Introduction
Cancer is caused by mutations in oncogenes and
tumor-suppressor genes. In addition to the identification of
genetic mutations, progress has been made in understanding cancer
epigenetics in oncology (1). DNA
methylation and covalent histone modifications are prominent
components of epigenetic regulation. Aberrant hypermethylation of
regulatory regions in tumor-suppressor genes and global
hypomethylation are found in cancerous cells. Perturbation of
histone modification patterns is another hallmark of cancer. Loss
of acetylation at lysine 16 in histone H4 (H4K16) and
trimethylation of H4K20 is reported in many tumor types (2). Therefore, regulators of epigenetics
have received attention as molecular targets, and several
inhibitors of DNA methyltransferase and histone deacetylase have
been approved for cancer treatment (3). However, regulators of epigenetics and
their roles in the early stages of hepatocarcinogenesis remain
poorly understood.
To identify factors concerned with tumorigenesis in
the early stages of hepatocarcinogenesis, hepatic preneoplastic
lesions were chemically induced in rats (4–6). We
found that several histone modification enzymes were upregulated in
livers highly expressing the tumor marker glutathione
S-transferase placental form (GST-P) (7–9).
Neoplastic transformation of GST-P-positive foci guides the
formation of hepatocellular carcinomas. GST-P expression is
completely repressed in normal rat liver. As specific induction of
the Gst-p gene in the pre-neoplastic lesions is induced by
almost all chemical carcinogens, analysis of the regulatory
mechanism of Gst-p expression will lead to a better
understanding of the early stages of hepatocarcinogenesis (10,11).
Analyses of transgenic rats harboring the regulatory region of
Gst-p gene and in vitro studies revealed that a
strong enhancer element, GST-P enhancer 1 (GPE1), located 2.5 kb
upstream from the transcription start site, was responsible for the
hepatocarcinogenic specific expression and was recognized by a
nuclear factor E2-related factor 2 (Nrf2)/musculoaponeurotic
fibrosarcoma oncogene homolog K (MafK) heterodimer (11).
We previously found that the expression of
coactivator-associated arginine methyltransferase 1 (CARM1), also
termed protein arginine methyltransferase 4 (PRMT4), was elevated
in the early stages of hepatocarcinogenesis in microarray
experiments (5). CARM1 was
originally identified as a factor associated with p160 coactivators
of nuclear receptor (12). CARM1
also interacts with several transcription factors including
cAMP-response element-binding protein-binding protein (CBP),
β-catenin, and the nuclear factor-κB (NF-κB) subunit p65 (13–15).
CARM1 functions as a secondary cofactor and activates transcription
by methylating arginine residues 17 and 26 of histone H3, cofactors
and itself (16–18). CARM1 contributes not only to
transcription but also RNA splicing (19). However, the functions of CARM1 in
the early stages of hepatocarcinogenesis currently are unclear.
In the present study, we demonstrated that CARM1 was
highly expressed in GST-P-positive foci and activated the
Gst-p promoter. Furthermore, knockdown of Carm1 by
shRNA in a hepatocellular carcinoma cell line inhibited cell
proliferation. These findings suggest that aberrantly expressed
CARM1 in GST-P-positive cells promotes tumorigenesis in the early
stages of hepatocarcinogenesis.
Materials and methods
Animal experiments
Five-week-old male F344 rats were obtained from
Charles River Japan, Inc. (Atsugi, Japan). At the age of 6 weeks,
they were randomly divided into 2 groups, which continuously
received either 0 or 50 ppm diethylnitrosamine (DEN) (Tokyo Kasei
Co. Ltd., Tokyo, Japan) in their drinking water for up to 18 weeks.
Rats were sacrificed at 12 or 18 weeks into the DEN treatment
(6). The livers were immediately
excised for analysis. Slices were fixed in 10% buffered formalin
for immunohistochemical examination and hematoxylin and eosin
staining, and the remaining liver tissue was immediately frozen in
liquid nitrogen and stored at −80°C until processed for RNA
extraction. All animal care and handling procedures were approved
by the Institutional Animal Care and Use Committee of the Nagoya
City University School of Medical Sciences.
RNA preparation and quantitative reverse
transcription coupled PCR (qRT-PCR)
Total RNA was isolated with TriPure Isolation
Reagent (Roche Applied Science, Indianapolis, IN, USA) according to
the manufacturer’s instructions and converted to cDNA using a
random primer and ReverTra Ace (Toyobo, Osaka, Japan). Three
adenomas (from 3 treated rats at 12 weeks), 3 carcinomas (from 3
treated rats at 18 weeks) and normal livers from 3 rats drinking
water without DEN for 18 weeks were used. For qRT-PCR, the
pre-designed primers and probe sets for Carm1 and 18S rRNA
were obtained from Applied Biosystems (Foster City, CA, USA). Data
collection was performed with an ABI PRISM 7000 sequence detection
system (Applied Biosystems).
Immunohistochemistry
Paraffin-embedded specimens were sectioned (3 μm)
and stained with rabbit anti-rat GST-P antibody (MBL, Nagoya,
Japan), CARM1 antibody (Millipore, Billerica, MA, USA) or Ki67
antibody (SP6; Acris Antibodies GmbH, Herford, Germany), and then
with anti-mouse or anti-rabbit secondary antibody and avidin-biotin
complex (Vectastatin Elite ABC kit; Vector Laboratories,
Burlingame, CA, USA), and binding sites were visualized with
diaminobenzidine. The sections were then counterstained lightly
with hematoxylin for microscopic examination. The number of CARM1-
and Ki67-labeled cells in at least 500 liver cells was counted to
determine a labeling index. The staining intensity of CARM1 in the
nucleus was quantitatively assessed with an Image Processor for
Analytical Pathology (IPAP-WIN; Sumika Technoservice, Takarazuka,
Japan) to provide the optical densities.
Luciferase reporter assay
Reporter plasmids, −2.5GST-luciferase containing the
regulatory region of the Gst-p gene, −2.5 kb to +59 bp, and
−2.15GST-luciferase, were described previously (9). A reporter plasmid including the GPE1
element, GPE1-50GST-luciferase, was kindly provided by Dr M. Sakai
(Hokkaido University) (20). The
Myc-tagged Nrf2 expression plasmid (pCMV-Myc-Nrf2) was constructed
by subcloning of the rat Nrf2 open reading frame into the
SalI-NotI site of pCMV-Myc (Clontech Laboratories,
Mountain View, CA, USA). The Flag-tagged CARM1 expression plasmid
(Flag-CARM1) was kindly provided by Dr N. Ohkura (Osaka University)
(19).
Rat hepatoma H4IIE cells, purchased from Dainippon
Sumitomo Pharma (Osaka, Japan), were maintained in α-medium
supplemented with 10% (v/v) fetal bovine serum. Transfection of
H4IIE cells in 24-well plates was performed using HilyMax (Dojindo,
Kumamoto, Japan) in accordance with the manufacturer’s
instructions. The cells were transfected with 50 ng of reporter
plasmid, 50 ng of pCMV-Myc-Nrf2, and 400 ng of the Flag-tagged
Carm1 expression plasmid. The amount of plasmid during transfection
was kept constant by using an empty vector. Fifty nanograms of the
Renilla luciferase reporter plasmid phRL-TK (Promega
Corporation, Madison, WI, USA), was used as the internal control.
At 40 h after transfection, cells were harvested and assayed for
luciferase activity by using the Dual-Luciferase Reporter Assay
System (Promega Corporation) according to the manufacturer’s
recommendations.
Transfection of shRNA expression plasmid
and cell counting
To generate shRNA expression plasmids, rat
Carm1 shRNA was designed using the Qiagen siRNA online
design tool. The selected region spanning base pairs 1339–1359 of
Carm1 was subcloned with a 5′-TTCAAGAGA-3′ loop into the
ApaI-EcoRI site of the plasmid pSilencer 1.0-U6
(Ambion, Inc., Austin, TX, USA). H4IIE cells (2.6×106)
were transfected with 6.5 μg of the shRNA expression plasmid using
the Neon transfection system (Invitrogen Life Technologies,
Carlsbad, CA, USA) and plated at 2×104 cells in 24-well
plates. Cells were trypsinized on days 1, 2, 3 and 4, and cell
numbers were measured by a hemocytometer.
Results
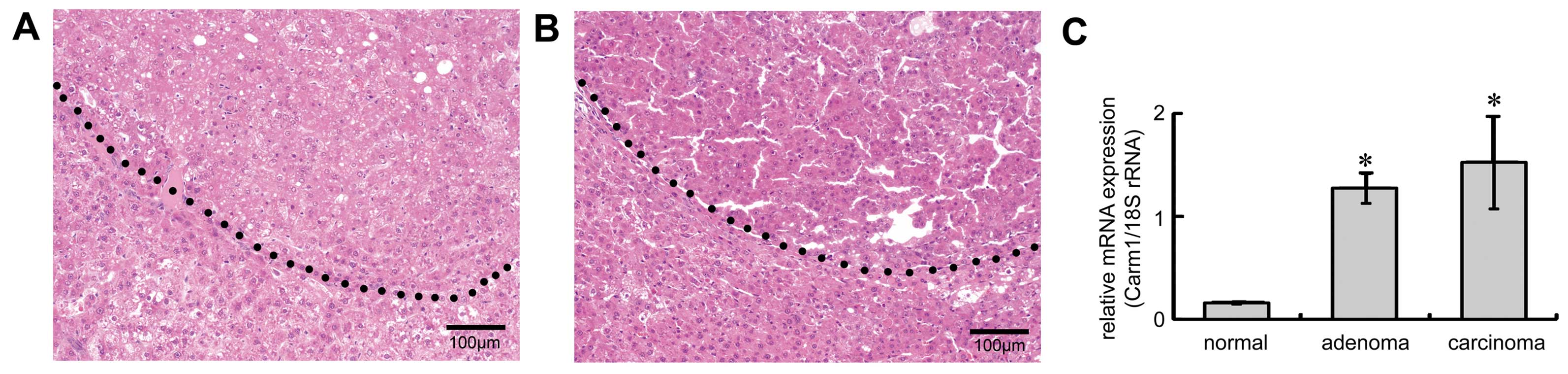
Expression profile of Carm1 during
hepatocarcinogenesis
Carm1 was identified as one of the genes
induced in GST-P-positive foci and involved in transcriptional
regulation (5). To investigate the
expression profile of Carm1 in the early stages of
hepatocarcinogenesis, adenomas and carcinomas were generated by
giving rats DEN in their drinking water. After 12 weeks of DEN
treatment, adenomas, round nodular lesions compressing adjacent
normal hepatocytes, were generated (right-upper side, Fig. 1A). Carcinomas, which had aberrant
trabeculae and were circumferentially infiltrated, formed after 18
weeks of treatment (right-upper side, Fig. 1B). RNA was prepared from dissected
adenomas and carcinomas, and qRT-PCR was performed. Expression of
Carm1 was elevated in the adenomas when compared with
expression in the normal liver, and expression of Carm1
remained aberrant in the carcinomas (Fig. 1C).
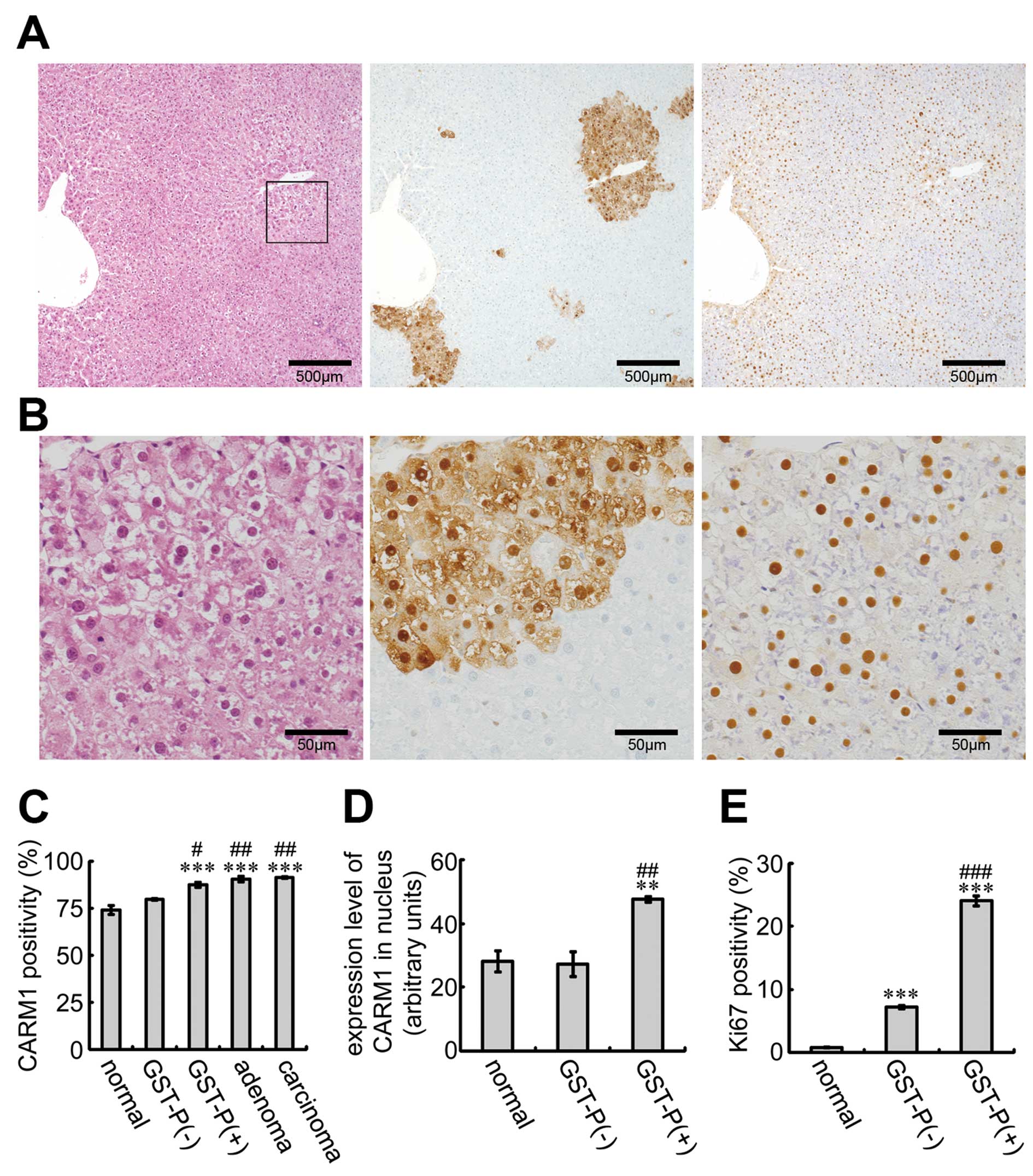
Expression of CARM1 in GST-P-positive
cells
To examine the expression of CARM1
immunohistochemically, sections from rats treated with DEN for 12
weeks were stained with the anti-CARM1 and anti-GST-P antibodies
(Fig. 2A and B). CARM1 was
expressed in the nucleus in many liver cells (Fig. 2B, right panel). Notably, CARM1 was
detected in the nucleus in almost all GST-P-positive foci. CARM1
positivity in several types of liver cells revealed that
CARM1-positive cells with GST-P staining were significantly induced
compared to both normal liver cells and GST-P-negative cells
(Fig. 2C). In addition, high CARM1
positivity was retained in the adenomas and carcinomas. To compare
CARM1 expression in the nucleus, we measured the staining intensity
of CARM1 in several types of cells (Fig. 2D). The expression level of CARM1 in
the nucleus was higher in GST-P-positive cells than that in the
normal liver cells and GST-P-negative-cells. We also examined Ki67,
a marker of cell proliferation, in the GST-P-positive and -negative
cells (Fig. 2E). Ki67 positivity
was greater in the GST-P-positive cells than that in the negative
cells. These results suggest that the elevated expression of CARM1
in GST-P-positive foci is associated with tumorigenesis and that
CARM1 is involved in cell proliferation.
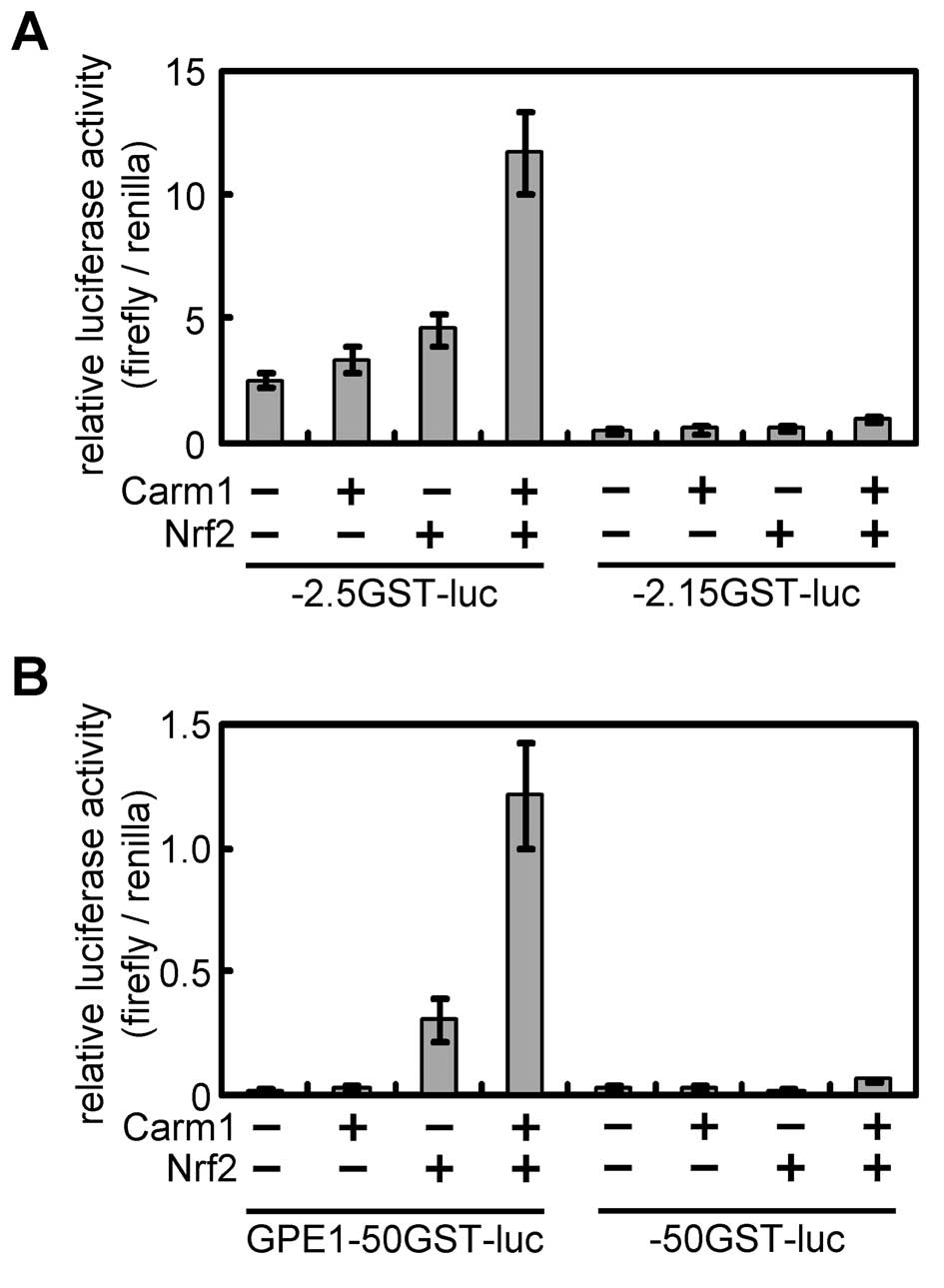
Effects of ectopic expression of CARM1 on
Gst-p promoter activity
Immunohistochemical staining of liver sections
revealed that nuclear CARM1 was overexpressed in GST-P-positive
foci. Next, we investigated the effect of the ectopic expression of
CARM1 on Gst-p promoter activity. The regulatory region of
the Gst-p gene is well characterized, and GST-P enhancer 1
(GPE1), located −2.5 kb upstream from the cap site, is responsible
for the hepatocarcinogenic specific gene expression (11). GPE1 is recognized by the Nrf2/MafK
heterodimer. When the CARM1 expression plasmid was transfected into
H4IIE cells with −2.5GST-luciferase, which has the entire
Gst-p regulatory region and promoter, the promoter was not
activated (Fig. 3A). However,
Gst-p promoter activity was enhanced when the CARM1
expression plasmid was introduced with the Nrf2 expression plasmid.
This activation was not observed when −2.15GST-lucifearse lacking
GPE1, was introduced. To restrict the element responding to CARM1,
a reporter plasmid containing the minimal promoter sequence of the
Gst-p gene with or without GPE1 was used. CARM1 enhanced the
Gst-p promoter activity dependent on GPE1 and Nrf2 (Fig. 3B).
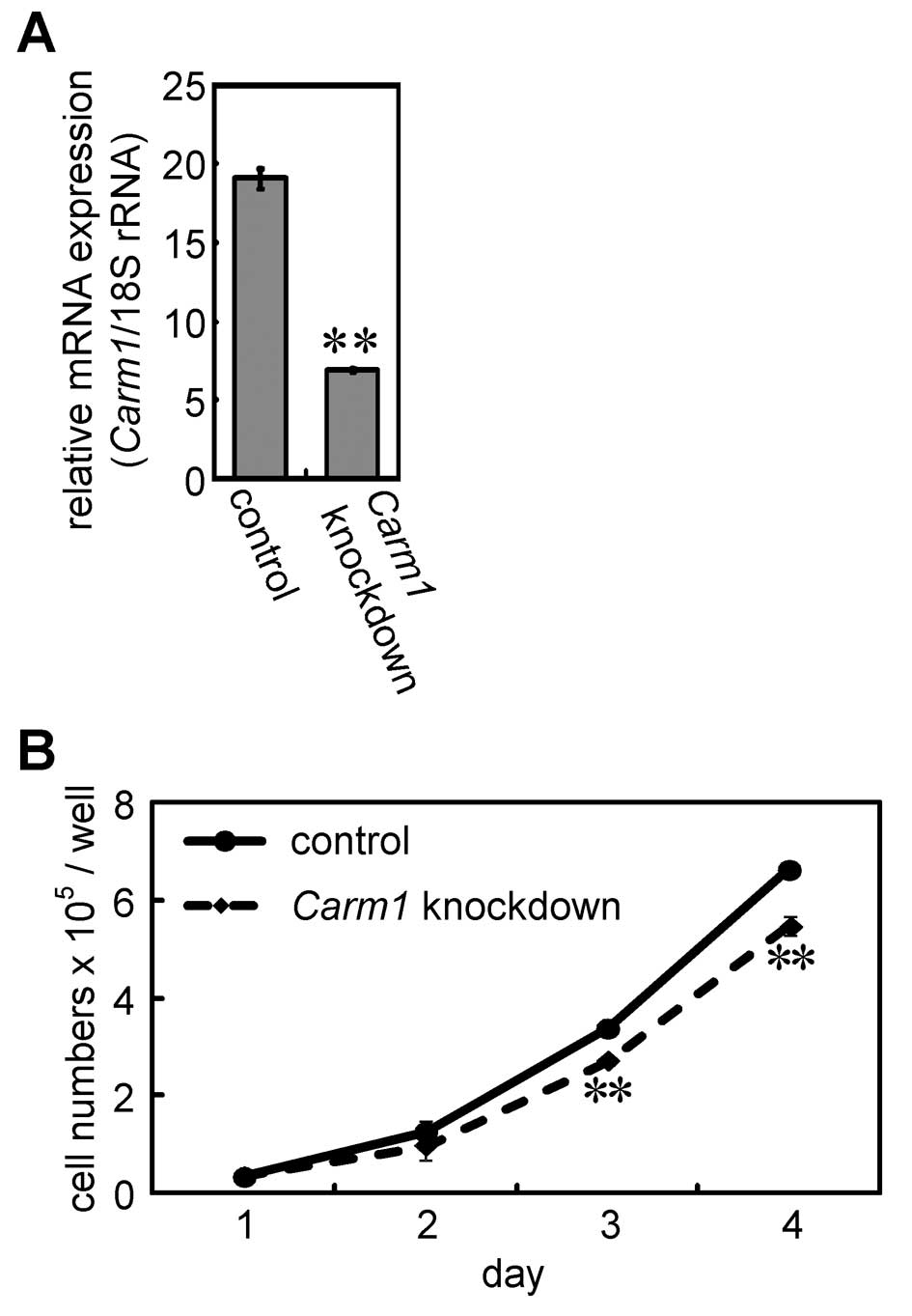
Effects of shRNA-mediated knockdown of
Carm1 on cell proliferation in a hepatocellular carcinoma cell
line
The ratio of Ki67-positive cells in the
GST-P-positive foci was higher than that in the GST-P-negative
cells (Fig. 2E). To investigate the
effects of the depletion of Carm1 on cell proliferation, we
constructed shRNA expression plasmid for Carm1. Cells
transfected with the plasmid were plated in 24-well plates, and the
expression level of the target gene was measured 3 days following
transfection (Fig. 4A).
Furthermore, the cell numbers were counted using a hemocytometer
(Fig. 4B). The expression of the
target gene was suppressed by the shRNA expression plasmid for
Carm1. Under these conditions, the number of cells
transfected with the shRNA expression plasmid for Carm1 were
reduced when compared with the number of cells in the control at
days 3 and 4. These results suggest that Carm1 is involved
in the cell proliferation of H4IIE cells.
Discussion
We demonstrated here that CARM1 expression is
upregulated in GST-P-positive foci during hepatocarcinogenesis. In
addition, we demonstrated that CARM1 activated the Gst-p
promoter through Nrf2. Furthermore, knockdown of Carm1 by
shRNA in H4IIE cells inhibited cell proliferation. These
observations suggest that aberrantly expressed CARM1 in
GST-P-positive cells promotes tumorigenesis in the early stages of
hepatocarcinogenesis.
Analysis of the expression of CARM1 in human cancers
using a tissue microarray containing various tumor types including
brain tumors, melanoma, colorectal cancer, prostate cancer and
breast cancer, but not liver cancer, revealed that CARM1 expression
was elevated in colorectal cancer, but not in prostate or breast
cancer (21). In contrast, elevated
expression of CARM1 in prostate cancer was previously reported
(22,23). These observations indicate that
CARM1 is involved in the tumorigenesis of various types of cancers.
Although CARM1 is known as a coactivator for nuclear receptors
including the androgen receptor, our results and previous
observations suggest that it is also involved in sex
hormone-independent cancer.
We showed here that CARM1 activated Nrf2-mediated
Gst-p promoter activity. As no interaction between Nrf2 and
CARM1 or localization of CARM1 to GPE1 was detected in H4IIE cells
in the immunoprecipitation and chromatin immunoprecipitation
experiments, respectively (data not shown), the chromatin-based
localization of CARM1 to GPE1 may be weak or transient. Nrf2
possesses a degron common to both Keap1-independent and -dependent
degradation in the nucleus (24,25).
Proteasome and ubiquitin-conjugated enzymes are distributed
throughout the cytoplasm and nucleus and they are important for the
regulation of gene expression (26). Nrf2 interaction with CARM1 may be
rapidly degraded after it functions as a transcriptional activator.
Nrf2 is crucial for the transactivation of the Gst-p
promoter mediated by GPE1. It is known that Nrf2 interacts with
several cofactors including CBP (27). Depletion of Carm1 did not
decrease the expression of Gst-p (data not shown). The loss
of CARM1 may be compensated for by the recruitment of other Nrf2
cofactors including CBP on the Gst-p promoter.
Nrf2 upregulates expression of the anti-apoptotic
gene Bcl-2 and prevents apoptosis (28). NF-κB, one of the transcription
factors targeted by CARM1, directly activates anti-apoptotic genes
including the genes for tumor necrosis factor receptor-associated
factor 1 (TRAF1), TRAF2 and the inhibitor-of-apoptosis (IAP)
proteins c-IAP1 and cIAP2 (29). We
demonstrated that anti-apoptotic genes, including Bcl-2,
were upregulated in GST-P-positive foci (5). These observations indicate that CARM1
may promote tumorigenesis by enhancing cell proliferation as well
as through its anti-apoptotic function. To better understand the
molecular mechanism of CARM1-mediated tumorigenesis, the
identification of CARM1 target gene(s) in GST-P-positive foci is
required.
Acknowledgements
We thank Dr Masaharu Sakai (Hokkaido University) and
Dr Naganari Ohkura (Osaka University) for providing the reporter
plasmids and the expression plasmid for Carm1, respectively. This
research was supported in part by grants from the Long-Range
Research Initiative (LRI) by the Japan Chemical Industry
Association (JCIA) and the Japan Society for the Promotion of
Science (JSPS).
References
|
1
|
Rodriguez-Paredes M and Esteller M: Cancer
epigenetics reaches mainstream oncology. Nat Med. 17:330–339. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fraga MF, Ballestar E, Villar-Garea A, et
al: Loss of acetylation at Lys16 and trimethylation at Lys20 of
histone H4 is a common hallmark of human cancer. Nat Genet.
37:391–400. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kelly TK, De Carvalho DD and Jones PA:
Epigenetic modifications as therapeutic targets. Nat Biotechnol.
28:1069–1078. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Suzuki S, Asamoto M, Tsujimura K and
Shirai T: Specific differences in gene expression profile revealed
by cDNA microarray analysis of glutathione S-transferase placental
form (GST-P) immunohistochemically positive rat liver foci and
surrounding tissue. Carcinogenesis. 25:439–443. 2004. View Article : Google Scholar
|
|
5
|
Osada S, Naganawa A, Misonou M, et al:
Altered gene expression of transcriptional regulatory factors in
tumor marker-positive cells during chemically induced
hepatocarcinogenesis. Toxicol Lett. 167:106–113. 2006. View Article : Google Scholar
|
|
6
|
Suzuki S, Takeshita K, Asamoto M, et al:
High mobility group box associated with cell proliferation appears
to play an important role in hepatocellular carcinogenesis in rats
and humans. Toxicology. 255:160–170. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ohta K, Ohigashi M, Naganawa A, et al:
Histone acetyltransferase MOZ acts as a co-activator of Nrf2-MafK
and induces tumour marker gene expression during
hepatocarcinogenesis. Biochem J. 402:559–566. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yura T, Hashizume H, Suzuki E, Imagawa M
and Osada S: Promotion of anchorage-independent growth by
cytoplasmic and nuclear histone deacetylase 9. J Health Sci.
56:581–588. 2010. View Article : Google Scholar
|
|
9
|
Hashizume H, Gomita U, Imagawa M and Osada
S: Histone methyltransferase PR-Set7 and histone variant H2A.Z,
induced during hepatocarcinogenesis, repress the promoter activity
of the tumor marker gene and the Ras-induced colony formation
activity. J Health Sci. 57:264–273. 2011. View Article : Google Scholar
|
|
10
|
Morimura S, Suzuki T, Hochi S, et al:
Trans-activation of glutathione transferase P gene during chemical
hepatocarcinogenesis of the rat. Proc Natl Acad Sci USA.
90:2065–2068. 1993. View Article : Google Scholar
|
|
11
|
Sakai M and Muramatsu M: Regulation of
glutathione transferase P: a tumor marker of hepatocarcinogenesis.
Biochem Biophys Res Commun. 357:575–578. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen D, Ma H, Hong H, et al: Regulation of
transcription by a protein methyltransferase. Science.
284:2174–2177. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu W, Chen H, Du K, et al: A
transcriptional switch mediated by cofactor methylation. Science.
294:2507–2511. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Koh SS, Li H, Lee YH, Widelitz RB, Chuong
CM and Stallcup MR: Synergistic coactivator function by
coactivator-associated arginine methyltransferase (CARM) 1 and
β-catenin with two different classes of DNA-binding transcriptional
activators. J Biol Chem. 277:26031–26035. 2002.PubMed/NCBI
|
|
15
|
Covic M, Hassa PO, Saccani S, et al:
Arginine methyltransferase CARM1 is a promoter-specific regulator
of NF-κB-dependent gene expression. EMBO J. 24:85–96. 2005.
|
|
16
|
Ma H, Baumann CT, Li H, et al:
Hormone-dependent, CARM1-directed, arginine-specific methylation of
histone H3 on a steroid-regulated promoter. Curr Biol.
11:1981–1985. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schurter BT, Koh SS, Chen D, et al:
Methylation of histone H3 by coactivator-associated arginine
methyltransferase 1. Biochemistry. 40:5747–5756. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kuhn P, Chumanov R, Wang Y, Ge Y, Burgess
RR and Xu W: Automethylation of CARM1 allows coupling of
transcription and mRNA splicing. Nucleic Acids Res. 39:2717–2726.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ohkura N, Takahashi M, Yaguchi H, Nagamura
Y and Tsukada T: Coactivator-associated arginine methyltransferase
1, CARM1, affects pre-mRNA splicing in an isoform-specific manner.
J Biol Chem. 280:28927–28935. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ikeda H, Nishi S and Sakai M:
Transcription factor Nrf2/MafK regulates rat placental glutathione
S-transferase gene during hepatocarcinogenesis. Biochem J.
380:515–521. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim YR, Lee BK, Park RY, et al:
Differential CARM1 expression in prostate and colorectal cancers.
BMC Cancer. 10:1972010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hong H, Kao C, Jeng MH, et al: Aberrant
expression of CARM1, a transcriptional coactivator of androgen
receptor, in the development of prostate carcinoma and
androgen-independent status. Cancer. 101:83–89. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Majumder S, Liu Y, Ford OH III, Mohler JL
and Whang YE: Involvement of arginine methyltransferase CARM1 in
androgen receptor function and prostate cancer cell viability.
Prostate. 66:1292–1301. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
McMahon M, Itoh K, Yamamoto M and Hayes
JD: Keap1-dependent proteasomal degradation of transcription factor
Nrf2 contributes to the negative regulation of antioxidant response
element-driven gene expression. J Biol Chem. 278:21592–21600. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Malloy MT, McIntosh DJ, Walters TS, Flores
A, Goodwin JS and Arinze IJ: Trafficking of the transcription
factor Nrf2 to promyelocytic leukemia-nuclear bodies: implications
for degradation of NRF2 in the nucleus. J Biol Chem.
288:14569–14583. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kwak J, Workman JL and Lee D: The
proteasome and its regulatory roles in gene expression. Biochim
Biophys Acta. 1809:88–96. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Katoh Y, Itoh K, Yoshida E, Miyagishi M,
Fukamizu A and Yamamoto M: Two domains of Nrf2 cooperatively bind
CBP, a CREB binding protein, and synergistically activate
transcription. Genes Cells. 6:857–868. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Niture SK and Jaiswal AK: Nrf2 protein
up-regulates antiapoptotic protein Bcl-2 and prevents cellular
apoptosis. J Biol Chem. 287:9873–9886. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang CY, Mayo MW, Korneluk RG, Goeddel DV
and Baldwin AS Jr: NF-κB antiapoptosis: induction of TRAF1 and
TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation.
Science. 281:1680–1683. 1998.
|