Introduction
In recent years, the association between chronic
inflammation and tumor has gained increasing attention (1). Diverse roles have been reported for
chemokines in tumor biology, including transformation,
proliferation and angiogenesis as well as recruiting lymphocytes to
the tumor sites (2). Lymphoma,
which can be divided into Hodgkin lymphoma (HL) and non-Hodgkin
lymphoma (NHL), is a type of tumor derived from malignant
lymphocytes and HL, in particular, is closely related with immune
functions and inflammation (3).
Different from NHL, the classical Hodgkin lymphoma
(cHL), which is the main HL type, is an unusual type of malignancy
in which the clonal B-cell population, known as the Hodgkin
Reed-Sternberg (H/RS) cells, contributes to less than 1% of the
tumor tissue. H/RS cells survive in a large amount of background
inflammatory cells, which are composed of lymphocytes, plasma
cells, eosinophils, histiocytes and others (4,5). The
transformation of H/RS cells has been reported to be related to the
loss of cell differentiation antigen 99 (CD99) from B-lymphocytes
(6,7) and was recently confirmed by our team
(8), who also established the CD99
overexpressing L428 cell line (L428-CD99+) and confirmed
that L428-CD99+ cells lost their nature of H/RS cells.
Furthermore, mouse CD99 antigen-like 2 (mCD99L2), a widely
expressed antigen with moderate sequence homology to human CD99
(9), was investigated by our group
in our recently published study (10), in which the stable mCD99L2
downregulated A20 cell line (A20-mCD99L2−) was
established by lentivirus conducted stable mCD99L2 RNA
interference. During the investigation of small hairpin RNA (shRNA)
targeting mCD99L2 effects in vitro and in vivo, CXC
chemokine ligand 16 (CXCL16) was noted among the differentially
expressed cytokines. In the present study, A20-mCD99L2−
tumor cells were inoculated into homologous BALB/c mice and the
differentially expressed cytokines/chemokines in tumor tissues were
extensively analyzed, and we focused on CXCL16 again.
CXCL16 is a newly found chemokine belonging to the
CXC chemokine family (11,12). The human CXCL16 gene is located on
chromosome 17p13 and is normally expressed on the antigen
conferring cells (11), occurring
both as transmembrane form (TM-CXCL16) as well as soluble form
(sCXCL16). The TM-CXCL16 is converted by a disintegrin and
metalloproteinase (ADAM) 10 or ADAM 17 into sCXCL16, which is
secreted into the supernatants of cell cultures and functions as
chemotactic factors for its effective cells (13). High expression of CXCL16 has been
associated with a number of human inflammatory diseases, including
rheumatoid arthritis, atherosclerosis, interstitial lung diseases,
liver injury, coronary artery disease and inflammatory bowel
disease (14–17).
CXCL16 was recently reported to be overexpressed in
several types of cancer such as colorectal cancer (18), prostate cancer (19) and correlated with lymph node
metastasis in epithelial ovarian carcinoma (20) and it may also be involved in the
self renewal of tumor stem cells (21). Darash-Yahana et al(22) mentioned positive CXCL16 expression
in some lymphomas, but the types and subtypes have not been
clarified. Lymphomas can be classified into more than 80 types or
subtypes and the characteristics of CXCL16 in lymphomas require
further elucidation. Although CXCL16 was previously noted in our
work, in the present study we extended our findings to an
association between CXCL16 and CD99 expression, and, in addition,
relevant signaling pathways were assessed. Furthermore, the
characteristics of CXCL16 expressions in a broad range of human
lymphoma cell lines and clinical samples were investigated, which
may contribute to further research of the complex lymphoma
microenvironments.
Materials and methods
Ethics statement
All the experimental protocols and human samples
obtained from patients undergoing lymphoma treatments were approved
by the Clinical Research Ethics Committee of Nanfang Hospital,
Southern Medical University.
Case selection
A representative series of 45 cHL and 35 NHL
samples, which were diagnosed between 2009 and 2012 and classified
according to the World Health Organization (WHO) criteria, were
selected from Nanfang Hospital, Southern Medical University,
Guangzhou, China (Table I). In
addition, 7 reactive and 7 normal lymph node sections were selected
as controls. All clinical samples were paraffin-embedded
blocks.
 | Table IThe selected samples of different
lymphoma types. |
Table I
The selected samples of different
lymphoma types.
| Subtype of cHL | n | Subtype of NHL | n |
|---|
| Nodular sclerosis
(NSHL) | 12 | Diffuse large
B-cell lymphoma (DLBCL) | 20 |
| Mixed cellularity
(MCHL) | 13 | Burkitt’s lymphoma
(BL) | 5 |
| Lymphocyte-rich
(LRCHL) | 15 | Anaplastic large
cell lymphoma (ALCL) | 5 |
| Lymphocyte-depleted
(LRCHL) | 5 | Peripheral T-cell
lymphoma, unspecified (U-PTL) | 5 |
Cells and culture
Nine cell lines with different origins and
significantly different clinical characteristics were conserved by
the Guangdong Provincial Key Laboratory of Molecular Oncopathology
and selected for the present study (Table II). The sub-clones of A20 cells
transfected with shRNAs targeting mCD99L2 (A20-mCD99L2−)
or negative control vectors (A20-CTR) and the sub-clones of L428
cells transfected with the human CD99 gene (L428-CD99+)
or negative control vectors (L428-CTR) were previously constructed
by our team. Cells were cultured in RPMI-1640 medium supplemented
with 10% heat-inactivated fetal bovine serum (FBS) (HyClone, Logan,
UT, USA) at 37°C and 5% CO2.
 | Table IINames and origins of the nine
lymphoma cell lines. |
Table II
Names and origins of the nine
lymphoma cell lines.
| Cell line | Origins |
|---|
| L428 | An H/RS cell line
from Hodgkin lymphoma (HL) |
| OCI-Ly1, OCI-Ly8,
OCI-Ly10 | Three cell lines
from diffuse large B-cell lymphoma (DLBCL) |
| Raji | An EB virus
positive cell line from Burkitt’s lymphoma (BL) |
| Karpass299 | A CD30 positive
cell line from anaplastic large cell lymphoma (ALCL) |
| Jurkat | T-leukemia derived
cell line |
| RPMI-8226 and
KM3 | Two multiple plasma
cell lines from multiple myeloma (MM) |
Immunochemistry analyses
Immunohistochemistry (IHC) of paraffin sections from
clinical samples and immunocytochemistry (ICC) of fixed cells from
cultured cell lines were carried out, using the ChemMate™ EnVision™
Detection kit (Dako, Carpinteria, CA, USA). Heat-induced antigen
retrieval was performed in 10 mM citrate buffer (pH 6.0) for 2 min
at 100°C. Endogenous peroxidase activity and non-specific antigen
were blocked with peroxidase blocking reagent containing 3%
hydrogen peroxide and serum, followed by incubation with the rabbit
anti-human CXCL16 antibody (ab101404, 1:100, Abcam, Cambridge, UK)
overnight at 4°C. After washing, the sections were incubated with
biotin-labeled goat anti-rabbit antibody (Zhongshan Inc.,
Zhongshan, China) for 10 min at room temperature, and were
subsequently incubated with streptavidin-conjugated horseradish
peroxidase (HRP) (Maixin Inc., Shenzhen, China). The peroxidase
reaction was developed using 3,3-diaminobenzidine chromogen
solution in DAB buffer substrate. Sections were visualized with DAB
and counterstained with hematoxylin, mounted in neutral gum and
analyzed using a bright field microscope.
Evaluations of the immunohistochemical staining
results were conducted independently by two independent
pathologists (T.Z. and HP.T.) with blinding of clinical data.
Staining results were evaluated semi-quantitatively as follow: (−),
negative or positive <10%; (+), moderate positive 10%–50%; (++),
strong positive >50% for intensity of immunostaining.
Immunofluorescence and confocal microscopy were
performed as previously described (8). The cells were labeled with goat
anti-human CXCL16 (SC-27344, 1:100; Santa Cruz Biotechnology, Santa
Cruz, CA, USA) followed by PE-conjugated donkey anti-goat IgG
(1:100; Proteintech Group Inc., Chicago, IL, USA). Negative
controls were performed by replacing the primary antibodies with
PBS.
RNA isolation and quantitative
RT-PCR
The extraction of total RNA, generation of cDNA and
real-time reverse transcription PCR (RT-PCR) were performed as
previously described (10). The
following primers were used: CXCL16,
5′-CGTCACTGGAAGTTGTTATTGTGGT-3′ (forward) and
5′-TGGTAGGAAGTAAATGCTTCTGGTG-3′ (reverse); GAPDH,
5′-ACAGTCAGCCGCATCTTCTT-3′ (forward) and 5′-GACAAGCTTCCCGTTCTCAG-3′
(reverse). Results were analyzed using the software installed in
the 7500 Real-Time PCR system (Applied Biosystems) and the relative
expression ratio was determined by the formula
2−ΔΔCt.
Western blot analysis
The western blot analyses were performed as
previously described (10). Rabbit
anti-human CXCL16 antibody (ab101404, 1:1,000; Abcam), goat
anti-mouse CXCL16 antibody (ab84435, 1:500; Abcam) and mouse
anti-human β-actin antibody (TA-09, 1:2,000; Zhongshan) were
applied, respectively. Specific binding was correspondingly
revealed by HRP-conjugated goat anti-rabbit IgG (ZB-2301, 1:5,000,
Zhongshan), rabbit anti-goat IgG (ZB-2306, 1:5,000; Zhongshan) and
goat anti-mouse IgG (ZB-2305, 1:5,000), respectively, with an
enhanced chemiluminescence system (ECL-Plus, Amersham).
Enzyme-linked immunosorbent assay
(ELISA)
Secreted CXCL16 concentrations of cell supernatants
were measured using commercially available kits (900-M230 Human
CXCL16 Mini EDK kit; Mini EDK buffer kit; Peprotech) following the
procedures recommended by the manufacturer. The range of
sensitivity for human sCXCL16 was 0–2,000 pg/ml.
Isolation of human peripheral blood
CD4+ T cells
Peripheral blood mononuclear cells (PBMCs) from
healthy donors were prepared using a Lymphocyte Separation kit
(Weijia Co., China). CD4+ T cells were isolated using
immunomagnetic beads flow cytometry (23). The CD4+ T cells
(2×104 cells/well) were cultured with LPS (2 μg/ml, BD
Biosciences) stimulation in anti-CD3-coated plates.
Cell proliferation assays
Cell Counting Kit-8 (CCK-8, Tongren Shanghai Co.,
Shanghai, China) was used to detect the cell proliferation. L428
cells or CD4+ T cells were seeded in 96-well plates at a
density of 1,000 cells/well with 100 μl of complete culture medium.
After 24 h, the medium was changed to DMEM supplemented with 5%
FBS. Recombinant human CXCL16 (300-55, Peprotech, USA) was added to
the medium in final concentrations ranging from 10 to 100 ng/ml.
The cells were then cultured for another 1–6 days. Cells not
exposed to CXCL16 were used as controls, and wells to which only
culture medium was added served as blanks. At the end of the CXCL16
stimulations, the supernatant was removed and 100 μl of DMEM medium
containing 10 μl of CCK-8 was added to each well for another 3 h at
37°C. The culture plates were then shaken for 10 min and the
optical density (OD) values were read at 450 nm.
Animals and in vivo tests
BALB/c mice (n=14) (6–8-week-old female/male) were
purchased from the Central Laboratory of Animal Science at the
Southern Medical University (Guangzhou) and divided into two groups
randomly. Tumor cells (2×107) (A20-mCD99L2−
or A20-CTR) in 0.1 ml growth medium were injected into the left
axillary fossa of the mice subcutaneously. When exhibiting external
signs of suffering, the mice were sacrificed and the tumor tissues
were conserved in liquid nitrogen. All procedures were conducted
under sterile conditions.
Mouse cytokine antibody arrays
RayBiotech mouse cytokine antibody arrays were used
for detecting the expression of cytokine/chemokine in tumor tissues
from BALB/c mice according to the manufacturer’s protocol and as
previously described (10). The
cytokine expressions were monitored and the differences were
analyzed.
Statistical analysis
SPSS 13.0 software was used for statistical
analysis. Results are expressed as means ± SD. One-way ANOVA was
used to determine the differences between groups for all in
vitro analyses. A P-value of <0.05 was considered to
indicate a statistically significant result.
Results
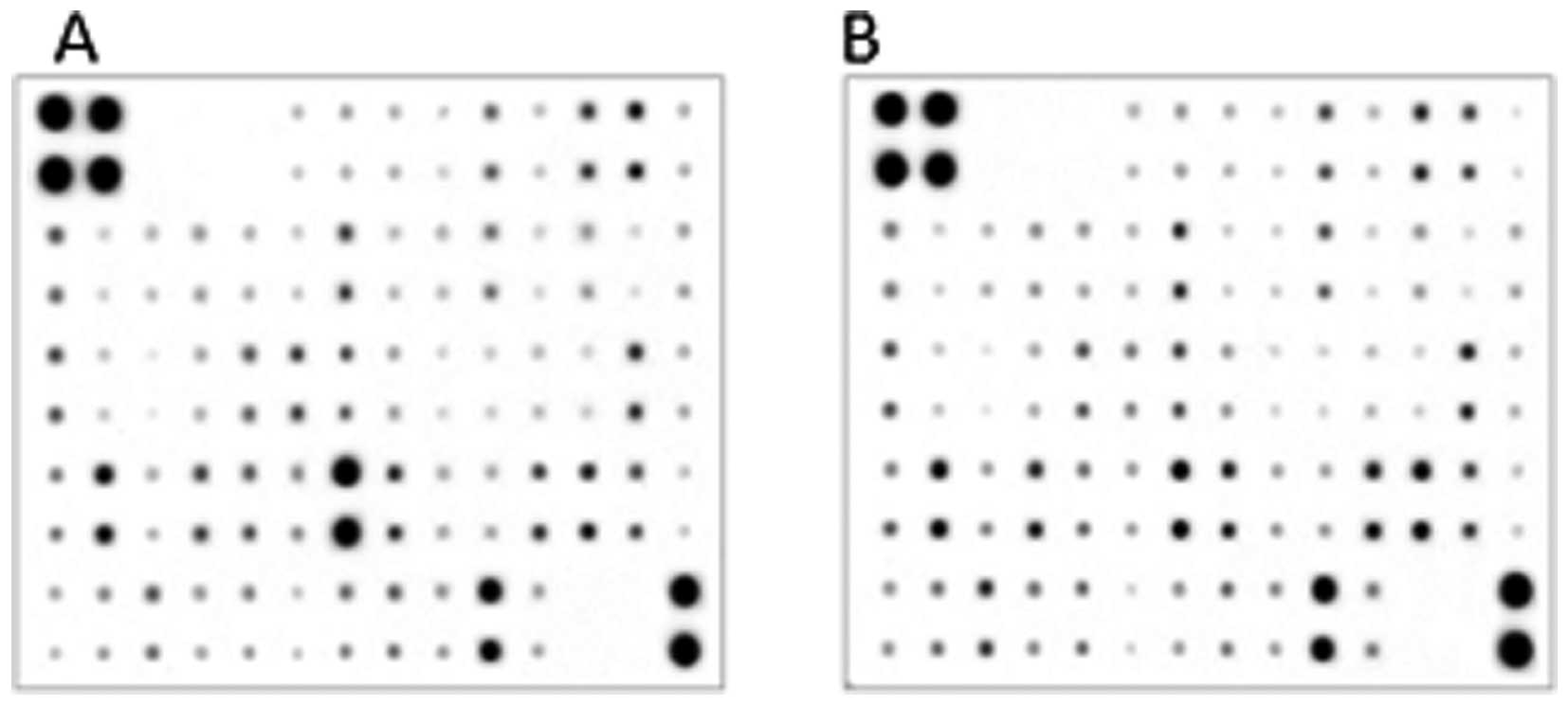
Multiple cytokines are involved in tumor
growth in vivo
The tumor tissues exhibited different
cytokine/chemokine profiles compared to the corresponding in
vitro tumor cell culture conditions (Fig. 1). Compared with the cultured
A20-mCD99L2− cells, A20-mCD99L2−-induced
tumor tissues exhibited upregulated cytokine/chemokine levels,
including vascular cell adhesion molecule 1 (VCAM-1), macrophage
inflammatory protein-1γ (MIP-1γ), monokine induced by interferon-γ
(MIG), interleukin-10 (IL-10), regulated upon activation normal
T-cell expressed and secreted (RANTES), platelet factor 4 (PF4),
and CXCL16 (Table III), whereas
the downregulated cytokine/chemokines included interleukin-6
(IL-6), eotaxin and tumor necrosis factor-α (TNF-α). Among the
above mentioned upregulated cytokines/chemokines, CXCL16 caught our
attraction, as it was already noted to be upregulated in
A20-mCD99L2− cells in our previous study (10) and was now further upregulated in
tumor tissues of BALB/c mice inoculated with
A20-mCD99L2− cells.
 | Table IIICytokines upregulated in excess of
1.5-fold in tissue from A20-mCD99L2− cell-induced BALB/c
mouse tumor compared with cultured A20-mCD99L2−
cells. |
Table III
Cytokines upregulated in excess of
1.5-fold in tissue from A20-mCD99L2− cell-induced BALB/c
mouse tumor compared with cultured A20-mCD99L2−
cells.
| Row | Col | Col | Name | 2-primary | 4-primary | 2-standard | 4-standard |
4-standard/2-standard |
|---|
| 1,2 | 13 | m | CXCL16 | 14359.5 | 17044.5 | 0.212270472 | 0.387308078 | 1.825 |
| 5,6 | 5 | e | IL-10 | 11541.5 | 15544 | 0.15395003 | 0.345131159 | 2.242 |
| 7,8 | 5 | e | MIG | 9185 | 13276 | 0.105180647 | 0.281380907 | 2.675 |
| 7,8 | 7 | g | MIP-1γ | 9780 | 32543.5 | 0.11749458 | 0.822962908 | 7.004 |
| 7,8 | 10 | j | MIP-3α | 9521 | 9629.5 | 0.112134398 | 0.178882982 | 1.595 |
| 7,8 | 11 | k | PF4 | 20683 | 25677 | 0.343139639 | 0.629955366 | 1.836 |
| 7,8 | 12 | l | P-selectin | 29052 | 32292.5 | 0.516341831 | 0.815907655 | 1.5806 |
| 7,8 | 13 | m | RANTES | 14570.5 | 18204.5 | 0.216637262 | 0.419914027 | 1.9386 |
| 9,10 | 10 | j | VCAM-1 | 11018.5 | 39538.5 | 0.143126187 | 1.019582402 | 7.124 |
| 9,10 | 11 | k | VEGF | 12655 | 13621.5 | 0.176994676 | 0.29109242 | 1.645 |
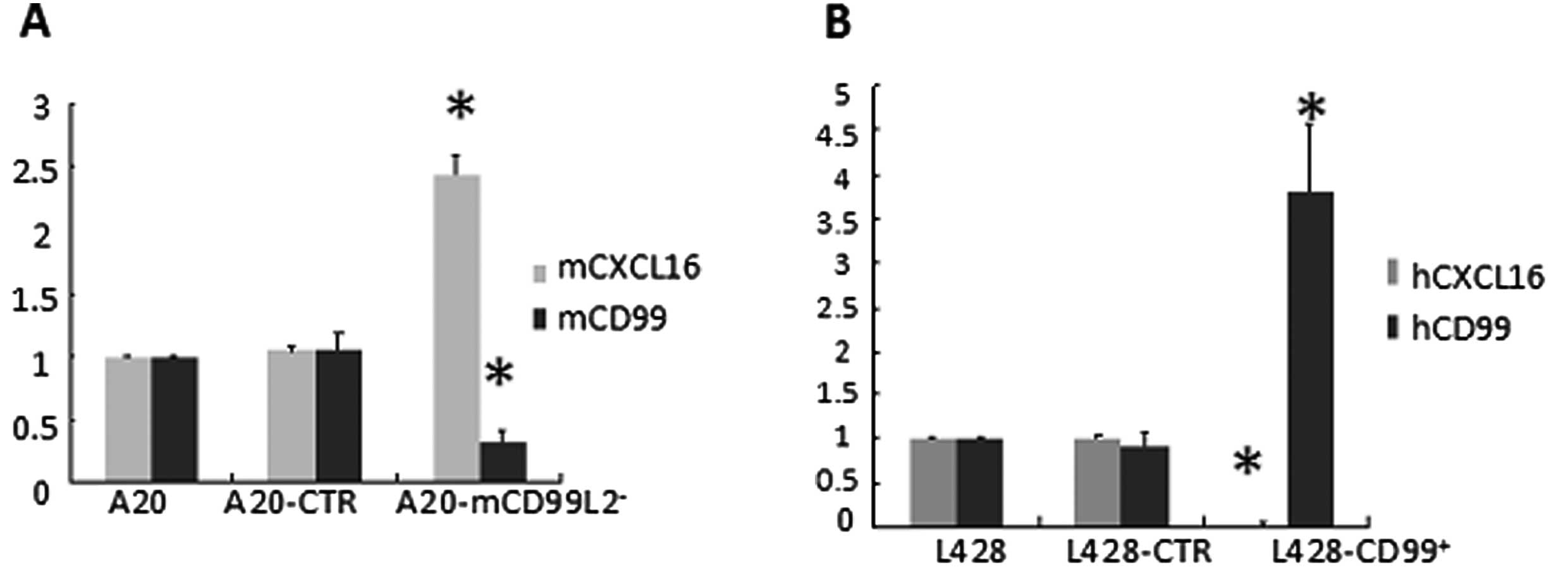
CXCL16 inversely correlates with
human/mouse CD99 expression
The mRNA and protein expression of CXCL16 was
measured by qRT-PCR and western blot analysis. As CXCL16 can exist
as transmembrane form (TM-CXCL16) and soluble form (sCXCL16),
supernatants of serum-free cultured cells were collected and the
secreted sCXCL16 was quantified by ELISA. In the murine cell lines,
results indicated that the expression of mouse CXCL16, as well as
sCXCL16, was markedly higher in A20-mCD99L2− cells than
in the control (Fig. 2A, Table IV; P<0.05), suggesting that
increased CXCL16 was correlated with downregulated mCD99L2
expression. In addition, in the previously established human cell
lines, the expression of human CXCL16 and sCXCL16 was significantly
lower in L428-CD99+ cells than in the control (Fig. 2B, Table
IV; P<0.05), corroborating the suggestion that decreased
CXCL16 is correlated with upregulated CD99 expression.
 | Table IVSecreted sCXCL16 detected by
ELISA. |
Table IV
Secreted sCXCL16 detected by
ELISA.
| Group | hCXCL16 (ng/l) | Group | mCXCL16 (ng/l) |
|---|
| L428 | 1075.9±203.1 | A20 | 323.8±28.3 |
| L428-CTR | 081.5±118.4 | A20-CTR | 335.5±25.7 |
|
L428-CD99+ | 144.1±20.9a |
A20-mCD99L2− | 854.9±23.6b |
Mouse CXCL16 is affected by the NF-κB
pathway in mouse lymphoma A20 cells as well as in the CD99
downregulated subcell type line
Subsequently, since CXCL16 is inversely correlated
with human/mouse CD99 expression, the corresponding mechanism is of
concern. The NF-κB pathway is focused on not only because of its
important role in the pathology of HL, but because it can also act
as a factor affecting cytokines/chemokines and pIκB, which
represents the activated state of the NF-κB pathway analyzed by
western blot analysis. The inhibitor (BAY) and the activator (LPS)
of the NF-κB signaling pathway were applied and sCXCL16 was
analyzed by ELISA. The results indicated that in
A20-mCD99L2− cells the expression of CXCL16 protein was
high and expression of p-IκB protein was somewhat higher than in
A20 and A20-control cells (Fig.
3A). When A20-mCD99L2− cells were treated with
inhibitor BAY, both expressions of p-IκB protein and CXCL16
decreased (Fig. 3A), suggesting
that inhibiting the NF-κB pathway decreases the CXCL16 expression
in the CD99 downregulated cells. By contrast, when A20 cells were
treated with the activator LPS, the expression of p-IκB protein and
CXCL16 increased (Fig. 3A),
suggesting that activating the NF-κB pathway increases the CXCL16
expression in the wild-type A20 lymphoma cells. The results from
the ELISA quantification were consistent with the above data
(Fig. 3B).
 | Figure 3NF-κB signaling is involved in the
regulation of CXCL16 expression. (A) Expression of mouse CXCL16
protein (mCXCL16), phosphorylation of IκB (p-IκB) and mCD99L2
protein in A20 cells, A20 cells treated with LPS (A20+LPS),
A20-control (A20-CTR), A20-mCD99L2− cells and
A20-mCD99L2− cells treated with BAY (A20-mCD99L2+BAY)
were detected by western blotting, respectively. (B) ELISA results
of mouse sCXCL16 secreted by A20-CTR cells, A20-mCD99L2−
cell, A20-CTR cells treated with LPS and A20-mCD99L2−
cells treated with BAY. (C) Western blotting results of human
CXCL16 protein, phosphorylation of IκB (p-IκB) and human CD99
protein in L428 cells, L428 cells treated with BAY (L428+BAY), L428
control (L428-CTR), L428-CD99+ cell treated with LPS
(L428-CD99+LPS) and L428-CD99+ cells, respectively. (D)
ELISA results of human sCXCL16 secreted by L428 control cells
(L428-CTR), L428-CD99+ cells, L428-CTR cells treated
with BAY (L428-CTR+BAY) and L428-CD99+ cells treated
with LPS (L428-CD99++LPS). ELISA data are presented as
means ± SD from 3 independent experiments in triplicate. |
Human CXCL16 is affected by the NF-κB
pathway in human L428 cells as well as in the CD99 upregulated
subcell type line
In human L428-CD99+ cells, CXCL16 was
lower expressed and the expression of p-IκB protein was weaker than
in the control (Fig. 3C).
When L428 cells were treated with the inhibitor BAY,
the expression of p-IκB protein and CXCL16 decreased (Fig. 3C), suggesting that inhibiting the
NF-κB pathway decreases the CXCL16 expression in the CD99
upregulated cells. When L428-CD99+ cells were treated
with the activator LPS, the expression of p-IκB protein and CXCL16
increased (Fig. 3C), suggesting
that activation of the NF-κB pathway increases the CXCL16
expression in human L428 cells. The ELISA results were in agreement
with the above findings (Fig. 3D).
Taken together, our data indicated that the NF-κB signaling pathway
affects the expression and secretion of CXCL16.
CXCL16 is expressed in a series of
lymphoma cell lines
Although CXCL16, including sCXCL16, were measured
above and were highly expressed in human L428 as well as in mouse
A20-mCD99L2− cells, CXCL16 expression in a series of
lymphoma cell lines with other origins was unclear. Therefore,
qRT-PCR and western blotting were used to analyze the relative
expression levels of CXCL16 in 9 lymphoma cell lines. The results
indicated that all 9 lymphoma cell lines expressed CXCL16 mRNA
(Fig. 4A). Compared with the L428
cells, RPMI-8226, KM3 and Jurkat cells transcribed higher CXCL16
mRNA amounts, while the other 5 cell lines (OCI-Ly1, OCI-Ly8,
OCI-Ly10, Karpass299 and Raji cells) transcribed relatively lower
levels of CXCL16 mRNA. CXCL16 protein was expressed higher in L428,
RPMI-8226, KM3 and Jurkat cells while it was expressed lower in the
other 5 cell lines (Fig. 4B).
Functional sCXCL16 is secreted by
different lymphoma cell lines
Since CXCL16 occurs as TM-CXCL16 and sCXCL16, the
secreted sCXCL16 fractions in the 9 cell lines were analyzed by
ELISA. The sCXCL16 in RPMI-8226, KM3 cell supernatants were
significantly lower than in L428 cells (P<0.05), while Jurkat,
Raji, Karpass299, OCI-Ly1, OCI-Ly8 and OCI-Ly10 cell lines secreted
higher amounts of sCXCL16 than the L428 cells (Fig. 4C).
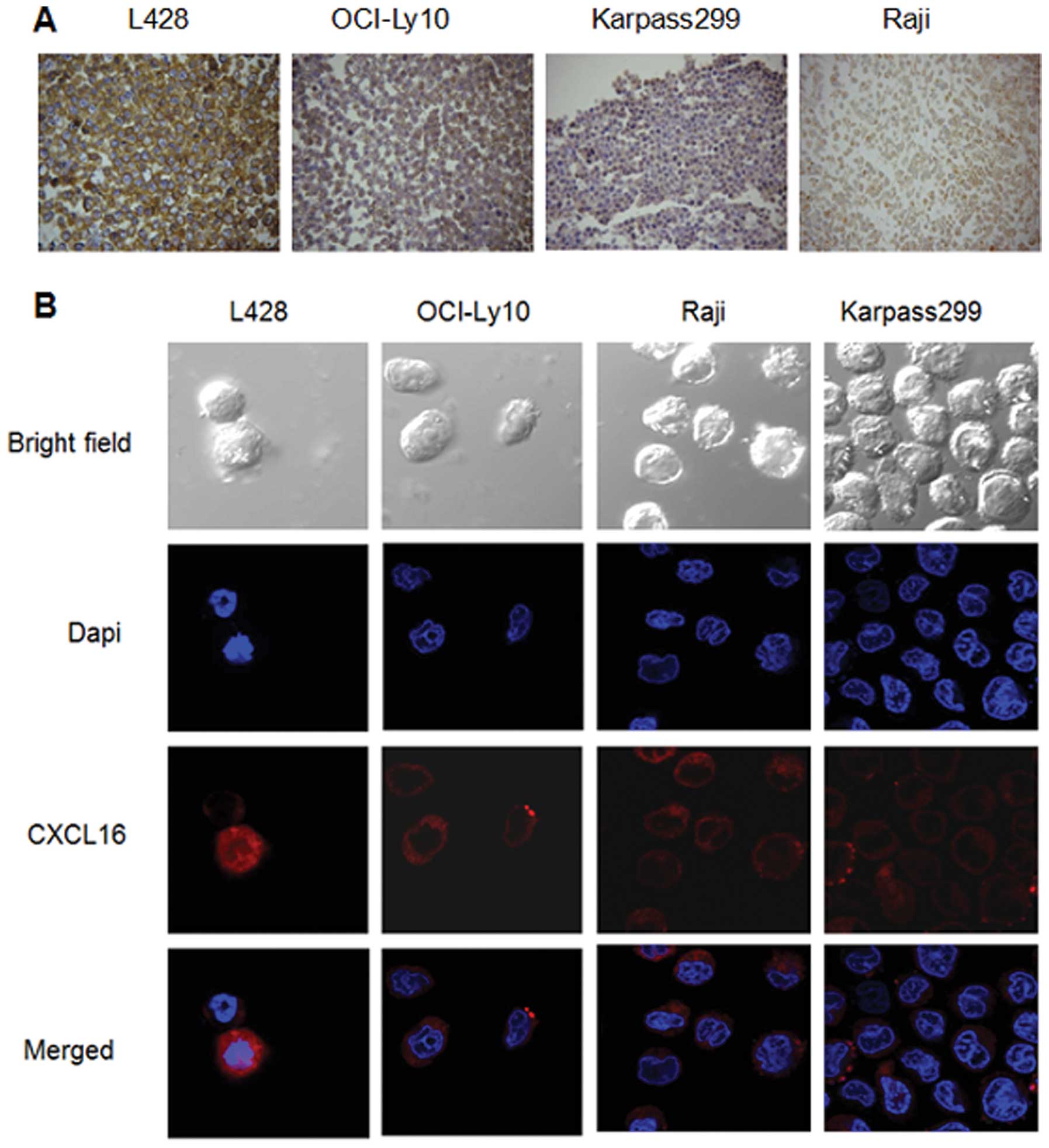
Localization of CXCL16 on the lymphoma
cells
As observed in ICC experiments, specific CXCL16
protein staining was visible in the cytoplasm and membrane
(TM-CXCL16) of malignant lymphoma cells (Fig. 5A). In L428 and OCI-Ly10 cells, the
TM-CXCL16 was significantly predominant, while in Karpass299 and
Raji cells, CXCL16 was expressed mainly in the cytoplasms. These
results were also observed in the confocal microscopy analysis
(Fig. 5B).
CXCL16 is expressed in different types of
lymphoma samples
As the in vitro environment may be different
from in vivo conditions, which involves the interaction
between tumor cells and reactive immune cells, CXCL16 expressions
in clinical samples of different lymphoma types were investigated.
Using IHC staining, we measured the expression levels and
subcellular localizations of CXCL16 protein in 80 archived
paraffin-embedded lymphoma samples (Table V) as well as in 7 reactive and 7
normal lymph node sections. The results showed that the total
positive rate of CXCL16 expression in the lymphomas was 68.8%
(55/80); it was moderately expressed in 37.5% (30/80), strongly
expressed in 31.25% (25/80) and negative in 31.25% (25/80) of the
samples. In comparison, only 14.2% (1/7) of the 7 reactive lymph
node samples and none of the normal lymph nodes expressed CXCL16
protein, which was significantly less than in the lymphoma samples
(P<0.05). In addition, the expression in HL samples was
relatively higher than that in NHL (Table V, Fig.
6B; P<0.05).
 | Table VImmunohistochemical analysis of
CXCL16 expression in clinical lymphoma samples. |
Table V
Immunohistochemical analysis of
CXCL16 expression in clinical lymphoma samples.
| n | − | + | ++ |
|---|
| cHL | 45 | 10 | 16 | 19b |
| NHL | 35 | 15a | 14 | 6 |
Specific CXCL16 protein staining was found positive
in the cytoplasm and membrane of malignant lymphoma tissues,
particularly in the golgi complex where strong staining was visible
(Fig. 6A). In the stromal tissues
and the infiltrated inflammatory cells, CXCL16 expression was
observed only in a few cells and was weak.
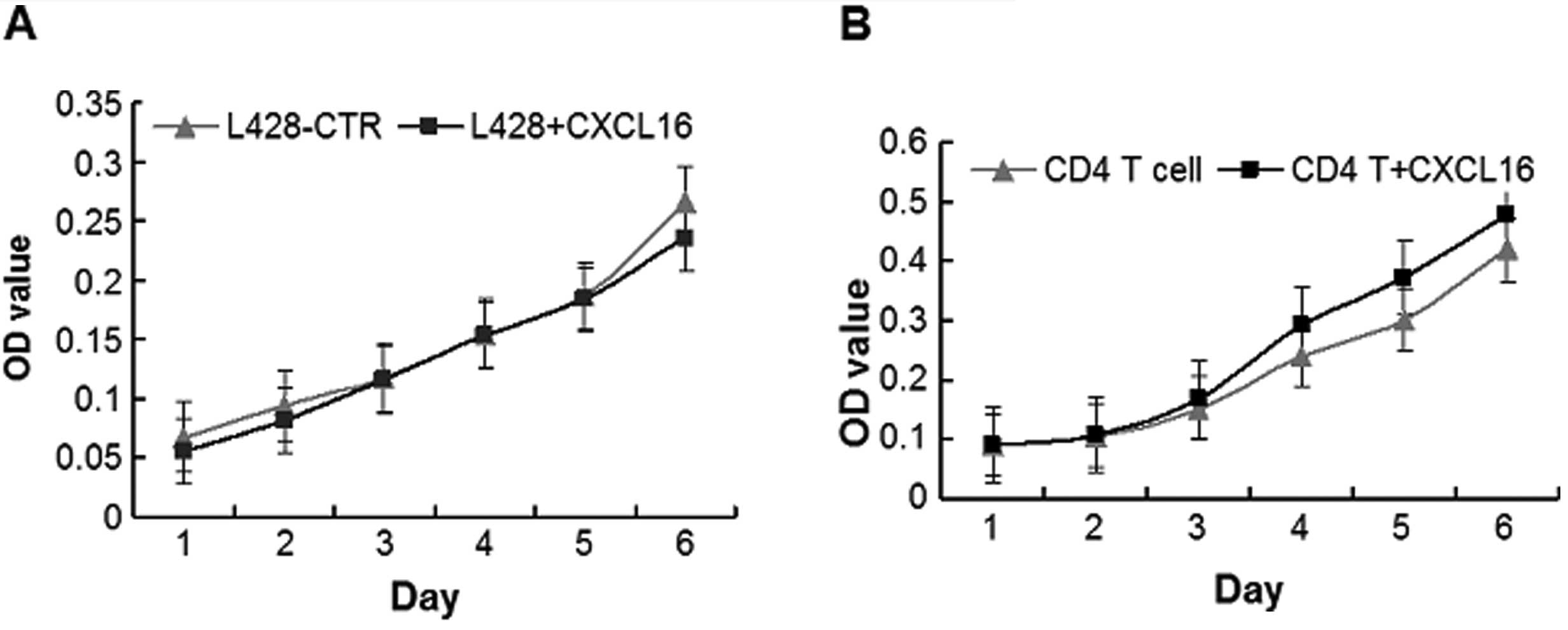
Proliferation of L428 cells and
CD4+ T lymphocyte under stimulation of recombinant human
CXCL16
Since the expression of CXCL16 was high in HL, we
hypothesized a proliferation-promoting effect of sCXCL16 in
vitro, but CCK-8 proliferation assays indicated that there were
no significant changes in the proliferation rates of L428 cells
following incubation with recombinant human CXCL16 from day 1 to
day 6 (Fig. 7A; P>0.05).
Since CD4+ T cells play important roles
in the composition of cHL microenvironments (24), the effect of CXCL16 on the
proliferation ability of CD4+ T cells in vitro
was further analyzed. As a result, the proliferation rate of
CD4+ T cells increased during stimulation with
recombinant human CXCL16, with significant changes from day 4 to
day 6 (Fig. 7B; P<0.05). Our
results suggested that CXCL16 might act indirectly on the H/RS
cells by promoting the crosstalk between CD4+ T and H/RS
cells.
Discussion
Several chemokines were recently reported to be
expressed in tumor tissues affecting the proliferation or
hemostasis of cells, particularly in inflammatory-related tumors.
The role of chemokines in the biology of lymphoma has been
investigated, as lymphomas are considered to be
inflammatory-related tumors (25).
Particularly in the cHL, the neoplastic H/RS cells construct their
unique microenvironment by secreting large amounts of cytokines and
chemokines (26,27) and it is beneficial to elucidate the
significance of the expression and pathological role of chemokines
for lymphoma-related mechanisms. We focused on the CXCL16 as it was
upregulated in the previously established A20-mCD99L2−
cells and was extensively further upregulated in the corresponding
tumor tissues in the present study, both results suggesting that
there may be an association between CD99 and CXCL16. As CD99 may
play a role during the differentiation of B-lymphocytes and may
tend to induce the phenotypes of H/RS cells, which secrete large
amounts of cytokines/chemokines to maintain their unique
microenvironment, it was necessary to confirm a correlation between
CXCL16 and CD99 expression. Our results indicated that the
expression of CXCL16 was inversely correlated with mCD99L2
expression in murine cell lines and with CD99 in human cell lines,
which provides additional insight into the role of CD99 in
phenotypes of H/RS cells. The NF-κB pathway has been focused on due
to its important role in the pathology of HL, but also as a factor
affecting cytokines/chemokines. In the present study, the effect of
the NF-κB pathway on the activation of CXCL16 and CD99 revealed
that NF-κB signaling is involved in the altered CXCL16 expression,
which is inversely correlated with CD99. Since the activated NF-κB
pathway promotes the production of several inflammatory cytokines
and chemokines, CXCL16 might be a downstream target of activated
NF-κB.
CXCL16 is a newly cloned chemokine which belongs to
the CXC family and is expressed both as a membrane and as a
transmembrane protein (TM-CXCL16) or in the supernatants in its
soluble form (sCXCL16) (13).
CXCL16 was found to be overexpressed in several types of cancer,
such as colorectal cancer (CRC) (18), prostate cancer (19), epithelial ovarian carcinoma
(20) and melanoma (21), while in pancreatic ductal
adenocarcinoma patients CXCL16 is also increased in the sera
(28). Preoperative high serum
levels of CXCL16 were associated with metachronous liver recurrence
and poor prognosis in CRC patients (30), and in prostate cancer CXCL16
production is particularly increased in aggressive cancer cells
(29). Although CXCL16 has been
shown to be involved in several inflammatory conditions and
inflammation-related tumors, little is known about the
characteristic and function of CXCL16 on lymphomas, which can be
classified into several types and subtypes. The present study
indicated for the first time that CXCL16 and sCXCL16 were expressed
in diverse types of lymphoma cell lines. Both TM-CXC16 and sCXCL16
are highly expressed in the HL cell line (L428), which is
consistent with a report of Hanamoto et al(31). sCXCL16 was highly secreted from some
cell lines of T origin, but rather low expressed in cell lines of
plasma cell origin, indicating that the secretion of CXCL16 may be
different according to the origins and the differentiation states
of the lymphocytes. The role of both forms during tumor progression
that was only described as TM-CXCL16 seems to be a signal of good
prognosis, whereas high serum sCXCL16 is a signal of poor prognosis
(32). The diverse expression
pattern as TM-CXCL16 or sCXCL16 in lymphomas indicated that the
chemokine might play diverse roles in different types of
lymphomas.
The in vitro conditions were quite different
from the in vivo conditions, in which the microenvironment
is affected by infiltrating lymphocytes and the tumor cells are
involved in complex interactions between tumor and body
immunoreactions. In the present study, we observed the expression
of CXCL16 in 80 clinical lymphoma samples and found that the
expression of CXCL16 was relatively higher in HL than in NHL
specimens. The clinical prognosis of NHL and HL are quite
different; compared with NHL, most patients with HL have a good
prognosis after a therapy (33).
Although there was also expression of CXCL16 in some of the NHL
samples, the difference of CXCL16 expression between HL and NHL
may, to some extent, reflect the different microenvironments of the
two types of lymphoma. Our cell line and clinical sample research
indicated a close relationship between CXCL16 expression and HL.
Consistent with in vitro results in cHL, CXCL16 was
especially expressed in the neoplastic H/RS cells instead of the
infiltrating lymphocytes, indicating that the tumor cells can
produce and secrete the chemokine by themselves. Reports showed
that CXCL16 might facilitate survival, adhesion and migration of
tumor cells. For example, sCXCL16 could also promote the
proliferation of adenocarcinoma (28) and may play a role in liver
metastases through the induction of EMT in CRC patients (30). CXCL16 could enhance the
proliferative capacity of melanoma cells by its receptor CXCR6,
which was recently shown to be involved in stem cell self-renewal
(21). By contrast, our results
showed, that CXCL16 has no direct effect on H/RS cells, but
promotes CD4+ T-cell proliferation, which in turn might
indirectly influence the survival of H/RS cells, similar to the
role of radiation-induced CXCL16 in the attraction of effector T
cells in breast cancer (34).
Whether in HL CXCL16 may be considered a potential molecule for
T-cell homing (35) requires
further research of the relationship between CXCL16 cells and
CD4+ T lymphocytes.
In conclusion, the present study extended our
previous results by finding a correlation and signaling pathway
between CD99 and CXCL16. The expressions of CXCL16 and functional
sCXCL16 were investigated systematically in various cell lines and
clinical samples of different origins for the first time. The
diversity of CXCL16 expressions suggested a high complexity of
chemokine secretions in subtypes of lymphomas. The highly expressed
CXCL16 in cHL and its effect on CD4+ T-lymphocyte
proliferation provide a potential target for a biological cHL
therapy.
Acknowledgements
This study was supported by a grant for the National
Natural Science Foundation of China (grant nos. 81101537, 81272552
and 81071941). The authors thank Professor K.C. Chan, the Nebraska
Medical Center, USA, Professor Xiaoyan Zhou, Pathology Department
of Fudan University Shanghai Cancer Center, Professor Longjun Gu,
Xinhua Hospital of Medicine School, Shanghai Jiao Tong University,
and Department of Hematology of the Zhujiang Hospital for providing
the cell lines.
Abbreviations:
|
ALCL
|
anaplastic large cell lymphoma
|
|
CD99
|
cell differentiation antigen 99
|
|
cHL
|
classical Hodgkin lymphoma
|
|
CXCL16
|
CXC chemokine ligand 16
|
|
DLBCL
|
diffuse large B-cell lymphoma
|
|
HL
|
Hodgkin lymphoma
|
|
H/RS
|
Hodgkin Reed-Sternberg
|
|
mCD99L2
|
mouse CD99 antigen-like 2
|
|
MM
|
multiple myeloma
|
|
NHL
|
non-Hodgkin lymphoma
|
|
IL-6
|
interleukin-6
|
|
IL-10
|
interleukin-10
|
|
VCAM-1
|
vascular cell adhesion molecule 1
|
|
MIP-1γ
|
macrophage inflammatory protein-1γ
|
|
PF4
|
platelet factor 4
|
|
MIG
|
monokine induced by interferon-γ
|
|
TNF-α
|
tumor necrosis factor-α
|
|
U-PTL
|
peripheral T-cell lymphoma,
unspecified
|
|
RANTES
|
regulated upon activation normal
T-cell expressed and secreted
|
References
|
1
|
Aggarwal BB, Shishodia S, Sandur SK, et
al: Inflammation and cancer: how hot is the link? Biochem
Pharmacol. 72:1605–1621. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
O’Hayre M, Salanga CL, Handel TM, et al:
Chemokines and cancer: migration, intracellular signalling and
intercellular communication in the microenvironment. Biochem J.
409:635–649. 2008.PubMed/NCBI
|
|
3
|
Yamashita H, Takahashi Y, Kano T, et al:
Malignant lymphoma presenting as inflammation of unknown origin.
Nihon Rinsho Meneki Gakkai Kaishi. 35:136–143. 2012.(In
Japanese).
|
|
4
|
Aldinucci D, Gloghini A, Pinto A, et al:
The classical Hodgkin’s lymphoma microenvironment and its role in
promoting tumor growth and immune escape. J Pathol. 221:248–263.
2010.
|
|
5
|
Küppers R: The biology of Hodgkin’s
lymphoma. Nat Rev Cancer. 9:15–27. 2009.
|
|
6
|
Kim SH, Choi EY, Shin YK, et al:
Generation of cells with Hodgkin’s and Reed-Sternberg phenotype
through downregulation of CD99 (Mic2). Blood. 92:4287–4295.
1998.
|
|
7
|
Kim SH, Shin YK, Lee IS, et al: Viral
latent membrane protein1 (LMP-1)-induced CD99 down-regulation in B
cells leads to the generation of cells with Hodgkin’s and
Reed-Sternberg phenotype. Blood. 95:294–300. 2000.
|
|
8
|
Huang X, Zhou X, Wang Z, et al: CD99
triggers upregulation of miR-9-modulated PRDM1/BLIMP1 in
Hodgkin/Reed-Sternberg cells and induces redifferentiation. Int J
Cancer. 131:E382–E394. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Suh YH, Shin YK, Kook MC, et al: Cloning,
genomic organization, alternative transcripts and expression
analysis of CD99L2, a novel paralog of human CD99, and
identification of evolutionary conserved motifs. Gene. 307:63–76.
2003. View Article : Google Scholar
|
|
10
|
Liu F, Zhang G, Liu F, et al: Effect of
shRNA targeting mouse CD99L2 gene in a murine B cell lymphoma in
vitro and in vivo. Oncol Rep. 29:1405–1414.
2013.PubMed/NCBI
|
|
11
|
Matloubian M, David A, Engel S, et al: A
transmembrane CXC chemokine is a ligand for HIV-coreceptor Bonzo.
Nat Immunol. 1:298–304. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wilbanks A, Zondlo SC, Murphy K, et al:
Expression cloning of the STRL33/BONZO/TYMSTR ligand reveals
elements of CC, CXC, and CX3C chemokines. J Immunol. 166:5145–5154.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hundhausen C, Schulte A, Schulz B, et al:
Regulated shedding of transmembrane chemokines by the disintegrin
and metalloproteinase 10 facilitates detachment of adherent
leukocytes. J Immunol. 178:8064–8072. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ruth JH, Haas CS, Park CC, et al:
CXCL16-mediated cell recruitment to rheumatoid arthritis synovial
tissue and murine lymph nodes is dependent upon the MAPK pathway.
Arthritis Rheum. 54:765–778. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Morgan AJ, Guillen C, Symon FA, et al:
Expression of CXCR6 and its ligand CXCL16 in the lung in health and
disease. Clin Exp Allergy. 35:1572–1580. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sheikine Y, Bang CS, Nilsson L, et al:
Decreased plasma CXCL16/SR-PSOX concentration is associated with
coronary artery disease. Atherosclerosis. 188:462–466. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li X, Conklin L and Alex P: New
serological biomarkers of inflammatory bowel disease. World J
Gastroenterol. 14:5115–5124. 2008. View Article : Google Scholar
|
|
18
|
Verbeke H, Struyf S, Laureys G, et al: The
expression and role of CXC chemokines in colorectal cancer.
Cytokine Growth Factor Rev. 22:345–358. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ha HK, Lee W, Park HJ, et al: Clinical
significance of CXCL16/CXCR6 expression in patients with prostate
cancer. Mol Med Rep. 4:419–424. 2011.PubMed/NCBI
|
|
20
|
Guo L, Cui ZM, Zhang J, et al: Chemokine
axes CXCL12/CXCR4 and CXCL16/CXCR6 correlate with lymph node
metastasis in epithelial ovarian carcinoma. Chin J Cancer.
30:336–343. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Taghizadeh R, Noh M, Huh YH, et al: CXCR6,
a newly defined biomarker of tissue-specific stem cell asymmetric
self-renewal, identifies more aggressive human melanoma cancer stem
cells. PLoS One. 5:e151832010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Darash-Yahana M, Gillespie JW, Hewitt SM,
et al: The chemokine CXCL16 and its receptor, CXCR6, as markers and
promoters of inflammation-associated cancers. PLoS One.
4:e66952009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Feng MJ, Qiu C, Lai YJ, et al: Application
of immunomagnetic screening strategy for separation of
CD4+and CD8+T cell subpopulations of
peripheral blood. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 13:205–209.
2005.(In Chinese).
|
|
24
|
Zhang Y, Li XZ, Wen ZH, et al:
Relationship between lymphocyte immunophenotypes and histologic
subtypes of Hodgkin’s lymphoma and its significance. Zhongguo Shi
Yan Xue Ye Xue Za Zhi. 17:888–893. 2009.(In Chinese).
|
|
25
|
Bracci PM, Skibola CF, Conde L, et al:
Chemokine polymorphisms and lymphoma: a pooled analysis. Leuk
Lymphoma. 51:497–506. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Aldinucci D, Lorenzon D, Cattaruzza L, et
al: Expression of CCR5 receptors on Reed-Sternberg cells and
Hodgkin lymphoma cell lines: involvement of CCL5/Rantes in tumor
cell growth and microenvironmental interactions. Int J Cancer.
122:769–776. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hnátková M, Mociková H, Trnený M, et al:
The biological environment of Hodgkin’s lymphoma and the role of
the chemokine CCL17/TARC. Prague Med Rep. 110:35–41. 2009.
|
|
28
|
Wente MN, Gaida MM, Mayer C, et al:
Expression and potential function of the CXC chemokine CXCL16 in
pancreatic ductal adenocarcinoma. Int J Oncol. 33:297–308.
2008.PubMed/NCBI
|
|
29
|
Hu W, Zhen X, Xiong B, et al: CXCR6 is
expressed in human prostate cancer in vivo and is involved in the
in vitro invasion of PC3 and LNCap cells. Cancer Sci. 99:1362–1369.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Matsushita K, Toiyama Y, Tanaka K, et al:
Soluble CXCL16 in preoperative serum is a novel prognostic marker
and predicts recurrence of liver metastases in colorectal cancer
patients. Ann Surg Oncol. 19(Suppl 3): S518–S527. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hanamoto H, Nakayama T, Miyazato H, et al:
Expression of CCL28 by Reed-Sternberg cells defines a major subtype
of classical Hodgkin’s disease with frequent infiltration of
eosinophils and/or plasma cells. Am J Pathol. 164:997–1006.
2004.PubMed/NCBI
|
|
32
|
Deng I, Chen N, Li Y, et al: CXCR6/CXCL16
functions as a regulator in metastasis and progression of cancer.
Biochim Biophys Acta. 1806:42–49. 2010.PubMed/NCBI
|
|
33
|
Jakovic LR, Mihaljevic BS, Perunicic
Jovanovic MD, et al: The prognostic relevance of tumor associated
macrophages in advanced stage classical Hodgkin lymphoma. Leuk
Lymphoma. 52:1913–1919. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Matsumura S, Wang B, Kawashima N, et al:
Radiation-induced CXCL16 release by breast cancer cells attracts
effector T cells. J Immunol. 181:3099–3107. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Machado L, Jarrett R, Morgan S, et al:
Expression and function of T cell homing molecules in Hodgkin’s
lymphoma. Cancer Immunol Immunother. 58:85–94. 2009.
|