Introduction
In thoracic tumor radiotherapy, radiation-induced
pulmonary injury (RIPI) is the main dose restrictive and common
complication which seriously impairs the local control of thoracic
tumors, particularly non-small cell lung cancer that is unsuitable
for surgery (1,2). Moreover, RIPI affects the prognosis of
lymphoma or breast cancer patients who were confirmed to have
extended survival following radiotherapy (2,3). Thus,
it is necessary to overcome the restraint of RIPI, in order to
improve the efficacy of thoracic radiotherapy in the future.
The transducer of erbB2, 1 (TOB1) gene is located on
chromosome 17q21, and was originally discovered as a member of the
antiproliferative protein family TOB/BTG (4). Numerous studies have revealed the
tumor-suppressor properties of TOB1 in different types of human
malignancies (5–9). Recently, our laboratory also confirmed
that TOB1 may radiosensitize human cancer cell lines such as
cervical cancer cell line HeLa, breast cancer cell lines MCF-7 and
MDA-MB-231 (10,11), and human lung cancer cell line A549
(unpublished data). However, few studies have investigated the
influence of TOB1 on the radiosensitivity of normal tissues or cell
lines.
In the present study, parental normal human
epithelial cell line HBE and HBE-overexpressing TOB1 were used as
cell models. RT-PCR, western blot analysis, clonogenic assay and
immunofluorescence assay were used to reveal the effects of TOB1 in
regards to the radiation protective effects on normal cells. Flow
cytometric assay, western blot analysis and pathway-specific
inhibitors were applied to elucidate the related mechanisms.
Together with previous studies, we report the theoretical and
experimental data on clinical lung cancer radiotherapy and
radiation protection.
Materials and methods
Cell culture, transfection and
irradiation
Human bronchial epithelial cells were originally
obtained from the American Type Culture Collection (ATCC, Manassas,
VA, USA), maintained in our laboratory and cultured with Dulbecco’s
modified Eagle’s medium (Invitrogen, Carlsbad, CA, USA) with 10%
fetal bovine serum (Invitrogen), at 37°C in an atmosphere of 5%
CO2. Medium was replaced every 2 days, and cells were
subcultured every 3 days. Recombinant plasmid pcDNA3.0/TOB1 was
constructed by our laboratory as previously described (10). HBE cells were transfected with the
vector and pcDNA3.0/TOB1 using Lipofectamine (Invitrogen) and
maintained in medium containing 500 μg/ml G418 (Sigma Aldrich, MO,
USA) until resistant cell clones were formed. Irradiation was
performed with 6-MeV X-ray linear accelerator (Siemens KD2,
Germany) with a dose rate of 200 cGy/min, with an irradiating area
of 20×20 cm and 100-cm source-skin distance.
Clonogenic assay
The exponentially growing cells were plated at
different cell densities and irradiated with 0, 0.5, 1, 2, 4, 6 Gy
of X-rays. After 12–14 days of incubation at 37°C, cells were fixed
in methanol followed by Giemsa staining. The number of colonies per
dish was counted, and the surviving fractions were calculated as
the ratio of plating efficiencies for irradiated and unirradiated
cells. Plating efficiency is defined as the colony number divided
by the number of cells plated for unirradiated controls.
Experiments were conducted in triplicate, and data are presented as
means ± standard deviation (SD) from three independent experiments.
All surviving fractions were fitted into the linear quadratic model
using GraphPad Prism 5.0 software (GraphPad Software Inc., La
Jolla, CA, USA).
Flow cytometric assay
Cells were removed with trypsin and collected into
centrifuge tubes together with the culture medium. The detailed
methods for flow cytometry and Annexin V-FITC apoptosis analysis
have been previously described (10). The cell cycle distribution and
apoptotic rate were calculated from 10,000 cells using ModFit LT
software (Becton-Dickinson, San Jose, CA, USA) using FACSCalibur
(Becton-Dickinson).
Immunofluorescence detection for
phosphorylated γ-H2AX
Cells were grown on sterile glass chambers
(Becton-Dickinson and Company, Franklin Lakes, NJ, USA). After a
single dose of X-ray exposure, all cells were fixed in 4%
formaldehyde before being permeabilized in 0.25% Triton X-100. The
chamber was blocked with 1% BSA for at least 1 h. The primary
anti-phosphorylation monoclonal antibody γ-H2AX (1:200, Epitomics,
Burlingame, CA, USA) was added and incubated for 2 h at room
temperature. Specific staining was visualized with a secondary
antibody conjugated to FITC 488. Hochest 33342 (Sigma Aldrich, St.
Louis, MO, USA) was utilized for nuclear staining. Fluorescence was
observed using a laser scanning confocal microscope at a wavelength
of 488 nm within 30 min. The number of foci in 20 cells in each
sample was counted randomly.
Western blot analysis
Western blot analysis was performed as previously
described (10). Briefly, the
following primary antibodies were used for immunoblotting: β-actin
(C-4), TOB1 (N-20), XRCC 1 (33-2-5), FEN1 (B-4), ATM (C-20), MRE11
(C-16) (1:1000 dilution; all from Santa Cruz Biotechnology, Santa
Cruz, CA, USA). The protein bands were visualized using an enhanced
chemiluminescence system (Hangzhou Youither Bioscience Co., Ltd.,
Hangzhou, China) with prestained markers as molecular size
standards.
RT-PCR assay
TOB1 mRNA expression was determined using
semi-quantitative RT-PCR assays. The PCR reaction conditions and
cycle numbers were rigorously adjusted so that each reaction
occurred within the linear range of amplification. The detailed
methods for RNA isolation, cDNA synthesis and RT-PCR analyses have
been previously described (10,12).
For specific intent genes, the PCR primers were as follows: GAPDH
sense, 5′-CAA CTA CAT GGT CTA CAT GTT CC-3′ and antisense, 5′-CAA
CCT GGT CCT CAG TGT AG-3′; TOB1 sense, 5′-GGA TCG ACC CAT TTG AGG
TTT CT-3′ and anti-sense-5′-CTA CCC AAG CCA AGC CCA TAC AG-3′. The
PCR products were analyzed via electrophoresis through 1% agrose
gels containing 0.1 mg/ml ethidium bromide (EB). The gels were
photographed under ultraviolet light.
Statistical comparisons
The data are presented as means and standard
deviations. Statistical comparisons of the experimental results
between the treated group and the control group were carried out
using the two-tailed Student’s t-test. All statistical tests were
performed using the SPSS version 17.0. P-value <0.05 between
groups was considered statistically significant.
Results
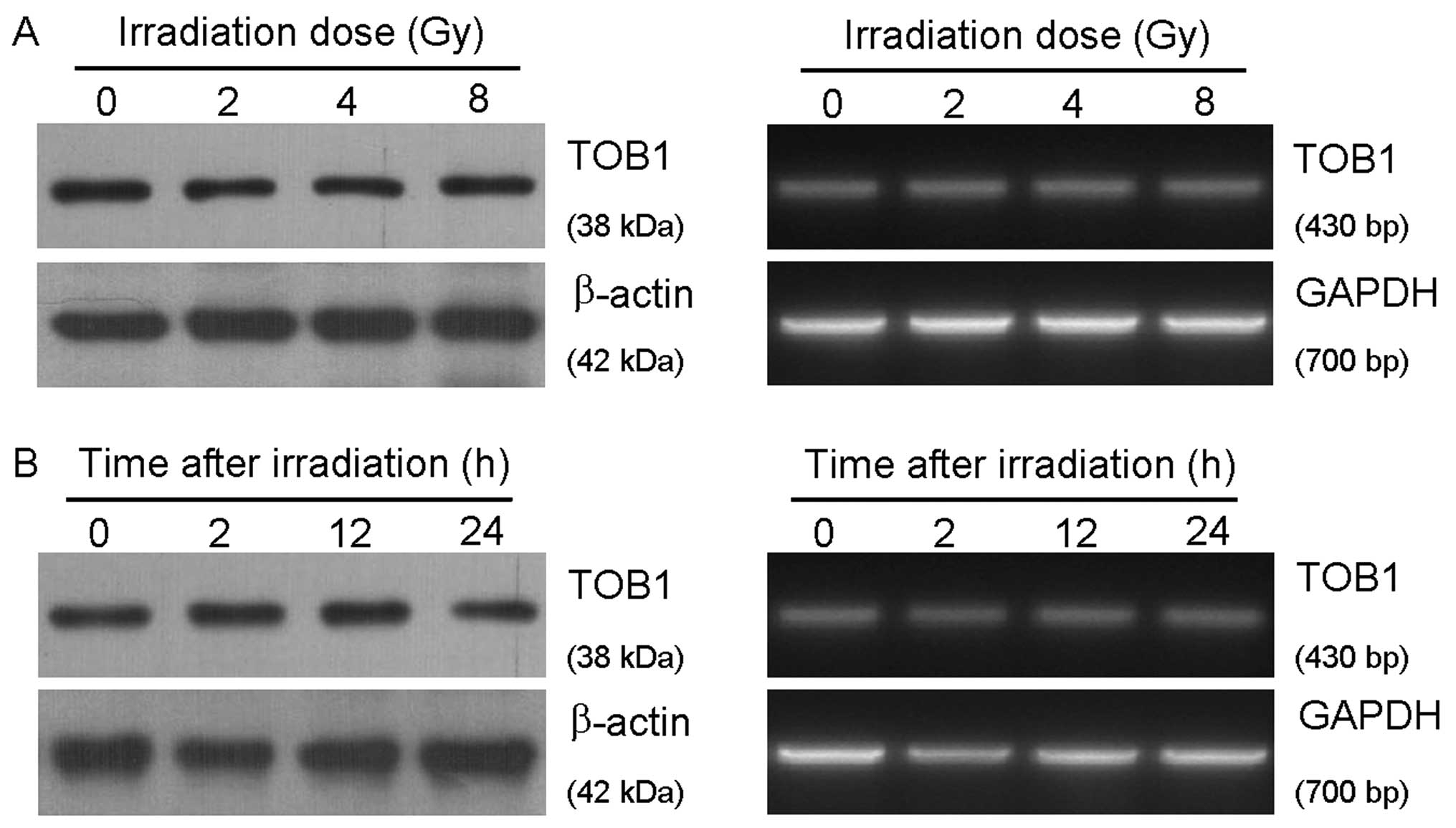
TOB1 expression in the normal human
bronchial epithelial cell line HBE is not induced by ionizing
radiation (IR)
HBE cells were respectively irradiated with 0, 2, 4
and 8 Gy of X-rays generated by a linear accelerator and harvested
after 24 h. The cell samples were also collected 2, 12 and 24 h
after exposed to 6-Gy irradiation. The relevant expression level of
TOB1 mRNA and protein was determined by semi-quantitative RT-PCR
and western blot analysis. As shown in Fig. 1, IR did not increase the expression
level of TOB1, neither in a dose-dependent nor in a time-dependent
manner.
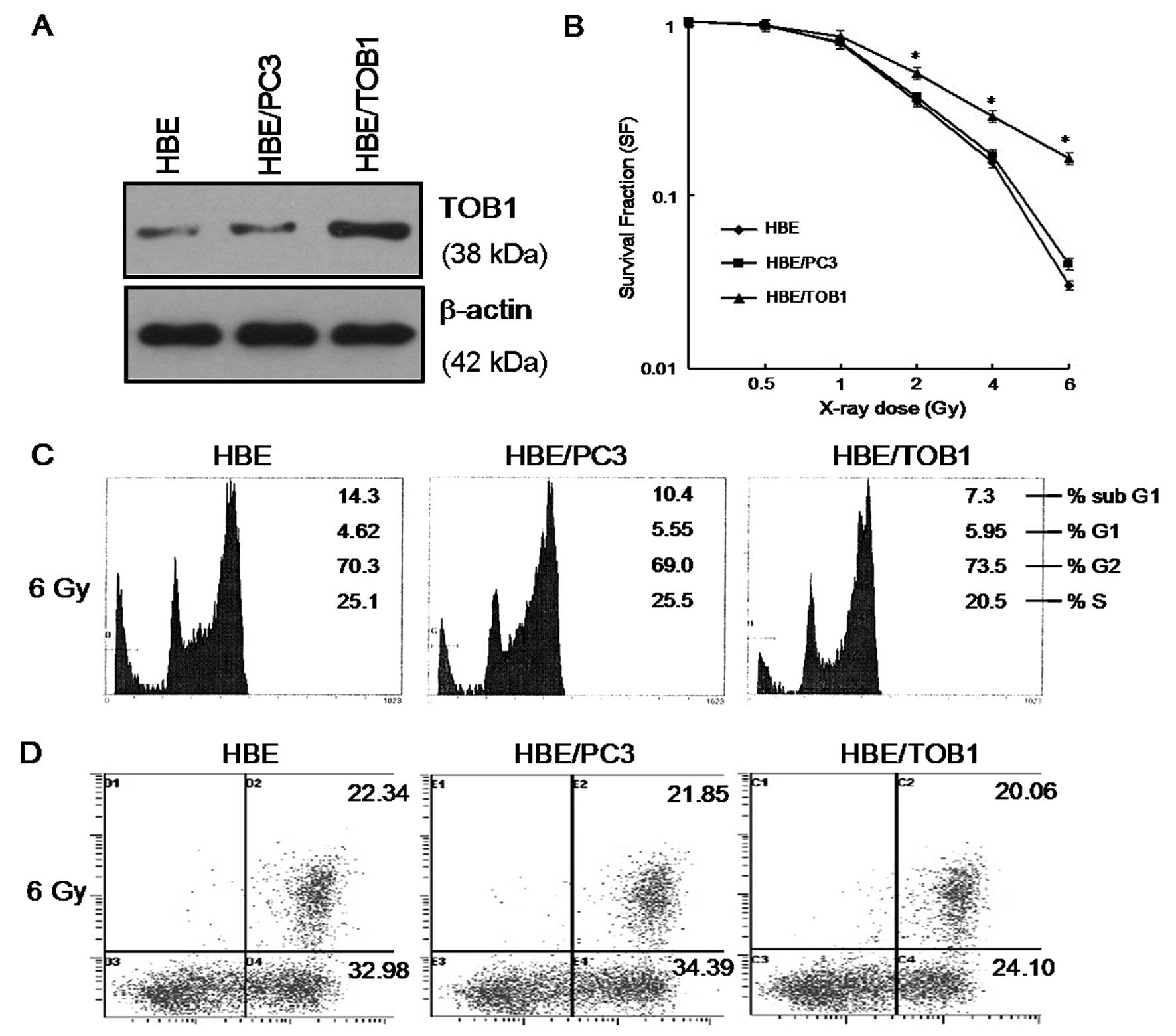
Overexpression of TOB1 reduces the
sensitivity of HBE cells to IR
To investigate whether TOB1 overexpression affects
the radiosensitivity of HBE cells, the TOB1 stable transfectant
(HBE/TOB1) was used. The TOB1 expression was determined by western
blot analysis (Fig. 2A). As shown
in Fig. 2B, the parental HBE cell
line and ‘mock’ transfectant HBE/PC3 both showed a typical
clonogenic survival curve with a shoulder representing the
potential DNA damage repair, while exogenous TOB1 significantly
protected HBE cells from the IR-induced damage.
Flow cytometry assay was also performed 24 h after a
single-dose irradiation of 6-Gy of X-rays. As shown in Fig. 2C, all cell lines exhibited typical
IR-induced cell cycle G2/M arrest without significant difference
(P>0.05). However, the apoptotic cell portion of HBE/TOB1
(24.1%) was obviously less than HBE (32.98%) and HBE/PC3 (34.39%)
after IR (P<0.05) (Fig. 2D).
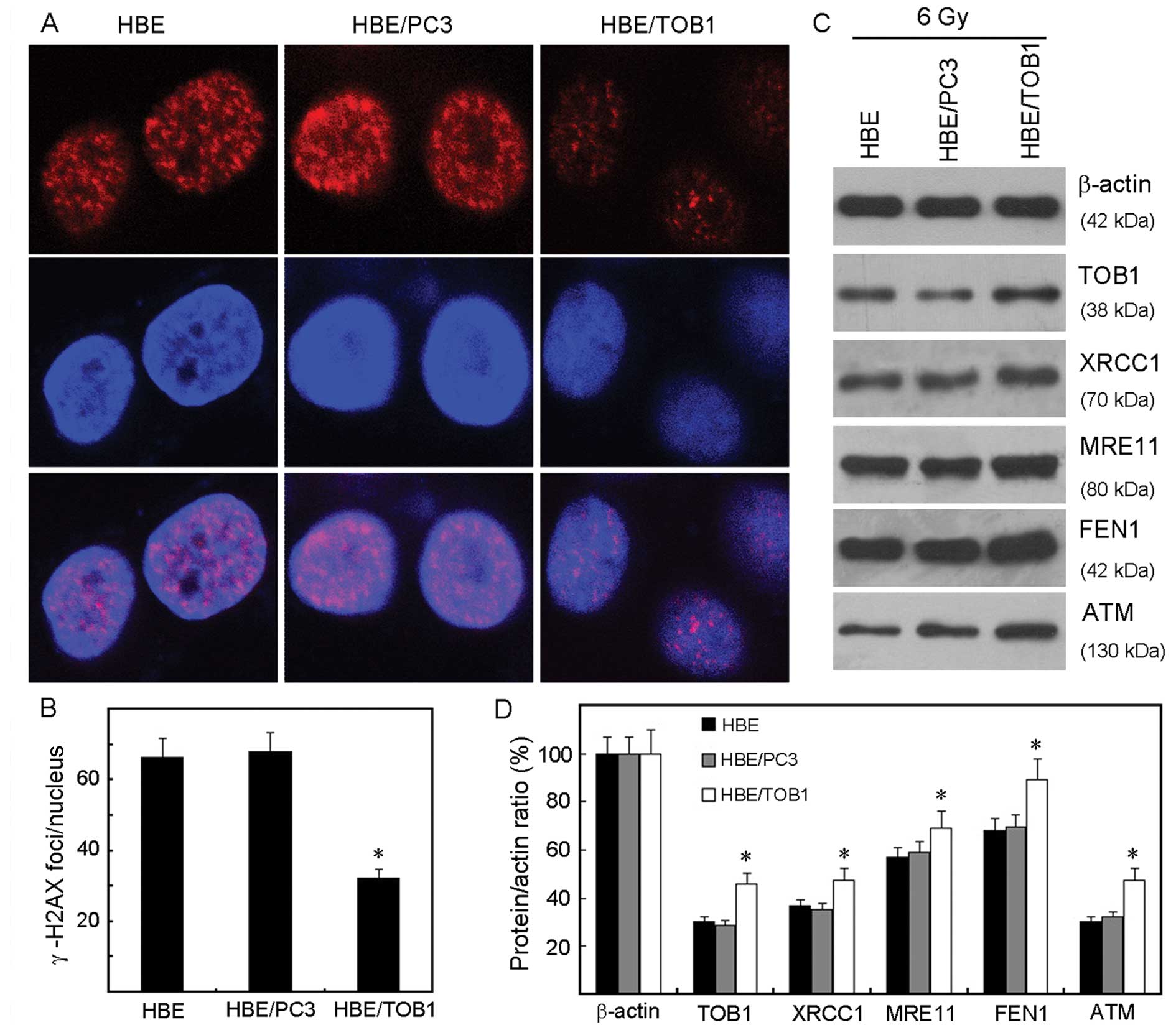
Exogenous TOB1 increases DNA damage
repair in HBE cells
In order to gain insight into the molecular
mechanisms of the radioprotective effect of TOB1 and to investigate
its effect on the initial DNA damage response to IR, the induction
of DNA double-strand breaks (DSBs), as analyzed by the formation of
phospho-γ-H2AX foci, was measured 2 h after irradiation of the HBE
cells, HBE/PC3, and HBE/TOB1 cells. The results showed that
IR-induced γ-H2AX foci were decreased in HBE/TOB1 cells 2 h
post-radiation, in contrast to the control cells (Fig. 3A and B) suggesting facilitation of
DNA damage repair.
Furthermore, we explored the underlying molecular
mechanisms related to different IR-induced DNA damage responses due
to TOB1 status. As shown in Fig. 3C and
D, compared with the HBE/PC3 and parental cells, the expression
levels of important DNA repair proteins such as XRCC1, MRE11, FEN1
and ATM were increased in the HBE/TOB1 cells after IR.
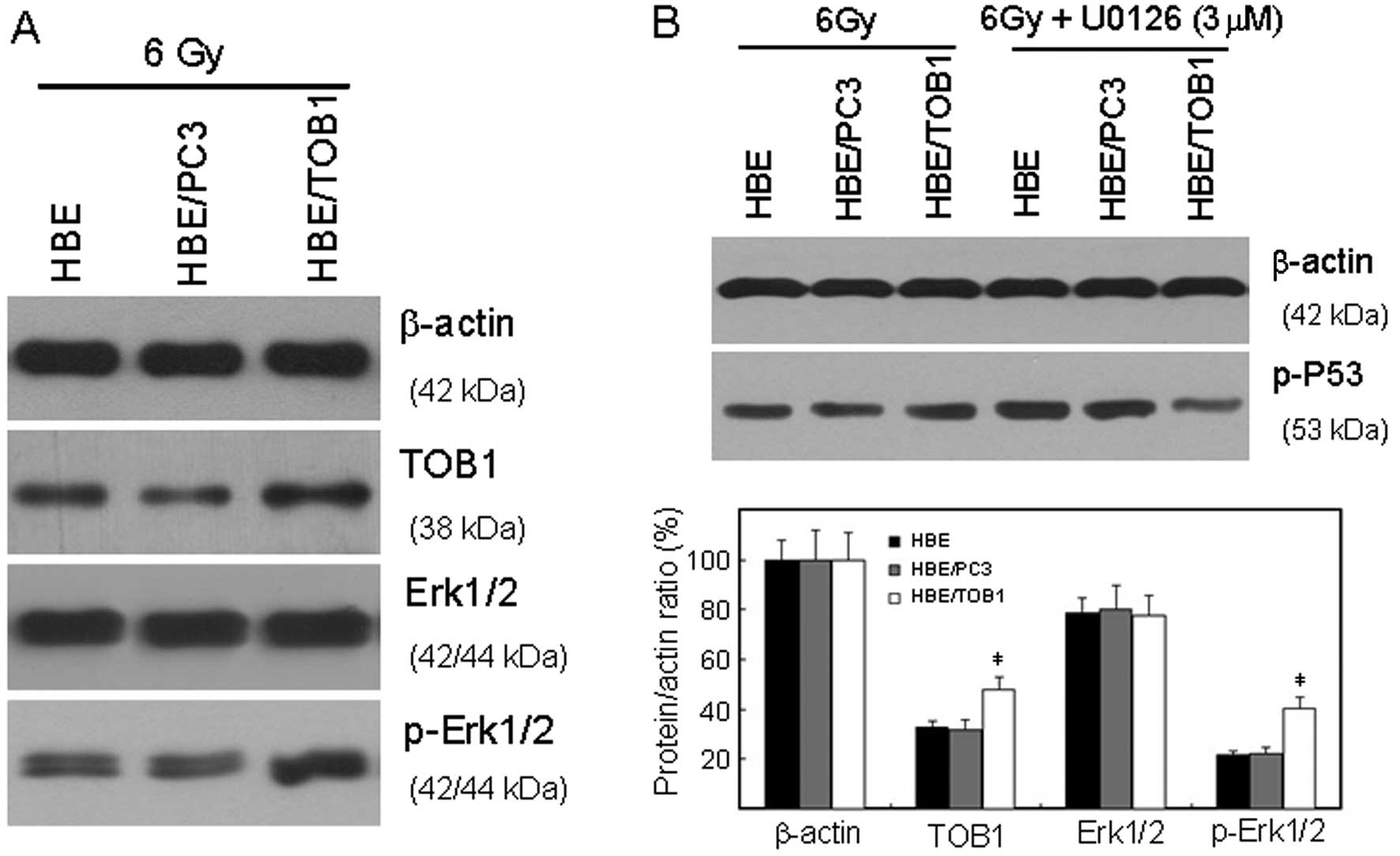
TOB1 regulates the radiosensitivity of
HBE via the MAPK/ERK signaling pathway
Phosphorylation and subsequent activation of p53, as
well as its transcription induction, are key initial responses to
stress responses (13). Therefore,
western blot analysis was used to investigate the related
mechanisms through which TOB1 is involved in radioprotection of
normal cells. In the present study, our results revealed that
overexpression of TOB1 significantly increased IR-induced
phosphorylation of p53 and ERK1/2 (Fig.
4).
We then explored the specific signaling cascade
involved in this response by using the MEK1/2 inhibitor (U0126)
specific to the MAPK/ERK signaling pathway. The increased
phosphorylation of p53 increased by TOB1 overexpression after IR
was blocked by U0126 (Fig. 4B).
Discussion
At present, although 70% of cancer patients require
radiotherapy, it is still a difficult task to protect adjacent
normal tissue and to increase the precision of dose delivery to the
target tumor for radiotherapy (14,15).
Gene therapy targeted by radiation may be a promising solution in
which the complementary DNA for a cytotoxic gene is ligated
downstream of IR-inducible promoters, which involve the expression
of immediate early genes (16). In
our previous study, it was demonstrated that TOB1 was IR-inducible
in lung cancer cell lines A549 and NCI-H1975, and that TOB1
radiosensitized lung cancer cells through certain signaling
pathways (unpublished data). However, for the first time, we
revealed the different biological functions of TOB1 in the
immortalized normal cell line HBE. The expression of TOB1 was
neither induced by X-rays, nor did it increase the radiosensitivity
of HBE cells (Figs. 1 and 2). On the contrary, TOB1 overexpression
obviously decreased the sensitivity of HBE to X-ray irradiation
(Fig. 2B). All of these results
together provide us with clues to the potential gene therapy
application of TOB1 in clinical radiotherapy.
A double-strand break (DSB) of DNA, the main
biological target of IR, determines mutation or cell death after IR
(17–19). The ability to repair DSBs determines
the sensitivity of mammalian cells to IR (18,20).
The recruitment of several proteins to the damage site is the
initial cellular response to DSBs, through which γ-H2AX focus
formation is one of the earliest event (19,21).
In our study, exogenous TOB1 reduced the amount of γ-H2AX foci
induced by IR in the HBE cells (Fig. 3A
and B), and decreased the percentage of apoptotic HBE cells
after IR (Fig. 2D), which indicated
either a decrease in DNA damage or an increase in DNA repair.
In response to IR-induced DSBs, DNA damage signals
are transmitted through several pathways, from which ataxia
telangiectasia-mutated (ATM) signaling plays perhaps the most
important role (13,22,23).
IR-induced ATM activation leads to the phosphorylation not only of
γ-H2AX, but also of other important downstream effectors of DDR
(apoptosis-related p53 and MDM2; cell cycle checkpoint-related
CHK1/2; DNA repair-related MRE11) (13,24–26).
In our study, exogenous TOB1 obviously increased the expression of
ATM and its downstream DNA repair effectors XRCC1, MRE11, and FEN1
(Fig. 3C and D). These data suggest
that TOB1 overexpression promotes IR-induced DNA repair, thus,
further confirming the feasibility of TOB1 application for thoracic
radiotherapy.
Our previous studies demontrated that the MAPK/ERK
pathway plays a significant role in cell survival after IR
regulated by TOB1 (unpublished data). We demonstrated in this study
that TOB1 overexpression enhanced the phosphorylation status of
ERK1/2 in HBE cells, which was mainly associated with MAPK
activation (Fig. 4A). After
exposure to IR the activated MAPK pathway leads to the
phosphorylation of p53 at multiple sites, which are key initial
responses to cell cycle distribution and DNA damage (27,28).
Our results revealed that exogenous TOB1 strongly enhanced
IR-induced p53 phosphorylation in normal HBE cells, suggesting
these cells have a more robust DNA damage surveillance and repair
mechanism helping them either adapt to or overcome critical
IR-induced DNA damage (Fig. 4A).
Furthermore, the MAPK/ERK pathway-specific inhibitor U0126 was used
just following IR. As shown in Fig.
4B, the serine 15 phosphorylation of p53 after IR was
significantly inhibited by U0126 in the HBE/TOB1 cells.
In conclusion, although the mechanisms of how TOB1
is involved in IR-induced DNA damage are still to be fully
elucidated, the results of the present study together with previous
results in lung cancer cell lines, suggest that gene therapeutic
approaches enhancing TOB1 expression may alleviate the side effects
of radiotherapy as well as enhance radiotherapeutic benefit.
Acknowledgements
The present study was supported by grants from the
Program for the Doctoral Fund of the Ministry of Education of China
(20103201120016), the Hospital Management Center of Medical Science
and Technology Development Foundation of Wuxi (YGM1101), the
National Science Foundation of China (81170468) and the PAPD.
References
|
1
|
Hunter NR, Valdecanas D, Liao Z, Milas L,
Thames HD and Mason KA: Mitigation and treatment of
radiation-induced thoracic injury with a cyclooxygenase-2
inhibitor, celecoxib. Int J Radiat Oncol Biol Phys. 85:472–476.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Eldh T, Heinzelmann F, Velalakan A, Budach
W, Belka C and Jendrossek V: Radiation-induced changes in breathing
frequency and lung histology of C57BL/6J mice are time- and
dose-dependent. Strahlenther Onkol. 188:274–281. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ma J, Zhang J, Zhou S, et al: Regional
lung density changes after radiation therapy for tumors in and
around thorax. Int J Radiat Oncol Biol Phys. 76:116–122. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jia S and Meng A: Tob genes in development
and homeostasis. Dev Dyn. 236:913–921. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yanagie H, Tanabe T, Sumimoto H, et al:
Tumor growth suppression by adenovirus-mediated introduction of a
cell-growth-suppressing gene tob in a pancreatic cancer model.
Biomed Pharmacother. 63:275–286. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tzachanis D and Boussiotis VA: Tob, a
member of the APRO family, regulates immunological quiescence and
tumor suppression. Cell Cycle. 8:1019–1025. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kitagawa K, Kotake Y and Kitagawa M:
Ubiquitin-mediated control of oncogene and tumor suppressor gene
products. Cancer Sci. 100:1374–1381. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Helms MW, Kemming D, Contag CH, et al:
TOB1 is regulated by EGF-dependent HER2 and EGFR signaling, is
highly phosphorylated, and indicates poor prognosis in
node-negative breast cancer. Cancer Res. 69:5049–5056. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
O’Malley S, Su H, Zhang T, Ng C, Ge H and
Tang CK: TOB suppresses breast cancer tumorigenesis. Int J Cancer.
125:1805–1813. 2009.
|
|
10
|
Jiao Y, Ge CM, Meng QH, Cao JP, Tong J and
Fan SJ: Adenovirus-mediated expression of Tob1 sensitizes breast
cancer cells to ionizing radiation. Acta Pharmacol Sin.
28:1628–1636. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jiao Y, Xu JY, Che J and Fan SJ: Study on
the effects of anti-proliferative protein Tob1 on the
radio-sensitivity of human cervix cancer cell line HeLa. J Radiat
Res Radiat Proc. 28:193–196. 2010.(In Chinese).
|
|
12
|
Jiao Y, Sun KK, Zhao L, Xu JY, Wang LL and
Fan SJ: Suppression of human lung cancer cell proliferation and
metastasis in vitro by the transducer of ErbB-2.1 (TOB1). Acta
Pharmacol Sin. 33:250–260. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gannon HS, Woda BA and Jones SN: ATM
phosphorylation of Mdm2 Ser394 regulates the amplitude and duration
of the DNA damage response in mice. Cancer Cell. 21:668–679. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guckenberger M, Baier K, Polat B, et al:
Dose-response relationship for radiation-induced pneumonitis after
pulmonary stereotactic body radiotherapy. Radiother Oncol.
97:65–70. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hart JP, McCurdy MR, Ezhil M, et al:
Radiation pneumonitis: correlation of toxicity with pulmonary
metabolic radiation response. Int J Radiat Oncol Biol Phys.
71:967–971. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nokisalmi P, Rajecki M, Pesonen S, et al:
Radiation-induced upregulation of gene expression from adenoviral
vectors mediated by DNA damage repair and regulation. Int J Radiat
Oncol Biol Phys. 83:376–384. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Parplys AC, Petermann E, Petersen C,
Dikomey E and Borgmann K: DNA damage by X-rays and their impact on
replication processes. Radiother Oncol. 102:466–471. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Neumaier T, Swenson J, Pham C, et al:
Evidence for formation of DNA repair centers and dose-response
nonlinearity in human cells. Proc Natl Acad Sci USA. 109:443–448.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hlatky L: Double-strand break motions
shift radiation risk notions. Proc Natl Acad Sci USA. 109:351–352.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schneider L, Fumagalli M and d’Adda dFF:
Terminally differentiated astrocytes lack DNA damage response
signaling and are radioresistant but retain DNA repair proficiency.
Cell Death Differ. 19:582–591. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zlobinskaya O, Dollinger G, Michalski D,
et al: Induction and repair of DNA double-strand breaks assessed by
gamma-H2AX foci after irradiation with pulsed or continuous proton
beams. Radiat Environ Biophys. 51:23–32. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Khoronenkova SV, Dianova II, Ternette N,
Kessler BM, Parsons JL and Dianov GL: ATM-dependent downregulation
of USP7/HAUSP by PPM1G activates p53 response to DNA damage. Mol
Cell. 45:801–813. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shouse GP, Nobumori Y, Panowicz MJ and Liu
X: ATM-mediated phosphorylation activates the tumor-suppressive
function of B56gamma-PP2A. Oncogene. 30:3755–3765. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cheng Q, Cross B, Li B, Chen L, Li Z and
Chen J: Regulation of MDM2 E3 ligase activity by phosphorylation
after DNA damage. Mol Cell Biol. 31:4951–4963. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gajjar M, Candeias MM, Malbert-Colas L, et
al: The p53 mRNA-Mdm2 interaction controls Mdm2 nuclear trafficking
and is required for p53 activation following DNA damage. Cancer
Cell. 21:25–35. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chiu YJ, Hour MJ, Lu CC, et al: Novel
quinazoline HMJ-30 induces U-2 OS human osteogenic sarcoma cell
apoptosis through induction of oxidative stress and up-regulation
of ATM/p53 signaling pathway. J Orthop Res. 29:1448–1456. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang Y, Xia F, Hermance N, et al: A
cytosolic ATM/NEMO/RIP1 complex recruits TAK1 to mediate the
NF-kappaB and p38 mitogen-activated protein kinase
(MAPK)/MAPK-activated protein 2 responses to DNA damage. Mol Cell
Biol. 31:2774–2786. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rutkowski R, Dickinson R, Stewart G, et
al: Regulation of Caenorhabditis elegans p53/CEP-1-dependent
germ cell apoptosis by Ras/MAPK signaling. PLoS Genet.
7:e10022382011.PubMed/NCBI
|