while staining intensity was assessed using a 0–3 scale, as
previously described (27).
Patients with a final pathology of primary UCB in situ
(pTis) were censored from the analysis testing for association with
concomitant CIS. The Kaplan-Meier method was used to analyze
disease-free, cancer-specific and overall survival curves
stratified by ERβ immunostaining scores. Time to recurrence or
death was compared between groups using a log-rank test. A
multivariable Cox proportional hazards model tested the ability of
immunostaining scores to predict survival outcomes after adjusting
for pathologic tumor stage. A second Cox proportional hazards model
was also constructed which included ERβ staining, pathologic tumor
stage, LVI and lymph node stage. For in vitro growth assays,
two-tailed t-tests were utilized to compare the effects on cellular
proliferation of individual drug treatments relative to solvent
control and between drug combinations. Statistical analyses were
conducted using IBM SPSS Statistics (version 19.0.1.80), Prism
(GraphPad Software Inc., La Jolla, CA, USA) or JMP version 8 (SAS
Institute Inc., Cary, NC, USA). In all cases, P≤0.05 was considered
to indicate a statistically significant result.
 | Table ICharacteristics of the cystectomy
patients. |
Table I
Characteristics of the cystectomy
patients.
| Characteristics | |
|---|
| Total patients, n
(%) | 72 (100) |
| Age (years) |
| Mean ± SD | 66.4±10.0 |
| Median | 66.6 |
| Range | 43.3–88.8 |
| Gender, n (%) |
| Female | 21 (29) |
| Male | 51 (71) |
| Race, n (%) |
| Caucasian | 66 (92) |
| Other | 6 (8) |
| BMI,
kg/m2 |
| Mean ± SD | 26.8±4.8 |
| Median | 26.5 |
| Smoking history, n
(%) | 56 (74) |
| Active smoker, n
(%) | 15 (21) |
| Prior pelvic
irradiation, n (%) | 8 (11) |
| Prior intravesical
chemotherapy, n (%) | 21 (29) |
| Prior neoadjuvant
systemic chemotherapy, n (%) | 11 (15) |
| Pre-operative
hydronephrosis, n (%) | 20 (28) |
| Pathological stage,
n (%) |
| pT0 | 13 (18) |
| pTa | 9 (13) |
| pTis | 11 (15) |
| pT1 | 7 (10) |
| pT2 | 10 (15) |
| pT3 | 11 (15) |
| pT4 | 11 (15) |
| Lymph node
metastases, n (%) | 18 (25) |
| N-stage |
| N1 | 10 (14) |
| N2 | 5 (7) |
| N3 | 3 (4) |
| Lymphovascular
invasion (LVI), n (%) | 15 (21) |
| Perineural
invasion, n (%) | 6 (8) |
| Concomitant
carcinoma in situ (CIS), n (%) | 34 (47) |
Results
Association of ERβ immunostaining with
bladder cancer histopathology and clinical risk factors
The majority of cases had only low (1+) intensity
ERβ staining (Fig. 1). Staining
positivity (percentage of tissue) was significantly elevated in
cancer tissues compared to benign bladder tissues (P<0.001)
(Fig. 1, Table II). For example, ~80% of cancer
specimens had >10% tissue positivity, compared to a minority of
benign specimens (Fig. 1). High
tissue positivity (>70%) was observed exclusively in cancer
specimens (Fig. 1). ERβ staining
was not associated with tumor stage (Fig. 1), including different substage
comparisons (Table II). However,
ERβ positivity in cancer specimens was strongly associated with
aggressive bladder cancer histological features, namely LVI and
perineural invasion (P<0.01 each; Table II). All cancers with LVI had at
least low (>10%) ERβ positivity, and all cancers with perineural
invasion had at least moderate (>40%) ERβ positivity. The
presence of LVI was also associated with ERβ positivity in the
adjacent benign urothelium (Table
II).
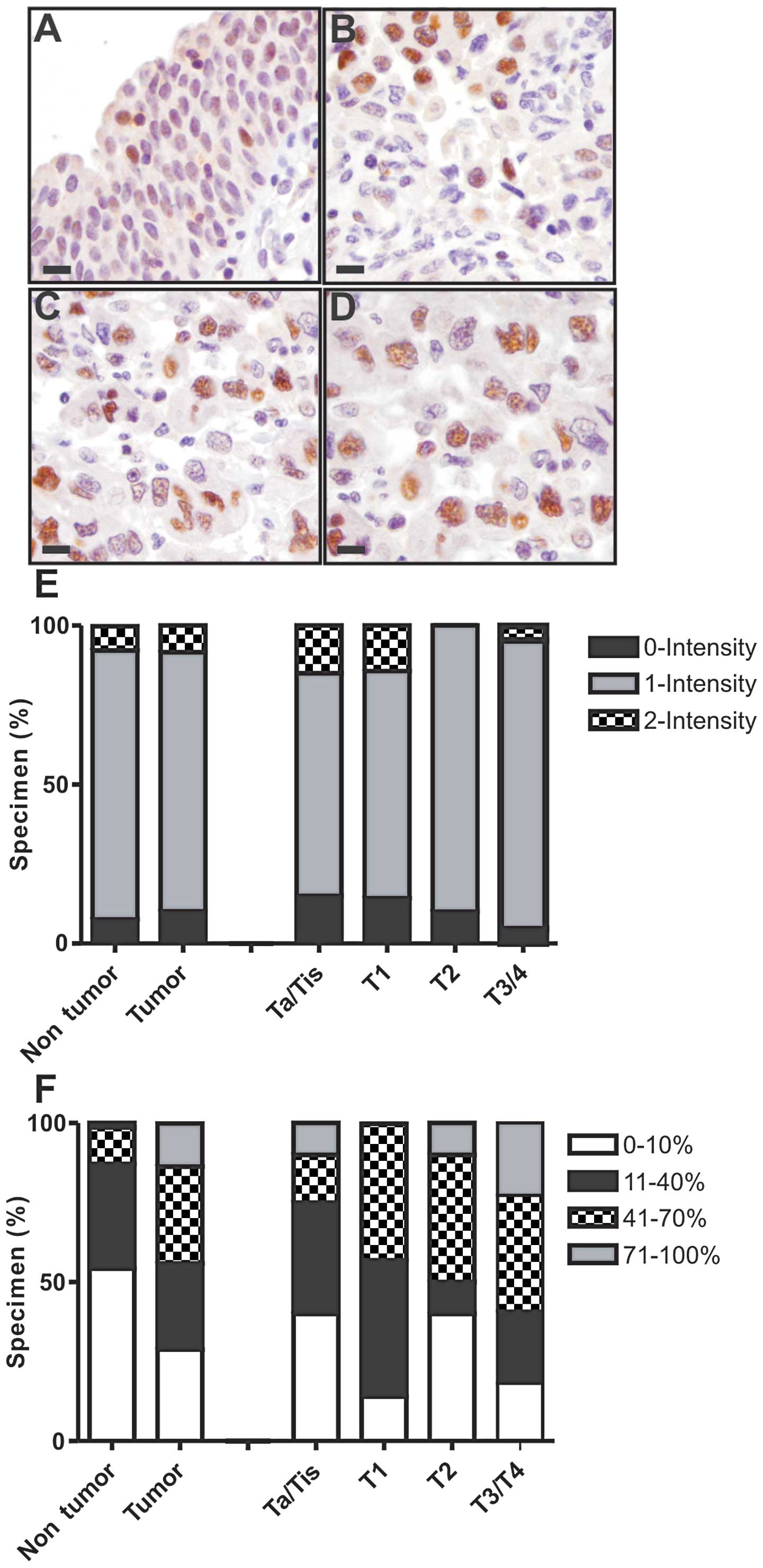 | Figure 1Immunohistochemical staining.
Representative immunohistochemical staining for ERβ in (A) benign
urothelium, (B) non-muscle invasive urothelial carcinoma (pTis,
pTa, pT1), (C) muscle invasive carcinoma (pT2) and (D) extravesical
(pT3) carcinoma. (All images were captured at ×400 total
magnification; bar, 20 μm). (E and F) Distribution of staining
positivity and intensity according to pathological stage. Separate
scores were assigned to the stained tissue sections based on (E)
the intensity of positively staining cells (0, none; 1, weak; 2,
moderate; and 3, strong) and on (F) tissue positivity, i.e., the
percentage of tissue staining positive (0–100%). Only nuclear
staining was considered positive, and a minimum of 100 tumor nuclei
were counted for each case. Please refer to Table II for detailed statistical
analysis. |
 | Table IIAssociation of ERβ with patient
histopathological traits, disease recurrence and cancer-specific
mortality in tumor specimens. |
Table II
Association of ERβ with patient
histopathological traits, disease recurrence and cancer-specific
mortality in tumor specimens.
| Tumor (n=59) | Benign urothelium
(n=70) |
|---|
|
|
|
|---|
| Histopathological
parameters | ERβ positivity
% | ERβ intensity | ERβ positivity
% | ERβ intensity |
|---|
| Stage
(overall) | 0.448 | 0.326 | 0.499 | 0.694 |
| Non-muscle
invasive vs. invasive | 0.197 | 0.290 | 0.540 | 0.288 |
| NMI vs. MI | 0.218 | 0.149 | 0.425 | 0.493 |
| BC vs. EVE | 0.249 | 0.457 | 0.105 | 0.043 |
| Lymphovascular
invasion | 0.008 | 0.389 | 0.033 | 0.317 |
| Perineural
invasion | 0.006 | 1 | 0.493 | 1 |
| Comcomitant
carcinoma in situ | 0.230 | 0.323 | 0.628 | 0.823 |
| Positive bladder
margin | 0.769 | 1 | 0.841 | 1 |
ERβ levels were also tested for association with
clinical variables, including risk factors for UCB diagnosis or
UCB-specific mortality (Table
III). ERβ positivity in cancer specimens was associated with
prior pelvic radiation and hydronephrosis (Table III), with >70% positivity found
in ~50 and ~33% of cancer specimens with these traits,
respectively. ERβ positivity was inversely associated with a
history of intravesical chemotherapy, with minimal or low staining
in the vast majority (82%) of cancer specimens from these patients.
ERβ positivity was associated with race in benign specimens.
 | Table IIIAssociation of ERβ with patient
clinicopathological traits, disease recurrence and cancer-specific
mortality in tumor specimens. |
Table III
Association of ERβ with patient
clinicopathological traits, disease recurrence and cancer-specific
mortality in tumor specimens.
| Tumor (n=59) | Non-tumor
(n=70) |
|---|
|
|
|
|---|
| Clinical
parameters | ERβ %
positivity | ERβ intensity | ERβ %
positivity | ERβ intensity |
|---|
| Age (years) | 0.283 | 0.630 | 0.156 | 0.373 |
| Gender | 0.791 | 1 | 0.952 | 0.199 |
| Race | 0.183 | 0.801 | 0.018 | 0.398 |
| Smoking |
| Any history | 0.399 | 0.819 | 0.813 | 1 |
| Active | 0.478 | 1 | 0.441 | 1 |
| Body mass
index | 0.198 | 0.928 | 0.139 | 0.677 |
| Prior pelvic
radiation | 0.005 | 0.310 | 0.418 | 0.771 |
| Hydronephrosis | 0.022 | 0.122 | 0.393 | 1 |
| Intravesical
chemotherapy | 0.038 | 0.194 | 0.083 | 0.330 |
| Neoadjuvant
chemotherapy | 0.292 | 0.462 | 0.701 | 0.385 |
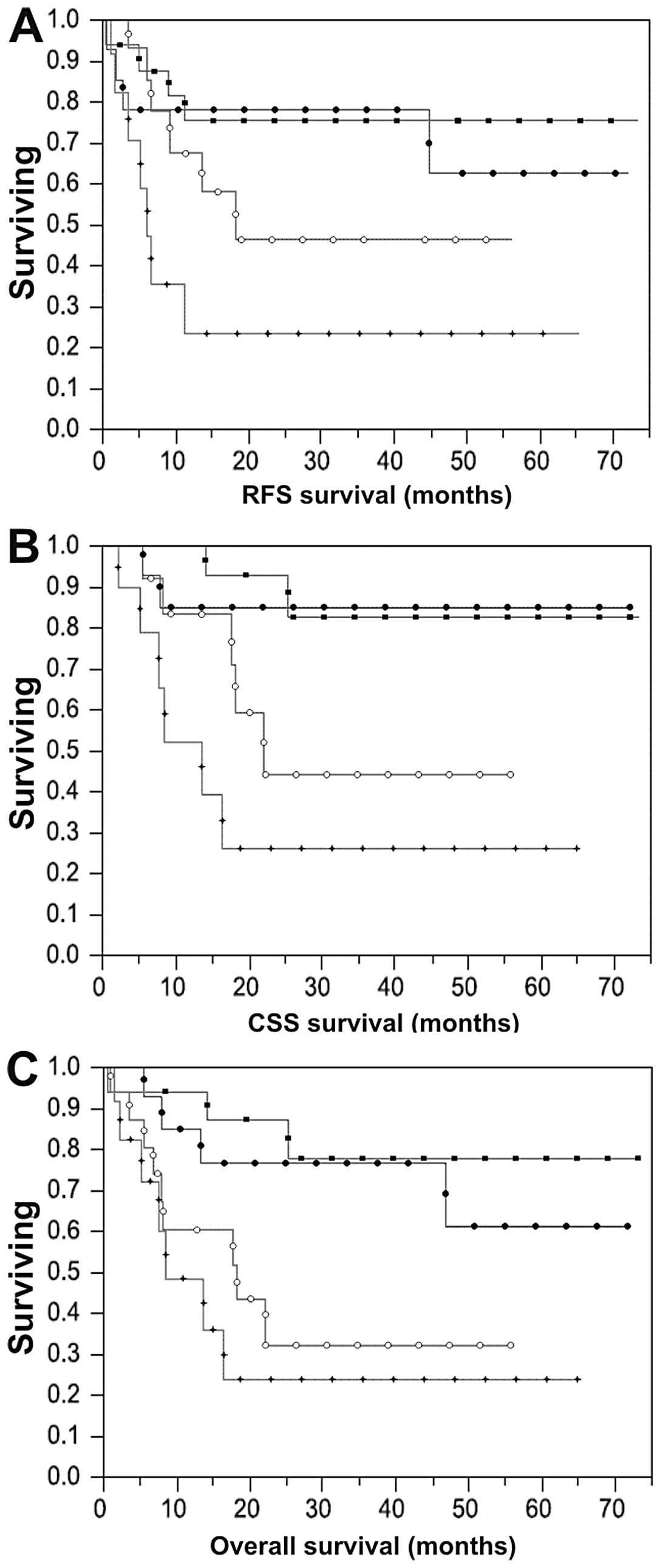
Association of ERβ immunostaining with
bladder cancer patient survival outcomes
Median and mean patient follow-up after radical
cystectomy was 21 and 26 months, respectively. Recurrence occurred
in 21/72 (29%) patients, 15/72 (21%) died from their disease, and
an additional 10/72 (14%) patients died of other causes. All
patients with a high (>70%) percentage of ERβ positivity in
tissue at >3-month follow-up developed recurrent disease. Tumor
stage, lymph node stage (P=−0.0001), LVI (P=0.03) and elevated ERβ
positivity (P=0.009) were significantly inversely associated with
recurrence-free, cancer-specific and overall survival in the Cox
univariable analysis (Table IV).
Each of these variables also correlated with shorter time to
recurrence, cancer-specific or all-cause mortality on log-rank
tests, as depicted for ERβ in Fig.
2. No association between ERβ intensity in cancer specimens and
time to recurrence, cancer-specific or all-cause mortality was
observed (P=0.59, 0.32, 0.54, respectively). There was also no
association between ERβ intensity or positivity in adjacent benign
urothelium and time to recurrence, cancer-specific or all-cause
mortality (P=0.62, 0.08, 0.28 and 0.28, 0.07, 0.18,
respectively).
 | Table IVMultivariable associations of
survival outcomes. |
Table IV
Multivariable associations of
survival outcomes.
| Univariate
analysis | Multivariable
analysis 1 | Multivariable
analysis 2 |
|---|
|
|
|
|
|---|
| (P-value) | pT stage
(P-value) | pT stage, N stage
and LVI (P-value) |
|---|
| Recurrence-free
survival |
| Percentage of ERβ
positivity in tissue | 0.0090 | 0.029 | 0.017 |
| pT stage | <0.0001 | <0.0001 | 0.0004 |
| N stage | 0.0001 | - | 0.14 |
| Lymphovascular
invasion | 0.030 | - | 0.91 |
| Cancer-specific
survival |
| Percentage of ERβ
positivity in tissue | 0.0014 | 0.11 | 0.26 |
| pT stage | <0.0001 | 0.016 | 0.071 |
| N stage | 0.0012 | - | 0.83 |
| Lymphovascular
invasion | 0.0017 | - | 0.89 |
| Overall
survival |
| Percentage of ERβ
positivity in tissue | 0.0061 | 0.041 | 0.067 |
| pT stage | <0.0001 | <0.0001 | 0.017 |
| N stage | 0.0003 | - | 0.58 |
| Lymphovascular
invasion | <0.0001 | - | 0.035 |
In Cox multivariable analysis including ERβ
positivity and tumor stage, both variables remained significantly
inversely associated with recurrence-free and overall survival;
tumor stage (P=0.02) but not ERβ (P=0.07) remained significantly
associated with cancer-specific survival, likely because of the
limited number of cancer-specific death events. A second
multivariable analysis of survival outcomes was performed with ERβ
positivity, tumor stage, lymph node stage and LVI. In this second
analysis, increased ERβ positivity still remained significantly
inversely associated with recurrence-free survival (P=0.02)
independent of these variables (Table
IV).
Effects of ERβ activation and selective
ER modulation on bladder cancer cell growth
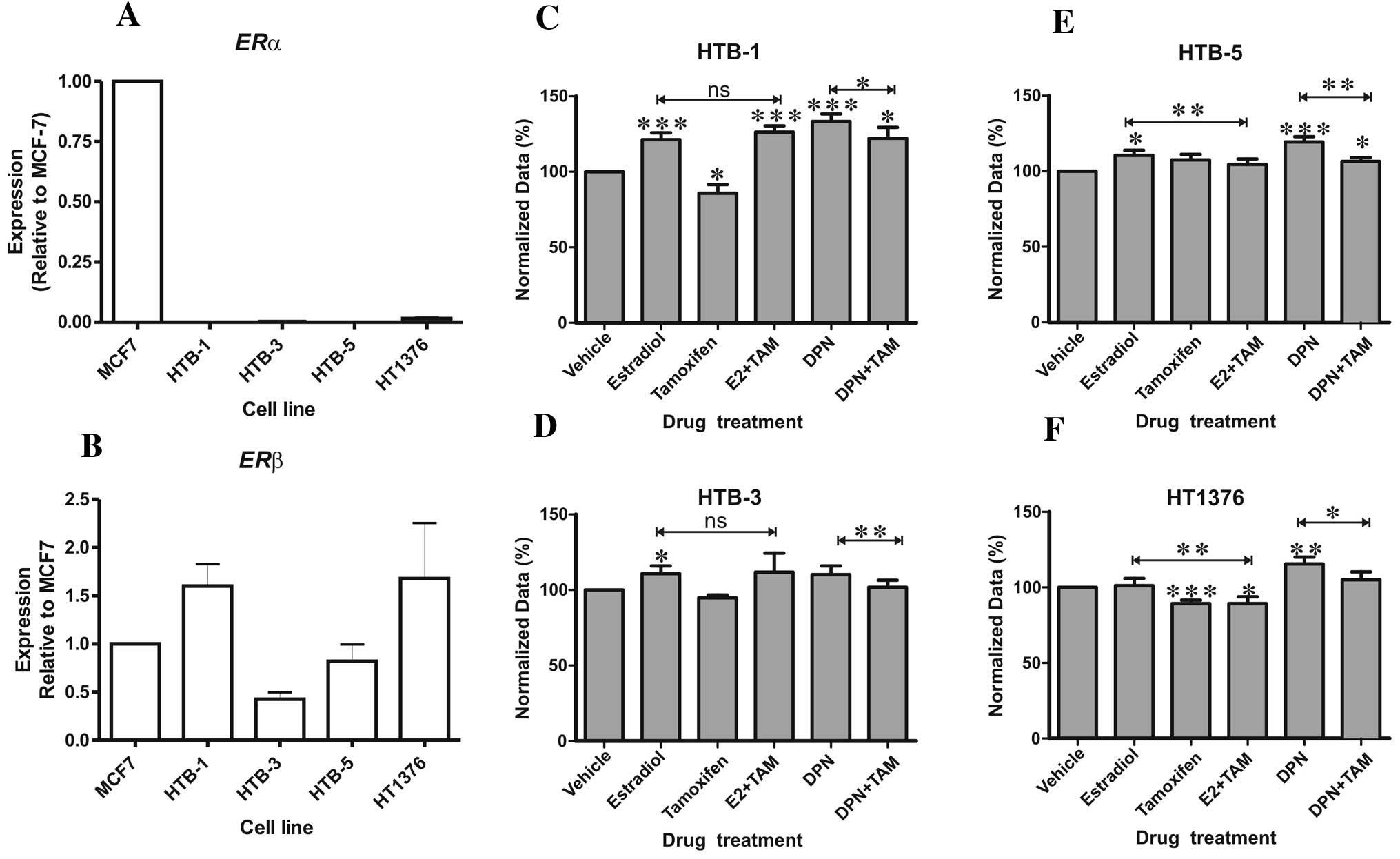
ERα and ERβ mRNA expression was evaluated in the
HTB-1 (J82), HTB-3 (SCaBER), HTB-5 (TCC-SUP) and HT1376 (CRL-1472)
bladder cancer cell lines. All four cell lines expressed
transcripts for ERβ but not ERα as compared to the MCF-7 breast
cancer cell line (Fig. 3A and B).
Treatment with estradiol resulted in a significant increase in
HTB-1, HTB-3 and HTB-5 proliferation, whereas the selective ER
modulator and ERβ antagonist tamoxifen inhibited proliferation of
HTB-1 and HT1376 cells, the two cell lines with the highest ERβ
expression (Fig. 3B). The
ERβ-selective agonist DPN induced a significant increase in
proliferation in all three UCB cell lines tested (Fig. 3C, E and F) relative to the control
treated cells. DPN did not affect proliferation of the squamous
HTB-3 cell line (Fig. 3D), which
exhibited the lowest ERβ expression (Fig. 3B). DPN-induced cellular
proliferation was inhibited by tamoxifen in all cell lines
(Fig. 3). These results indicate
that hormonal activation of ERβ promotes UCB cell proliferation and
that this can be blocked by tamoxifen.
Discussion
Identification of the molecular mechanisms governing
bladder carcinogenesis is critical for the development of novel
pharmacotherapies and biomarkers for this disease. Despite clinical
observations of more advanced UCB and shorter survival among female
patients, in addition to an established role for ERβ in bladder
development and function (14),
controversy remains over the contributions of estrogen signaling to
bladder carcinogenesis (5–13,15–18).
ERβ has been implicated in endocrine-related human malignancies,
notably invasive prostate cancers (19). Previous studies of ERα and ERβ
expression in UCB specimens yielded inconsistent results (22,25,26,29,30)
and have been limited to a subset of available UCB preclinical
models (22,28,29).
We sought to clarify these findings by examining ERβ
in a well-described UCB patient cohort (27) and additional bladder cancer cell
lines. Among the UCB patients, we found no association between ERβ
and tumor stage, but uncovered a novel association between elevated
ERβ and aggressive bladder histology characterized by perineural
invasion and LVI, the latter being a well-established independent
predictor of worse oncologic outcomes. In addition, our findings
demonstrated that elevated ERβ predicts reduced survival among
cystectomy patients with all UCB stages, and provide the first
demonstration that this association is independent of tumor stage.
ERβ expression remained significantly associated with
recurrence-free survival after adjusting for other established
independent predictors of cystectomy patient outcomes, including
lymph node stage and LVI. We thus identified ERβ as an independent
biomarker for poor cystectomy patient outcome, suggesting a model
in which estrogen signaling promotes aggressive UCB histology. We
additionally found an association between LVI and higher ERβ levels
in the adjacent benign urothelium, raising the possibility of
paracrine signaling effects in promoting this aggressive UCB
histology. We also investigated for the first time the association
between ERβ expression and clinical risk factors for UCB diagnosis
and/or cancer-specific death. We identify a significant association
between elevated ERβ and preoperative hydronephrosis and prior
pelvic radiation, both linked to aggressive bladder cancers,
suggesting a potential role for ERβ in these processes. Finally, we
noted lower ERβ levels in patients who had prior intravesical
chemotherapy, consistent with a role of ERβ in promoting cell cycle
progression.
To corroborate our patient findings, we determined
the effect of ERβ activation on the in vitro proliferation
of a panel of bladder cancer cell lines. Shen and colleagues
(22) previously reported increased
proliferation in the RT4 UCB cell line treated with the
non-selective ER agonist estradiol; but higher expression of ERα
than ERβ in this cell line raises the possibility that ERα
activation was responsible. Another study investigating estradiol
in a UCB cell line expressing predominantly ERβ observed no growth
effects (31). Here, we
demonstrated that selective ERβ activation by DPN in the bladder
cancer cells predominantly expressing ERβ increased proliferation
in all cases (Fig. 3C, E and F),
whereas DPN had no positive effect on the proliferation of HTB-3
cells with low ERβ expression (Fig.
3D). These data provide mechanistic evidence that ERβ
activation promotes UCB cell growth, although contrasting with a
recent report from Han et al(29) where ERβ activation reduced UCB
migration and invasion.
To explore ERβ as a therapeutic target, we measured
the growth effects of the selective ER modulator (SERM), tamoxifen,
in UCB cell lines, with or without simultaneous ERβ activation
using DPN. Several prior studies of SERMs have described
conflicting growth inhibitory effects in bladder cancer cell lines
or murine xenografts (21–23,31).
We found that tamoxifen treatment alone significantly inhibited
growth (Fig. 3C and F) of the two
cell lines (HTB-1 and HT1376) with high ERβ expression (Fig. 3B). Tamoxifen also significantly
inhibited the proliferative effects of DPN in all 4 bladder cancer
cell lines (Fig. 3C-F) when the two
treatments were combined, supporting the therapeutic efficacy of
selective ER modulators in bladder cancer.
In conclusion, we demonstrated ERβ upregulation in
UCB compared to benign urothelium, consistent with an oncogenic
function. Furthermore, we uncovered a stage-independent association
between ERβ levels and: i) aggressive UCB histology and ii)
clinical traits carrying a poor prognosis. We provide the first
demonstration of ERβ as an independent predictor of poor cystectomy
patient outcomes after adjustment for tumor stage, lymph node
involvement and LVI. We demonstrated that ERβ activation increases
UCB cell proliferation but can be effectively blocked by SERM
pharmacotherapy. Although the relationship between ERβ and tumor
stage and patient outcomes is disputed (22,25,26),
our research supports a role for ERβ in promoting aggressive
bladder cancer and suggests that pharmacological targeting of
estrogen signaling pathways may be useful in treating this
disease.
Acknowledgements
The authors gratefully acknowledge the institutional
financial support of Weill Cornell Medical College, the University
of Nottingham and an F31 Fellowship from NIH (NIDCR) (K.M.).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar
|
|
2
|
Stein JP, Lieskovsky G, Cote R, et al:
Radical cystectomy in the treatment of invasive bladder cancer:
long-term results in 1,054 patients. J Clin Oncol. 19:666–675.
2001.PubMed/NCBI
|
|
3
|
Messing E: Urothelial tumors of the
bladder. Campbell-Walsh Urology. Wein AJ: 9th edition. Saunders
Elsevier; Philadelphia: pp. 2407–2446. 2007
|
|
4
|
Shariat SF, Karakiewicz PI, Palapattu GS,
et al: Outcomes of radical cystectomy for transitional cell
carcinoma of the bladder: a contemporary series from the Bladder
Cancer Research Consortium. J Urol. 176:2414–2422. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Madeb R and Messing EM: Gender, racial and
age differences in bladder cancer incidence and mortality. Urol
Oncol. 22:86–92. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tilki D, Svatek RS, Karakiewicz PI, et al:
Characteristics and outcomes of patients with pT4 urothelial
carcinoma at radical cystectomy: a retrospective international
study of 583 patients. J Urol. 183:87–93. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
May M, Bastian PJ, Brookman-May S, et al:
Gender-specific differences in cancer-specific survival after
radical cystectomy for patients with urothelial carcinoma of the
urinary bladder in pathologic tumor stage T4a. Urol Oncol. Nov
4–2011.(Epub ahead of print).
|
|
8
|
Palou J, Sylvester RJ, Faba OR, et al:
Female gender and carcinoma in situ in the prostatic urethra are
prognostic factors for recurrence, progression, and
disease-specific mortality in T1G3 bladder cancer patients treated
with bacillus Calmette-Guérin. Eur Urol. 62:118–125. 2012.
|
|
9
|
Scosyrev E, Noyes K, Feng C and Messing E:
Sex and racial differences in bladder cancer presentation and
mortality in the US. Cancer. 115:68–74. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shariat SF, Chromecki TF, Cha EK, et al:
Risk stratification of organ-confined bladder cancer after radical
cystectomy using cell cycle-related biomarkers. J Urol.
187:457–462. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tilki D, Reich O, Svatek RS, et al:
Characteristics and outcomes of patients with clinical carcinoma in
situ only treated with radical cystectomy: an international study
of 243 patients. J Urol. 183:1757–1763. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tilki D, Svatek RS, Novara G, et al: Stage
pT0 at radical cystectomy confers improved survival: an
international study of 4,430 patients. J Urol. 184:888–894. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
van Rhijn BW, van der Kwast TH, Alkhateeb
SS, et al: A new and highly prognostic system to discern T1 bladder
cancer substage. Eur Urol. 61:378–384. 2012.
|
|
14
|
Imamov O, Yakimchuk K, Morani A, et al:
Estrogen receptor beta-deficient female mice develop a bladder
phenotype resembling human interstitial cystitis. Proc Natl Acad
Sci USA. 104:9806–9809. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
May M, Stief C, Brookman-May S, et al:
Gender-dependent cancer-specific survival following radical
cystectomy. World J Urol. 30:707–713. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Davis-Dao CA, Henderson KD,
Sullivan-Halley J, et al: Lower risk in parous women suggests that
hormonal factors are important in bladder cancer etiology. Cancer
Epidemiol Biomarkers Prev. 20:1156–1170. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
McGrath M, Michaud DS and De Vivo I:
Hormonal and reproductive factors and the risk of bladder cancer in
women. Am J Epidemiol. 163:236–244. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Prizment AE, Anderson KE, Harlow BL and
Folsom AR: Reproductive risk factors for incident bladder cancer:
Iowa Women’s Health Study. Int J Cancer. 120:1093–1098. 2007.
|
|
19
|
Thomas C and Gustafsson JA: The different
roles of ER subtypes in cancer biology and therapy. Nat Rev Cancer.
11:597–608. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim HT, Kim BC, Kim IY, et al: Raloxifene,
a mixed estrogen agonist/antagonist, induces apoptosis through
cleavage of BAD in TSU-PR1 human cancer cells. J Biol Chem.
277:32510–32515. 2002. View Article : Google Scholar
|
|
21
|
Pu YS, Hsieh TS, Cheng AL, et al: Combined
cytotoxic effects of tamoxifen and chemotherapeutic agents on
bladder cancer cells: a potential use in intravesical chemotherapy.
Br J Urol. 77:76–85. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shen SS, Smith CL, Hsieh JT, et al:
Expression of estrogen receptors-α and -β in bladder cancer cell
lines and human bladder tumor tissue. Cancer. 106:2610–2616.
2006.
|
|
23
|
Sonpavde G, Okuno N, Weiss H, et al:
Efficacy of selective estrogen receptor modulators in nude mice
bearing human transitional cell carcinoma. Urology. 69:1221–1226.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kontos S, Papatsoris A, Kominea A, et al:
Expression of ERβ and its co-regulators p300 and NCoR in human
transitional cell bladder cancer. Urol Int. 87:151–158. 2011.
|
|
25
|
Kontos S, Kominea A, Melachrinou M,
Balampani E and Sotiropoulou-Bonikou G: Inverse expression of
estrogen receptor-β and nuclear factor-κB in urinary bladder
carcinogenesis. Int J Urol. 17:801–809. 2010.
|
|
26
|
Tuygun C, Kankaya D, Imamoglu A, et al:
Sex-specific hormone receptors in urothelial carcinomas of the
human urinary bladder: a comparative analysis of
clinicopathological features and survival outcomes according to
receptor expression. Urol Oncol. 29:43–51. 2011. View Article : Google Scholar
|
|
27
|
Kauffman EC, Robinson BD, Downes MJ, et
al: Role of androgen receptor and associated lysine-demethylase
coregulators, LSD1 and JMJD2A, in localized and advanced human
bladder cancer. Mol Carcinog. 50:931–944. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Teng J, Wang ZY, Jarrard DF and Bjorling
DE: Roles of estrogen receptor α and β in modulating urothelial
cell proliferation. Endocr Relat Cancer. 15:351–364. 2008.
|
|
29
|
Han B, Cui D, Jing Y, Hong Y and Xia S:
Estrogen receptor β (ERβ ) is a novel prognostic marker of
recurrence survival in non-muscle-invasive bladder cancer
potentially by inhibiting cadherin switch. World J Urol.
30:861–867. 2012.
|
|
30
|
Miyamoto H, Yao JL, Chaux A, et al:
Expression of androgen and oestrogen receptors and its prognostic
significance in urothelial neoplasm of the urinary bladder. BJU
Int. 109:1716–1726. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Waliszewski P, Waliszewska MK, Hemstreet
GP III and Hurst RE: Expression of sex steroid receptor genes and
comodulation with retinoid signaling in normal human uroepithelial
cells and bladder cancer cell lines. Urol Oncol. 3:141–147. 1997.
View Article : Google Scholar : PubMed/NCBI
|