Introduction
Pancreatic cancer is the fourth leading cause of
cancer-related mortality and patients suffer a poor prognosis with
a total 5-year survival rate <5% among all major types of cancer
(1). Surgical resection is the only
curative treatment for pancreatic cancer; however, extensive local
invasion and early metastasis to regional lymph nodes or other
sites in pancreatic cancer lead to unresectable diseases.
Therefore, molecules which are involved in tumor metastasis are
critical for staging and may be unique therapeutic targets in
pancreatic cancer.
miRNAs are small non-coding RNAs, approximately
20–25 nucleotides in length. They lead to inhibition of translation
or direct degradation of their target mRNAs by binding to the 3′UTR
regions of these mRNAs, which participate in various biological
processes, such as proliferation, apoptosis and cell
differentiation. miRNAs also play a role in the progression of
tumor and are termed as oncomiR or anti-oncomiR. In pancreatic
cancer, investigators have found some miRNAs, including miR-96,
miR-217, let-7 and miR-34 (2–5), that
serve as tumor suppressors, and another class of miRNAs, including
miR-21, miR-155 and miR-200 family members (6–9), that
promote tumor progression.
Previous studies have reported their miRNA profiling
results (10–12) and the majority are involved in tumor
growth, apoptosis, invasion and chemotherapy resistance (9,13–18);
however, miRNAs related to lymphatic metastasis of pancreatic
cancer have yet to be fully clarified. According to the miRNA array
profiling of both lymphatic metastasis cell lines and clinical
tissue samples, we investigated the gene related to lymphatic
metastasis of pancreatic cancer and explored its target genes.
Materials and methods
Cell lines
The human pancreatic cancer cell lines BxPC-3,
SW1990 and Panc-1 were obtained from the Cell Library of the
Chinese Academy of Sciences. BxPC-3-LN was a human pancreatic cell
line with relative pure nature of lymphatic metastasis, which was
derived from a metastatic lymph node of an ectopically implanted
tumor model described in our previous studies (19,20).
Briefly, we implanted the parental cell line BxPC-3 (106
cells) in the foot pads of nude mice (BALB/C) and harvested the
metastatic lymph nodes, followed by primary culturing of the tumor
cells. We repeated the procedure and purified the nature of the
lymphatic metastasis of the cell line BxPC-3 and found BxPC-3-LN.
BxPC-3 and BxPC-3-LN were cultured in RPMI-1640 medium (Thermo
Fisher Scientific, Shanghai, China) supplemented with 10% fetal
bovine serum (FBS; Gibco, Grand Island, NY, USA). SW1990 and Panc-1
were cultured in Leibovitz's L-15 medium (Gibco) and DMEM medium
(Thermo Fisher Scientific) supplemented with 10% FBS, respectively.
All cell lines were maintained at 37°C with 5% CO2.
Clinical tissue samples
Patients with pancreatic tumors who received
curative resections of tumors at the Department of Pancreatic
Surgery, Huashan Hospital, between 2009 and 2011, were enrolled in
the present study. For microarray analysis, PCR and western
blotting, clinical tissue samples from the resected pancreatic
tumors were snap frozen in liquid nitrogen and stored at −80°C
until the extractions of RNA and protein. Sections adjacent to the
fresh frozen tissue blocks were used to confirm the diagnosis of
pancreatic ductal adenocarcinoma (PDAC) by two experienced
pathologists. A total of 98 fresh frozen tissue samples from 61
patients consisted of 6 PDAC samples with matched adjacent benign
tissues (MAT), 51 PDAC samples and 35 unmatched adjacent benign
tissue samples. For in situ hybridization, paraffin-embedded
tissue samples included 10 PDACs, 3 intraductal papillary mucinous
neoplasms (IPMNs), 4 metastatic lymph nodes and 13 MATs from 13
patients. All procedures were performed with the written informed
consent of patients and in accordance with the policies and
practices of the Institution's Ethics Committee. Tumor stage was
evaluated according to the American Joint Committee on Cancer
(AJCC) Cancer Staging, 6th edition. Clinical data of the patients
were collected.
miRNA microarray analysis
miRNAs were extracted from cells and tissues using
the mirVana™ miRNA Isolation kit (Applied Biosystems, p/n AM1556)
following the manufacturer's instructions. The quality control
criteria of RNA were RIN≥6.0, 28S/18S>0.7 and A260/A280>1.8
(Agilent 2100 Bioanalyzer). Human miRNA microarray (Agilent,
version 14.0 and illumina miRNA array) was used to examine the
differential expression profile of miRNAs in groups BxPC-3 vs.
BxPC-3-LN and PDAC vs. MAT (6 paired fresh frozen tissue samples).
Briefly, the RNA sample was dephosphorylated with calf intestinal
phosphatase, degenerated with DMSO, labeled with T4 RNA ligase and
cyanine-3-pCp, hybridized at 55°C using Microarray Hybridization
Chamber and Hybridization oven, washed and scanned.
Array-comparative genomic hybridization (array-CGH) analysis was
carried out and the differential extents of miRNAs between groups
were denoted as Diff Pval, Diff Score or fold-change.
RT-PCR
The expression levels of miRNA and mRNAs were
quantitatively analyzed by RT-PCR in cells and fresh frozen tissue
samples. miRNAs and mRNAs were extracted from cells and tissues
using TRIzol (Invitrogen, Carlsbad, CA, USA). The purity and
concentration of RNA were evaluated by A260/A280 using NanoDrop
ND-1000 Spectrophotometer (Thermo Fisher Scientific, Courtaboeuf,
France). miRNAs and mRNAs were converted to cDNAs using the
TaqMan® MicroRNA Reverse Transcription kit (Applied
Biosystems, Foster City, CA, USA) and the PrimeScript RT Master Mix
Kit (Takara, Dalian, China) respectively, by Mastercycler
(Eppendorf, USA). The real-time PCR was performed by Applied
Biosystems 7500 Real-Time PCR System, using TaqMan MicroRNA assays
(Applied Biosystems) for miRNAs and SYBR Premix Ex Taq II (Takara)
for mRNAs, respectively, following the manufacturer's instruction.
U6 snRNA and GAPDH were selected for normalization to the
expression levels of miRNAs and mRNAs. Relative quantitations (RQs)
of miRNAs and mRNAs were calculated using the 2−ΔΔCt
method. The sequences of RT-PCR primers are listed in Table I.
 | Table IPrimer sequences for RT-PCR. |
Table I
Primer sequences for RT-PCR.
| Gene | Sequence
(5′→3′) |
|---|
| BIRC5 | F:
ACTTGGCCCAGTGTTTCTTCT
R: TCTTGGCTCTTTCTCTGTCCA |
| EGFR | F:
AGCCTCCAGAGGATGTTCAA
R: GGAATTCGCTCCACTGTGTT |
| PAXILLIN | F:
ACGTCTACAGCTTCCCCAACAA
R: AGCAGGCGGTCGAGTTCA |
| CETN2 | F:
GGCTTTGAACCCAAGAAAGA
R: TTTGAACGAAATCTTCCCAGTT |
| LAMB3 | F:
GGCCTGCTATCCACCTGTT
R: GCCACATTCTCTACTCGGTGA |
| HOXA1 | F:
AGCCACCAAGAAGCCTGTC
R: TCCTTCTCCAGTTCCGTGAG |
| LEF1 | F:
GATCAGTCATCCCGAAGAGG
R: GTGTTCTCTGGCCTTGTCGT |
| ROBO-1 | F:
GCGTGCAGTACTAAGGGAACA
R: GGCTTCTTACATGAACATAATGAA |
In situ hybridization (ISH)
Double-DIG labeled locked nucleic acid microRNA
probe, complementary to hsa-miR-218 (P/N AM17100; Exiqon, Vedbaek,
Denmark) was designed for ISH of 13 paired paraffin-embedded PDAC
and MAT samples, conforming to the manufacturer's instructions. The
scrambled microRNA probe was used as negative control. In addition,
4-μm tissue sections were deparaffinized in xylene and ethanol,
incubated with Proteinase-K (15 μg/ml, 10 min at 37°C), washed
twice in PBS, dehydrated in ethanol, hybridized (1 h at 49°C) with
hybridization mix (1:500 dilution of probe), washed in standard
saline citrate buffer, and blocked. Subsequently, staining was
developed with sheep anti-digoxin-horseradish peroxidase antibody
and visualized with diaminobenzidine followed by hematoxylin
counter staining of nuclear. The results were evaluated
independently by two experienced pathologists. In brief, 10 HPFs of
each section were chosen randomly. Staining intensity was ranked as
negative staining (0), weak staining (1+) and strong staining (2+).
The mean positive-staining cells (PSCs) were scored as 0 (PSC
<1%), 1 (PSC 1–25%), 2 (PSC 25–50%), 3 (PSC >50%).
Target genes of miRNA
Eight genes (BIRC5, EGFR, PAXILLIN, CETN2, LAMB3,
HOXA1, LEF1 and ROBO-1) which were predicted by biological
databases (www.targetscan.org and
pictar.mdc-berlin.de) and reported to be the target genes of
miRNA-218 in various tumors in the literature, were validated in
pancreatic cancer. The transcriptional levels of theses target
genes were investigated in PDAC and adjacent benign tissue by
RT-PCR.
Western blotting
Protein from fresh frozen tissue samples was
extracted and quantified. Western blotting was performed according
to the standard procedure. The primary antibody, anti-Robo-1
(rabbit polyclonal antibody, sc-25672) was purchased from Santa
Cruz Biotechnology (Santa Cruz, CA, USA). GAPDH (rabbit monoclonal
antibody, 2251-1) was purchased from Epitomics (Burlingame, CA,
USA) and used for control. Chemiluminescent substrate (34079;
Thermo Fisher Scientific, Rockford, IL, USA) was used for detection
of immunoblots. The images were developed by Fujifilm Las-3000.
Statistical analysis
Measurement variable was summed as means ± SD and
compared by the Student's t-tests, unless otherwise indicated. SPSS
16.0 software was employed for statistical analysis and P<0.05
was considered to indicate a statistically significant
difference.
Results
Differential expression of miRNAs in
array-CGH analysis
Profiling analysis of group (PDAC and MAT) was
performed to examine the differential miRNAs in pancreatic cancer,
which may be involved in several important processes of tumor
biology. Seventy miRNAs were found differentially expressed
(P<0.05 and Diff Score >13 or <13), including 43
downregulated miRNAs and 27 upregulated miRNAs in pancreatic cancer
compared to adjacent benign tissue. Differential expression of
miRNAs between the cell lines BxPC-3 and BxPC-3-LN were also
characterized by array-CGH, which may include genes responsible for
lymphatic metastasis of pancreatic cancer. Sixty-three miRNAs
differentially expressed in BxPC-3-LN compared to BxPC-3, including
33 downregulated miRNAs and 30 upregulated miRNAs. Combined
analysis of tissue-based profiling and cell-based profiling results
revealed 4 co-differentially expressed miRNAs (Fig. 1). Among these co-differentially
expressed miRNAs, miRNA-218 was the most notable gene (fold-change
>10) in BxPC-3-LN.
Downregulation of miRNA-218 in pancreatic
cancer
Quantitative real-time PCR was performed in 37 PDAC
samples, 35 adjacent benign tissue samples and cell lines to
confirm the expression level of miRNA. miRNA-218 was found
downregulated in pancreatic cancer and the cell line BxPC-3-LN
(Fig. 2). In tissue samples, the RQ
of miRNA-218 was significantly lower (P=0.001) in pancreatic cancer
(0.0684±0.0339) than in benign tissues (0.2055±.0208). In cell
lines, the RQ of miRNA-218 was 1.0005±0.0429 in BxPC-3-LN and
1.4961±0.0362 in BxPC-3 (P<0.001). Furthermore, the cell lines
SW1990 and Panc-1 showed higher RQ of miRNA-218 (26.9468±2.1649 and
9.8628±0.4647, respectively) compared to BxPC-3-LN5
(P<0.001).
Expression of miRNA-218 and
clinicopathological characteristics
The relationship between the expression levels of
miRNA-218 and clinicopathological data of PDAC patients was
analyzed in a total of 37 PDAC cases (Table II). The differentiation level was
underdetermined in 1 case and 7 cases were without definite status
of lymph nodes. The young patients (age ≤64 years) showed a
relatively higher expression level of miRNA-218 (P=0.002) than the
older patients (age >64 years). Tumor stage tended to correlate
with miRNA-218 expression (P>0.05). The group with lymph node
metastasis showed lower expression level of miRNA-218 than the
group without (P=0.003). No significant differences in the
expression levels of miRNA-218 were found grouped by the factors of
gender, differentiation grade, tumor size, vascular involvement
status, perineural invasion status and neoadjuvant
chemotherapy.
 | Table IIRelationship between
clinicopathological characteristics and miRNA-218 expression in
PDAC. |
Table II
Relationship between
clinicopathological characteristics and miRNA-218 expression in
PDAC.
| Clinicopathological
characteristics | No. of patients
(37) | RQ of
miRNA-218 | P-value |
|---|
| Gender |
| Male | 26 | 0.0747±0.0552 | 0.207a |
| Female | 11 | 0.0533±0.0495 | |
| Age (years) |
| >64 | 17 | 0.0414±0.0290 | 0.002a,b |
| ≤64 | 20 | 0.0912±0.0599 | |
| Stage |
| I | 11 | 0.0949±0.0691 | 0.348c |
| IIA | 6 | 0.0456±0.0246 | |
| IIB | 19 | 0.0607±0.0480 | |
| III | 1 | 0.0585 | |
| IV | 0 | | |
| Differentiation
grade |
| I–II | 20 | 0.0613±0.0526 | 0.610a |
| III | 16 | 0.0692±0.0481 | |
| Size (cm) |
| ≤3 | 21 | 0.0684±0.0487 | 0.602a |
| >3 | 16 | 0.0683±0.0616 | |
| Lymph node
metastasis |
| Positive | 16 (20) | 0.0398±0.0154 | 0.003a,b |
| Negative | 14 (17) | 0.0912±0.0589 | |
| Vascular
involvement |
| Positive | 9 | 0.0527±0.0468 | 0.288a |
| Negative | 28 | 0.0734±0.0557 | |
| Perineural
invasion |
| Positive | 17 | 0.0728±0.0576 | 0.648a |
| Negative | 20 | 0.0645±0.0516 | |
| Neoadjuvant
chemotherapy |
| Yes | 6 | 0.0733±0.0419 | 0.484a |
| No | 31 | 0.0674±0.0564 | |
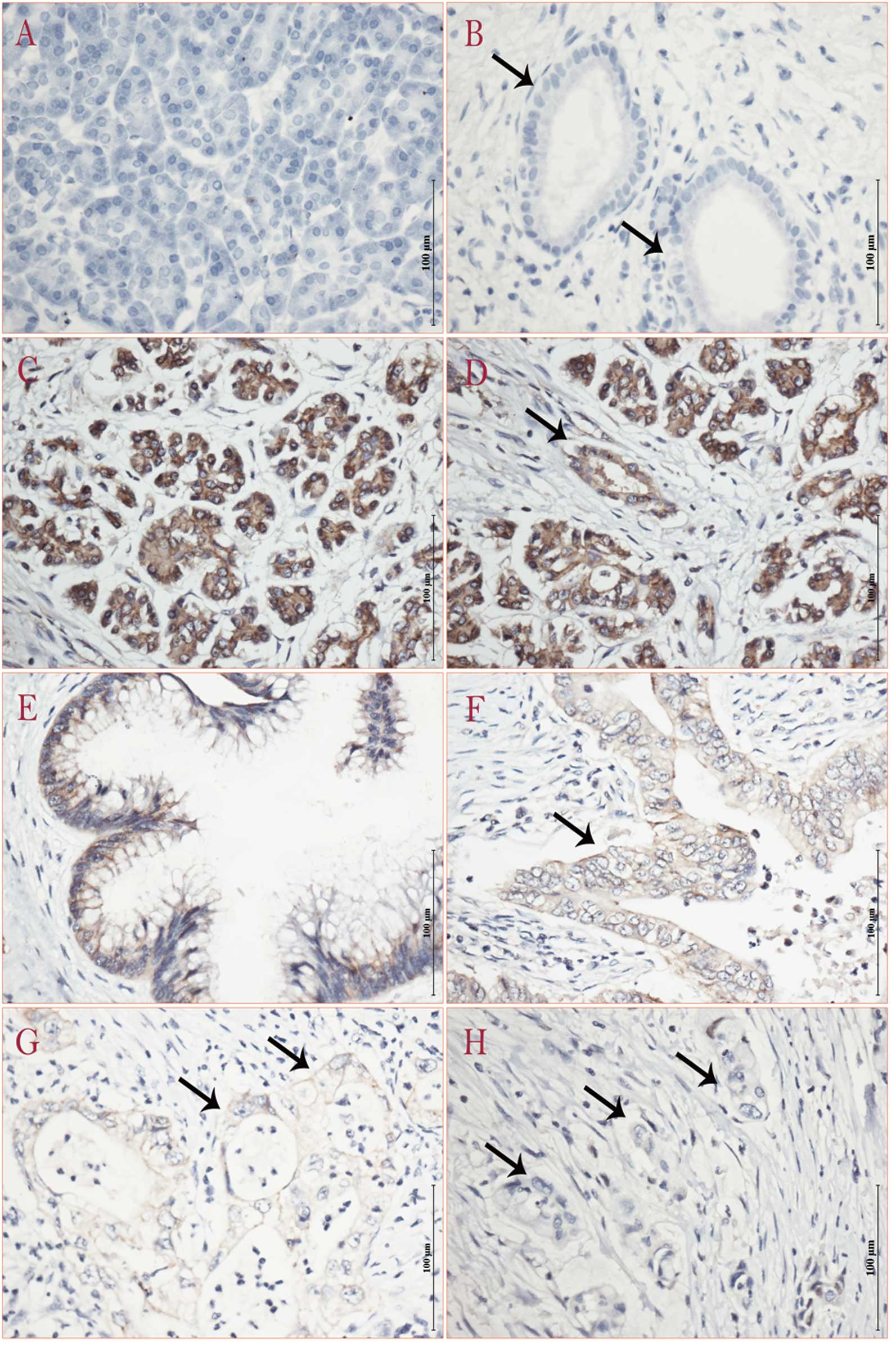
ISH of miRNA-218
Locked nucleic acid ISH was performed to
characterize the expressing features of miRNA-218 in pancreatic
cancer. The intensity and abundance of miRNA-218 in MAT, IPMN and
PDAC was analyzed (Table III).
Widespread and high expression of miRNA-218 was found in both
normal acinar and ductal epithelium, whereas focal or low
expression of miRNA-218 was found in PDAC. The precursor lesion of
pancreatic cancer, IPMN expressed less miRNA-218 than normal acinar
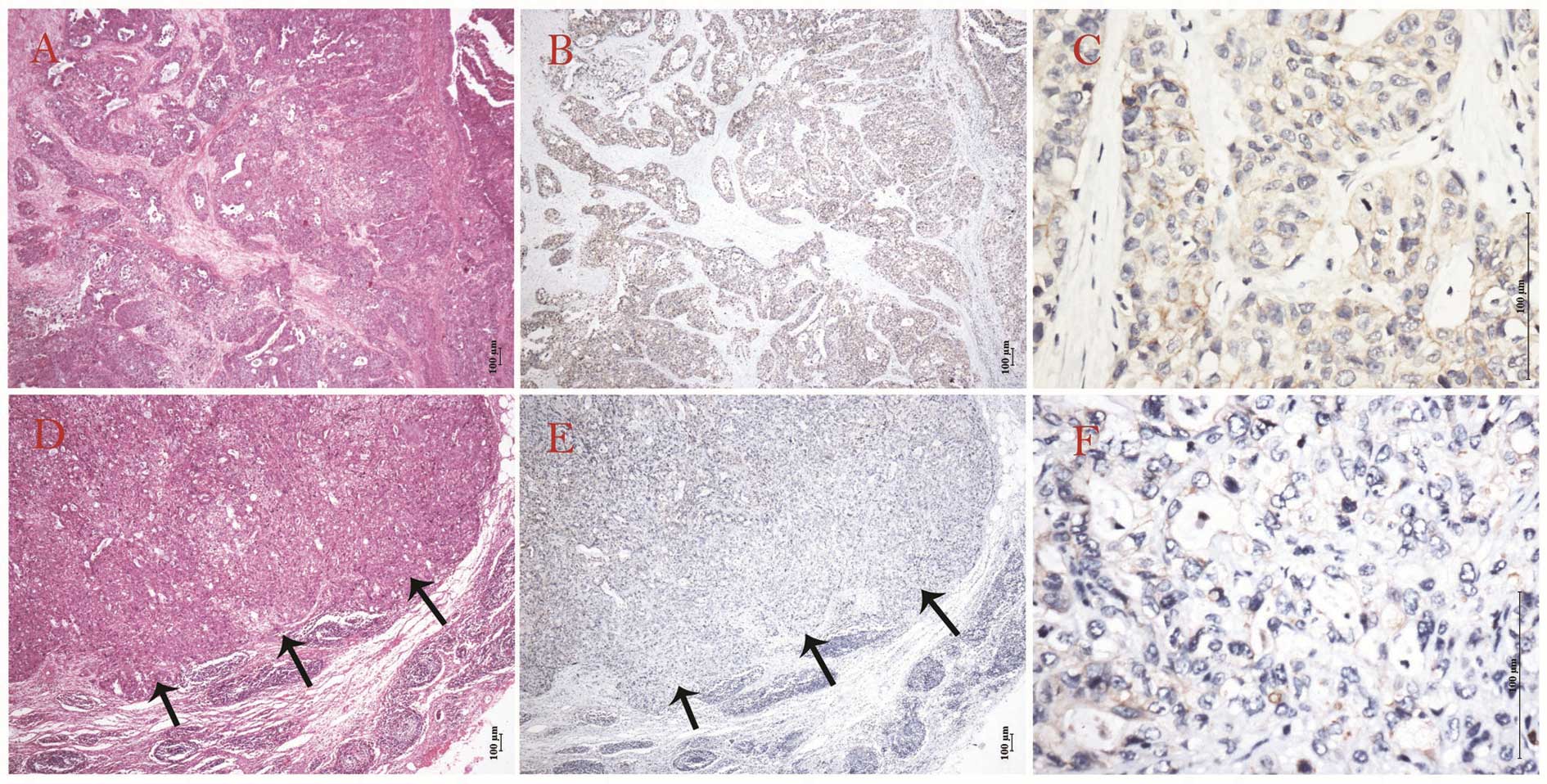
or ductal epithelium, but more compared to PDAC (Fig. 3). The expression features of
miRNA-218 according to tumor grade revealed that the lower cell
differentiation showed lower expression of miRNA-218. Almost
negative expression was demonstrated in metastatic lymph nodes,
compared to corresponding primary lesions in PDAC (Fig. 4).
 | Table IIIISH of miRNA-218. |
Table III
ISH of miRNA-218.
| Intensity rank | Mean PSC score |
|---|
|
|
|
|---|
| 0 | 1+ | 2+ | 0 | 1 | 2 | 3 |
|---|
| PDAC (case) | 3 | 7 | 0 | 3 | 4 | 2 | 1 |
| IPMN (case) | 0 | 3 | 0 | 0 | 0 | 1 | 2 |
| MAT (case) | 0 | 2 | 11 | 0 | 0 | 1 | 12 |
| P-value | <0.001 | <0.001 |
Target genes of miRNA-218 in pancreatic
cancer
Eight target genes of miRNA-218 were quantitatively
analyzed in 31 PDAC samples and 13 adjacent benign tissue samples.
The expression level of ROBO-1 was found upregulated in PDAC
compared to adjacent benign tissues (P=0.0066, 26.75±36.60 vs.
4.849±2.865), which was consistent with the regulatory pattern of
miRNA-218. BIRC5, EGFR, PAXILLIN, CETN2, LAMB3, HOXA1 and LEF1
showed no statistical significance (Fig. 5A). It was confirmed that the protein
level of ROBO-1 was significantly upregulated in PDAC compared to
adjacent benign tissues by western blotting (Fig. 5B).
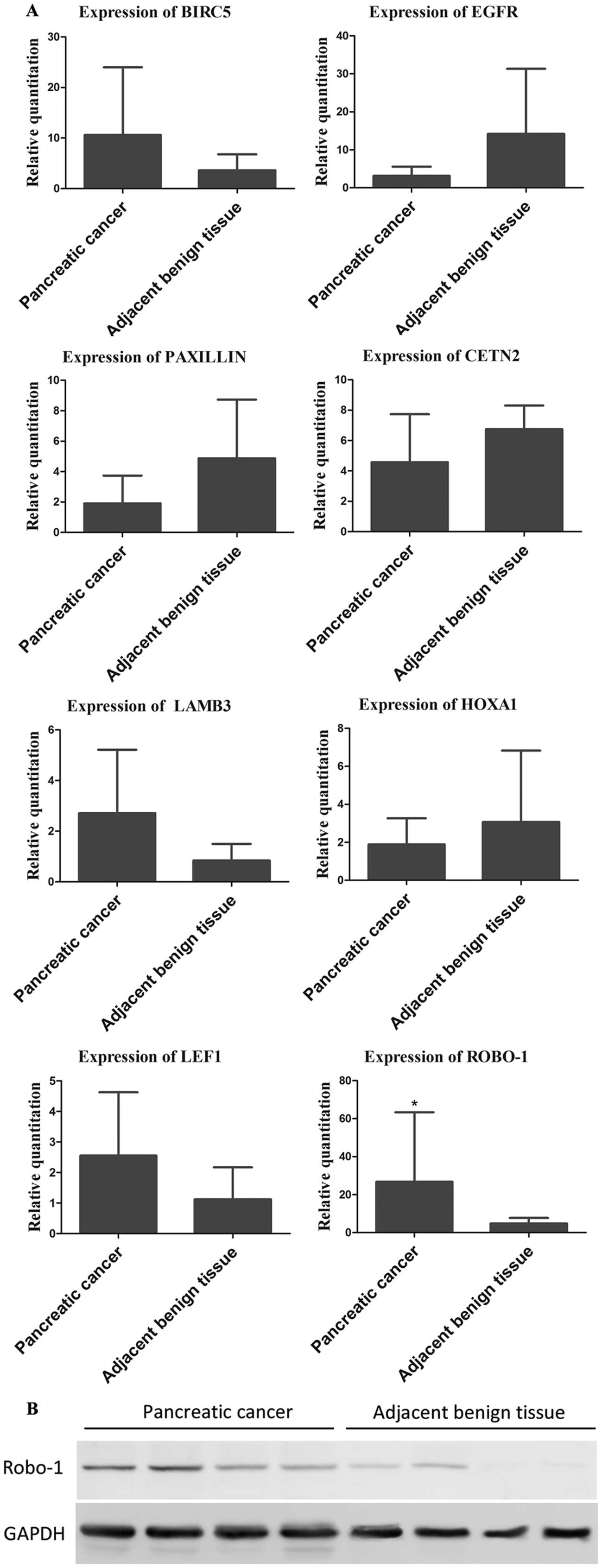 | Figure 5(A) Relative quantitation of target
genes of miRNA-218 in tissue samples. The expression of ROBO-1 was
significantly higher in pancreatic ductal adenocarcinoma (PDAC)
compared to adjacent benign tissue (*P<0.05). The
expression of BIRC5, EGFR, PAXILLIN, CETN2, LAMB3, HOXA1 and LEF1
showed no significant difference between PDAC and adjacent benign
tissue (P=0.3306, 0.7479, 0.0513, 0.1650, 0.1579, 0.5425 and
0.2216, respectively). (B) Western blot result of the protein level
of ROBO-1 in PDAC and adjacent benign tissue. |
Discussion
miRNAs are a series of small non-coding RNAs and up
to 30% of human genes are regulated by miRNAs. Since the first
lin-4 miRNA was discovered in 1993 (21), the family of miRNAs has been
highlighted and the role of miRNAs in tumor biology has also been
revealed. In pancreatic cancer, pioneer studies described
aberrantly expressed miRNAs (2–3,5,9,18,22).
Among these miRNAs, miRNA-21 and miRNA-155 are the most notable
genes, which regulate proliferation, apoptosis and
therapy-resistance of pancreatic cancer in principal (7,9,18,22,23).
Lymphatic metastasis is an unfavorable event in patients with
pancreatic cancer (24,25). Nevertheless, conventional profiling
results cannot trace genes related to the lymphatic metastasis of
pancreatic cancer. We previously established the ectopic-implanted
pancreatic cancer model and found the cell line BxPC-3-LN
metastasized to regional or distant lymph nodes much earlier than
the parental cell line BxPC-3 (19,20).
The genetic heterogeneity of primary carcinoma is preserved in
pancreatic cancer and clonal population that gives rise to
metastasis is genetically evolved from the original parental clone
(26). Therefore, we performed
miRNA profiling of these two cell lines, to find the aberrantly
expressed miRNAs potentially promoting lymphatic metastasis.
Combined analysis of profiling results between clinical tissue
samples and cell lines confirmed 4 coincident differentially
expressed miRNAs. Of these 4 miRNAs, miRNA-218 was the most
significantly altered gene in BxPC-3-LN compared to the parental
cell line BxPC-3, which may be correlated to the lymphatic
metastasis of pancreatic cancer and was also reported to be
downregulated in pancreatic cancer in another profiling study
(12).
We confirmed that miRNA-218 was downregulated in
human PDAC and BxPC-3-LN by RT-PCR. Consistent with our results,
miRNA-218 was found downregulated in several human solid tumors,
including cervical carcinoma, lung cancer, gastric cancer, glioma,
nasopharyngeal cancer, prostate cancer, bladder cancer and oral
cancer, but it only indicated that miRNA-218 might function as a
tumor suppressor in these tumors (8,27–33).
In the present study, BxPC-3-LN with decreased expression of
miRNA-218 compared to BxPC-3 provided insight that miRNA-218 may be
involved in the lymphatic metastasis of pancreatic cancer.
Moreover, we analyzed the clinicopathological
characteristics of PDAC patients and found that the group with
lymph node metastasis showed lower expression of miRNA-218 than the
group without. This is consistent with the results in gastric
cancer (34) and supports the
hypothesis that miRNA-218 promotes the lymphatic metastasis of
PDAC. Furthermore, we found miRNA-218 decreased in the elder age
group and hypothesized that miRNA-218 might also act as a tumor
suppressor, since pancreatic cancer and several other tumors
usually develop in middle-aged and older patients, whose tumor
suppressors are lost or mutated. However, there was a negative
correlation between miRNA-218 and age in gastric and lung cancer
(34,35).
Results of ISH revealed that miRNA-218 is mainly
expressed in normal acinar epithelium and ductal epithelium,
whereas the mesenchyma seldom expressed miRNA-218. We speculate
that miRNA-218 may regulate the differentiation of normal
pancreatic acinar epithelium and ductal epithelium. Therefore, if
the expression of miRNA-218 is impaired, it may induce aberrant
differentiation of normal epithelium and even lead to
carcinogenesis. Lower expression of miRNA-218 in IPMN compared to
normal acinus or duct, suggested that the downregulation of
miRNA-218 might be an early sign in the precursor lesions of
pancreatic cancer. Additionally, PDAC showed even lower expression
of miRNA-218 than IPMN demonstrating that the decreased expression
of miRNA-218 advances as the precursor lesions transform to
pancreatic cancer. Thus, the insight into the role of miRNA-218 as
a marker of diagnosis or follow-up is promising. Furthermore,
miRNA-218 might regulate the differentiation of tumor cells
(8) and it was found that the
expression of miRNA-218 was correlated with tumor grades in the
present study, although it was not demonstrated by RT-PCR. Low or
even negative expression of miRNA-218 in metastatic lymph nodes of
PDAC was further evidence that decreased expression of miRNA-218
might promote tumor cells migrating into the lymphatic system.
A detailed mechanism of the involvement of miRNA-218
in tumor lymphatic metastasis remains to be confirmed. The target
genes of miRNA-218 were hallmarks of different pathways. BIRC5,
EGFR, PAXILLIN, CETN2, LAMB3, HOXA1, LEF1 and ROBO-1 were reported
to be the direct targets of miRNA-218 and are involved in the
carcinogenesis and tumor progression of various tumors (30,34–36).
Here, we investigated these target genes of miRNA-218 in pancreatic
cancer and found ROBO-1 showed higher expression of both mRNA and
protein in PDAC, compared to adjacent benign tissues. ROBO-1
(Rodent Bone) which plays a novel role in bone metabolism, was
first discovered by Noel et al(37) and it is a member of the emerging
multifunctional uPAR/CD59/Ly-6/snake toxin family (38). It has been proved that miRNA-218
regulates ROBO-1 by binding to its 3′UTR and participates in neural
development, angiogenesis and cell migration. Additionally,
previous studies also found miRNA-218-ROBO-1 might promote tumor
metastasis in several tumors such as nasopharyngeal cancer, gastric
cancer and breast cancer (30,34,39,40).
Collectively, we considered that an miRNA-218-ROBO-1 dependent
pathway might be involved in the lymphatic metastasis of pancreatic
cancer according to the expressing features of miRNA-218 and ROBO-1
in pancreatic cancer.
In conclusion, we established a pioneer screening
strategy through combined analysis of profiling results from cell
lines and tissue samples, to trace the genes involved in the
lymphatic metastasis of pancreatic cancer. We successfully found
miRNA-218 differentially expressed in both BxPC-3-LN and pancreatic
cancer which potentially promoted the lymphatic metastasis of
pancreatic cancer. The downregulation of miRNA-218 and upregulation
of ROBO-1 in pancreatic cancer were confirmed and we hypothesized
that miRNA-218-ROBO-1 may regulate the process of metastasis,
particularly lymphatic metastasis, in pancreatic cancer. The
downstream mechanism of miRNA-218-ROBO-1 remains unclear and
further studies regarding the lymphatic metastasis of pancreatic
cancer are currently being performed.
Acknowledgements
The authors thank Feng Tang and Zunguo Du,
Department of Surgical Pathology, Huashan Hospital, for their
histological assistance. The study was supported by a grant from
the National Natural Science Foundation of China (no. 81172274), a
grant from the Shanghai Committee of Science and Technology, China
(no. 11JC1401600) and a grant from the Shanghai Municipal Health
Bureau, China (no. 20114v141).
References
|
1
|
Jemal A, Siegel R, Xu JQ and Ward E:
Cancer statistics, 2010. CA Cancer J Clin. 60:277–300. 2010.
View Article : Google Scholar
|
|
2
|
Zhao WG, Yu SN, Lu ZH, Ma YH, Gu YM and
Chen J: The miR-217 microRNA functions as a potential tumor
suppressor in pancreatic ductal adenocarcinoma by targeting KRAS.
Carcinogenesis. 31:1726–1733. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yu SN, Lu ZH, Liu CZ, et al: miRNA-96
suppresses KRAS and functions as a tumor suppressor gene in
pancreatic cancer. Cancer Res. 70:6015–6025. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Torrisani J, Bournet B, du Rieu MC, et al:
Let-7 microRNA transfer in pancreatic cancer-derived cells inhibits
in vitro cell proliferation but fails to alter tumor progression.
Hum Gene Ther. 20:831–844. 2009. View Article : Google Scholar
|
|
5
|
Ji Q, Hao X, Zhang M, et al: MicroRNA
miR-34 inhibits human pancreatic cancer tumor-initiating cells.
PLoS One. 4:e68162009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Radhakrishnan P, Mohr AM, Grandgenet PM
and Hollingsworth MA: MicroRNA-200c modulates the expression of
MUC4 and MUC16 in human pancreatic cancer. The 42nd Annual Meeting
of the American Pancreatic Association Pancreas. 40:13502011.
|
|
7
|
Bhatti I, Lee A, James V, et al: Knockdown
of microRNA-21 inhibits proliferation and increases cell death by
targeting programmed cell death 4 (PDCD4) in pancreatic ductal
adenocarcinoma. J Gastrointest Surg. 15:199–208. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Song L, Huang Q, Chen K, et al: miR-218
inhibits the invasive ability of glioma cells by direct
downregulation of IKK-β. Biochem Biophys Res Commun. 402:135–140.
2010.PubMed/NCBI
|
|
9
|
Ryu JK, Hong SM, Karikari CA, Hruban RH,
Goggins MG and Maitra A: Aberrant MicroRNA-155 expression is an
early event in the multistep progression of pancreatic
adenocarcinoma. Pancreatology. 10:66–73. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Szafranska AE, Davison TS, John J, et al:
MicroRNA expression alterations are linked to tumorigenesis and
non-neoplastic processes in pancreatic ductal adenocarcinoma.
Oncogene. 26:4442–4452. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee EJ, Gusev Y, Jiang J, et al:
Expression profiling identifies microRNA signature in pancreatic
cancer. Int J Cancer. 120:1046–1054. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bloomston M, Frankel WL, Petrocca F, et
al: MicroRNA expression patterns to differentiate pancreatic
adenocarcinoma from normal pancreas and chronic pancreatitis. JAMA.
297:1901–1908. 2007. View Article : Google Scholar
|
|
13
|
Srivastava SK, Bhardwaj A, Singh S, et al:
MicroRNA-150 directly targets MUC4 and suppresses growth and
malignant behavior of pancreatic cancer cells. Carcinogenesis.
32:1832–1839. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ohuchida K, Mizumoto K, Kayashima T, et
al: MicroRNA expression as a predictive marker for gemcitabine
response after surgical resection of pancreatic cancer. Ann Surg
Oncol. 18:2381–2387. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nakata K, Ohuchida K, Mizumoto K, et al:
MicroRNA-10b is overexpressed in pancreatic cancer, promotes its
invasiveness, and correlates with a poor prognosis. Surgery.
150:916–922. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liffers ST, Munding JB, Vogt M, et al:
MicroRNA-148a is down-regulated in human pancreatic ductal
adenocarcinomas and regulates cell survival by targeting CDC25B.
Lab Invest. 91:1472–1479. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hao J, Zhang SY, Zhou YQ, Liu C, Hu XG and
Shao CH: MicroRNA 421 suppresses DPC4/Smad4 in pancreatic cancer.
Biochem Biophys Res Commun. 406:552–557. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Giovannetti E, Funel N, Peters GJ, et al:
MicroRNA-21 in pancreatic cancer: correlation with clinical outcome
and pharmacologic aspects underlying its role in the modulation of
gemcitabine activity. Cancer Res. 70:4528–4538. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Luo GP, Yu XJ, Jin C, et al:
LyP-1-conjugated nanoparticles for targeting drug delivery to
lymphatic metastatic tumors. Int J Pharm. 385:150–156. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang F, Jin C, Yang D, et al: Magnetic
functionalised carbon nanotubes as drug vehicles for cancer lymph
node metastasis treatment. Eur J Cancer. 47:1873–1882. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee RC, Feinbaum RL and Ambros V: The
C. elegans heterochronic gene lin-4 encodes small
RNAs with antisense complementarity to lin-14. Cell.
75:843–854. 1993.
|
|
22
|
Hwang JH, Voortman J, Giovannetti E, et
al: Identification of microRNA-21 as a biomarker for
chemoresistance and clinical outcome following adjuvant therapy in
resectable pancreatic cancer. PLoS One. 5:e106302010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim HR, Jang SE, Beik WH, Lee SH and Hwang
JH: Chemosensitivity of indole-3-carbinol via downregulation of
microRNA-21 in gemcitabine-resistant pancreatic cancer cells. Asian
Pacific Digestive Week, Oct, 2011. J Gastroen Hepatol.
26:2322011.
|
|
24
|
Massucco P, Ribero D, Sgotto E, Mellano A,
Muratore A and Capussotti L: Prognostic significance of lymph node
metastases in pancreatic head cancer treated with extended
lymphadenectomy: not just a matter of numbers. Ann Surg Oncol.
16:3323–3332. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Riediger H, Keck T, Wellner U, et al: The
lymph node ratio is the strongest prognostic factor after resection
of pancreatic cancer. J Gastrointest Surg. 13:1337–1344. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yachida S, Jones S, Bozic I, et al:
Distant metastasis occurs late during the genetic evolution of
pancreatic cancer. Nature. 467:1114–1117. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Uesugi A, Kozaki K, Tsuruta T, et al: The
tumor suppressive microRNA miR-218 targets the mTOR component
Rictor and inhibits AKT phosphorylation in oral cancer. Cancer Res.
71:5765–5778. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tatarano S, Chiyomaru T, Kawakami K, et
al: miR-218 on the genomic loss region of chromosome 4p15.31
functions as a tumor suppressor in bladder cancer. Int J Oncol.
39:13–21. 2011.
|
|
29
|
Leite KRM, Sousa-Canavez JM, Reis ST, et
al: Change in expression of miR-let7c, miR-100, and miR-218 from
high grade localized prostate cancer to metastasis. Urol Oncol.
29:265–269. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Alajez NM, Lenarduzzi M, Ito E, et al:
MiR-218 suppresses nasopharyngeal cancer progression through
downregulation of survivin and the SLIT2-ROBO1 pathway. Cancer Res.
71:2381–2391. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gao CP, Zhang ZY, Liu WZ, Xiao SD, Gu WQ
and Lu H: Reduced microRNA-218 expression is associated with high
nuclear factor kappa B activation in gastric cancer. Cancer.
116:41–49. 2010.PubMed/NCBI
|
|
32
|
Davidson MR, Larsen JE, Yang IA, et al:
MicroRNA-218 is deleted and downregulated in lung squamous cell
carcinoma. PLoS One. 5:e125602010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Martinez I, Gardiner AS, Board KF, Monzon
FA, Edwards RP and Khan SA: Human papillomavirus type 16 reduces
the expression of microRNA-218 in cervical carcinoma cells.
Oncogene. 27:2575–2582. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tie J, Pan YL, Zhao LN, et al: MiR-218
inhibits invasion and metastasis of gastric cancer by targeting the
Robo1 receptor. PLoS Genet. 6:e10008792010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wu DW, Cheng YW, Wang J, Chen CY and Lee
H: Paxillin predicts survival and relapse in non-small cell lung
cancer by microRNA-218 targeting. Cancer Res. 70:10392–10401. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhou XY, Chen XJ, Hu LM, et al:
Polymorphisms involved in the miR-218-LAMB3 pathway and
susceptibility of cervical cancer, a case-control study in Chinese
women. Gynecol Oncol. 117:287–290. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Noel LS, Champion BR, Holley CL, et al:
RoBo-1, a novel member of the urokinase plasminogen activator
receptor/CD59/Ly-6/snake toxin family selectively expressed in rat
bone and growth plate cartilage. J Biol Chem. 273:3878–3883. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ploug M and Ellis V: Structure-function
relationships in the receptor for urokinase-type plasminogen
activator. Comparison to other members of the Ly-6 family and snake
venom α-neurotoxins. FEBS Lett. 349:163–168. 1994.PubMed/NCBI
|
|
39
|
Yang L, Li Q, Wang Q, Jiang Z and Zhang L:
Silencing of miRNA-218 promotes migration and invasion of breast
cancer via Slit2-Robo1 pathway. Biomed Pharmacother. 66:535–540.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Woo S, Fish JE, Wythe JD, Bruneau BB,
Stainier DY and Srivastava D: A novel Slit-Robo-miR-218 signaling
axis regulates VEGF-mediated heart tube formation in zebrafish. The
69th Annual Meeting of the Society for Developmental
Biology/Japanese Society of Developmental Biologists. Dev Biol.
344:4332010. View Article : Google Scholar
|