Introduction
Matrix metalloproteinases (MMPs), zinc- and
calcium-dependent metalloproteinases are involved in the
degradation of the various components of the extracellular matrix
(ECM), such as collagen, laminin, fibronectin, vitronectin, elastin
and proteoglycans (1). Among the
previously reported human MMPs, gelatinase-A (MMP-2) and
gelatinase-B (MMP-9) are key enzymes involved in the degradation of
type IV collagen, a major component of the basement membrane
(2,3). Recent studies have reported a positive
correlation between expression of MMP-9 and tumor metastasis in
cervical cancer as well as several types of epithelial cancer
(4–7); thus, inhibition of MMP activity has
been adopted as an anticancer therapeutic strategy.
Withaferin A (Wit A), a bioactive compound isolated
from Withania somnifera, is a cell permeable steroidal
lactone, and exhibits anti-inflammatory and immunomodulatory
activities. Wit A suppresses growth of human cancer cells of the
prostate, breast, and soft tissue sarcoma origin in vitro
and in vivo(8–10). In addition, Wit A is a potent
inhibitor of angiogenesis in vivo via inhibition of
proliferation of HUVECs (11). More
recently, Wit A was reported to trigger apoptosis and to inhibit
cell migration and invasion of breast cancer cells (12).
Wit A inhibits the motility and invasiveness of
carcinoma cells in vitro, yet the inhibitory mechanism(s) of
TGF-β-induced motility and invasiveness as well as the
TGF-β-induced MMP-9 activation of carcinoma cells by Wit A are not
well defined. The aim of this study was to evaluate the inhibitory
effect of Wit A on TGF-β-mediated invasion as well as MMP-9
expression and activity in Caski cells. In addition, we attempted
to determine whether inhibition of these MMPs by treatment with Wit
A is mediated through cellular signaling pathways, such as by
inhibition of the phosphorylation of the Akt pathway in Caski
cells.
Materials and methods
Cells and materials
Human Caski and SK-Hep-1 cells were obtained from
the American Type Culture Collection (Rockville, MD, USA).
Dulbecco’s modified Eagle’s medium (DMEM), supplemented with 2 mM
L-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, and 10%
fetal bovine serum (FBS) was the culture medium used throughout the
experiments. Wit A was purchased from Calbiochem (La Jolla, CA,
USA). Lipofectamine 2000 reagent was obtained from Life
Technologies, Inc. (Rockville, MA, USA). Luciferase assay and
β-galactosidase assay systems were purchased from Promega (Madison,
WI, USA).
Cell viability assay
The 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl
tetrazolium bromide] (MTT) assay was used for determination of the
effect of Wit A on cell viability. Cells were seeded into 96-well
plates at a density of 3×104 cells/100 μl/well. After 24
h of growth, cells were treated with different concentrations of
Wit A ranging from 0.8 to 1.2 μM for 24 h. After the exposure
period, the medium was removed, and the cells were washed with PBS.
The medium was then replaced, followed by incubation with the MTT
solution (Promega) for 4 h. The number of viable cells was directly
proportional to the production of formazan, which was dissolved in
DMSO, followed by spectrophotometric measurement at 563 nm. The
percentage of viable cells was estimated by comparison with that of
the untreated control cells.
Gelatin substrate gel zymography
To determine the effect of Wit A on TGF-β- or
PMA-induced MMP-9 activity, cells were treated with various
concentrations of Wit A in the presence of 50 ng/ml TGF-β or 75 nM
PMA, followed by performance of zymography for evaluation of MMP-9
expression. Zymography was performed using the procedure described
by Overall et al(13) with
minor modifications. The human cell lines were suspended in their
respective medium containing 10% FBS and plated at 8×105
cells. Dishes were incubated until reaching ~80% confluency, and
the medium was aspirated, followed by addition of fresh serum-free
medium to each dish, with or without Wit A. Supernatants were
collected after incubation for 24 h. Supernatants were subjected to
SDS-PAGE in 10% polyacrylamide gels, which were copolymerized with
1 mg/ml of gelatin. After electrophoresis, the gels were washed
several times in 2.5% Triton X-100 for 1 h at room temperature for
removal of SDS, followed by incubation for 24–48 h at 37°C in
buffer containing 5 mM CaCl2 and 1 μM ZnCl2.
The gels were stained with Coomassie blue (0.25%) for 30 min,
followed by destaining for 1 h in a solution of acetic acid and
methanol. The proteolytic activity was evidenced as clear bands
(zones of gelatin degradation) against the blue background of the
stained gelatin.
Plasmids, transfection, and luciferase
gene assays
The MMP-9 reporter construct was kindly provided by
Dr T.K. Kwon (Keimyung University, School of Medicine, Korea). In
brief, cells were plated onto 6-well plates at a density of
5×105 cells/well and grown overnight. Cells were
transfected with 2 μg of the MMP-9 promoter construct and 1 μg of
the pCMV-β-galactosidase plasmid for 5 h using the Lipofectamine
2000 method. After transfection, cells were cultured in 10% FBS
medium with vehicle (DMSO) or drugs for 24 h. Luciferase and
β-galactosidase activities were assayed according to the
manufacturer’s protocol (Promega). Luciferase activity was
normalized for β-galactosidase activity in cell lysate and
expressed as an average of three independent experiments.
RNA isolation and RT-PCR
To determine whether the reduced amounts of MMP-9
activity were a result of decreased levels of mRNA encoding this
collagenase, we compared the levels of MMP-9 in several types of
cancer cells treated with or without various concentrations of Wit
A in the presence of TGF-β or PMA. RT-PCR was performed for
determination of MMP-9 mRNA expression. Total cellular RNA was
extracted from Caski cells using TRIzol reagent (Life Technologies,
Inc.). cDNA was synthesized from 2 μg of total RNA using Moloney
murine leukemia virus reverse transcriptase (Life Technologies,
Inc.). The sequences of the sense and antisense primers for MMP-9
were 5′-CACTGTCCACCCCTCAGA GC-3′ and 5′-GCCACTTGTCGGCGATAAGG-3′,
respectively. For analysis of PCR products, agarose gel
electrophoresis was performed, followed by visualization using
ethidium bromide.
Invasion assay
Each invasion assay was performed using
5×104 cells/chamber. Invasion assays were performed
using modified Boyden chambers with a polycarbonate nucleopore
membrane (Corning, Corning, NY, USA). Precoated filters (6.5-mm in
diameter, 8-μm pore-size, Matrigel 100 μg/cm2) were
rehydrated with 250 μl of medium, and 5×104 cells in 200
μl medium with or without Wit A in the presence of TGF-β or PMA
were seeded into the upper part of each chamber. After incubation
for 24 h at 37°C, nonmigratory cells on the upper surface of the
filter were wiped with a cotton swab and cells that had migrated to
the lower surface of the filter were fixed and stained with 0.125%
Coomassie blue in a methanol:acetic acid:water mixture (45:10:45,
v/v/v). Random fields were counted under a light microscope.
Results
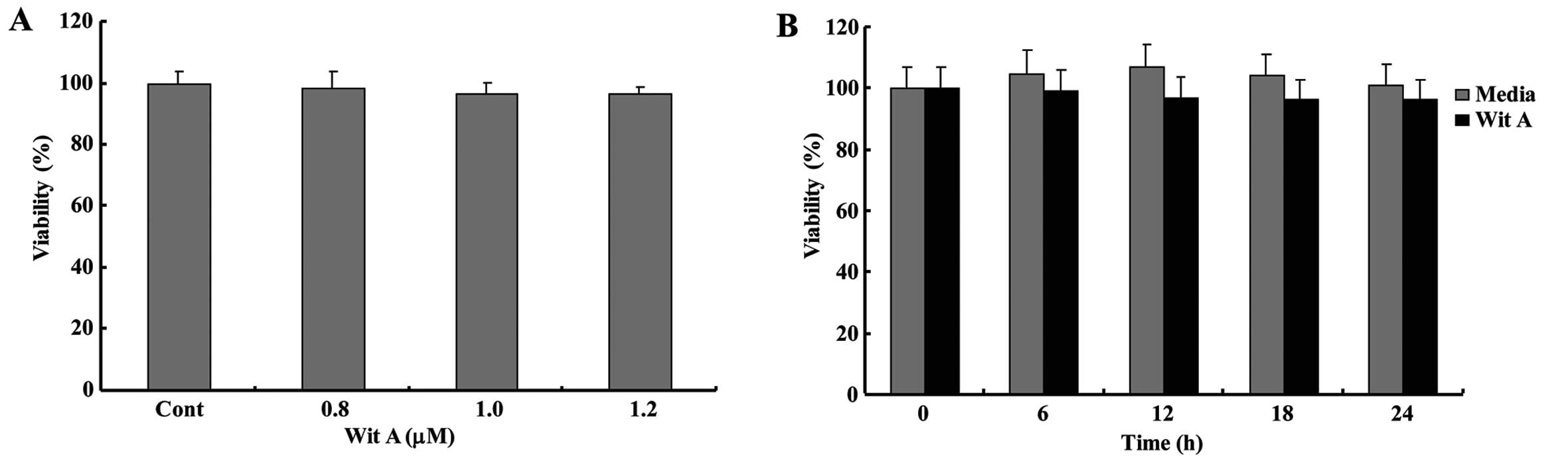
Effect of Wit A on the cell viability of
Caski cells
We assessed the cell viability of human cervical
cancer Caski cells following treatment with various concentrations
(0.8–1.2 μM) of Wit A. Compared with the controls, the cell
viability in Caski cells was not markedly altered following
treatment with Wit A, even at a concentration up to 1.2 μM
(Fig. 1). As shown in Fig. 1B, Wit A did not markedly influence
cell viability in Caski cells from 6 to 24 h following treatment
with 1.2 μM Wit A. Therefore, it was clear that treatment of Caski
cells with Wit A for 24 h, at concentrations ranging from 0.8 to
1.2 μM, has no cytotoxic effects.
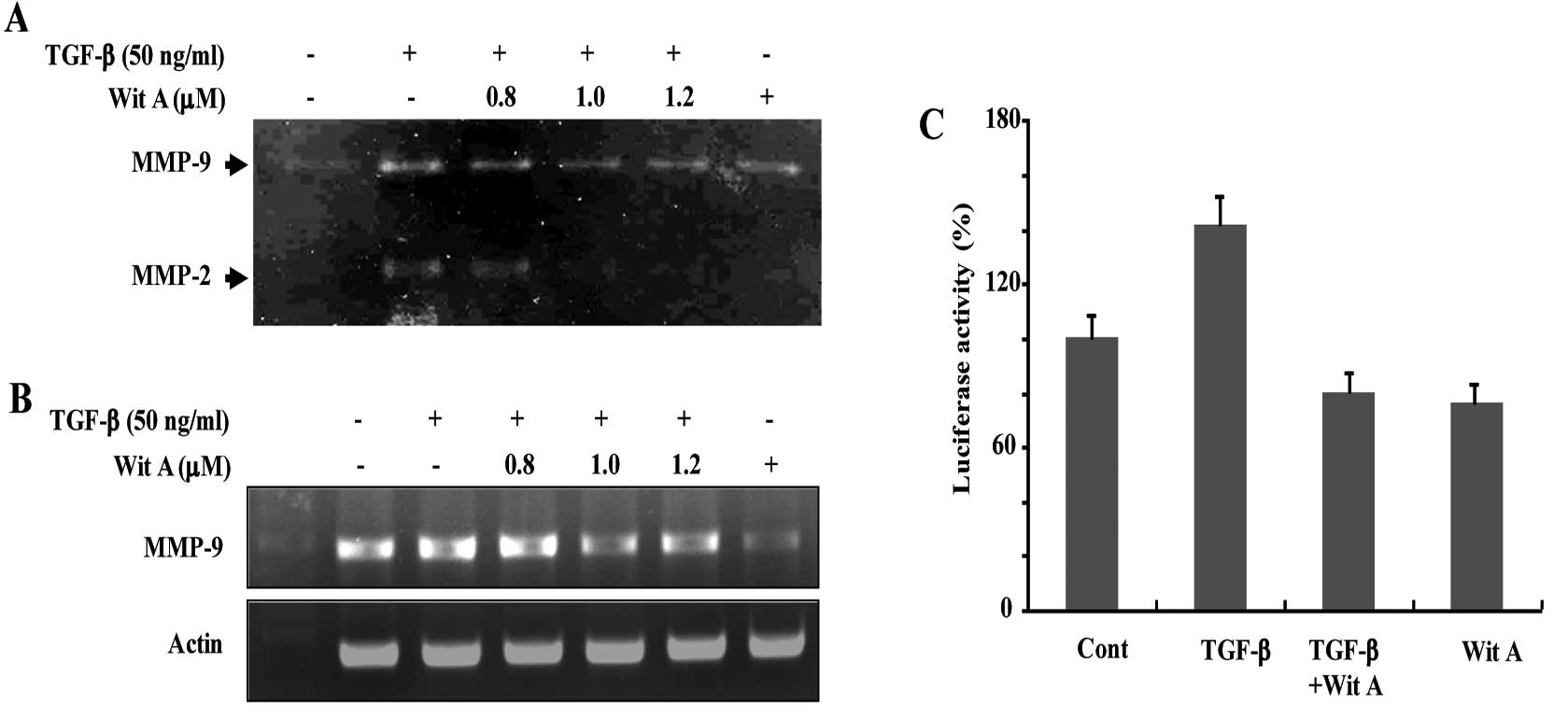
Effect of Wit A on MMP-9 activity
Caski cells, which release basal levels of MMP-9
when cultured in serum-free medium, were treated with TGF-β for 24
h. Although the level of MMP-2 expression was not significantly
altered by TGF-β, TGF-β induced the expression and secretion of
large amounts of latent MMP-9, as determined by gelatin zymography
(Fig. 2A). As shown in Fig. 2A, treatment with Wit A resulted in
decreased TGF-β-induced MMP-2 and MMP-9 activities in a
dose-dependent manner.
Suppression of TGF-β-induced MMP-9
transcription by Wit A
Treatment of Caski cells with Wit A induced a
decrease in the levels of TGF-β-stimulated MMP-9 mRNA (Fig. 2B). Results of RT-PCR showed that
steady-state levels of MMP-9 mRNA were lower in Wit A-treated
cells, compared with non-treated cells. The effect of treatment
with Wit A on expression of MMP-9 was additionally investigated
using Caski cells transiently transfected with a luciferase
reporter gene linked to the 0.7-kb fragment of the MMP-9 promoter
sequence. Activation of luciferase gene expression up to 1.6-fold
was observed in cells treated with TGF-β, compared with untreated
cells. Treatment of cells with Wit A (1.2 μM) resulted in a
decrease in TGF-β-mediated luciferase activity (Fig. 2C).
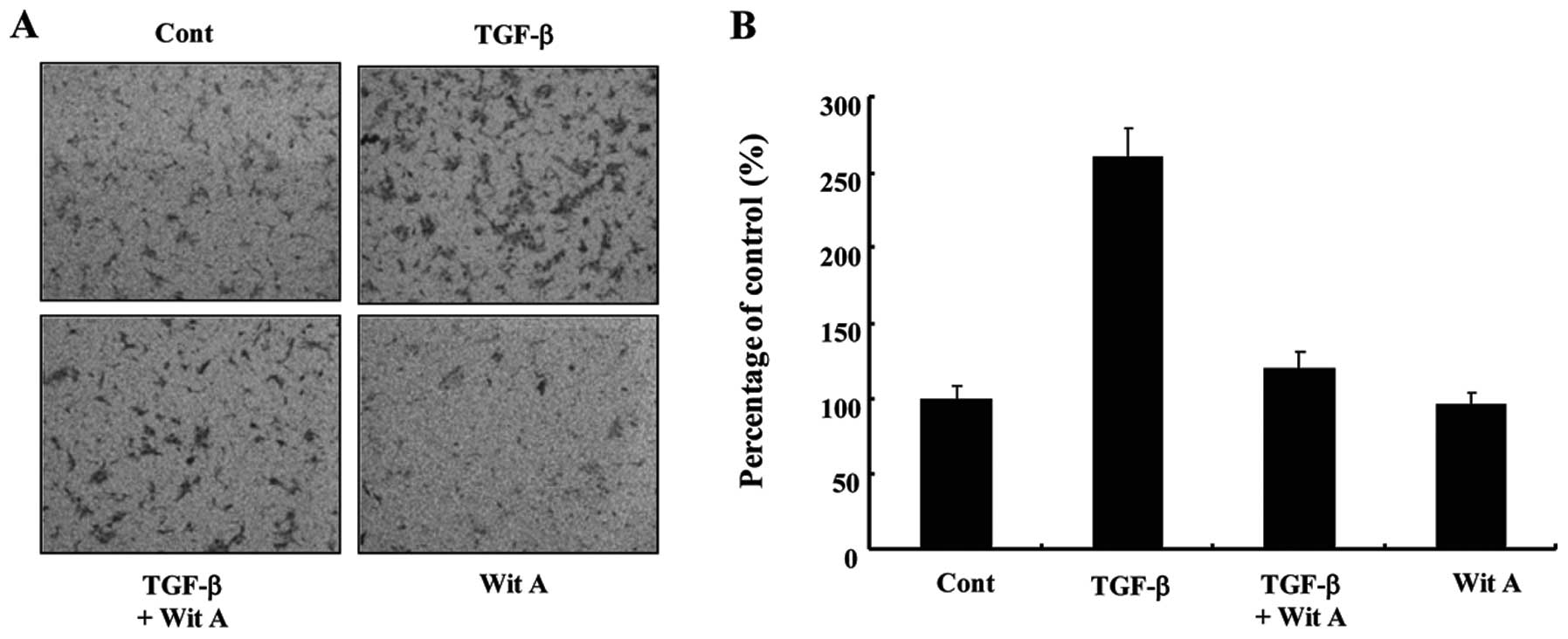
Effects of Wit A on migration of Caski
cells
We next examined the effect of Wit A on migration of
Caski cells using the cell invasion assay. As shown in Fig. 3, treatment with 1.2 μM Wit A
resulted in a significant reduction in TGF-β-induced invasiveness
of these cells by ~44%, compared with the TGF-β-treated cells,
although reduced invasion was still observed in the presence of Wit
A. Therefore, the effect of Wit A on in vitro invasion
inhibition showed a correlation with its effect on MMP-9
inhibition.
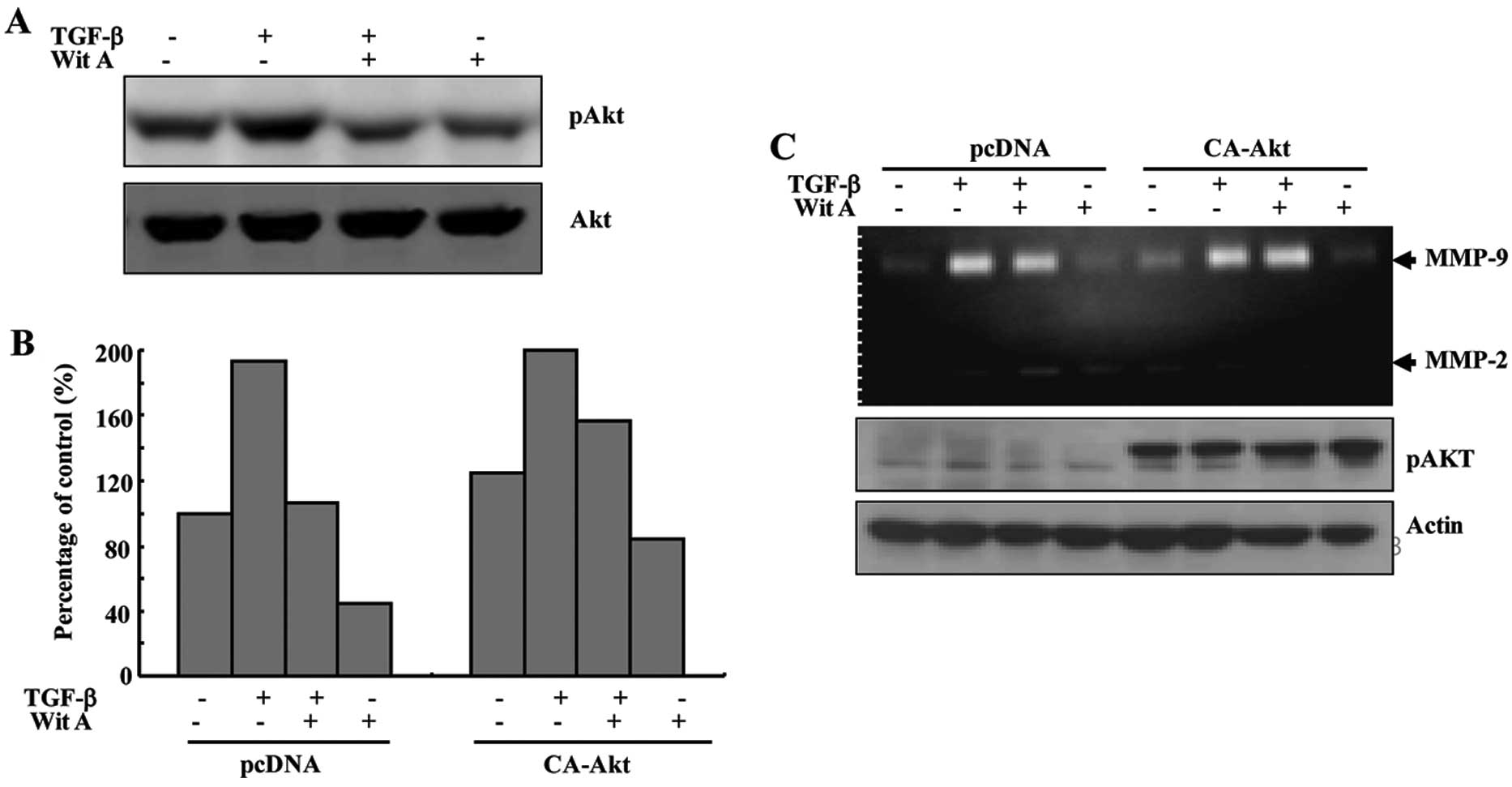
Wit A inhibits TGF-β-induced secretion of
MMP-9 and phosphorylation of Akt in Caski cells
Treatment of Caski cells with TGF-β also resulted in
an increase in the levels of phosphorylation of Akt, compared with
the control, which was markedly attenuated by treatment with Wit A.
In order to determine whether the decrease in TGF-β-induced
invasiveness of the cells following treatment with Wit A was
related to the Akt signaling pathway, Caski cells were transiently
transfected with an empty vector and constitutively active (CA)-Akt
expression vector. As shown in Fig.
4B, following exposure of Wit A, TGF-β-treated Caski cells
transfected with the empty vector exhibted a decrease in cell
invasion, which was slightly blocked by ectopic expression of
CA-Akt. For determination of whether the decrease in TGF-β-induced
MMP-9 secretion by Wit A was related to the Akt signaling pathway,
Caski cells were transiently transfected with an empty vector and
CA-Akt expression vector. Treatment of TGF-β-treated Caski cells
transfected with the empty vector led to a decrease in MMP-9
secretion, which was slightly blocked by ectopic expression of
CA-Akt (Fig. 4C). This result
indicated that the decrease in TGF-β-induced MMP-9 secretion by Wit
A was related to inhibition of the Akt signaling pathway in Caski
cells. Taken together, treatment with Wit A resulted in decreased
inhibition of TGF-β-induced secretion of MMP-9 and TGF-β-stimulated
MMP-9 secretion via blockade of the Akt signaling pathway in Caski
cells.
Effect of Wit A on MMP-9 activity and
expression
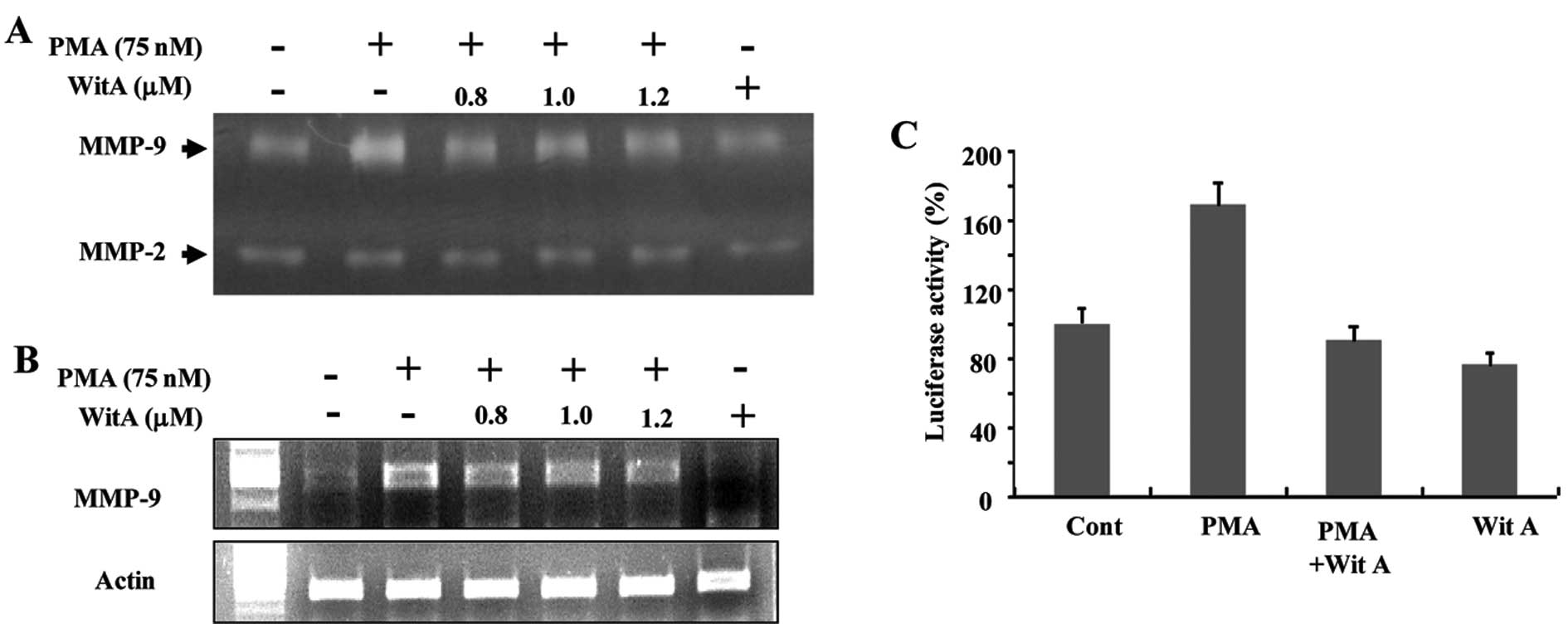
Caski cells were treated with 75 nM PMA for 24 h.
PMA induced expression and secretion of large amounts of latent
MMP-9, as determined by gelatin zymography (Fig. 5A). As shown in Fig. 5A, treatment with Wit A resulted in a
decrease in PMA-induced MMP-9 activity in a dose-dependent manner.
Treatment of Caski cells with Wit A also induced a decrease in the
levels of PMA-stimulated MMP-9 mRNA (Fig. 5B). The effect of Wit A on MMP-9
expression was additionally investigated using Caski cells
transiently transfected with an MMP-9 promoter plasmid. As shown in
Fig. 5C, activation of luciferase
gene expression up to 1.8-fold was observed in cells treated with
PMA, when compared with the untreated cells. Treatment of cells
with Wit A (1.2 μM) resulted in a decrease in PMA-mediated
luciferase activity.
Wit A inhibits PMA-induced MMP-9
expression in a metastatic cancer cell line
We further attempted to determine whether Wit A
attenuates PMA-induced MMP-9 expression and activity in another
human metastatic cancer cell line, SK-Hep1. As shown in Fig. 6A, treatment with Wit A resulted in a
decrease in PMA-induced MMP-9 activity in SK-Hep1 cells in a
dose-dependent manner. In addition, treatment of SK-Hep1 cells with
Wit A also induced a decrease in the levels of PMA-stimulated MMP-9
mRNA or luciferase activity in a dose-dependent manner (Fig. 6 and B).
Discussion
Tumor invasion and metastasis involve a complex
multi-step process, and are the leading causes of death of cancer
patients. Therefore, inhibition of invasion and metastasis by
chemopreventive agents has been suggested as a potential
therapeutic strategy for the treatment of cancers (14,15).
MMPs play a major role in the promotion of metastasis in cancer
development. Currently, there are <20 known human MMPs; MMP-2
and -9 have been studied extensively due to their key roles in
degradation of type IV collagen and gelatin, as well as the strong
association between MMP-2 and -9 in human lung and cervical cancers
(16,17). Therefore, an efficient therapeutic
molecule that efficiently inhibits expression or activity of MMP-9
could interfere with the invasiveness of cancer cells, and, thus,
provide a therapeutic target against human metastatic cancer.
The usefulness of low-molecular-weight Wit A for the
prevention of tumor growth has been reported in many different
types of human cancers (8–10). However, the precise actions of Wit A
in invasion and migration of human cervical cancer cells and the
associated signaling pathways have not been reported. In this
study, we found that treatment with Wit A resulted in suppression
of TGF-β-enhanced MMP-9 secretion and mRNA expression, and
invasiveness in cervical cancer cells.
Accumulating evidence has indicated close
involvement of the Akt pathway in angiogenesis via activation of
proliferation (18,19). In addition, activation of MMP-9 can
occur via PI3K-AKT signaling pathways in ovarian cancer cells
(20). In the present study, we
found that pre-treatment with Wit A resulted in markedly inhibited
TGF-β-triggered activation of Akt in human cervical cancer cells.
In addition, introduction with CA-Akt resulted in a partial
increase in the secretion of TGF-β-induced MMP-9 attenuated by
treatment of Caski cells with Wit A. These results indicate that
treatment with Wit A resulted in inhibition of the invasive and
migratory abilities of Caski cells by reducing MMP-9 expression via
suppression of the Akt signaling pathway. Further extensive study
of the role of Akt activation in TGF-β-induced MMP-9 expression is
warranted.
We also demonstrated the inhibition of TGF-β-induced
MMP-9 mRNA expression and its activity by treatment with Wit A in
SK-Hep1 cells, suggesting the possibility that Wit A may commonly
regulate MMP-9 induction by TGF-β at the transcriptional level in
different types of cancer cells. In addition, treatment with Wit A
also inhibited PMA-induced MMP-9 mRNA expression and its activity
in Caski cells. Thus, these studies indicate MMP-9 as a potential
target molecule for the anti-invasive and anti-migratory activities
of Wit A in cancer cells.
In conclusion, we showed that Wit A inhibited
TGF-β-induced invasion and metastasis through a reduction in
expression and secretion of MMP-9 through the Akt pathway in Caski
human cervical cancer cells. In addition, the inhibitory effect on
MMP-9 induction by treatment with Wit A was also observed in human
hepatoma SK-Hep1 cells. Overall, these findings demonstrate the
potential of Wit A as a powerful candidate for use in the
development of chemopreventive agents for cancer metastasis.
Acknowledgements
This research was supported by the Basic Science
Research Program through the National Research Foundation of Korea
(NRF) funded by the Korean government (MEST) (no. 2010-0011228) and
by the National Research Foundation of Korea (NRF) grant funded by
the Korean government (MEST) (2012-0000286).
References
|
1
|
Westermarck J and Kähäri VM: Regulation of
matrix metalloproteinase expression in tumor invasion. FASEB J.
13:781–792. 1999.PubMed/NCBI
|
|
2
|
Johansson N, Ahonen M and Kähäri VM:
Matrix metalloproteinases in tumor invasion. Cell Mol Life Sci.
57:5–15. 2000. View Article : Google Scholar
|
|
3
|
Murphy G and Nagase H: Progress in matrix
metalloproteinase research. Mol Aspects Med. 29:290–308. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yu W, Liu J, Xiong X, Ai Y and Wang H:
Expression of MMP9 and CD147 in invasive squamous cell carcinoma of
the uterine cervix and their implication. Pathol Res Pract.
205:709–715. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Piao S, Zhao S, Guo F, et al: Increased
expression of CD147 and MMP-9 is correlated with poor prognosis of
salivary duct carcinoma. J Cancer Res Clin Oncol. 138:627–635.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Libra M, Scalisi A, Vella N, et al:
Uterine cervical carcinoma: role of matrix metalloproteinases
(Review). Int J Oncol. 34:897–903. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sakata K, Shigemasa K, Nagai N and Ohama
K: Expression of matrix metalloproteinases (MMP-2, MMP-9, MT1-MMP)
and their inhibitors (TIMP-1, TIMP-2) in common epithelial tumors
of the ovary. Int J Oncol. 17:673–681. 2000.PubMed/NCBI
|
|
8
|
Srinivasan S, Ranga RS, Burikhanov R, Han
SS and Chendil D: Par-4-dependent apoptosis by the dietary compound
withaferin A in prostate cancer cells. Cancer Res. 67:246–253.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hahm ER, Moura MB, Kelley EE, Van Houten
B, Shiva S and Singh SV: Withaferin A-induced apoptosis in human
breast cancer cells is mediated by reactive oxygen species. PLoS
One. 6:e233542011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang X, Samadi AK, Roby KF, Timmermann B
and Cohen MS: Inhibition of cell growth and induction of apoptosis
in ovarian carcinoma cell lines CaOV3 and SKOV3 by natural
withanolide Withaferin A. Gynecol Oncol. 124:606–612. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mohan R, Hammers HJ, Bargagna-Mohan P, et
al: Withaferin A is a potent inhibitor of angiogenesis.
Angiogenesis. 7:115–122. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee J, Hahm ER and Singh SV: Withaferin A
inhibits activation of signal transducer and activator of
transcription 3 in human breast cancer cells. Carcinogenesis.
31:1991–1998. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Overall CM, Wrana JL and Sodek J:
Independent regulation of collagenase, 72-kDa progelatinase, and
metalloendoproteinase inhibitor expression in human fibroblasts by
transforming growth factor-beta. J Biol Chem. 264:1860–1869.
1989.PubMed/NCBI
|
|
14
|
Kohn EC and Liotta LA: Molecular insights
into cancer invasion: strategies for prevention and intervention.
Cancer Res. 55:1856–1862. 1995.PubMed/NCBI
|
|
15
|
Sporn MB: The war on cancer. Lancet.
347:1377–1381. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ylisirniö S, Höyhtyä M and
Turpeenniemi-Hujanen T: Serum matrix metalloproteinases -2, -9 and
tissue inhibitors of metalloproteinases -1, -2 in lung cancer -
TIMP-1 as a prognostic marker. Anticancer Res. 20:1311–1316.
2000.PubMed/NCBI
|
|
17
|
Li Y, Wu T, Zhang B, Yao Y and Yin G:
Matrix metalloproteinase-9 is a prognostic marker for patients with
cervical cancer. Med Oncol. 29:3394–3399. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fang J, Ding M, Yang L, Liu LZ and Jiang
BH: PI3K/PTEN/AKT signaling regulates prostate tumor angiogenesis.
Cell Signal. 19:2487–2497. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cai WJ, Wang MJ, Moore PK, Jin HM, Yao T
and Zhu YC: The novel proangiogenic effect of hydrogen sulfide is
dependent on Akt phosphorylation. Cardiovasc Res. 76:29–40. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Thant AA, Nawa A, Kikkawa F, et al:
Fibronectin activates matrix metalloproteinase-9 secretion via the
MEK1-MAPK and the PI3K-Akt pathways in ovarian cancer cells. Clin
Exp Metastasis. 18:423–428. 2000. View Article : Google Scholar
|