Introduction
Newcastle disease virus (NDV), known as avian
paramyxovirus of serotype 1 (APMV-1), is assigned as the type
species member of the genus Avulavirus belonging to the
Paramyxoviridae family within the order
Mononegavirales(1). NDV is
an enveloped virus which consists of a nucleocapsid harboring a
non-segmented, negative sense single-stranded RNA genome consisting
of 6 transcriptional units in the order of 3′-NP-P-M-F-HN-L-5′
which encodes at least 6 major proteins, i.e. the nucleocapsid
(NP), the phosphoprotein (P), the matrix protein (M), the large
polymerase protein (L), and 2 types of trans-membrane
glycoproteins, the hemagglutinin-neuraminidase (HN) and the fusion
(F) proteins (2,3). Two additional proteins, V and W, are
expressed by mRNAs, which are derived from the P gene via RNA
editing (4).
The natural reservoir of NDV is wild birds and NDV
is an economically considerable avian pathogen causing extensive
morbidity, mortality and losses in the poultry industry in several
countries (5). On the other hand,
NDV is non-pathogenic to humans and its oncotherapeutic benefits
have been used in preclinical studies making it a promising
non-conventional oncovirotherapeutic agent (6). NDV is as highly suitable as an
oncolytic agent and is also considered a potential agent in the
treatment of cancer as it selectively kills tumor cells (7). AF2240 is a viscerotropic-velogenic NDV
(VVNDV) Malaysian strain isolated in the 1960s during a local field
outbreak (8).
The HN glycoprotein protrudes from the viral
envelope in the non-fusion virion and in NDV-infected cells where
it is expressed at the cell surface (9). HN glycoprotein is a multifunctional
protein; it plays a major role in NDV infection, pathogenesis and
is responsible for the immunogenic properties of NDV (10). HN protein is responsible for NDV
attachment to sialic acid receptor and it contains the
neuraminidase (sialidase) activity (11).
NDV is known as a naturally occurring oncolytic
virus and it is cytotoxic against several types of human tumor
cells (12,13). NDV replicates selectively in tumor
cells but not in the normal cells (14). The oncolytic activity of NDV has
been exploited in the development of various types of antitumor
vaccines (15) and is mediated by
apoptosis induction (14,16,17).
The oncolytic- and apoptotic-induced activities of NDV have been
well characterized but their molecular mechanisms are not fully
understood. Earlier investigators suggested that NDV induced
apoptosis through signal molecules such as IFN-α and TNF-α
(18) and TRAIL (17). TNF-α and TRAIL are well known
apoptosis inducers and simultaneously exert an antitumor activity
(19,20).
Little is known about the HN glycoprotein oncolytic
activity. As for parental NDV, HN oncolytic activity may be
mediated by apoptosis. HN glycoprotein was shown to induce
apoptosis in human hepatoma SMMC-7721 cells (21). Similar to their parental NDV, the
molecular mechanism of HN apoptosis or oncolysis induction has yet
to be elucidated. The antitumor properties of HN may be related to
IFN-α and TRAIL which were induced by HN expression in human blood
mononuclear cells (22). Based on
its binding and neuraminidase activities, the HN protein was able
to activate adhesion molecules and increase the tumor cytotoxic T
lymphocytes (CTL) responses (23).
The antitumor activity of HN appears to be dependent on its cell
surface localization within the tumor cell. A membrane anchored HN
showed enhanced antitumor effect compared to the cytoplasmic or the
secreted HN protein (24). In
vivo expression of HN protein reduced tumor growth and
stimulated innate antitumor activity in a mouse model (25). The expression of HN protein of an
Indian NDV strain was shown to induce apoptosis in chicken embryo
fibroblast (CEF) cells (26).
The current study is part of a major project aimed
at developing an anticancer vaccine based on NDV AF2240 strain for
the treatment of human breast cancer. However, it is imperative to
understand the oncolytic mechanism of NDV AF2240 strain which is a
prerequisite for efficient development of a cancer vaccine
candidate. Little is known about NDV AF2240 strain oncolytic
activity. Only in vitro cytotoxicity studies of NDV AF2240
have been carried out and it was found that NDV AF2240 strain
induced apoptosis in a number of tumor cell lines including WEHI-3B
leukemic (27), brain tumor
(28), HT-29 human colon
adenocarcinoma, HCT-11 Bax and wt colorectal carcinoma cells
(29). The oncolytic activity of
NDV AF2240 strain and several other local NDV strains (C, Ijuk, S,
F and V4) were screened on tumor cell lines including CEM-SS
(T-lymphoblastic leukemic cells) and HT-29 based on an MTT
cytotoxic assay and it was found that NDV AF2240 strain was more
cytotoxic to tumor cells than other strains (30).
The molecular mechanism of NDV AF2240-induced
apoptosis is not fully understood with the exception that NDV
AF2240 strain induced conformational changes of Bax protein which
in turn is translocated from the cytoplasm to mitochondria and this
leads to the release of cytochrome c in the cytoplasm
(31). However, neither the
signaling mechanism leading to the conformational changes of Bax
nor the type of apoptotic stimuli responsible for this
conformational change were identified. The current study is the
first to demonstrate that the expression of NDV AF2240 strain’s HN
alone induced apoptosis in MCF-7 cells. Based on similar reported
studies, we hypothesized that the expression of HN glycoprotein of
NDV AF2240 strain may not only induce apoptosis but it may also be
a stronger inducer of apoptosis in MCF-7 cells than the parental
virus.
The objective of the present study was to
demonstrate whether HN expression alone induced apoptosis in MCF-7
cells and to compare the potency of both HN glycoprotein and the
parental NDV AF2240 strain in inducing apoptosis in MCF-7 cells in
order to select the most suitable antitumor candidate for future
investigations.
Materials and methods
Experimental design
The complete HN gene of NDV AF2240 strain was
amplified, cloned and expressed at the MCF-7 cell surface. The
induction of apoptosis by both recombinant HN and parental NDV
AF2240 were demonstrated by flow cytometry analysis and were
statistically analyzed. The potency of apoptosis induction by the
recombinant HN and NDV AF2240 strain was analyzed.
Cell and virus
Human breast carcinoma MCF-7 cells (ATCC®
no. HTB-22™) were cultured in RPMI-1640 tissue culture medium
supplemented with 10% fetal bovine serum (FBS) and 1% of
antibiotic-antimycotic. The cells were maintained at 37ºC in 5%
CO2 atmosphere. The medium, serum and antibiotics were
purchased from Invitrogen Life Technologies (Carlsbad, CA,
USA).
Virus stock was prepared by propagation in 9-day old
embryonated SPF eggs, followed by purification as previously
described (32). The virus was
titrated by hemagglutination assay and stored as single-use
aliquots at −80ºC for all experiments.
NDV AF2240 strain-induced apoptosis
Flow cytometry analysis
MCF-7 cells (5×106) cultured in 25
cm2 tissue culture flasks were infected with various
concentrations of NDV AF2240 strain of including 50, 100, 250 and
500 hemagglutination units (HAUs). After 1 h adsorption, the virus
inoculums were removed and fresh RPMI-1640 medium was added. The
infected cells were incubated for 48 h at 37ºC in the presence of
5% CO2 atmosphere. NDV AF2240 strain-induced apoptosis
was assessed using flow cytometry according to a previously
described method (33). At the end
of the incubation time, both adherent cells and supernatant were
processed by 2 successive centrifugations of 1,000 rpm for 10 min,
followed by fixation in 80% cold ethanol for 2 h at 4ºC. After 3
successive centrifugations at 1,000 rpm for 10 min, the cells were
incubated for 5 min at 4ºC in 1X phosphate-buffered saline (PBS)
buffer containing 10 mM Triton X-100 and 50 μg/ml of RNase A
(Invitrogen Life Technologies). Following centrifugation, the cells
were incubated for 30 min at 4ºC in the dark in 1 ml of 1X PBS
buffer containing 5 μg/ml propidium iodide (PI) (BioResource
International, Morrisville, NC, USA). Apoptosis was assessed by
flow cytometry analysis. The stained cells were then analyzed with
a CyAn ADP (Beckman Coulter, Brea, CA, USA) flow cytometer. The
data were analyzed with Summit v4.3 software (Beckman Coulter).
Mitochondrial transition pore
assay
MCF-7 cells (105) grown in chamber slides
(Nalge Nunc International, Rochester, NY, USA) were infected with
250 HAUs of NDV AF2240 strain. After 1 h virus adsorption, the
cells were incubated for 1 h at 37ºC in the presence of 5%
CO2 atmosphere. The non-infected and infected MCF-7
cells were processed for the detection of the activation of
mitochondrial transition pore opening using Image-iT live
mitochondrial transition pore assay kit according to the
manufacturer’s protocol (Molecular Probes; Invitrogen Life
Technologies, Carlsbad, CA, USA). The cells were viewed under a
fluorescence microscope (Leica DMRA II, Germany).
Reverse transcription-polymerase chain
reaction (RT-PCR) amplification of HN gene
Total RNA was extracted from NDV AF2240
strain-infected allantoic fluid of specific-pathogen-free
embryonating chicken eggs using TRI Reagent according to the
manufacturer’s instructions (Promega Corporation, Madison, WI,
USA). The primer set for the amplification of the complete HN gene
was designed using the Primer premier 5.0™ software and based on
the published sequence of NDV AF2240 strain (accession number
X79092). Two restriction enzyme sites SalI and SacII
were included in the primer set. The primer set sequences were:
HNSF, 5′-AAT CCG CGG ATC ATG GAC CGT GCA GTT AG-3′ and HNSR, 5′-GGG
GTC GAC CTC TCA TGG TTG ACT CAA-3′. The amplification of the
complete HN gene was performed using access RT-PCR System (Promega
Corporation, Madison, WI, USA). The HN gene was amplified in a
reaction mixture containing 1.5 mM MgS04, 10 mM each of
dNTP mix, 0.2 units RNase inhibitor, 5 units of Avian
myeloblastosis virus (AMV) reverse transcriptase, 5 units of
Tf1 DNA polymerase, and 0.5 μM of each primer. A total RNA
of 420 ng was added. The reaction mixture was first incubated for
45 min at 42ºC followed by 95ºC for 5 min. Then, 40 cycles of 94ºC
for 45 sec, 65ºC for 1 min and 70ºC for 1 min were carried out. A
last step of 72ºC for 5 min was added. The amplified HN fragment
was fractionated on 1% Tris-borate-EDTA agarose gel, stained with
ethidium bromide solution (50 ng/ml) and analyzed on gel alpha
imaging system (Alpha Innotech Corp., San Leandro, CA, USA).
Plasmid constructs
The amplified HN fragments were purified using the
wizard SV gel and PCR clean up system according to the
manufacturer’s instructions (Promega Corporation). The purified HN
fragment was cloned into pCR 2.1 vector using the TOPO TA
Cloning® kit according to the manufacturer’s
instructions (Invitrogen Life Technologies). The positive
recombinants were analyzed by EcoRI restriction enzyme
digestion. The recombinant pCR 2.1-HN and the expression vector
pDisplay were purified by using the pure yield plasmid Midiprep
System (Promega Corporation) and double digested with SalI
and SacII. The linearized HN fragment was cloned into double
digested pDisplay vector and incubated for 1 h at 16ºC. The
orientations of the positive recombinants were examined by double
digestion with SalI and SacII and the authenticity of
the positive recombinant was confirmed by DNA sequencing.
Transfection
MCF-7 cell population of 0.5×105 was
plated in 500 μl of RPMI-1640 free antibiotic-antimycotic tissue
culture medium. MCF-7 cells were cultured to ~80% confluency at the
time of transfection. For each transfection, 0.3–1.2 μg of the
recombinant pDisplay-HN diluted in 100 μl of Opti-MEM® I
reduced serum medium was transfected into MCF-7 cells using 1.25–10
μl of Lipofectamine® LTX Reagent according to the
manufacturer’s instructions (both from Invitrogen Life
Technologies). The transfected cells were incubated for 48 h at
37ºC in a 5% CO2 incubator before assay of HN expression
and apoptosis induction.
Immunofluorescence assay
The detection and localization of HN glycoprotein
was carried out using indirect immunofluorescence assay. Briefly,
MCF-7 cells were transfected as previously described and incubated
for 48 h at 37ºC in a 5% CO2 atmosphere. The glass slide
containing the transfected MCF-7 cells was removed from the
Lab-Teck chamber slide and rinsed with 1X PBS buffer. The cells
were fixed in cold acetone for 10 min at room temperature. A
dilution of 1:200 of chicken polyclonal anti-NDV AF2240 serum
prepared previously was added to the fixed cells, incubated at room
temperature for 30 min and rinsed a few times with 1X PBS buffer. A
fluorescein-labeled affinity purified antibody to chicken IgG
(Kirkegaard & Perry Laboratories, Gaithersburg, MD, USA)
diluted at 1:100 was added to the cells and incubated at room
temperature for 30 min. The cells were rinsed with 1X PBS buffer
and dried. Finally, 1 drop of Antifade Solution (Chemicon
International, Temecula, CA, USA) was added and cells were
visualized using a fluorescence microscope (Leica DMRA II).
Flow cytometry analysis
The detection of HN induction of apoptosis was
carried out as previously described (33). Briefly, MCF-7 cells were transfected
as described above with 0.3, 0.6 and 1.2 μg of recombinant
pDisplay-HN and transfected cells were incubated for 48 h at 37ºC
in a 5% CO2 atmosphere. Both the transfected cells and
the supernatant were harvested and processed for flow cytometry
analysis as described above.
Statistical analysis
Data were analyzed and expressed as means ± SD.
Statistical analysis was performed using one-way analysis of the
percentage of apoptosis by the recombinant pDisplay-HN. The
comparison for the pairs was carried out by the Tukey-Kramer HSD
test from flow cytometry assays.
Results
NDV AF2240-induced apoptosis in MCF-7
cells
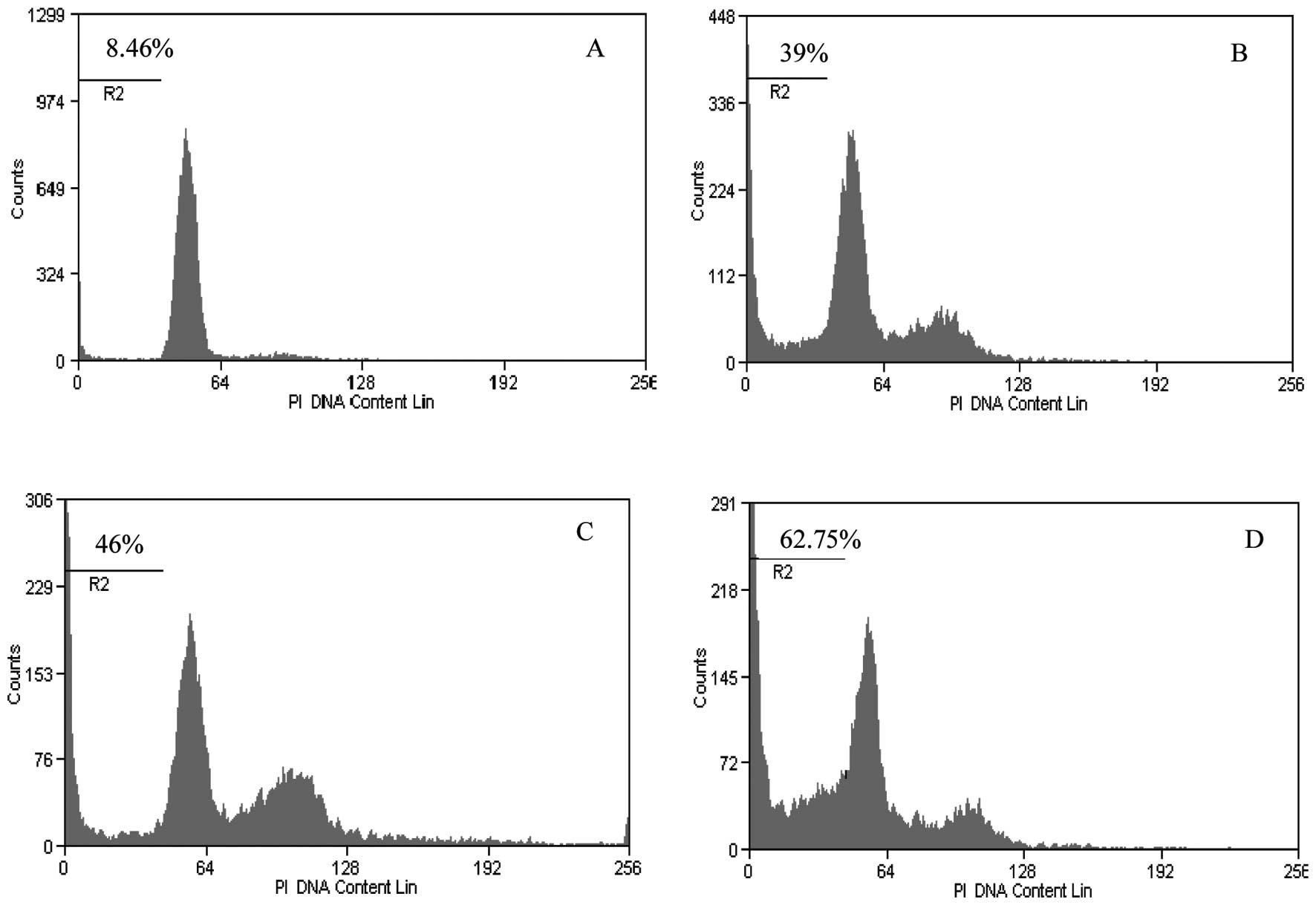
The result of apoptosis induction using various
titers of NDV AF2240 strain revealed that 250 HAUs induced the
highest apoptosis level in MCF-7 cells (Fig. 1). At higher titer of 500 HAUs and
above, the viral AF2240 strain was more cytotoxic to MCF-7 cells
grown in 25 cm2 tissue culture flasks in which the cells
detached from the surface of the flask in less than 24 h post
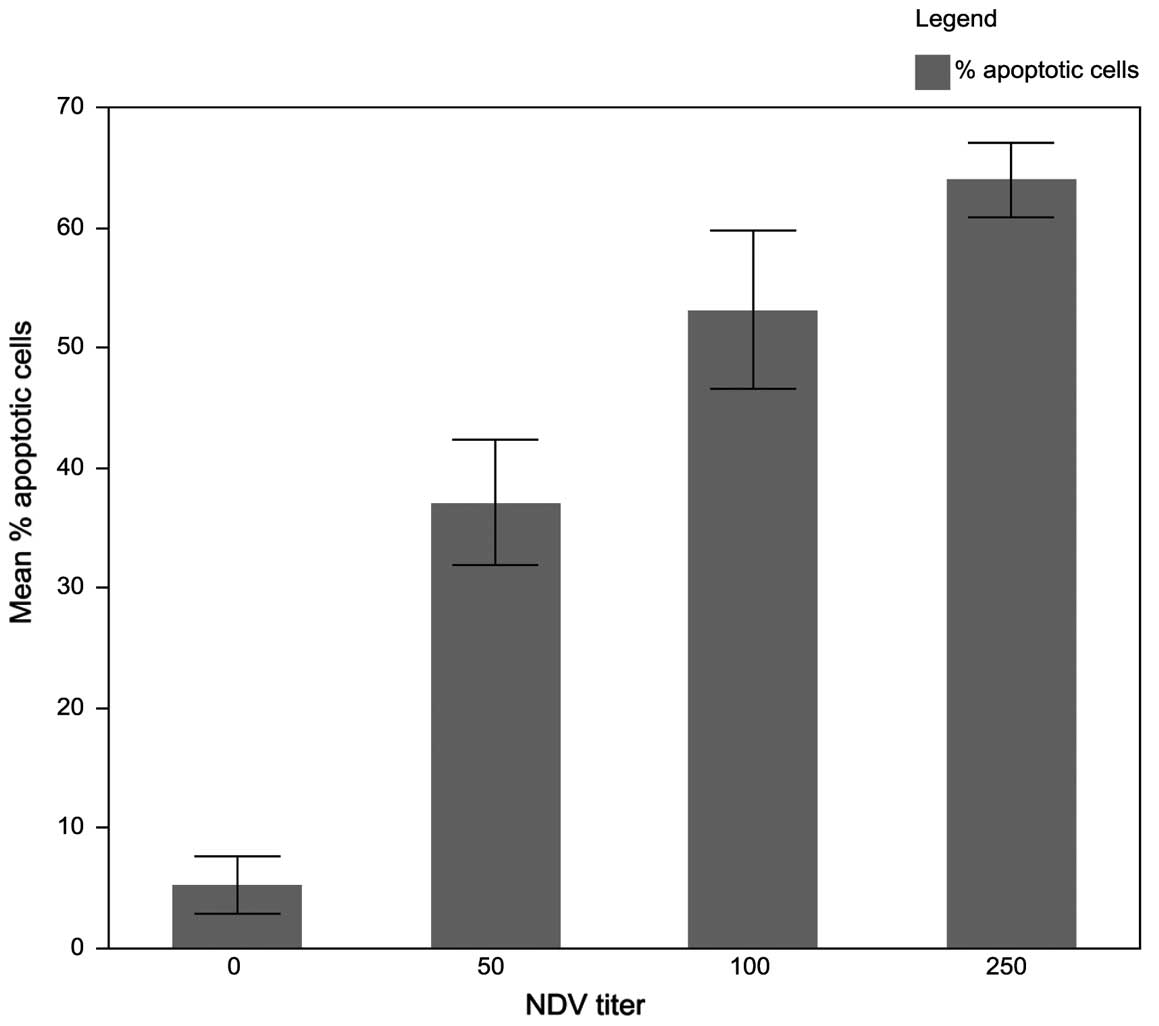
infection (pi). The apoptosis induction increased from 37% obtained
after infection with 50 HAUs to ~63% of dead cells with infection
with 250 HAUs. The increase of the percentage of apoptotic cells
was significant for all titers of NDV as compared to negative
control (p<0.0001), as shown in Fig.
2. The induced apoptosis was dose-dependent. There was a
positive correlation between the dose of NDV and the percentage of
apoptotic cells, suggesting that NDV AF2240 strain kills 100% of
MCF-7 cells if the dose is increased. The NDV AF2240 strain dose of
250 HAUs was selected based on its strong induction of apoptosis
for the detection of mitochondrial permeability transition pore
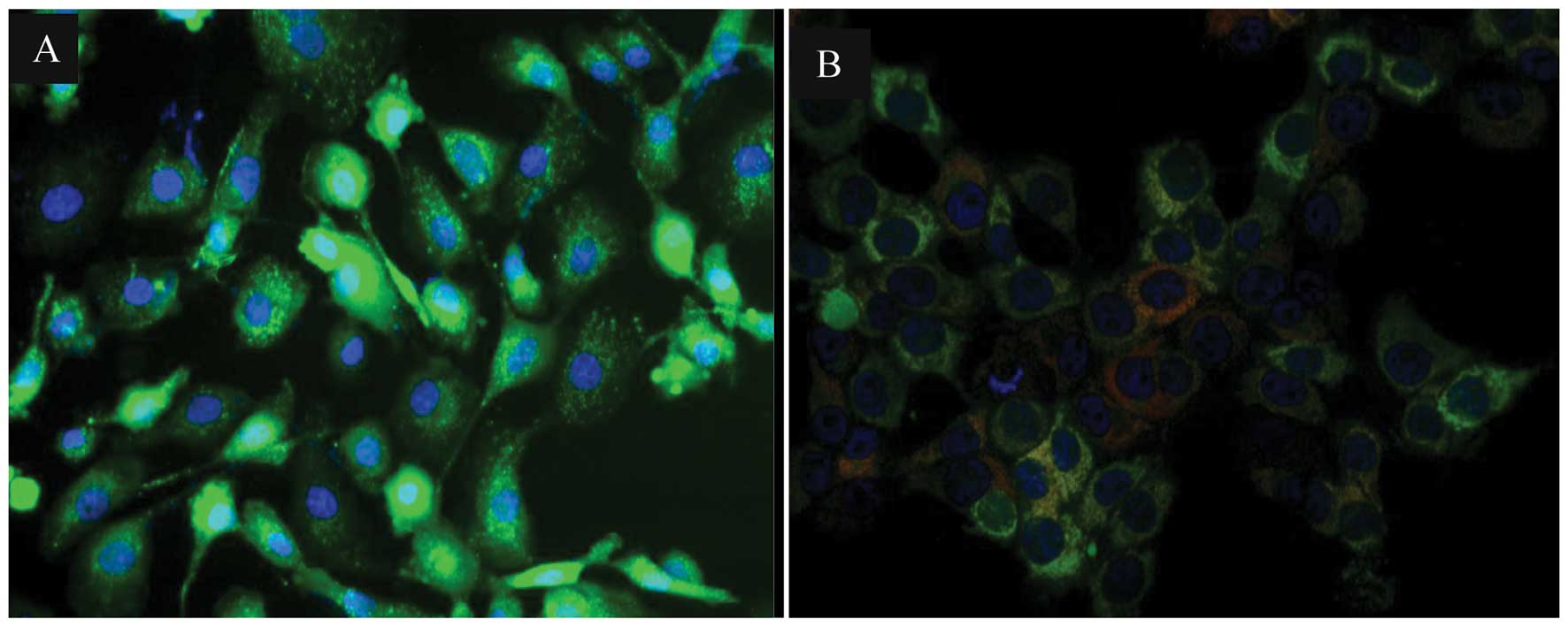
activation. MCF-7 cells infected with 250 HAUs of NDV AF2240 showed
a red fluorescence in the form of a comet which is a typical
characteristic of mitochondria permeability transition pore opening
activation while only green fluorescence was observed in
non-infected MCF-7 cells (Fig.
3).
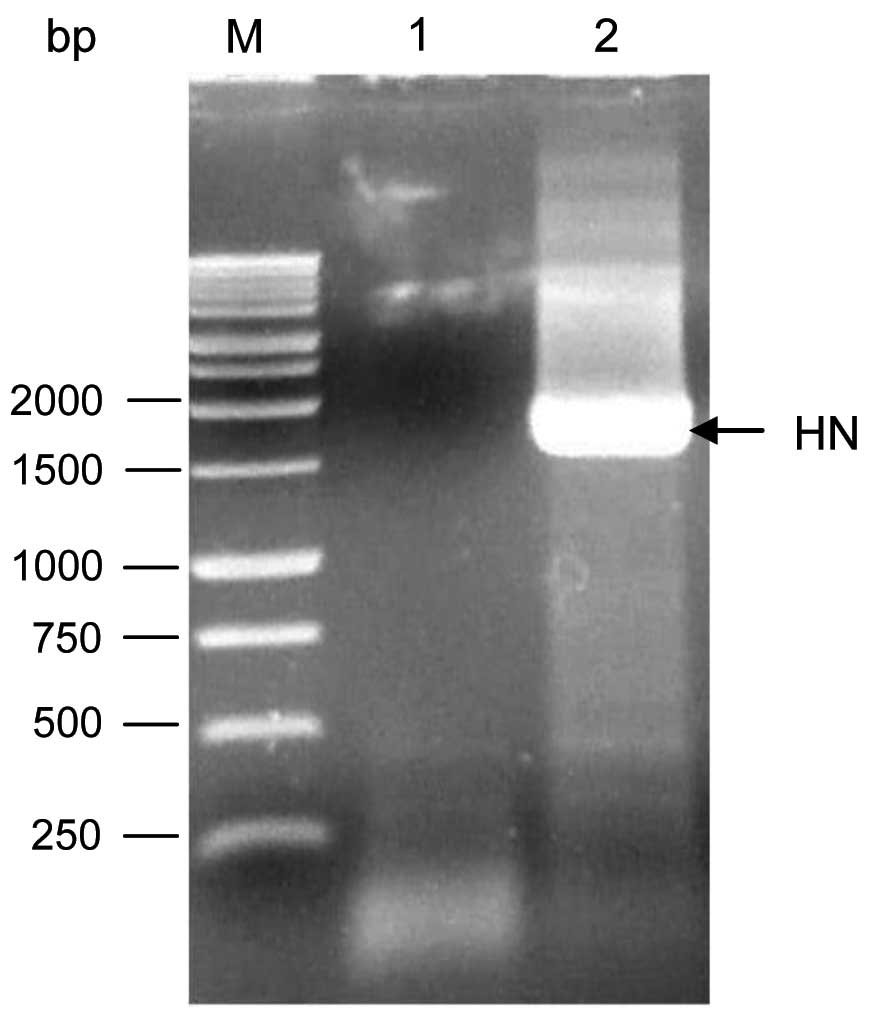
Amplification of the whole HN gene
The whole HN gene was amplified using RT-PCR at the
expected size of ~1.8 kb as shown in Fig. 4. To avoid introducing deleterious
mutations, the HN DNA fragment was fractionated on agarose gel
electrophoresis without exposing it to ethidium bromide and UV
light. Then, the HN DNA fragment was purified and stored at
−20ºC.
Plasmid constructs
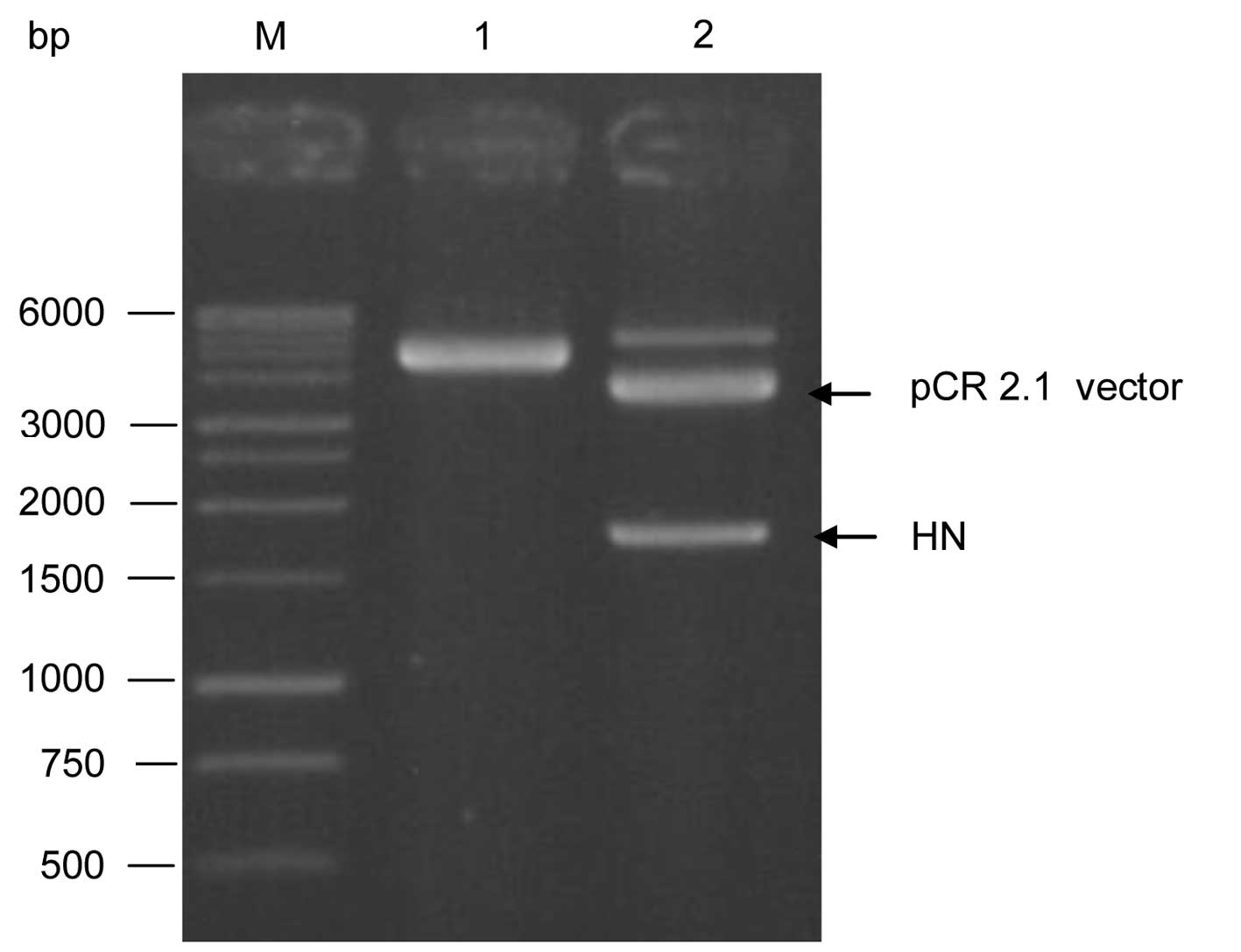
The pure HN DNA fragment (1 μl) was first cloned in
pCR 2.1-TOPO cloning vector. The positive recombinant pCR 2.1-HN
was analyzed by EcoRI restriction enzyme as shown in
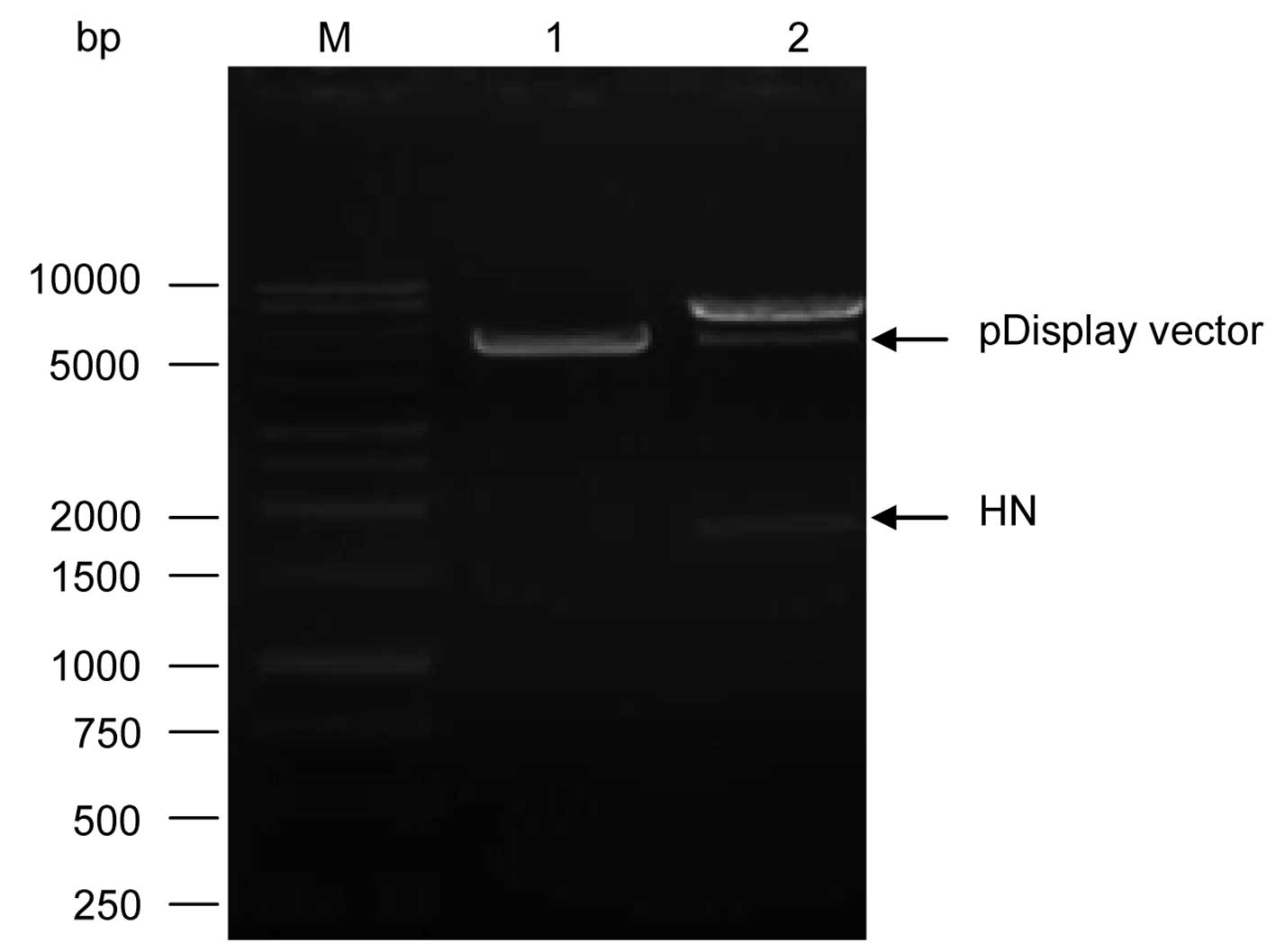
Fig. 5. The pCR 2.1-HN and the
pDisplay expression vector were purified and double digested with
SalI and SacII restriction enzymes. A ratio of
vector: insert of 1:5 was the most suitable for a successful
cloning of the HN fragment into pDisplay expression vector and the
successful recombinant pDisplay-HN was confirmed by the release of
HN gene by double digestion with the restriction enzymes
SalI and SacII which released the HN fragment as
shown in Fig. 6. The sequencing of
the cloned HN gene revealed a correct in-frame cloning.
Expression of HN gene induces apoptosis
in MCF-7 cells
It was revealed that apoptosis induced by NDV AF2240
strain in MCF-7 cells may be mediated by HN protein expression
alone. This hypothesis was investigated in 2 steps. First, the
positive recombinant pDisplay-HN harboring the complete HN gene was
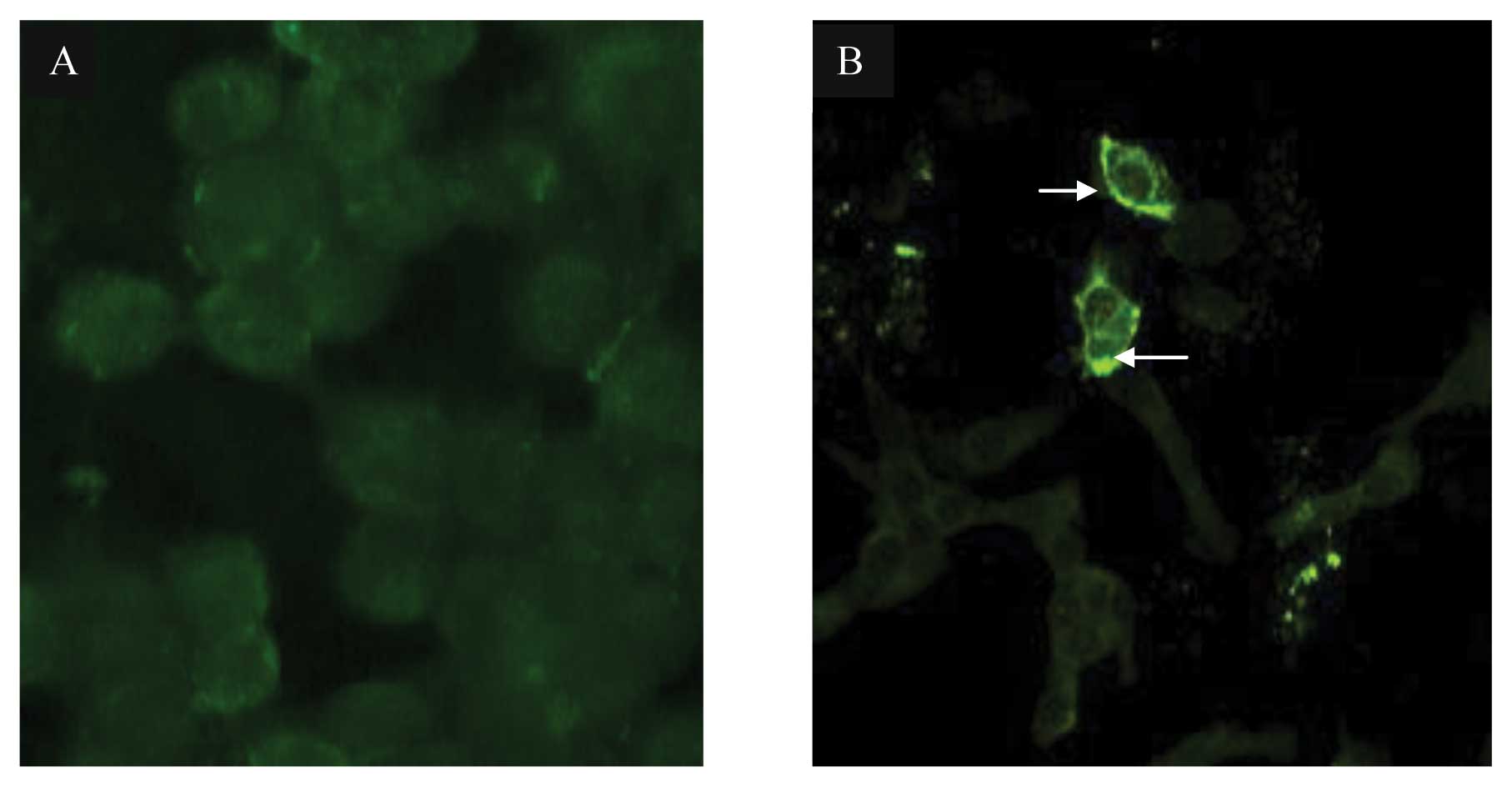
transfected into MCF-7 cells. The expression and localization of HN
protein were detected by indirect immunofluorescence assay as shown
in Fig. 7. HN expression-induced
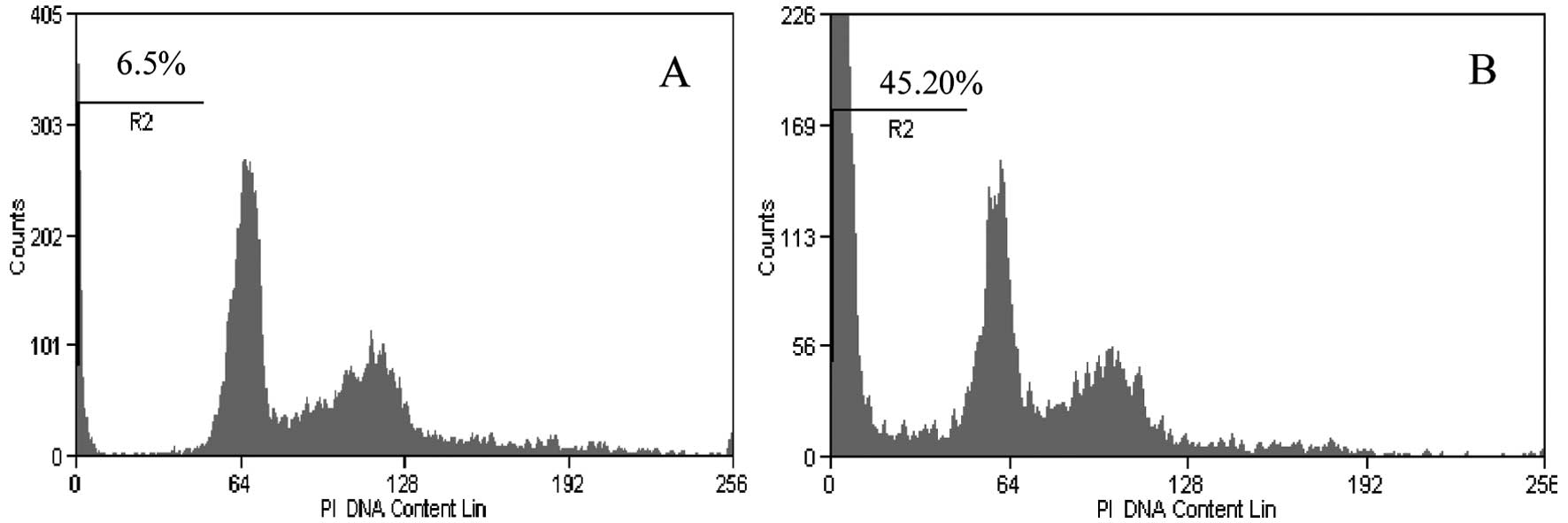
apoptosis was confirmed and quantified using flow cytometry. The
recombinant pDisplay-HN at a concentration of 1.2 μg induced the
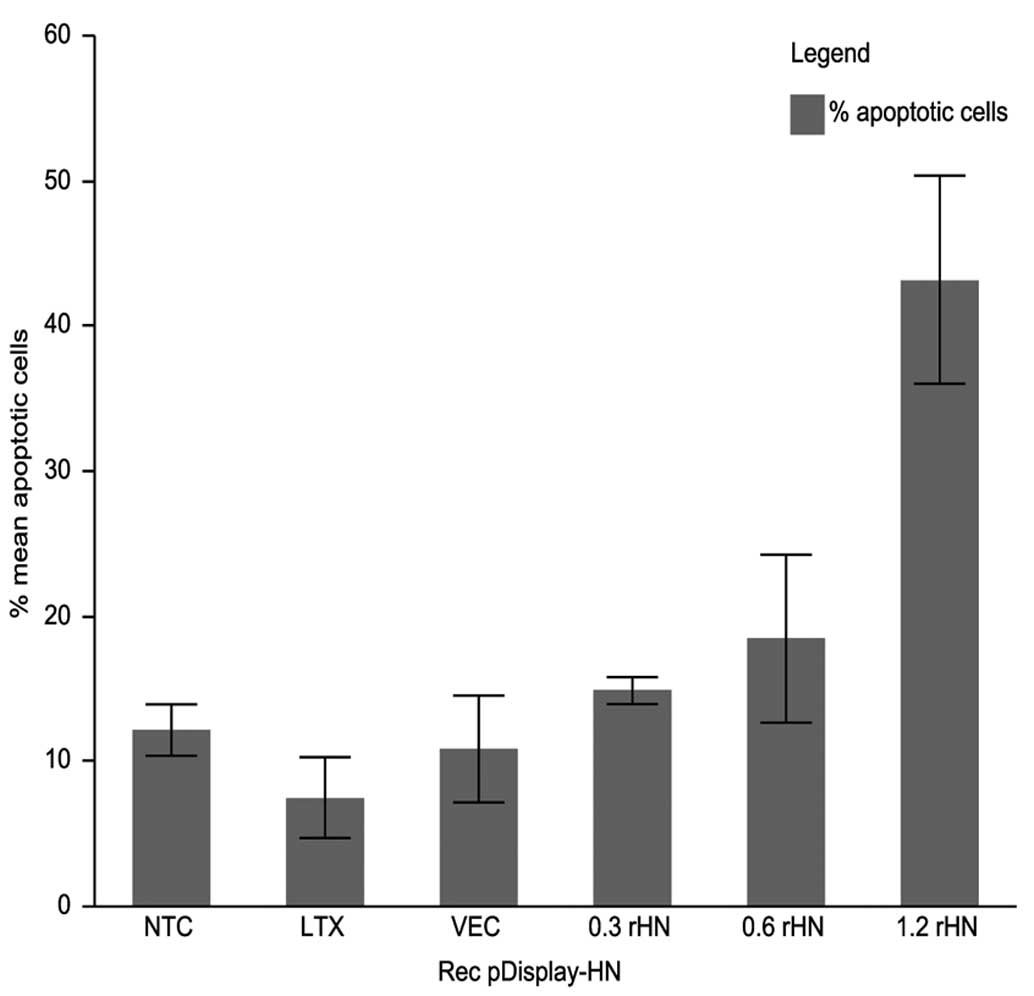
highest level of apoptosis as shown in Fig. 8. The percentage of apoptotic cells
increased from ~15% with transfection of 0.3 μg of recombinant
pDisplay-HN to 43% with transfection of 1.2 μg of recombinant
pDisplay. The significant increase of apoptosis was induced with
1.2 μg of recombinant HN (p<0.0001). Lower concentrations of 0.3
and 0.6 μg recombinant HN did not induce any significant increase
of apoptosis. Lipofectamine LTX treatment of MCF-7 cells and
transfection of MCF-7 cells with pDisplay vector only had no
significant effect on apoptosis induction as shown in Fig. 9. The apoptosis induced by
recombinant HN was dose-dependent.
Discussion
NDV strain AF2240 is known to induce apoptosis in a
number of human tumor cell lines, but induction of apoptosis in
MCF-7 cells was not well documented. In the present study, we first
evaluated the extent of NDV AF2240 strain-induced apoptosis using
flow cytometry analysis. Second, we confirmed NDV AF2240 induction
of apoptosis via mitochondrial permeability transition pore opening
assay. The mechanisms of apoptosis induction and regulation are not
fully elucidated. This is due, in part, to the existence of a wide
variety of stimulatory factors (34).
The majority of apoptosis research is focused on
these stimulatory and initiating signals due to their significance
in apoptosis modulation. Apoptosis is a physiological process used
as a defence or a self-destructive mechanism against infection and
neoplasm in which infected or tumor cells are eliminated by a cell
death suicide (35,36). Imbalance in apoptosis causes
diseases either when excess apoptosis occurs, as in the case of
degenerative diseases, or when a failure or decrease in apoptosis
happens, as in the case of autoimmune diseases and cancer (37). Most of the antitumor research takes
advantage of the apoptosis mechanism (38).
The antitumor activity of several NDV strains is
well documented (7,15). Some of these strains were tested in
a clinical setting while others are in an advanced clinical trial
(7,15,39–44).
In this study, we focused on NDV AF2240 strain as a
continuation of its previously reported cytotoxic activities in
vitro and apoptotic activity in tumor cell lines (27,28).
However, little is known about the molecular mechanism of NDV
AF2240-induced apoptosis. The role of NDV AF2240 gene(s) in
apoptosis has not been investigated. Earlier studies showed that HN
glycoprotein mediates the expression of TNF-α and TRAIL (17,22).
It appears that TRAIL-induced apoptosis is a general phenomenon
since it was shown in numerous viruses including, but not exclusive
to, avian influenza (45), reovirus
(46), measles (47) and respiratory syncytial virus
(48). However, the molecular
mechanism of HN induction of these cytokines remains to be fully
elucidated. HN may not be the only NDV gene inducing death ligands;
these latter can be induced by cellular stress proteins which can
also be induced by NDV infection (49). In addition, HN glycoprotein may
share similar effects with other viral envelope proteins which were
shown to induce cytotoxicity via apoptosis induction such as HIV
gp120 (50) and sigma-1 attachment
protein of type 3 reovirus (51).
HN glycoprotein of NDV is a well-known mediator of cytotoxicity
(10).
In another study, HN expression was shown to induce
apoptosis in normal chicken embryo fibroblast (CEF) cells (26). We were able to induce a higher level
of apoptosis (45.20%) with only a small amount of recombinant HN at
48 h post infection (pi) compared to the low level of apoptosis
induction (4.9%) induced using 5 μg of recombinant HN at 96 h pi as
previously reported (26). This
difference in apoptosis level may be due to differences in the cell
type, the use of different expression systems and different origin
of the HN glycoprotein derived from different NDV pathotypes. The
expression system may have played a greater role in inducing a high
level of apoptosis as it was demonstrated in a previous study in
which the Semliki forest virus (SFV) based expression system
harboring NDV HN gene which generated a stronger expression of HN
protein which in turn induced 90% of cell death (52).
In the present study, the lower level of apoptosis
induced by recombinant HN may be attributed to several factors
including, in particular, the pDisplay expression system used. The
induced apoptosis by HN protein was found to be dose-dependent to a
certain level but not as potent as the parental virus NDV AF2240
strain-induced apoptosis. This low level of apoptosis induced by
the recombinant HN may be partially explained by the insufficient
amount of recombinant HN DNA used to transfect MCF-7 cells. This is
due to the limiting factor of the transfection reagent used. It was
not possible to increase the level of apoptosis indefinitely by
increasing the amount of the recombinant HN DNA. An increased
amount of recombinant HN DNA requires an increased level of
transfection agent which becomes too toxic to MCF-7 cells. Second,
recombinant HN DNA cannot transfect every MCF-7 cell since it is
not an infectious or self replicating agent. These 2 shortcomings
did not apply for NDV AF2240 strain-induced apoptosis. NDV AF2240
is not only highly infectious but it is also possible to increase
the amount of the virus indefinitely to induce a massive and
complete cell death of the infected MCF-7 cells. In a clinical
setting, this fact has potential benefits in cancer treatment as
long as other limiting factors such as the development of antitumor
resistance, virus escape mutant and anti-NDV antibodies are kept in
control. The administration of massive amount of NDV has resulted
in encouraging outcomes in the past (39).
The use of parental NDV AF2240 strain in inducing
apoptosis was more efficient compared to recombinant HN protein.
This finding is in agreement with previous findings that
NDV-induced apoptosis depends on the virus particle itself
(17,22,53),
indicates that apoptosis is NDV dose-dependent as found in the
present study. Our observation that apoptosis level is NDV
dose-dependent is in agreement with previous findings (54). However, replication within the tumor
cell may have an additive greater effect in inducing a higher level
of apoptosis. In agreement with this suggestion, it was reported
that one infectious NDV particle was able to kill at least
104 tumor cells in 2–3 days (15). The reason that NDV particle induced
a higher level of apoptosis than recombinant HN protein may be due
to the contribution of other NDV genes. In fact, NDV M protein was
shown to have an additive role in apoptosis induction (55). Hence, the involvement of other NDV
genes either alone or in synergy with cellular genes cannot
completely be excluded.
It is known that NDV infection stimulates several
cellular genes and the production of chemokines and cytokines
(53). The involvement of cytokines
such as TRAIL are well known apoptosis inducers (20). In addition, this difference in
apoptosis induction level can be explained by the difference in the
binding avidity between parental NDV AF2240 and recombinant HN
protein as in the case of IFN-α induction where it was suggested
that IFN-α induction by recombinant HN was 70% lower than the
induction by the complete NDV virion (22). The binding avidity difference may be
due to the overall structural organization of the HN protein within
the virion and when HN is expressed alone. It is not known whether
all HN glycoproteins irrespective of their origin of NDV strains
and pathotypes induced apoptosis or induced similar levels of
apoptosis in a particular cell type or in all cell types. In other
viruses, such as rabies virus, only G protein of non-pathogenic
rabies virus (RV) strain ERA induced apoptosis while G protein from
highly neurotropic RV strain CVS did not induce apoptosis (56).
Whether the complete HN gene or part of it is
required to trigger apoptosis or induce a higher level of apoptosis
is not known. Notably, a small part of the HN gene (443 bp) was
able to induce apoptosis in CEF cells but at low level (26). In the present study, the complete HN
gene that was used to induce apoptosis is 1996 nucleotides long and
encodes a predicted HN protein of 581 amino-acids as previously
reported (57). In adenovirus type
5 E1A protein, only one of the different domains was responsible
for apoptosis induction (58). The
trans-membrane portion of the Sindbis virus surface glycoprotein E1
and E2 can induce apoptosis (59).
HIV envelope protein with truncated cytoplasmic domain was
sufficient to induce apoptosis (60). HN protein-induced apoptosis may be
dependent on host cellular membrane localization (53). In addition, a membrane anchored HN
protein was shown to have a better antitumor effect than the
cytoplasmic or secreted protein (61).
Induction of apoptosis is possibly independent of
the virus pathotype. Apoptosis was induced by very virulent NDV
AF2240 strain as shown in the present study in agreement with other
reports (27,28). Apoptosis was also induced by
avirulent, non-lytic NDV Ulster strain (53) and by moderately pathogenic Beaudette
C NDV strain and avirulent NDV LaSota strain (16). In the present study, we found that
MCF-7 cells were more sensitive to NDV AF2240 strain. MCF-7 cells
were also reported to be sensitive to NDV Ulster strain (72%
apoptotic cells at 72 h pi) (53).
However, MCF-7 cells were shown to be resistant to the recombinant
NDV LaSota and Beaudette C strains (16). The aforementioned suggestion assumed
that HN protein binding to the host cell surface receptor may be
responsible and sufficient to trigger apoptosis in NDV-infected
cells. However, this suggestion is not certain and how the binding
transduces stimuli into the cell or how it activates apoptotic
pathways remains unclear and requires further investigations.
Similarly, the binding of the reovirus surface attachment protein
sigma-1 to sialic acid receptor potentiated virus-induced apoptosis
(51) and the binding of HIV
surface envelope glycoprotein to its receptor triggered apoptosis
(60).
NDV AF2240 HN protein-induced apoptosis may also be
the result of the modification of the highly ordered lipid rafts
following the accumulation of HN glycoprotein at the MCF-7 cell
membranes. These changes have previously been reported to be
associated with apoptosis such as in the case of the accumulation
of gp120 protein on the membrane of HIV-infected cells (62) and the rabies virus G protein on cell
membranes (56).
Another explanation for HN-induced apoptosis may
include the involvement of oxidative stress where it was previously
reported that an increase in oxidative stress in CEF cells
transfected with recombinant HN was observed (26). An earlier study suggested that
oxidative stress induced by NDV may be involved in apoptosis
induction (63).
The mechanism of HN glycoprotein-induced apoptosis,
the binding of HN glycoprotein to cell receptor and the
accumulation of HN glycoprotein in cell membrane-induced apoptosis
are not known. HN glycoprotein of NDV AF2240 strain may be
responsible either alone or in association with other viral
proteins for inducing apoptosis and since the oncolytic activity of
NDV is mediated by apoptosis (16,17) HN
glycoprotein may then also be responsible for the oncolytic
activity of NDV as suggested elsewhere (22–24,52).
Since HN glycoprotein mediates NDV cytopathogenecity (10) and the difference in NDV cytotoxicity
and probably its oncolytic activity is due to differences in the
nucleotide sequence of the HN gene and the amino acid sequence of
the HN protein (16), we suggest
that apoptosis induced by HN is part or an end of the
cytopathogenic process. Previous evidence supports this suggestion.
First, NDV-induced apoptosis may be involved in NDV cytotoxicity
(64). Second, NDV-induced
cytopathic effect in infected cells is the result of NDV-induced
apoptosis (65). Third, NDV
antitumor cytotoxicity may be due to the expression of TRAIL-well
known cytotoxic molecule induced by HN expression (20,22).
The correlation between pathogenicity and oncolysis properties of
NDV was previously reported (66).
As a result, HN may represent a good candidate for
antitumor therapy if further improvements are included. In fact,
the antitumor property of HN glycoprotein has already been
exploited for antitumor therapy (24,67,68).
In conclusion, HN protein expression alone induced apoptosis in
MCF-7 cells but it was less efficient than the parental NDV AF2240
strain in inducing apoptosis. Therefore, NDV AF2240 strain-induced
apoptosis in MCF-7 cells was most probably mediated by HN protein
expression alone. Unless the apoptosis-inducing property of HN
protein is further improved, NDV AF2240 strain was shown to be more
efficient in inducing apoptosis and probably more oncolytic than
the recombinant HN protein. Currently, we are focusing on the
elucidation of the molecular mechanism of HN inducing apoptosis in
MCF-7 cells via investigation of the HN interaction with the
cellular membrane proteins and molecular machinery of MCF-7
cells.
Acknowledgements
This study was supported by a grant from the
Malaysian National Cancer Council (MAKNA). The authors thank Mr.
Rafiuz Zaman Haroun, the Microscopy Unit and Ms. Norsharina Ismail,
the Laboratory of Molecular Biomedicine, Institute of Bioscience,
UPM Serdang for their technical support in fluorescence and flow
cytometry analysis. Dr Mohamed Ezzat El Zowalaty, Lecturer,
Department of Microbiology and Immunology, Faculty of Pharmacy,
Zagazig University, Egypt, is a postdoctoral research fellow under
sponsorship of the Ministry of Higher Education, Malaysia.
Abbreviations:
|
ATCC
|
American Type Culture Collection
|
|
HAU
|
hemagglutination unit
|
|
HN
|
hemagglutinin-neuraminidase
|
|
IFN-α
|
interferon alpha
|
|
NDV
|
Newcastle disease virus
|
|
NP
|
nucleoprotein
|
|
pi
|
post infection
|
|
RT-PCR
|
reverse transcription-polymerase chain
reaction
|
|
TNF
|
tumor necrosis factor
|
|
TRAIL
|
TNF-related apoptosis-inducing
ligand
|
|
wt
|
wild-type
|
References
|
1
|
International Committee on Taxonomy of
Viruses (ICTV). http://ictvonline.org/taxonomyHistory.asp?taxnode_id=20114200&taxa_name=Newcastle%20disease%20virus.
2012
|
|
2
|
Choppin PW and Compans RW: Reproduction of
Paramyxoviruses. Comprehensive Virology. Fraenkel-Conrat H and
Wagner RR: 4. Plenum Press; New York: pp. 95–178. 1975, View Article : Google Scholar
|
|
3
|
Stone-Hulslander J and Morrison TG:
Detection of an interaction between the HN and F proteins in
Newcastle disease virus-infected cells. J Virol. 71:6287–6295.
1997.PubMed/NCBI
|
|
4
|
Steward M, Vipond IB, Millar NS and
Emmerson PT: RNA editing in Newcastle disease virus. J Gen Virol.
74:2539–2547. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Alexander DJ: Newcastle disease. Br Poult
Sci. 42:5–22. 2001. View Article : Google Scholar
|
|
6
|
Kumar R, Tiwari AK, Chaturvedi U, et al:
Velogenic Newcastle disease virus as an oncolytic virotherapeutics:
in vitro characterization. Appl Biochem Biotechnol. 167:2005–2022.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sinkovics JG and Horvath JC: Newcastle
disease virus (NDV): brief history of its oncolytic strains. J Clin
Virol. 16:1–15. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lai MC and Ibrahim AL: Velogenic
viscerotropic Newcastle disease virus. Newcastle Disease in
Poultry: a new food pellet vaccine. Copland JW: (Vol monograph 5).
ACIAR; Canberra: pp. 33–34. 1987
|
|
9
|
Millar NS, Chambers P and Emmerson PT:
Nucleotide sequence analysis of the haemagglutinin-neuraminidase
gene of Newcastle disease virus. J Gen Virol. 67:1917–1927. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang Z, Panda A, Elankumaran S,
Govindarajan D, Rockemann DD and Samal SK: The
hemagglutinin-neuraminidase protein of Newcastle disease virus
determines tropism and virulence. J Virol. 78:4176–4184. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Choppin PW and Scheid A: The role of viral
glycoproteins in adsorption, penetration, and pathogenicity of
viruses. Rev Infect Dis. 2:40–61. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schirrmacher V, Haas C, Bonifer R, Ahlert
T, Gerhards R and Ertel C: Human tumor cell modification by virus
infection: an efficient and safe way to produce cancer vaccine with
pleiotropic immune stimulatory properties when using Newcastle
disease virus. Gene Ther. 6:63–73. 1999. View Article : Google Scholar
|
|
13
|
Fábián Z, Csatary CM, Szeberényi J and
Csatary LK: p53-independent endoplasmic reticulum stress-mediated
cytotoxicity of a Newcastle disease virus strain in tumor cell
lines. J Virol. 81:2817–2830. 2007.PubMed/NCBI
|
|
14
|
Reichard KW, Lorence RM, Cascino CJ, et
al: Newcastle disease virus selectively kills human tumor cells. J
Surg Res. 52:448–453. 1992. View Article : Google Scholar
|
|
15
|
Schirrmacher V and Fournier P: Newcastle
disease virus: a promising vector therapy of cancer. Viral Therapy
of Cancer. Kevin JH, Richard GV and Hardev SP: Wiley; New Jersey:
pp. 171–186. 2008, View Article : Google Scholar
|
|
16
|
Elankumaran S, Rockemann D and Samal SK:
Newcastle disease virus exerts oncolysis by both intrinsic and
extrinsic caspase-dependent pathways of cell death. J Virol.
80:7522–7534. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Washburn B, Weigand MA, Grosse-Wilde A, et
al: TNF-related apoptosis-inducing ligand mediates tumoricidal
activity of human monocytes stimulated by Newcastle disease virus.
J Immunol. 170:1814–1821. 2003. View Article : Google Scholar
|
|
18
|
Lorence RM, Rood PA and Kelley KW:
Newcastle disease virus as an antineoplastic agent: induction of
tumor necrosis factor-α and augmentation of its cytotoxicity. J
Natl Cancer Inst. 80:1305–1312. 1988.
|
|
19
|
Pitti RM, Marsters SA, Ruppert S, Donahue
CJ, Moore A and Ashkenazi A: Induction of apoptosis by Apo-2
ligand, a new member of the tumor necrosis factor cytokine family.
J Biol Chem. 271:12687–12690. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Walczak H, Miller RE, Ariail K, et al:
Tumoricidal activity of tumor necrosis factor-related
apoptosis-inducing ligand in vivo. Nat Med. 5:157–163. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sun Y, Jin N, Mi Z, Li X, Lian H and Li P:
Induction of apoptosis in human hepatoma cell line SMMC7721 by
Newcastle disease virus HN gene. Zhonghua Zhong Liu Za Zhi.
27:279–282. 2005.(In Chinese).
|
|
22
|
Zeng J, Fournier P and Schirrmacher V:
Induction of interferon-α and tumor necrosis factor-related
apoptosis-inducing ligand in human blood mononuclear cells by
hemagglutinin-neuraminidase but not F protein of Newcastle disease
virus. Virology. 297:19–30. 2002.
|
|
23
|
Schirrmacher V, Haas C, Bonifer R and
Ertel C: Virus potentiation of tumor vaccine T-cell stimulatory
capacity requires cell surface binding but not infection. Clin
Cancer Res. 3:1135–1148. 1997.PubMed/NCBI
|
|
24
|
Sui H, Bai Y, Wang K, et al: The
anti-tumor effect of Newcastle disease virus HN protein is
influenced by differential subcellular targeting. Cancer Immunol
Immunother. 59:989–999. 2010. View Article : Google Scholar
|
|
25
|
Ni J, Galani IE, Cerwenka A, Schirrmacher
V and Fournier P: Antitumor vaccination by Newcastle disease virus
hemagglutinin-neuraminidase plasmid DNA application: changes in
tumor microenvironment and activation of innate anti-tumor
immunity. Vaccine. 29:1185–1193. 2011. View Article : Google Scholar
|
|
26
|
Ravindra P, Tiwari AK, Sharma B, et al: HN
protein of Newcastle disease virus causes apoptosis in chicken
embryo fibroblast cells. Arch Virol. 153:749–754. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Alabsi AM, Bakar SAA, Ali R, et al:
Effects of Newcastle disease virus strains AF2240 and V4-UPM on
cytolysis and apoptosis of leukemia cell lines. Int J Mol Sci.
12:8645–8660. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ali R, Alabsi AM, Ali AM, et al: Cytolytic
effects and apoptosis induction of Newcastle disease virus strain
AF2240 on anaplastic astrocytoma brain tumor cell line. Neurochem
Res. 36:2051–2062. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Molouki A and Yusoff K: NDV-induced
apoptosis in absence of Bax; evidence of involvement of apoptotic
proteins upstream of mitochondria. Virol J. 9:1792012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bakar SAA, Zawawi M, Ali AM and Ideris A:
Induction of apoptosis by Newcastle disease virus strains AF220 and
V4-UPM in human promyelocytic leukemia (HL60) and human
T-lymphoblastic leukemia (CEM-SS) cells. World Acad Sci Eng
Technol. 64:395–399. 2012.
|
|
31
|
Molouki A, Hsu YT, Jahanshiri F, Rosli R
and Yusoff K: Newcastle disease virus infection promotes Bax
redistribution to mitochondria and cell death in HeLa cells.
Intervirology. 53:87–94. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yusoff K, Tan WS, Lau CH, Ng BK and
Ibrahim AL: Sequence of the haemagglutinin-neuraminidase gene of
the Newcastle disease virus oral vaccine strain V4(UPM). Avian
Pathol. 25:837–844. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nicoletti I, Migliorati G, Pagliacci M,
Grignani F and Riccardi C: A rapid and simple method for measuring
thymocyte apoptosis by propidium iodide staining and flow
cytometry. J Immunol Methods. 139:271–279. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hale AJ, Smith CA, Sutherland LC, et al:
Apoptosis: molecular regulation of cell death. Eur J Biochem.
236:1–26. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Elmore S: Apoptosis: a review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
O’Brien V: Viruses and apoptosis. J Gen
Virol. 79:1833–1845. 1998.
|
|
37
|
Vaux DL: Toward an understanding of the
molecular mechanisms of physiological cell death. Proc Natl Acad
Sci USA. 90:786–789. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cohen JJ, Duke RC, Fadok VA and Sellins
KS: Apoptosis and programmed cell death in immunity. Annu Rev
Immunol. 10:267–293. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Csatary LK and Bakács T: Use of Newcastle
disease virus vaccine (MTH-68/H) in a patient with high-grade
glioblastoma. JAMA. 281:1588–1589. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Freeman AI, Zakay-Rones Z, Gomori JM, et
al: Phase I/II trial of intravenous NDV-HUJ oncolytic virus in
recurrent glioblastoma multiforme. Mol Ther. 13:221–228. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Karcher J, Dyckhoff G, Beckhove P, et al:
Antitumor vaccination in patients with head and neck squamous cell
carcinomas with autologous virus-modified tumor cells. Cancer Res.
64:8057–8061. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Pecora AL, Rizvi N, Cohen GI, et al: Phase
I trial of intravenous administration of PV701, an oncolytic virus,
in patients with advanced solid cancers. J Clin Oncol.
20:2251–2266. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Schirrmacher V: Clinical trials of
antitumor vaccination with an autologous tumor cell vaccine
modified by virus infection: improvement of patient survival based
on improved antitumor immune memory. Cancer Immunol Immunother.
54:587–598. 2005. View Article : Google Scholar
|
|
44
|
Steiner HH, Bonsanto MM, Beckhove P, et
al: Antitumor vaccination of patients with glioblastoma multiforme:
a pilot study to assess feasibility, safety, and clinical benefit.
J Clin Oncol. 22:4272–4281. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhou J, Law HKW, Cheung CY, Ng IHY, Peiris
JSM and Lau YL: Functional tumor necrosis factor-related
apoptosis-inducing ligand production by avian influenza
virus-infected macrophages. J Infect Dis. 193:945–953. 2006.
View Article : Google Scholar
|
|
46
|
Clarke P, Meintzer SM, Gibson S, et al:
Reovirus-induced apoptosis is mediated by TRAIL. J Virol.
74:8135–8139. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Vidalain PO, Azocar O, Lamouille B, Astier
A, Rabourdin-Combe C and Servet-Delprat C: Measles virus induces
functional TRAIL production by human dendritic cells. J Virol.
74:556–559. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kotelkin A, Prikhod’ko EA, Cohen JI,
Collins PL and Bukreyev A: Respiratory syncytial virus infection
sensitizes cells to apoptosis mediated by tumor necrosis
factor-related apoptosis-inducing ligand. J Virol. 77:9156–9172.
2003. View Article : Google Scholar
|
|
49
|
Collins PL and Hightower LE: Newcastle
disease virus stimulates the cellular accumulation of stress (heat
shock) mRNAs and proteins. J Virol. 44:703–707. 1982.PubMed/NCBI
|
|
50
|
Sunila I, Vaccarezza M, Pantaleo G, Fauci
AS and Orenstein JM: gp120 is present on the plasma membrane of
apoptotic CD4 cells prepared from lymph nodes of HIV-1-infected
individuals: an immunoelectron microscopic study. AIDS. 11:27–32.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Connolly JL, Barton ES and Dermody TS:
Reovirus binding to cell surface sialic acid potentiates
virus-induced apoptosis. J Virol. 75:4029–4039. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Fournier P, Zeng J and Schirrmacher V: Two
ways to induce innate immune responses in human PBMCs: Paracrine
stimulation of IFN-α responses by viral protein or dsRNA. Int J
Oncol. 23:673–680. 2003.PubMed/NCBI
|
|
53
|
Washburn B and Schirrmacher V: Human tumor
cell infection by Newcastle disease virus leads to upregulation of
HLA and cell adhesion molecules and to induction of interferons,
chemokines and finally apoptosis. Int J Oncol. 21:85–93.
2002.PubMed/NCBI
|
|
54
|
Szeberényi J, Fábián Z, Töröcsik B, Kiss K
and Csatary LK: Newcastle disease virus-induced apoptosis in PC12
pheochromocytoma cells. Am J Ther. 10:282–288. 2003.PubMed/NCBI
|
|
55
|
Molouki A, Hsu YT, Jahanshiri F, Abdullah
S, Rosli R and Yusoff K: The matrix (M) protein of Newcastle
disease virus binds to human bax through its BH3 domain. Virol J.
8:3852011. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Préhaud C, Lay S, Dietzschold B and Lafon
M: Glycoprotein of nonpathogenic rabies viruses is a key
determinant of human cell apoptosis. J Virol. 77:10537–10547.
2003.PubMed/NCBI
|
|
57
|
Tan W, Lau C, Ng B, Ibrahim A and Yusoff
K: Nucleotide sequence of the haemagglutinin-neuraminidase (HN)
gene of a Malaysian heat resistant viscerotropic-velogenic
Newcastle disease virus (NDV) strain AF2240. DNA Seq. 6:47–50.
1995.
|
|
58
|
Mymryk J, Shire K and Bayley S: Induction
of apoptosis by adenovirus type 5 E1A in rat cells requires a
proliferation block. Oncogene. 9:1187–1193. 1994.PubMed/NCBI
|
|
59
|
Joe AK, Foo HH, Kleeman L and Levine B:
The transmembrane domains of Sindbis virus envelope glycoproteins
induce cell death. J Virol. 72:3935–3943. 1998.PubMed/NCBI
|
|
60
|
Yao Q, Compans RW and Chen C: HIV envelope
proteins differentially utilize CXCR4 and CCR5 coreceptors for
induction of apoptosis. Virology. 285:128–137. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Wang K, Sui H, Li L, Li X and Wang L:
Anti-tumor immunity of Newcastle disease virus HN protein is
influenced by differential subcellular targeting. Zhongguo Fei Ai
Za Zhi. 13:773–776. 2010.(In Chinese).
|
|
62
|
Castedo M, Roumier T, Blanco J, et al:
Sequential involvement of Cdk1, mTOR and p53 in apoptosis induced
by the HIV-1 envelope. EMBO J. 21:4070–4080. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Lam KM, Kabbur MB and Eiserich JP:
Newcastle disease virus-induced functional impairments and
biochemical changes in chicken heterophils. Vet Immunol
Immunopathol. 53:313–327. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Kommers GD, King DJ, Seal BS and Brown CC:
Pathogenesis of chicken-passaged Newcastle disease viruses isolated
from chickens and wild and exotic birds. Avian Dis. 47:319–329.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Ravindra P, Tiwari AK, Ratta B, Chaturvedi
U, Palia SK and Chauhan R: Newcastle disease virus-induced
cytopathic effect in infected cells is caused by apoptosis. Virus
Res. 141:13–20. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Vigil A, Park MS, Martinez O, et al: Use
of reverse genetics to enhance the oncolytic properties of
Newcastle disease virus. Cancer Res. 67:8285–8292. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Bian H, Fournier P, Moormann R, Peeters B
and Schirrmacher V: Selective gene transfer to tumor cells by
recombinant Newcastle disease virus via a bispecific fusion
protein. Int J Oncol. 26:431–439. 2005.PubMed/NCBI
|
|
68
|
Ertel C, Millar NS, Emmerson PT,
Schirrmacher V and Von Hoegen P: Viral hemagglutinin augments
peptide-specific cytotoxic T cell responses. Eur J Immunol.
23:2592–2596. 1993. View Article : Google Scholar : PubMed/NCBI
|