Introduction
Cordyceps militaris (C. militaris)
also known as the rare Chinese caterpillar fungus has been used in
traditional medicine to maintain health and to treat numerous
diseases associated with circulatory, respiratory, glandular and
metabolic systems. Several studies demonstrated that a variety of
chemical constituents in C. militaris are involved in these
activities, with cordycepin, a nucleoside analog
(3′-deoxyadenosine), considered to be one of the most important
bioactive components mediating its beneficial effects (1,2). C.
militaris has been shown to display anti-inflammatory (3), antidiabetic (4), and anti-infectious activities
(5–7) conceivably being related to the immune
response. Although it has also been considered to have antitumor
activities (8–13), there is still considerable
uncertainty about the effect on tumor immunity.
The recent advance in the mechanisms responsible for
tumor progression have suggested the possibility of controlling
tumor growth through chemotherapy-induced cancer cell destruction
as well as by stimulating the antitumor immunity.
Tumor-infiltrating lymphocytes (TILs) are seen as a reflection of a
tumor-related immune response and are recognized as the principal
effectors of the local antitumor immune response (14). During the neoplastic process,
however, tumor cells acquire immunotolerance and cause an
accumulation of immunosuppressive infiltrates in the tumor
microenvironment (15). In our
previous study, we evaluated the effect of cordycepin purified from
C. militaris JLM 0636 strain on the production of cytokines
in lipopolysaccharide-stimulated mouse splenocytes (16). Th1 cytokines such as IL-12 and
IFN-γ, and Th2 cytokines such as IL-4 and IL-10, were significantly
increased by cordycepin; however, IL-2 was rather decreased with
time, indicating that cordycepin might induce change in the
regulatory subpopulation among CD4+ T lymphocytes (Treg
cells). Treg cell is one of the most potent and well-studied
suppressive phenotypes found in the tumor microenvironment,
constitutively expressing high levels of CD25, CTLA-4, GITR, and a
transcriptional factor forkhead box P3 (Foxp3) (17–19).
They play an important role in immune evasion mechanisms employed
by tumors which may differentiate, expand, recruit and activate
them and potently abrogate antitumor immunity. Indeed, higher
numbers of Treg cells are associated with malignancy progression in
various solid tumors (lung, breast and pancreas) as well as
hematological malignancies. The increased populations of
CD4+CD25+ Treg cells appear to correlate with
a poorer survival for several types of cancer (20).
In the present study, we demonstrated that C.
militaris induced the immunomodulatory and antitumor properties
controlled by the suppression of Treg cell population, which made
peritumoral microenvironment unfavorable to tumors and eventually
resulted in growth inhibition of tumor cells. We also showed that
these effects were dependent on the cordycepin content in C.
militaris by comparing wild-type C. militaris and
cordycepin-enriched C. militaris JLM 0636 strain.
Materials and methods
Preparation of C. militaris and
quantitative analysis of cordycepin
C. militaris used in the present study was
supplied by Chungwon-Industrial Farm (Gimhae, Korea) which had
constructed C. militaris JLM 0636 strain by single spore
fusion of various strains of C. militaris. For the
preparation of C. militaris contained food, the powder of
dried fruiting bodies was replaced with cornstarch 3% in the basal
food (casein 20%, cornstarch 15%, sucrose 44.5%, cellurose 5%, corn
oil 10%, AIN-93M-MX mineral mixture 4%, AIN-93-VX vitamin mixture
1%, choline bitartrate 0.2% and DL-methionine 0.3%). For the
preparation of C. militaris contained water, the powder of
dried fruiting bodies was extracted with distilled deionized water
for 3 h at 121°C, the insoluble materials were removed by
centrifugation at 10,000 g for 30 min and filtration with 0.45 μm
membrane filter, and the resulting water extract was added to the
standard water to 1%. Cordycepin content in the water extract was
analyzed by high performance liquid chromatography (PerkinElmer 200
Series System; PerkinElmer, Waltham, MA, USA) using a Bridge C18
column (4.6 × 250 mm, 3.5 mm; Waters, Taunton, MA, USA) and UV
Detector (260 nm). The mobile phase was 0.01 M phosphate buffer, pH
6.5 and linear gradient with MeOH: 0–15% for 10 min, 15–10% for 5
min, 10–20% for 2 min and 20% for 12 min. The column oven was kept
at 40°C, and the flow rate was 5 ml/min. The cordycepin content was
calculated from the peak area shown in the standard curve of
commercial cordycepin (Sigma, St. Louis, MO, USA).
Animal and experimental design
Female C3H/He mice, 6 weeks of age, were obtained
from Central Laboratory Animal Inc., (Seoul, Korea) and housed at
the animal maintenance facility of the Clinical Research Center of
Dong-A University Hospital. All animals were maintained in specific
pathogen-free conditions according to Good Laboratory Practices
OECD guidelines. All animal procedures were performed according to
approved protocols (Approval number; DIACUC-09-24) from the
Institutional Animal Care and Use Committee (IACUC) of Dong-A
University and in accordance with recommendations for the proper
use and care of laboratory animals. Animals were allowed free
access to basal food and standard water for 2 week before the
experiment. Animals were then randomly divided into four
experimental groups: normal mice group fed with basal food and
standard water (normal), tumor-bearing mice group fed with basal
food and standard water (control), tumor-bearing mice group fed
with wild-type C. militaris contained food and water
(CMloC), tumor-bearing mice group fed with C. militaris JLM
0636 strain contained food and water (CMhiC). FM3A murine breast
cancer cells originated from the mammary gland of the C3H/He mouse
and cells were cultured in RPMI-1640 medium (Invitrogen, Carlsbad,
CA, USA) supplemented with 2 mM L-glutamine, 100 U/ml penicillin,
100 μg/ml streptomycin, and 10% heat-inactivated fetal bovine serum
(FBS; Invitrogen) at 37°C in a humidified 5% CO2
atmosphere. Cells in logarithmic growth phase (2×106
cells/50 μl saline) were inoculated subcutaneously on the right
flank of female C3H/He mice. Mice were monitored daily for signs of
toxicity and survival, and tumor volumes were measured twice weekly
with a caliper. Tumor volumes were calculated as
(width)2 × length × 0.52. When the tumor-bearing mice
were sacrificed at day 30, upon reaching >100 mm3 of
tumor volume, the spleen was aseptically removed. Single cell
suspension was prepared by gently teasing the cells through sterile
stainless steel screen and the erythrocytes were lysed at room
temperature using ACK lysis buffer (NH4Cl,
KHCO3 and Na2EDTA). The isolated splenocytes
were suspended in complete medium (RPMI-1640 supplemented with 10%
FBS, 50 mM 2-mercaptoethanol, 100 U/ml penicillin and 100 μg/ml
streptomycin) and cultured at 37°C in a humidified 5%
CO2 atmosphere.
Cytokine production assay
The isolated splenocytes were cultured with
concanavalin A (ConA, 5 μg) for 24 h at 107 cells/ml in
serum-free RPMI medium containing 200 μg/ml BSA. IL-2, IL-4 and
TGF-β concentrations were determined in supernatant using
enzyme-linked immunosorbent assay kits (ELISA; BD Biosciences
Pharmingen, San Jose, CA, USA) according to the manufacturer’s
instructions.
Lymphocyte proliferation assay
Lymphocyte proliferation was determined by BrdU
(5-bromo-2-deoxyuridine) incorporation assay using ConA-stimulated
cell suspension at 5×105 cells/well in flat-bottom
96-well microculture plates. The cells were cultured for 48 h and
further incubated for 24 h in the presence of 10 μl of the BrdU
solution in RPMI medium (1:100 diluent). The BrdU incorporation was
measured by using the Cell Proliferation ELISA BrdU kit (Roche
Diagnostics-Applied Science, Mannheim, Germany), following the
supplier’s specifications.
Lymphocyte subpopulation analysis
Lymphocyte subpopulations among splenocytes were
determined by flow cytometric analysis. The freshly prepared
splenocytes were washed three times with ice cold PBS-containing
0.1% FBS and then stained with appropriately diluted labeled rat
anti-mouse antibodies [anti-CD4 FITC and anti-CD8 PE (BD
Biosciences Pharmingen); anti-CD25 PERCP-Cy5.5 (eBioscience) and
isotype controls (BD Biosciences Pharmingen)] at 0.1–0.5 μg/ml for
40 min on ice. For the identification of intracellular IFN-γ
expression in CD8+ T cells, the freshly prepared cells
were stimulated with PMA (50 ng/ml; Sigma) and ionomycin (500
ng/ml; Sigma) for 2 h and further incubated with protein transport
inhibitor brefeldin A (10 μg/ml; Sigma) for 2 h to accumulate for
intracellular cytokines. At the end of the incubation, Fc Block (BD
Biosciences Pharmingen) was added for 15 min at 4°C, and standard
surface staining procedures were performed with anti-CD8 PE. The
cells were then fixed, permeabilized and stained with anti-IFN-γ
FITC (BD Biosciences Pharmingen). Following staining, cells were
washed and analyzed immediately with an FC500 flow cytometer (BD
FACSAria; BD Biosciences). Two color flow cytometric analyses were
performed and data represent 50,000 events unless otherwise noted.
The phenotype of Treg cells
(CD4+CD25+FoxP3+) was evaluated by
three-color fluorescence flow cytometric analysis following
standard surface staining procedures combined with intracellular
FoxP3 staining method. Intracellular detection of FoxP3 was
performed by using PE anti-mouse FoxP3 staining buffer set
purchased from eBioscience. Briefly, the cells stained with
anti-CD4-FITC and anti-CD25-PERCP-Cy5.5 were washed, fixed and
permeabilized according to the manufacturer’s instructions, and
then incubated with PE-conjugated rat anti-mouse FoxP3 Ab for 40
min on ice.
Statistical analysis
All statistical analyses were performed using
commercially available statistical software (SPSS, Inc., Chicago,
IL, USA). Results are expressed as means ± standard deviation (SD),
and numerical data were analyzed by Student’s t-test or one-way
analysis of variance (ANOVA). For all analyses, a difference was
considered statistically significant at P<0.05.
Results
Induction of immunomodulation by
cordycepin-enriched C. militaris
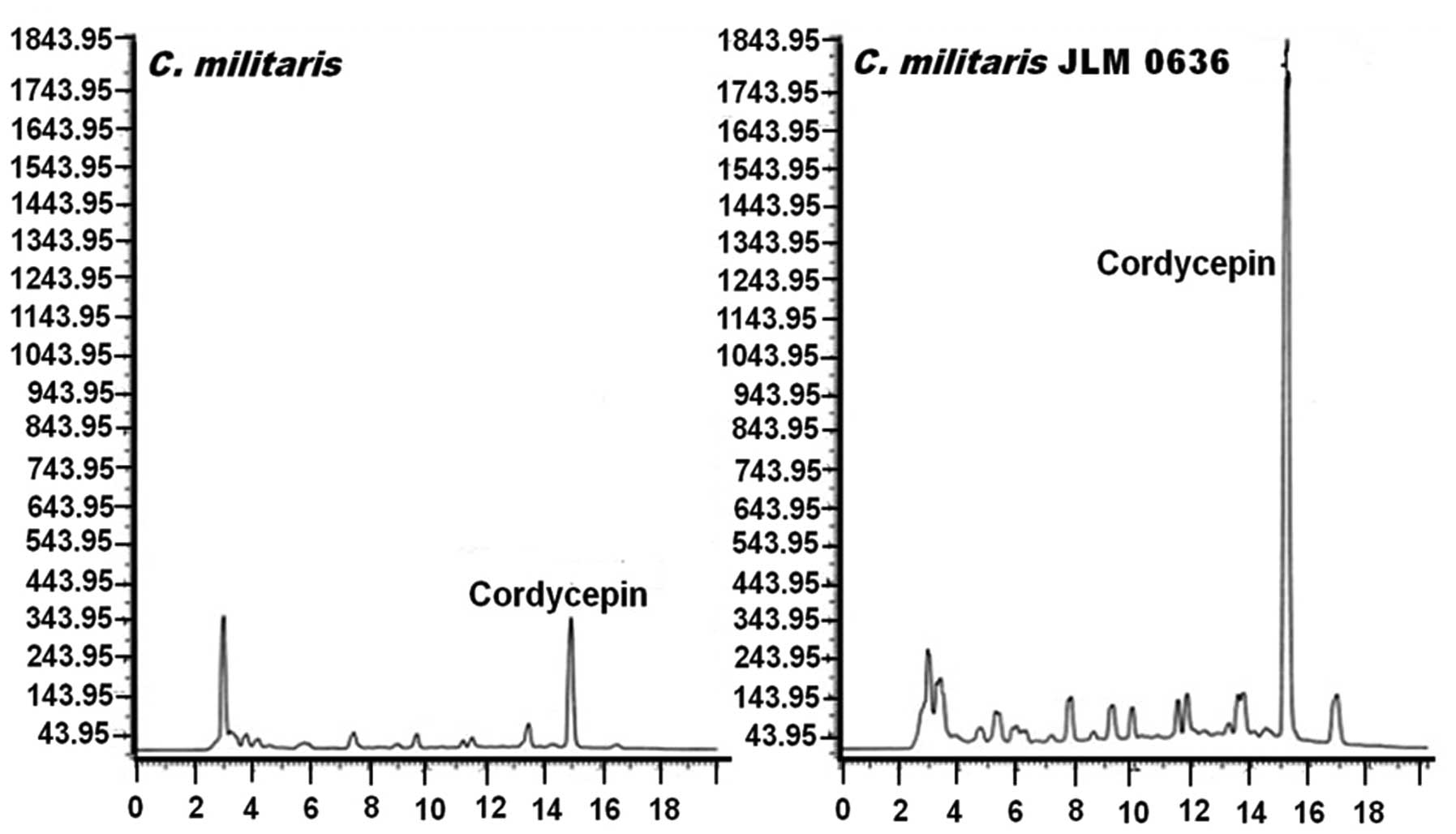
For the determination of cordycepin content, hot
water extract was prepared and subjected to HPLC analysis (Fig. 1). The concentration of cordycepin
contained in C. militaris JLM 0636 strain (7.42 mg/g dry
weight) was 7-fold higher than that of wild-type C.
militaris (1.21 mg/g dry weight), which was calculated from the
peak area shown in the standard curve of commercial cordycepin. To
examine whether C. militaris could lead modulation of
tumor-derived T cells, ConA-stimulated lymphocyte proliferative
response was tested by BrdU incorporation assay. The ConA response
of splenocytes from tumor-bearing mice was generally lower than
that from normal mice and was not significantly altered by the
feeding of C. militaris (Fig.
2). Having demonstrated the presence of cytokines for the
development of effector T cells and Treg cells, the cultured
supernatants of ConA-stimulated lymphocytes were analyzed for the
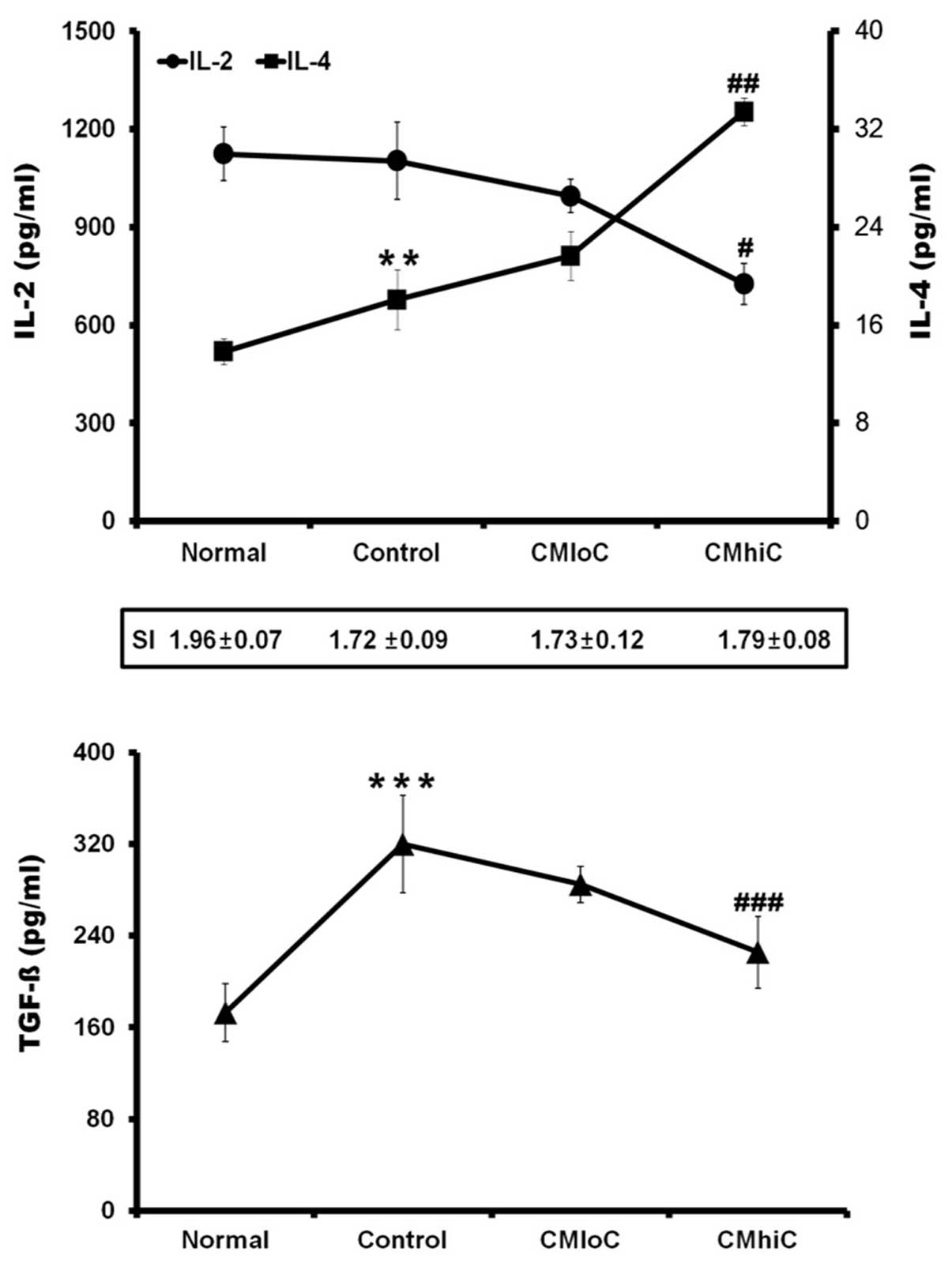
production of IL-2, IL-4 and TGF-β. Tumor-bearing mice fed with
C. militaris showed cordycepin-dependent decrease in IL-2
and TGF-β production and increase in IL-4 production (Fig. 2), which indicated that C.
militaris may induce changes in the subpopulations of
tumor-derived T lymphocytes. Thus, immunofluorescent analysis for
cell surface markers using flow cytometric analysis was performed
to investigate T lymphocyte subpopulations. The average frequencies
of CD4+ T cells among total splenocytes from
tumor-bearing mice groups were generally lower than the normal mice
group and were not significantly altered by the differences in the
feeding conditions (data not shown). The frequency of
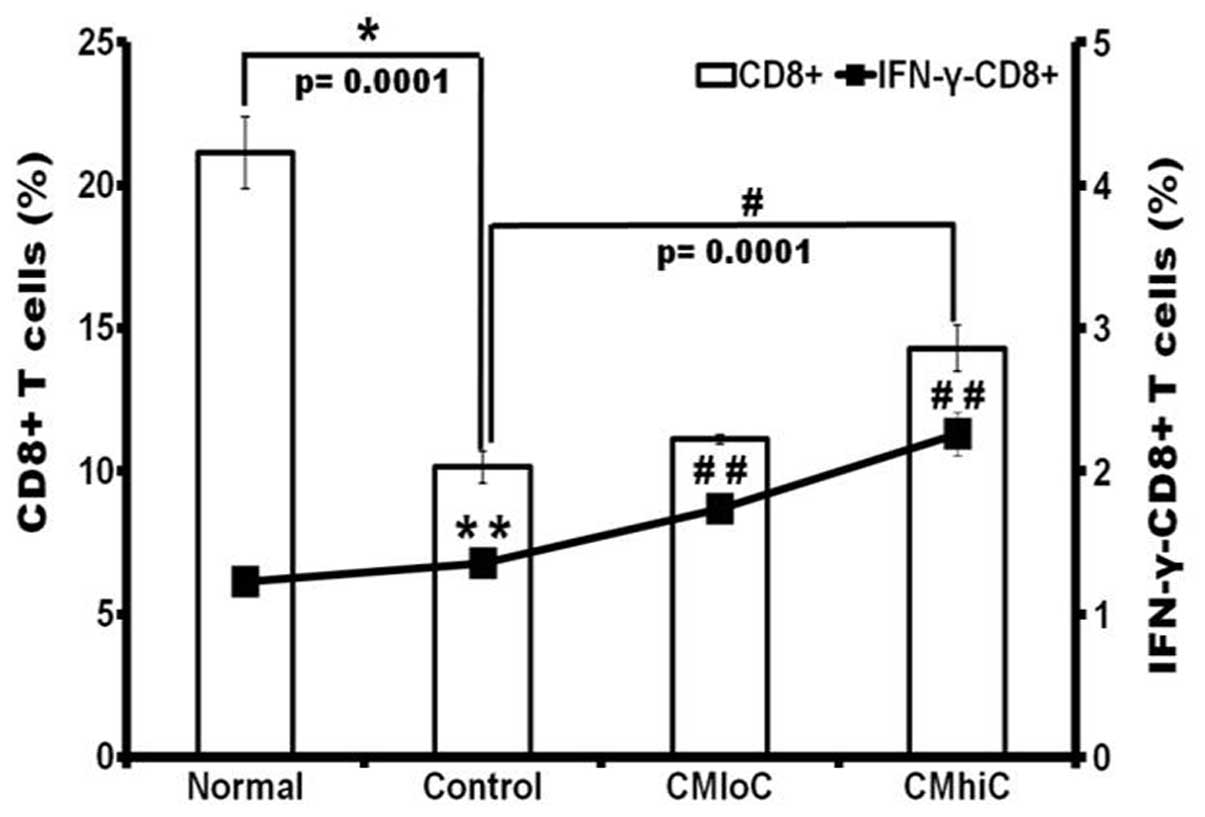
CD8+ T cells from the control tumor-bearing mice group
was also decreased compared with the normal mice group; however,
C. militaris feeding prominently recovered the frequencies
of CD8+ T cells particularly in the cordycepin-enriched
C. militaris JLM 0636 fed group (Fig. 4).
Suppression of Treg cells by
cordycepin-enriched C. militaris
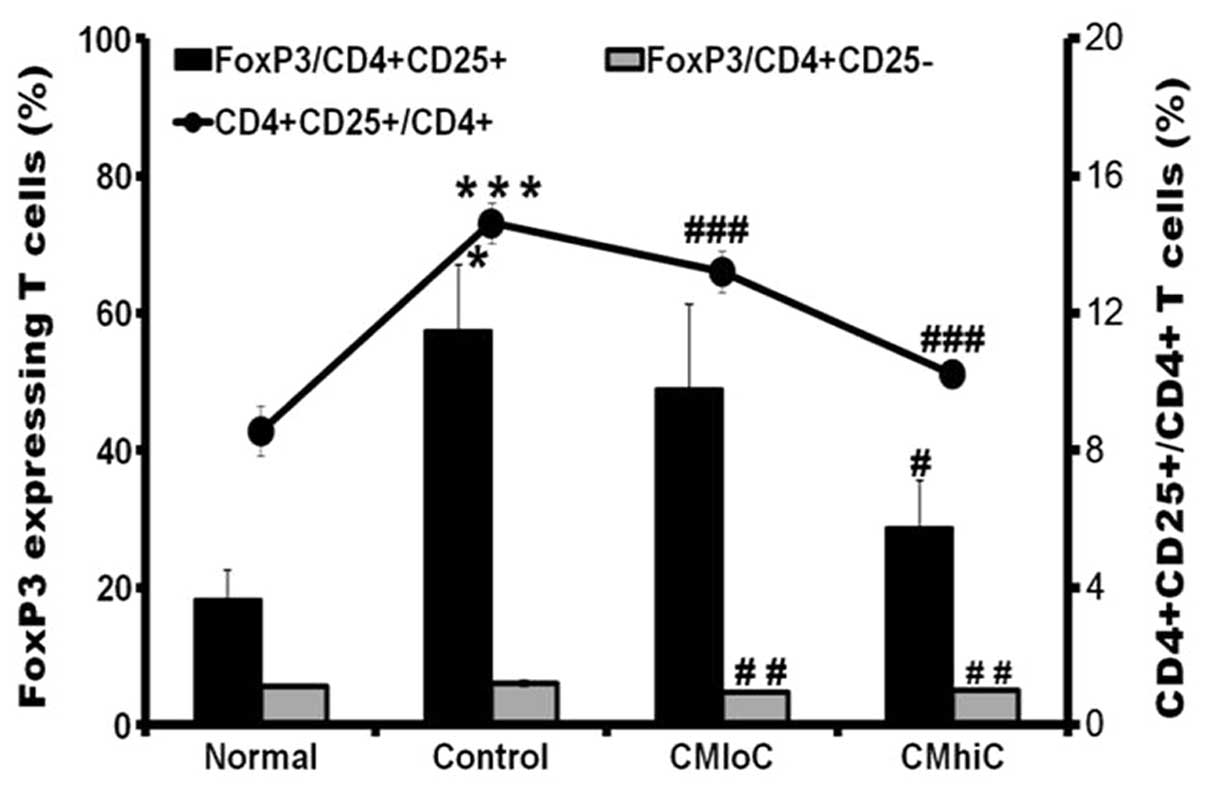
To investigate the effect of C. militaris on
Treg cell population, CD4+CD25+ cell
proportions were determined by flow cytometric analysis. Based on
the changes in the average frequency of CD4+ T cells
between the normal and tumor-bearing mice groups, comparison of
CD4+CD25+ to CD4+ cell ratio was
better to discriminate the differences between groups. The higher
ratio of the control tumor-bearing mice group compared to the
normal mice group was significantly decreased in the
cordycepin-enriched C. militaris JLM 0636 fed group
(Fig. 3). The expression of a
specific Treg cell marker, FoxP3, was further investigated to
confirm that the reduced CD4+CD25+ cells were
true Treg cells. The proportions of FoxP3+ cells among
CD4+CD25+ cells showed similar but more
prominent changes between the experimental groups (Fig. 3). On the other hand,
FoxP3+ cells among CD4+CD25− cells
were almost unchanged at minimal level (data not shown).
Enhancement of IFN-γ-expressing CD8 T
cells by cordycepin-enriched C. militaris
To investigate whether C. militaris could
lead to enhanced effector T cells involved in antitumor immunity in
tumor-bearing mice, IFN-γ-expressing CD8+ T cells were
examined by flow cytometric analysis. The number and percentage of
IFN-γ-expressing CD8+ T cells in tumor-bearing mice were
significantly increased in the experimental groups fed with C.
militaris, compared to those in the normal mice (Fig. 4). The degree of increase was much
more pronounced in the mice group fed with the C. militaris
JLM 0636 strain, which was in accordance with the recovery of the
frequencies of CD8+ T cells. This result indicates that
C. militaris enhanced IFN-γ secretion and effector T cell
function, which may suggest unfavorable microenvironmental change
to tumor and induction of effective antitumor immunity.
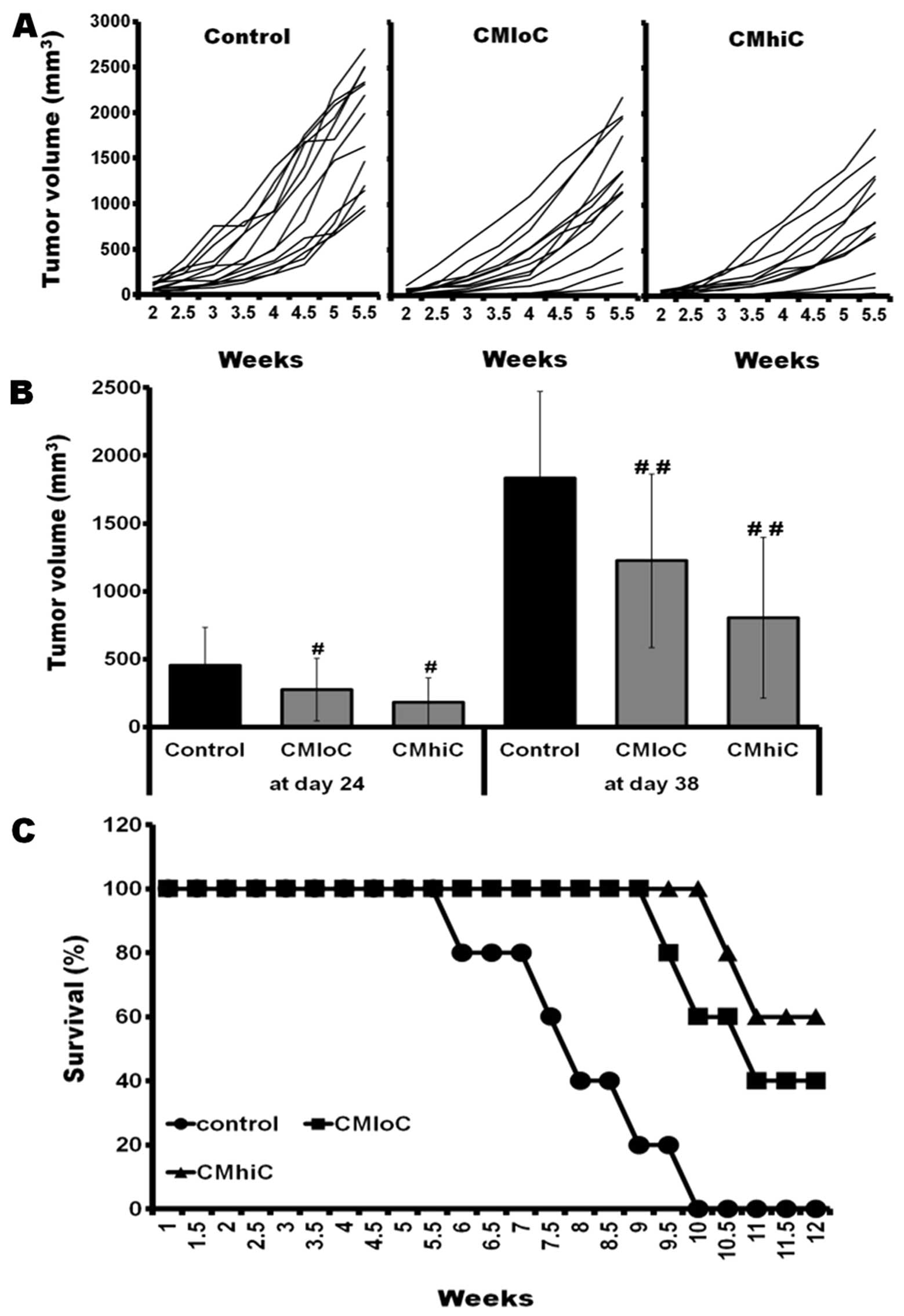
Tumor growth delay by cordycepin-enriched
C. militaris
To determine the ability of C. militaris to
induce a protective antitumor activity in vivo, mice were
fed systematically with C. militaris powder and water
extract contained diet during which mice were challenged
subcutaneously with FM3A murine breast cancer cells. When mice were
fed with C. militaris, tumor formations were delayed and the
survival rates were prolonged compared with control mice (Fig. 5). The mice group fed with C.
militaris JLM 0636 strain showed much slower tumor growth and
survived for longer time periods than the mice group fed with
wild-type C. militaris (Fig.
5). C. militaris at the dose used was not toxic to the
animals as we observed no differences in body weight gain, food
intake, water consumption and behaviors between experimental groups
until visible tumor formation. Although these parameters of
tumor-bearing mice were gradually decreased with the lapse of time
compared with the normal mice, there were no significant
differences between control and C. militaris-fed mice (data
not shown).
Discussion
There has been considerable interest in cordycepin
as a potential cancer chemotherapeutic agent. Due to the absence of
oxygen in the 30 position of its ribose moiety, the incorporation
of cordycepin during RNA synthesis results in termination of chain
elongation. This activity has been well described in vitro
with purified RNA polymerases and poly (A) polymerases from a
number of organisms, including yeast and mammals (21,22).
However, cordycepin is not a potent cytotoxic compound when
compared to other chemotherapeutic drugs, and the chemical
compounds including cordycepin contained in C. militaris
have been known to mediate unique properties related to the
immunity. As tumor growth can be controlled by destroying cancer
cells as well as by stimulating antitumor immunity, it is necessary
to explore the role of C. militaris in immune response
underlying antitumor activity. For this purpose, we constructed
C. militaris JLM 0636 strain with 7-fold higher cordycepin
content than wild-type C. militaris and fed each containing
food and water to mice inoculated with FM3A tumor cells. The
results presented in the present study showed that the feeding of
cordycepin-enriched C. militaris JLM 0636 strain for 30 days
had a considerable effect on in vivo growth and survival
rate of an FM3A breast tumor in CH3/He mice (Fig. 5), which demonstrated that it may be
one of the most appropriate candidate antitumor substances.
The antitumor effect can be explained to the
end-result of several interactions involving cytokines and immune
cells, provided by the stimulatory or suppressive effects on the
antitumor immunity (23). The lack
of an effective antitumor immune response in most cancer patients
with advanced disease would simply depend on the prevalence of
immunosuppressive mechanisms with respect to the immunostimulatory
ones. The suppression of the antitumor immune response is mediated
by several cytokines, and the various endogenous suppressive
factors would exert their inhibitory immune effect through a common
end-mechanism, consisting of the generation of a subtype of T
helper lymphocytes (Treg cell), which at present seems to
constitute the main mechanism responsible for cancer-related
immunosuppressive status (24).
In the present study, ConA-stimulated lymphocyte
proliferative response of splenocytes from tumor-bearing mice,
which was lower than that of normal mice, was unaltered by the
feeding of C. militaris (Fig.
2). However, it is noteworthy that IL-2, IL-4 and TGF-β
secretion of ConA-stimulated lymphocytes from tumor-bearing mice
was modulated in a cordycepin-dependent manner by the feeding of
C. militaris (Fig. 2). C.
militaris also induced changes in the subpopulations of
tumor-derived T lymphocytes. The average frequency of
CD4+ T cell population among total splenocytes from
tumor-bearing mice, which was lower than that from normal mice, was
not significantly altered by the feeding of C. militaris.
However, CD4+CD25+ cell population from the
tumor-bearing mice, which was higher than that in the normal mice,
exhibited a cordycepin-dependent decrease, and this decreasing
trend in the CD4+CD25+ cell population became
more definite when it was illustrated in terms of the
CD4+CD25+ to CD4+ cell ratio
(Fig. 3). The regulatory
subpopulation among CD4+ cells constitutively expressing
high levels of CD25 (Treg cells) is one of the most potent and
well-studied suppressive phenotypes found in the tumor
microenvironment. Indeed, IL-2, IL-4 and TGF-β are essential for
naturally occurring Treg cell homeostasis and activation. Treg
cells are refractory to TCR-induced proliferation (25,26)
and depend on IL-2 for survival (27,28).
The signaling via the high-affinity IL-2R complex, in combination
with TCR engagement, is essential for Treg cell proliferation as
well as the acquisition of their potent suppressive function
(28). IL-2 leads to
phosphorylation and activation of STAT5 and, consequently, binding
to the FoxP3 promotor, resulting in enhanced FoxP3 expression
(29). TGF-β induces
CD4+CD25+ T-cell proliferation (30,31)
and promotes the differentiation of CD4+CD25+
Treg cells as a costimulator of FoxP3 expression (32). By contrast, IL-4 inhibits
TGF-β-induced Foxp3 expression and thus suppresses the new
generation of Foxp3+ Treg cells (33). It may be assumed that C.
militaris inhibited IL-2 and TGF-β secretion and promoted IL-4
secretion of tumor-derived T lymphocytes, which downregulated
CD25+-expressing CD4+ T cells more
sensitively. As the transcriptional factor FoxP3 is crucial in the
developmental and functional factors expressed in Treg cells and is
regarded as a useful intracellular Treg cell marker, we
investigated the expression of FoxP3 to more clearly define Treg
cells. FoxP3 was expressed mainly in the
CD4+CD25+ cell population and was extensively
increased in cells from tumor-bearing mice. Moreover,
CD4+CD25+FoxP3+ cells were
decreased significantly after the feeding of C. militaris in
a cordycepin-dependent manner (Fig.
3).
Tumor-infiltrating lymphocytes are seen as a
reflection of a tumor-related immune response and are recognized as
the principal effectors of a local antitumor immune response,
particularly IFN-γ-producing CD8+ T cells (cytotoxic T
lymphocytes; CTL) which kill tumor cells and impede tumor growth
(14). As shown in the results of
our present study, the average frequency of CD8+ T cell
population among total splenocytes from the tumor-bearing mice,
which was lower than that from normal mice, was significantly
increased by the feeding of C. militaris. The
IFN-γ-producing CD8+ T cell population from
tumor-bearing mice also showed a cordycepin-dependent increase
(Fig. 4). IFN-γ plays a key role in
tumor surveillance and immunoediting and block TGF-β-mediated Treg
cell differentiation (34), while
Treg cells suppress tumor specific CD8+ T cell
cytotoxicity through TGF-β signals in vivo(35). Although the exact mechanism of
enhanced IFN-γ expression in CD8+ cells by the feeding
of C. militaris remains unclear, it may be related, in part,
to C. militaris-induced Treg cell depletion in tumor-bearing
mice.
In conclusion, the present study revealed that
cordycepin-enriched C. militaris effectively suppressed the
Treg cell population and enhanced IFN-γ production of
CD8+ T cells in FM3A breast cancer cell-bearing C3H/He
mice. Furthermore, another advantage of cordycepin-enriched C.
militaris involves the fact that the CD4+ cell pool
did not decrease, which may include tumor-specific CD4+
cells involved in antitumor immunity. Based on the results of our
present and previous studies, cordycepin-enriched C.
militaris itself could be applied as an adequate principal
therapeutic agent, facilitating the immune system against
pre-established cancer and inducing apoptosis of cancer cells. As
it is an ideal conditioning strategy to make the peritumoral
microenvironment unfavorable to tumor merely by manipulating Treg
cells without harming the host tissue, cordycepin-enriched C.
militaris may be applied as an adequate therapeutic agent
against the tumor, facilitating antitumor immunity via Treg cell
depletion and CTL enhancement. Furthermore, cordycepin-enriched
C. militaris-induced antitumor immunity may play a factor
via a bystander effect in eradicating chemotherapy or
radiotherapy-resistant cancer cells and may synergistically act
when treated with dendritic cell vaccine.
Acknowledgements
This research was supported by the 2013 National
R&D Program through the Dongnam Institute of Radiological and
Medical Sciences (DIRAMS) funded by the Ministry of Education,
Science and Technology (50493-2013 and 50592-2013) and the
Technology Development Program for Agriculture and Forestry
(610003-03-1-SB110), Ministry for Food, Agriculture, Forestry and
Fisheries, Republic of Korea.
References
|
1
|
Cunningham KG, Manson W, Spring FS and
Hutchinson SA: Cordycepin, a metabolic product isolated from
cultures of Cordyceps militaris (Linn.). Nature.
166:9491950. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Paterson RR: Cordyceps: a traditional
Chinese medicine and another fungal therapeutic biofactory?
Phytochemistry. 69:1469–1495. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kim HG, Shrestha B, Lim SY, et al:
Cordycepin inhibits lipopolysaccharide-induced inflammation by the
suppression of NF-κB through Akt and p38 inhibition in RAW 264.7
macrophage cells. Eur J Pharmacol. 545:192–199. 2006.PubMed/NCBI
|
|
4
|
Yun Y, Han S, Lee S, Ko S, Lee C, Ha N and
Kim K: Anti-diabetic effects of CCCA, CMESS, and cordycepin from
Cordyceps militaris and the immune responses in
streptozotocin-induced diabetic mice. Nat Prod Sci. 9:291–298.
2003.
|
|
5
|
Ahn YJ, Park SJ, Lee SG, Shin SC and Choi
DH: Cordycepin: selective growth inhibitor derived from liquid
culture of Cordyceps militaris against Clostridium
spp. J Agric Food Chem. 48:2744–2748. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sugar AM and McCaffrey RP: Antifungal
activity of 3′-deoxyadenosine (cordycepin). Antimicrob Agents
Chemother. 42:1424–1427. 1998.
|
|
7
|
Müller WE, Weiler BE, Charubala R, et al:
Cordycepin analogues of 2′,5′-oligoadenylate inhibit human
immunodeficiency virus infection via inhibition of reverse
transcriptase. Biochemistry. 30:2027–2033. 1991.
|
|
8
|
Kodama EN, McCaffrey RP, Yusa K and
Mitsuya H: Antileukemic activity and mechanism of action of
cordycepin against terminal deoxynucleotidyl transferase-positive
(TdT+) leukemic cells. Biochem Pharmacol. 59:273–281.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cho MA, Lee DS, Kim MJ, Sung JM and Ham
SS: Antimutagenicity and cytotoxicity of cordycepin isolated from
Cordyceps militaris. Food Sci Biotechnol. 12:472–475.
2003.
|
|
10
|
Nakamura K, Konoha K, Yoshikawa N,
Yamaguch Y, Kagota S, Shinozuka K and Kunitomo M: Effect of
cordycepin (3′-deoxyadenosine) on hematogenic lung metastatic model
mice. In Vivo. 19:137–141. 2005.
|
|
11
|
Nakamura K, Yoshikawa N, Yamaguchi Y,
Kagota S, Shinozuka K and Kunitomo M: Antitumor effect of
cordycepin (3′-deoxyadenosine) on mouse melanoma and lung carcinoma
cells involves adenosine A3 receptor stimulation. Anticancer Res.
26:43–48. 2006.
|
|
12
|
Thomadaki H, Scorilas A, Tsiapalis CM and
Havredaki M: The role of cordycepin in cancer treatment via
induction or inhibition of apoptosis: implication of
polyadenylation in a cell type specific manner. Cancer Chemother
Pharmacol. 61:251–265. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jeong JW, Jin CY, Park C, et al: Induction
of apoptosis by cordycepin via reactive oxygen species generation
in human leukemia cells. Toxicol In Vitro. 25:817–824. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Odunsi K and Old LJ: Tumor infiltrating
lymphocytes: indicators of tumor-related immune responses. Cancer
Immun. 7:32007.PubMed/NCBI
|
|
15
|
Zou W: Immunosuppressive networks in the
tumour environment and their therapeutic relevance. Nat Rev Cancer.
5:263–274. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jeong MH, Seo MJ, Park JU, et al: Effect
of cordycepin purified from Cordyceps militaris on Th1 and
Th2 cytokines in mouse splenocytes. J Microbiol Biotechnol.
22:1161–1164. 2012.PubMed/NCBI
|
|
17
|
Zou W: Regulatory T cells, tumour immunity
and immunotherapy. Nat Rev Immunol. 6:295–307. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Curiel TJ: Regulatory T cells and
treatment of cancer. Curr Opin Immunol. 20:241–246. 2008.
View Article : Google Scholar
|
|
19
|
Liu Z, Kim JH, Falo LDJ and You Z: Tumor
regulatory T cells potently abrogate antitumor immunity. J Immunol.
182:6160–6167. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Malmberg KJ: Effective immunotherapy
against cancer: a question of overcoming immune suppression and
immune escape? Cancer Immunol Immunother. 53:879–892. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Horowitz B, Goldfinger BA and Marmur J:
Effect of cordycepin triphosphate on the nuclear DNA-dependent RNA
polymerases and poly(A) polymerase from the yeast, Saccharomyces
cerevisiae. Arch Biochem Biophys. 172:143–148. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Muller WE, Seibert G, Beyer R, Breter HJ,
Maidhof A and Zahn RK: Effect of cordycepin on nucleic acid
metabolism in L5178Y cells and on nucleic acid-synthesizing enzyme
systems. Cancer Res. 37:3824–3833. 1977.PubMed/NCBI
|
|
23
|
Rosenberg SA: Karnofsky Memorial Lecture.
The immunotherapy and gene therapy of cancer. J Clin Oncol.
10:180–199. 1992.PubMed/NCBI
|
|
24
|
von Boehmer H: Mechanisms of suppression
by suppressor T cells. Nat Immunol. 6:338–344. 2005.PubMed/NCBI
|
|
25
|
Itoh M, Takahashi T, Sakaguchi N, Kuniyasu
Y, Shimizu J, Otsuka F and Sakaguchi S: Thymus and autoimmunity:
production of CD25+CD4+ naturally anergic and
suppressive T cells as a key function of the thymus in maintaining
immunologic self-tolerance. J Immunol. 162:5317–5326.
1999.PubMed/NCBI
|
|
26
|
Takahashi T, Kuniyasu Y, Toda M, et al:
Immunologic self-tolerance maintained by
CD25+CD4+ naturally anergic and suppressive T
cells: induction of autoimmune disease by breaking their
anergic/suppressive state. Int Immunol. 10:1969–1980. 1998.
|
|
27
|
de la Rosa M, Rutz S, Dorninger H and
Scheffold A: Interleukin-2 is essential for
CD25+CD4+ regulatory T cell function. Eur J
Immunol. 34:2480–2488. 2004.PubMed/NCBI
|
|
28
|
Furtado GC, Curotto de Lafaille MA,
Kutchukhidze N and Lafaille JJ: Interleukin 2 signaling is required
for CD4+ regulatory T cell function. J Exp Med.
196:851–857. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yao Z, Kanno Y, Kerenyi M, et al:
Nonredundant roles for Stat5a/b in directly regulating
Foxp3. Blood. 109:4368–4375. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yamagiwa S, Gray JD, Hashimoto S and
Horwitz DA: A role for TGF-β in the generation and expansion of
CD4+CD25+ regulatory T cells from human
peripheral blood. J Immunol. 166:7282–7289. 2001.
|
|
31
|
Ghiringhelli F, Puig PE, Roux S, et al:
Tumor cells convert immature myeloid dendritic cells into
TGF-β-secreting cells inducing CD4+CD25+
regulatory T cell proliferation. J Exp Med. 202:919–929.
2005.PubMed/NCBI
|
|
32
|
Chen W, Jin W, Hardegen N, et al:
Conversion of peripheral CD4+CD25− naive T
cells to CD4+CD25+ regulatory T cells by
TGF-β induction of transcription factor Foxp3. J Exp Med.
198:1875–1886. 2003.
|
|
33
|
Valérie D, Amit A, Hyoung K, et al: IL-4
inhibits TGF-β-induced Foxp3+ T cells and, together with
TGF-β, generates IL-9+ IL-10+
Foxp3−effector T cells. Nat Immunol. 9:1347–1355.
2008.
|
|
34
|
Xiaoyu H and Lionel BL: Cross-regulation
of signaling pathway by interferon-γ: implication for immune
response and autoimmune diseases. Immunity. 31:539–550. 2009.
|
|
35
|
Chen ML, Pittet MJ, Gorelik L, et al:
Regulatory T cells suppress tumor-specific CD8 T cell cytotoxicity
through TGF-β signals in vivo. Proc Natl Acad Sci.
102:419–424. 2005.PubMed/NCBI
|