Introduction
Tumor-associated antigens (TAAs) are a category of
proteins relevant to the occurrence, transformation and progression
of cancer, which evoke an immune response and elicit autoantibodies
to these antigens. TAAs and anti-TAA autoantibodies can be used as
biomarkers for diagnosing cancer or predicting the prognosis of
disease. Although there has been a rapid increase in the number of
TAAs identified using proteomics approach or serological analysis
of recombinant cDNA expression libraries (SEREX), a great
percentage of these identified TAAs, however, were found to have no
direct relevance to cancer. Thus, it is necessary to further
evaluate and validate these candidate TAAs as diagnostic markers or
therapeutic targets of immunotherapy (1). The humoral immune response of
candidate TAAs can be detected by using immunoassays such as ELISA
or western blotting in large scale sera from cancer patients and
controls under different clinical conditions when using recombinant
proteins as target antigens (2).
Peroxiredoxins (Prdxs) are a family of 22–27 kDa
non-selenium-dependent glutathione peroxidases which destroy
peroxides, organic hydroperoxides and peroxynitrite (3,4). Six
isoforms of Prdxs have been identified in mammals, and are divided
into 3 subclasses: typical 2-cysteine Prdxs (Prdx1-4), atypical
2-cysteine (Prdx5) and 1-cysterine Prdx (Prdx6) (5). They localize in different locations of
the cell; Prdx1, Prdx2 and Prdx6 are localized to the cytoplasm,
Prdx3 in the mitochondria, Prdx4 in the extracellular space, and
Prdx5 in the mitochondria and peroxisomes (4). Prdx1 has been viewed as a
tumor-suppressor as Prdx1-knockout mice exhibit a shortened life
span due to the development of hemolytic anemia and cancer. One
study demonstrated that Prdx1 inhibits the activation of oncogenes
such as c-Abl and c-myc, and it can also be considered as a
safeguard for the lipid phosphatase activity of PTEN, which is
essential for its tumor-suppressive function (6).
Prdx1 was recently identified as a candidate
esophageal squamous cell carcinoma (ESCC)-related TAA in a previous
study using a proteomics approach. It was found that Prdx1 was
overexpressed in ESCC tissues when compared to adjacent normal
tissues (7,8), and the expression level of this
protein was also elevated in other types of tumor tissues (9–16). In
addition, it was found that Prdx1 induces the production of an
autoantibody against this protein in the sera of patients with
non-small cell lung cancer (NSCLC), but to date there is no report
available regarding whether this protein induces an autoimmune
response in ESCC. In order to further characterize and validate the
identified tumor-associated protein Prdx1, recombinant Prdx1
protein was subsequently used as a target antigen to screen the
anti-Prdx1 autoantibody in sera from patients with ESCC and normal
individuals by ELISA and western blotting. Indirect
immunofluorescence assay with cancer cell lines and
immunohistochemistry with cancer tissue array slides were also
performed to analyze the protein expression profiles of Prdx1 in
cancer cells and tissues.
Materials and methods
Sera and general information
Sera from 68 patients with ESCC and 89 normal human
sera (NHS) were obtained from the serum bank of the Cancer
Autoimmunity and Epidemiology Research Laboratory at the University
of Texas, El Paso (UTEP), which were originally provided by Dr
X.-X. Peng of Sun Yat-sen University, Guangzhou, China. All ESCC
cases were confirmed by histopathological diagnosis. All ESCC sera
were collected at the time of initial cancer diagnosis, when the
patients had not yet received any chemotherapy or radiation
therapy. Normal human sera were collected during annual health
examinations from individuals who had no obvious evidence of
malignancy.
Cell lines and cell extracts
Nine different tumor cell lines [human epidermoid
carcinoma (Hep2), human hepatocellular carcinoma (HepG2), human
hepatocellular carcinoma (SUN449), human breast cancer (SKBR3),
human ovarian carcinoma (SKOV3), human lung epithelial
adenocarcinoma (A549), human urinary bladder carcinoma (T24), human
acute lymphoblastic leukemia (MOLT-4) and leukemia (KOPN63)] were
obtained from the tumor cell bank of our laboratory and cultured
following the specific protocol for each cell line. Cells grown in
monolayers were solubilized in Laemmli’s sample buffer containing
protease inhibitors after sonication. Solubilized lysates were
briefly denatured before electrophoresis on SDS-polyacrylamide
gels.
Enzyme-linked immunosorbent assay
(ELISA)
Standard protocol for ELISA was used as described in
our previous study (12). In brief,
a 96-well microtiter plate (Thermo Scientific, Waltham, MA, USA)
was coated overnight at 4°C with recombinant Prdx1 protein (Abcam,
Cambridge, MA, USA) at a final concentration of 0.5 μg/ml in
phosphate-buffered saline (PBS). The antigen-coated wells were
blocked with gelatin post-coating solution at room temperature for
2 h. Human sera diluted at 1:100 with serum diluent were incubated
for 2 h at room temperature in the antigen-coated wells, followed
by HRP-conjugated goat anti-human IgG (Santa Cruz Biotechnology,
Inc., Santa Cruz, CA, USA). The substrate
2,2′-azino-bis-3-ethylbenzo-thiazoline-6-sulfonic acid (ABTS;
Sigma-Aldrich, St. Louis, MO, USA) was used as the detecting
reagent. The average optical density (OD) value at a wavelength of
405 nm was applied as data analysis. The cutoff value designating a
positive reaction was the mean OD of 89 NHS + 3SD.
Western blotting
Denatured recombinant Prdx1 protein and tumor cell
lysates were electrophoresed on 12% SDS-PAGE and transferred to
nitrocellulose membranes, respectively. After blocking in PBS with
5% non-fat milk and 0.05% Tween-20 for 1 h at room temperature, the
nitrocellulose membranes were incubated overnight at 4°C with a
1:200 dilution of human sera, a 1:1,000 dilution of polyclonal
anti-Prdx1 antibody (GeneTex Inc., Irvine, CA, USA) and a 1:500
dilution of monoclonal anti-β-actin antibody (Cell Signaling
Technology, Inc., Danvers, MA, USA), separately. HRP-conjugated
goat anti-human IgG, HRP-conjugated goat anti-rabbit IgG and
HRP-conjugated goat anti-mouse IgG (Santa Cruz Biotechnology, Inc.)
were applied as secondary antibodies at a 1:3,000 dilution. The ECL
kit was used to detect immunoreactive bands according to the
manufacturer’s instructions (Thermo Scientific).
Absorption of antibodies with recombinant
protein
The diluted human sera (1:80) were incubated with
recombinant protein (final concentration of recombinant protein in
the diluted human sera was 0.01 μg/μl) overnight at 4°C, and then
centrifuged at 10,000 × g for 15 min. The supernatant was used for
immunofluorescence assay.
Indirect immunofluorescence assay
(IIFA)
Hep-2 antigen substrate for the IIFA test system was
incubated with a dilution of sera (1:80) and preabsorbed sera at
4°C overnight. FITC-conjugated goat anti-human IgG (Santa Cruz
Biotechnology, Inc.) was used as the secondary antibody at a 1:100
dilution. A fluorescence microscope (Leica DM1000, Germany) was
used for examination.
Immunohistochemical (IHC) analysis of the
tissue assay slides
ESCC tissue array slides with adjacent normal tissue
controls (16 ESCC tissue specimens, 14 adjacent tissue specimens
and 12 normal esophagus specimens) with information regarding
clinical stages and pathological grades) were commercially
purchased (US Biomax, Inc., Rockville, MD, USA), and used to detect
the expression of Prdx1 protein. There were 7 cases containing
completely self-paired ESCC, adjacent and normal specimens. Tissue
array slides were deparaffinized with xylene and dehydrated with
ethanol. Antigen retrieval was performed by microwave-heating
methods in Trilogy™ pretreatment solution for 20 min. Avidin/biotin
blocking solution was used to prevent nonspecific binding of the
antibodies. The sections were incubated with polyclonal anti-Prdx1
antibody (1:50 dilution) for 1 h at room temperature. HRP detection
system (HRP streptavidin labeled and polyvalent biotinylated
linked) and DAB substrate kit were used as detecting reagents.
After counterstaining with hematoxylin, the sections were
dehydrated and mounted. The slides were observed using a microscope
(Leica DM1000).
Statistical analysis
The mean OD value of each group of patient sera was
compared using the Mann-Whitney U test. The frequencies of
antibodies to Prdx1 in each group of patient sera were compared
using the χ2 test with Yate’s correction. Sensitivity
and specificity were calculated as previously described (17). The expression profile of Prdx1 in
the ESCC, adjacent and normal tissue groups was compared using
χ2 test and Fisher’s exact test, whereas self-paired
specimens of the different groups were compared using Cochran Q
test, and 2 levels of significance (0.01 and 0.05) were used.
Results
Frequency and the titer of the
autoantibody against Prdx1 in ESCC
The full length recombinant Prdx1 protein was used
as the coating antigen in ELISA to detect the autoantibody against
Prdx1 in sera from 68 patients with ESCC and 89 normal individuals.
As demonstrated in Table I, the
prevalence of an autoantibody against Prdx1 was 13.2% (9/68) in
ESCC, which was significantly higher than that in the NHS
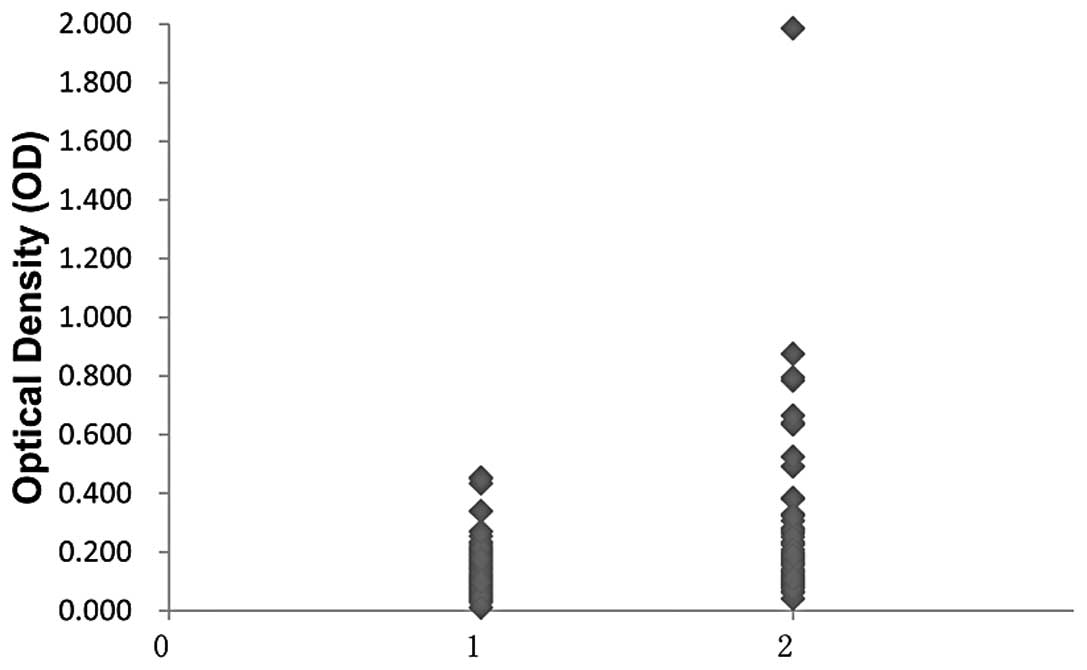
(P<0.01). The titer of the autoantibody against Prdx1 is shown
in Fig. 1. The average titer of the
autoantibody against Prdx1 in ESCC was significantly higher than
that in NHS (P<0.01). The ELISA result was also confirmed by
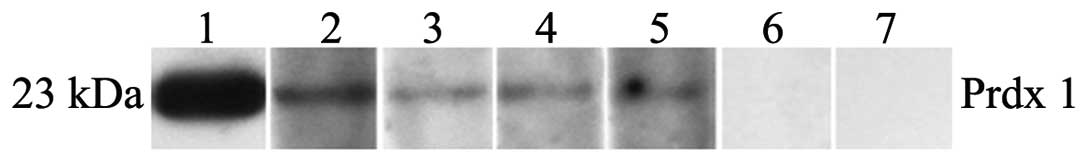
western blot analysis. Fig. 2 shows
four representative ESCC sera which were positive in ELISA, and
also had strong reactivity in the western blot analysis.
 | Table IFrequency of an autoantibody to Prdx1
in human sera by ELISA. |
Table I
Frequency of an autoantibody to Prdx1
in human sera by ELISA.
| Type of sera | No. tested | Autoantibody to
PRDX1, n (%) |
|---|
| ESCC | 68 | 9 (13.2)a |
| Normal | 89 | 0 (0.0) |
Perinuclear intense staining pattern in
Hep-2 cells by indirect immunofluorescence assay with
representative positive ESCC sera
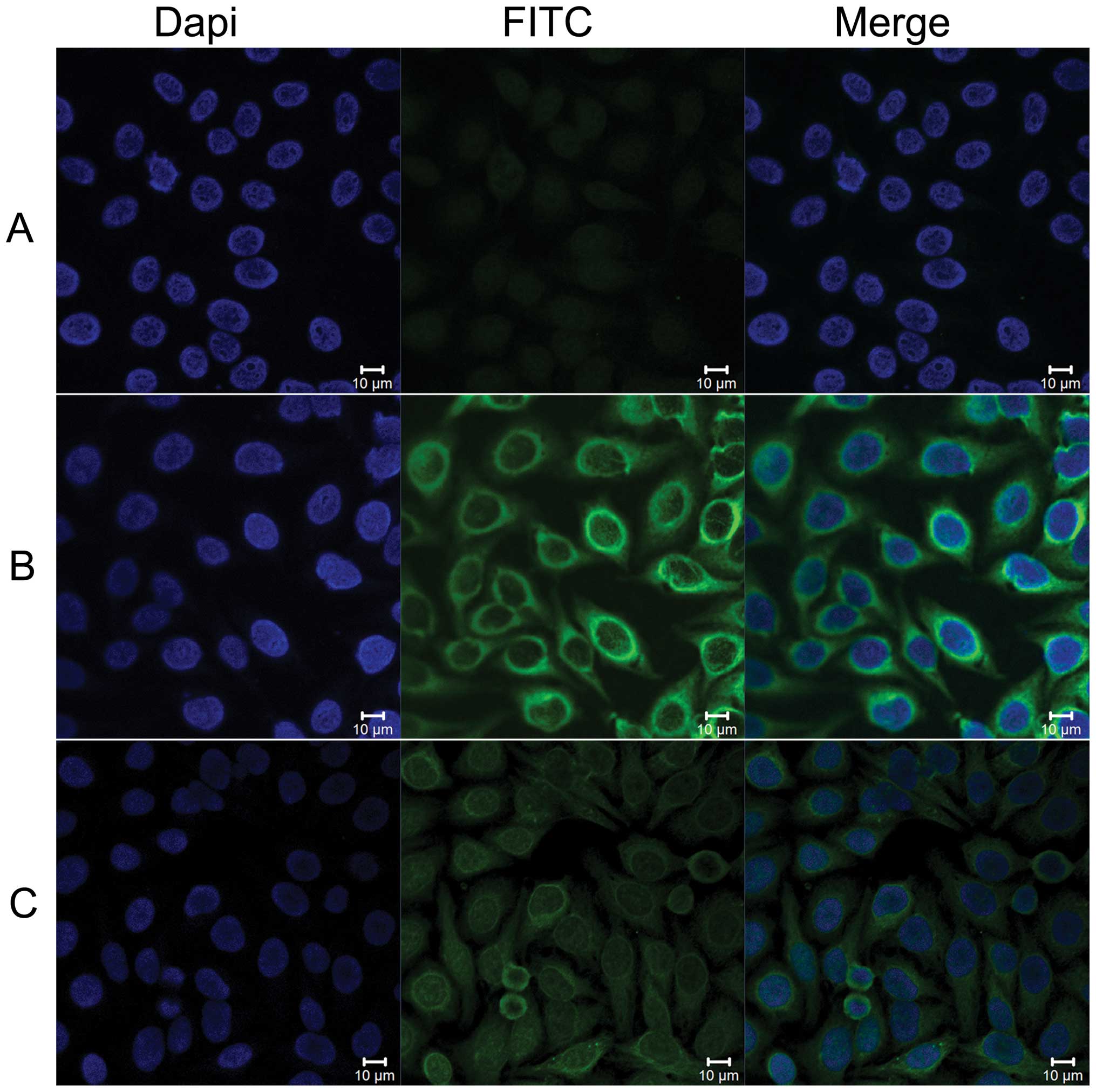
To further confirm the specificity of an
autoantibody response to Prdx1 in ESCC sera, ESCC sera with
anti-Prdx1 positivity in ELISA were also examined by indirect
immunofluoresence assay with commercially purchased Hep-2 cell
slides. As shown in Fig. 3, a
representative ESCC serum sample with anti-Prdx1 antibody
positivity in ELISA had a cytoplasmic staining pattern with more
intense staining in the perinuclear regions. The fluorescent signal
was significantly reduced after being preabsorbed with the
anti-Prdx1 antibobies with recombinant Prdx1 protein in the same
serum.
Expression of Prdx1 in ESCC tissues by
immunohistochemistry with tissue array
The expression profile of Prdx1 in ESCC, adjacent
and normal esophagus tissues was examined by immunohistochemistry
with tissue array slides. Tissue array slides containing 16 ESCC
tissue specimens, 14 adjacent tissue specimens and 12 normal
esophagus specimens, were commercially available for this study.
The polyclonal anti-Prdx1 antibody was used as the primary antibody
to detect the expression of Prdx1 in these tissues. As shown in
Table II, the result indicated
that there was an increased frequency of Prdx1 overexpression in
ESCC tissues (100%, 16/16) compared to the adjacent carcinoma
tissues (64.3%, 9/14) or normal tissues (50%, 6/12). The frequency
of Prdx1 expression in ESCC tissues was significantly higher than
that in the adjacent tissues (P<0.01) and normal tissues
(P<0.05). Among the 7 self-paired cases (Table III), the frequency of Prdx1 was
significantly higher in the ESCC tissues (7/7) than that in the
adjacent carcinoma tissues (3/7) and in the normal tissues (3/7)
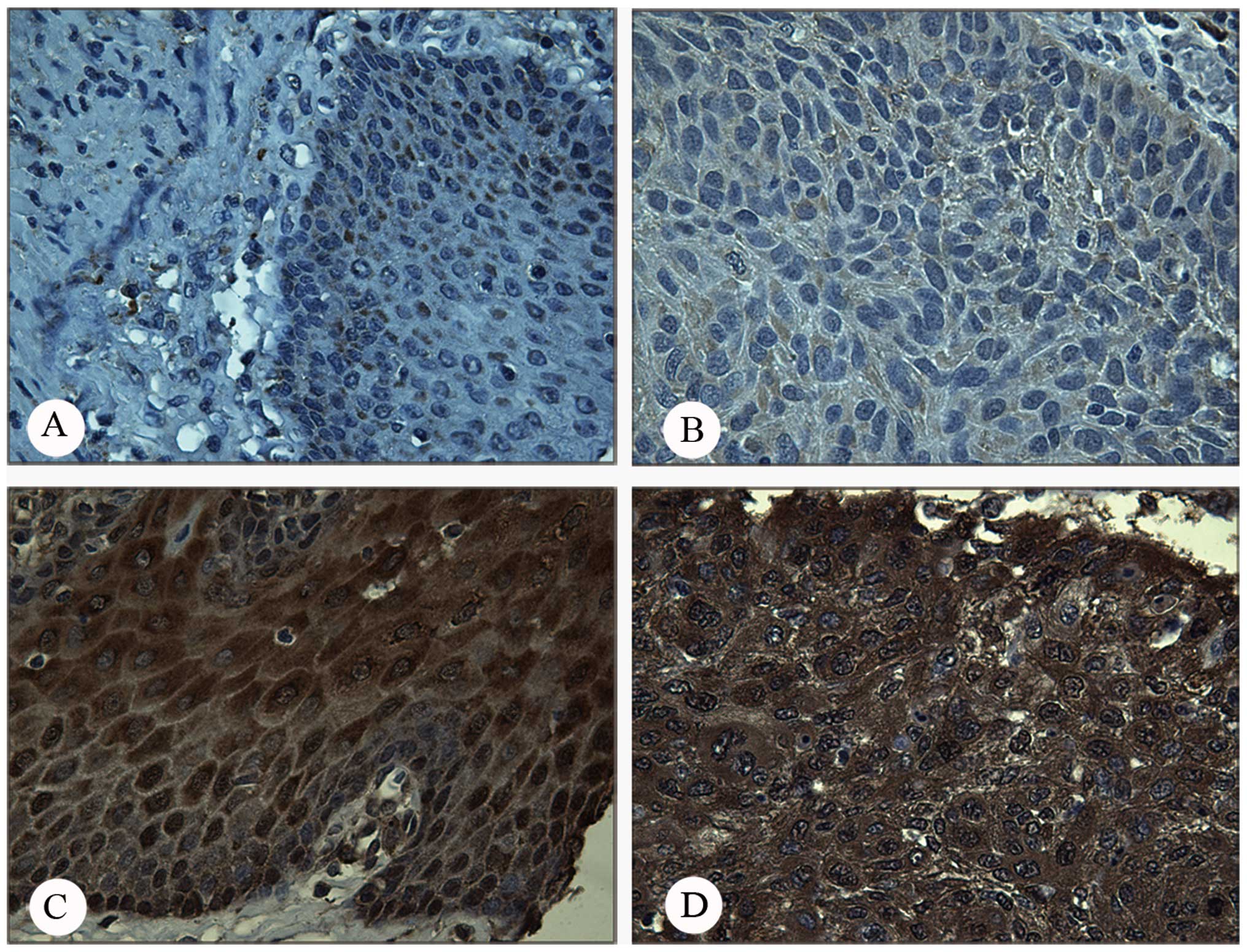
(P<0.05). Fig. 4 shows a
representative positive and negative immunostaining pattern of
Prdx1 in ESCC and normal tissue. Due to the small sample size of
the tissues examined, it was not possible to establish a
statistical correlation between Prdx1 expression and clinical stage
in the present study.
 | Table IIExpression profile of Prdx1 in ESCC,
adjacent carcinoma and normal tissues including the score and
intensity. |
Table II
Expression profile of Prdx1 in ESCC,
adjacent carcinoma and normal tissues including the score and
intensity.
| | | Score and
intensity |
|---|
| | |
|
|---|
| | | | 1 | 2 |
|---|
| | | |
|
|
|---|
| Type of
tissues | No. tested | Positive, n
(%) | 0 | + | ++ | +++ | + | ++ | +++ |
|---|
| ESCC | 16 | 16 (100) | 0 | 2 | 2 | 1 | 4 | 3 | 4 |
| Adjacent
carcinoma | 14 | 9 (64.3) | 5 | 1 | 3 | 2 | 0 | 0 | 3 |
| Normal | 12 | 6 (50.0) | 5 | 1 | 1 | 0 | 3 | 0 | 2 |
 | Table IIIExpression profile of Prdx1 in paired
ESCC, adjacent carcinoma and normal tissues. |
Table III
Expression profile of Prdx1 in paired
ESCC, adjacent carcinoma and normal tissues.
| Tissues |
|---|
|
|
|---|
| No. | ESCC | Adjacent | Normal |
|---|
| 1 | + | + | + |
| 2 | + | − | − |
| 3 | + | − | − |
| 4 | + | − | − |
| 5 | + | + | + |
| 6 | + | + | + |
| 7 | + | − | − |
Overexpression of Prdx1 in different
cancer cell lines
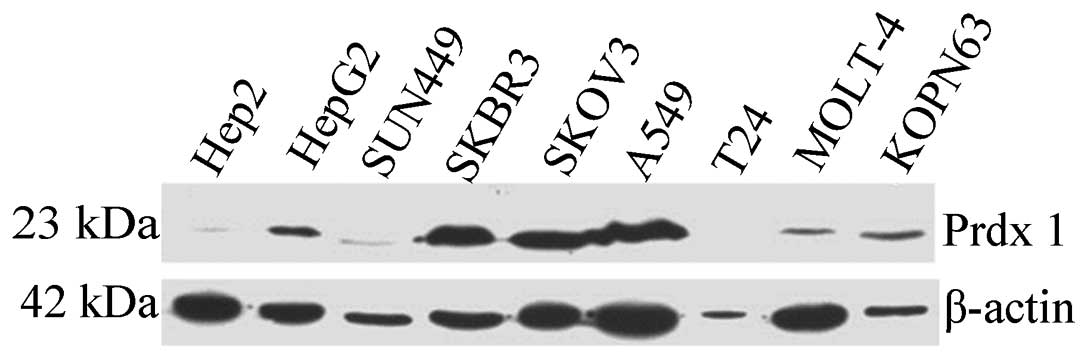
To explore the expression level of Prdx1 in
different types of cancers, 9 cancer cell lines (Hep2, HepG2,
SUN449, SKBR3, SKOV3, A549, T24, MOLT-4 and KOPN63) were analyzed
by western blotting. The polyclonal anti-Prdx1 antibody was used as
a probe for this study. As shown in Fig. 5, based on the expression level of
internal control β-actin, several human cancer cell lines such as
HepG2 (hepatocellular carcinoma), SKBR3 (breast cancer), SKOV3
(ovarian carcinoma) and A549 (lung epithelial adenocarcinoma)
exhibited a strong reactivity to anti-Prdx1. The SUN449
(hepatocellular carcinoma) cell line showed moderate reactivity,
while Hep2 (epidermoid carcinoma), MOLT-4 (acute lymphoblastic
leukemia) and KOPN63 (leukemia) cell lines exhibited weak
reactivity. The T-24 (urinary bladder carcinoma) cell line was
completely negative to the anti-Prdx1 antibody.
Discussion
The family of Prdxs is one of the 3 major peroxidase
consisting of catalase, Prdx and glutathione peroxidase, which
function as scavengers of H2O2(5). Under small amounts of cellular
H2O2, Prdx1 decomposes peroxides more
efficiently than catalases due to its high affinity and wide
cellular distribution, but it becomes over-oxidized under condition
of high levels of H2O2, while catalases
scavenge H2O2 rapidly and efficiently in this
case (5). It has been shown that
Prdxs and catalases function as peroxidases sequentially but not
synergistically. Prdx1 modulates cell signaling pathways not only
by influencing the intracellular levels of reactive oxygen species
(ROS) and deactivating MAPK phosphatases indirectly induced by ROS,
which results in c-Jun N-terminal kinase (JNK) activation, but also
by directly interacting and inhibiting stress protein kinases such
as c-Abl and JNK (18–20). Prdx1 was originally identified as a
tumor-suppressor (6,20,21)
based on the observation that the formation of sarcomas and blood
malignancies was increased in Prdx1 gene knockout mice (21). Although the function of Prdx1 in the
process of carcinogenesis is still not clear, various mechanisms
have been recently proposed and verified in certain types of
cancer. In cancer cells, the cellular level of
H2O2 is abnormally high (22). Under a high level of
H2O2, Prdx1 is over-oxidized and shifts the
function from peroxidase to a chaperone (23). Over-oxidized Prdx1 can result in
oligomerization and in losing its peroxidase activity, whereas it
functions as a chaperone and regulates cell signaling via
interacting with signaling proteins. Then it may lose the ability
to form a complex with signaling partners such as the kinases c-Ab1
(20), JNK (9), the oncoprotein c-myc (24) and the phosphatase PTEN (6), resulting in activation of c-Ab1, JNK,
c-myc and inactivation of PTEN’s phosphatase activity. Under normal
conditions, Prdx1 interacts with the SH3 and kinase domains of
c-Abl tyrosine kinase, thereby inhibiting the activity of c-Abl
kinase (20). Under increased
H2O2 stress, over-oxidized Prdx1 loses the
ability to interact with these domains, resulting in the activation
of c-Abl kinase (20). Prdx1
regulates c-myc signaling by binding to the myc box II, which is a
highly conserved region of c-myc, inducing inhibition of c-myc
which then causes a broad but selective loss of c-myc target gene
regulation (24,25). Prdx1 regulates the tumor-suppressive
function of PTEN by forming a complex with it, and inhibits
inactivation of the lipid phosphatase of PTEN induced by
H2O2. As two of its cysteine domains form a
disulfide bond after oxidation, over-oxidized Prdx1 can lose the
ability to combine with PTEN, resulting in hyperactive Akt
signaling and oncogenesis (6). In
addition, in prostate cancer, Prdx1 was found to be secreted into
the extracellular location and to interact with Toll-like receptor
4 (TLR4), subsequently promoting angiogenesis and VEGF production,
which eventually stimulated TLR4 and VEGF-dependent endothelial
cell proliferation, migration and differentiation (15).
The present study revealed that there was a high
level of expression of Prdx1 in the liver cancer cell line HepG2,
breast, ovarian and lung cancer cell lines (SKBR3, SKOV3 and A549),
and relatively weak expression in another type of liver cancer cell
line (SUN449) and in laryngeal cancer (Hep2), acute lymphoblastic
leukemia (MOLT-4) and lymphoma leukemia (KOPN63) cell lines. Our
results also indicate that Prdx1 was not only overexpressed in
certain solid tumors, but also in leukemia, suggesting the high
relevance of Prdx1 with malignancy. The elevated expression of
Prdx1 was also reported in numerous types of cancers by other
groups (7,10,14,16).
In the present study, the expression profile of Prdx1 in ESCC,
adjacent and normal tissues was examined and evaluated by
immunohistochemistry with tissue array slides. The expression level
of Prdx1 was highly elevated in ESCC tissues when compared to
adjacent and normal tissues. The data of paired ESCC with adjacent
and normal tissues provided a similar result confirming that Prdx1
was overexpressed in cancer tissues while the paired samples were
at a lower level. Hoshino et al(7) found that Prdx1 was overexpressed in
90% of the examined 114 ESCC samples. Due to the small sample size
in the present study, it could not be determined whether the
expression level was correlated with the pathological
classification. However, Hoshino et al(7) showed that the expression level of
Prdx1 was inversely correlated with depth of invasion and stage,
and reduced expression predicted shorter overall survival. While
comparing the expression profile of Prdx1 in ESCC tissues with
other tumor types, there may be various significant differences
which are notable. Prdx1 expression was increased in lung (10,11),
liver (12), gallbladder (13), bladder (14), prostate (15) and ovarian cancer (16,26),
and a high level of Prdx1 expression was significantly correlated
with tumor grade and clinical stage in some types of tumors such as
non-small cell lung cancer (NSCLC), gallbladder cancer and
cholangiocarcinoma (10,12,13),
but there was no correlation noted in ovarian carcinoma (16). Overexpression of Prdx1 was found to
be correlated with overall survival and prognosis. A high level of
Prdx1 expression was significantly correlated with poor overall
survival in most reported cancers, but inversely reduced Prdx1
expression was correlated with reduced overall survival and poor
clinical outcome in cholangiocarcinoma, suggesting that Prdx1 is a
valuable prognostic marker in predicting the outcome, recurrence
and overall survival in patients with certain types of tumors.
An anticancer agent, FK228, inhibits the growth of
esophageal squamous cell cancer and induces apoptosis in part
through Prdx1 activation. The sensitization of ESCC cells to FK228
was downregulated after silencing of Prdx1 gene expression
(27). In prostate cancer,
reduction in Prdx1 expression was found to lead to reduced tumor
vasculature formation, and further inhibition of tumor growth
(15). Therefore, the tumor growth
and augmentation of radio-sensitivity by decreasing Prdx1
expression in lung cancer cell lines was inhibited (28). Taken together, these results also
indicate that Prdx1 may be a potential therapeutic target for ESCC
and other cancers.
To date, an autoimmune response to Prdx1 in ESCC has
not been reported. A study from Korea showed that the positive rate
of autoantibody against Prdx1 was 47.0% in 53 sera from NSCLC
patients by western blot analysis, whereas it was only 8.0% for the
anti-Prdx1 antibody in 50 normal individual controls (11). In another study with HCC, only 2 of
the 70 (2.9%) HCC sera showed a positive response to Prdx1, and 1
of 70 (1.4%) normal human sera was positive to Prdx1, as detected
by ELISA with phage-expressed Prdx1 protein as the coating antigen
(29). In the present study, we
also tested the anti-Prdx1 antibody in HCC. The positive rate of
anti-Prdx1 antibody was only 3.8% (3/78) and 2.4% (2/82) in normal
human sera, respectively (data not shown). This preliminary data
also suggest that the autoantibody against Prdx1 may be used as a
potential biomarker in certain types of cancer but not for all
types of cancer.
Although it is still unclear how autoantibodies are
developed by the human immune system, many studies have
demonstrated that autoantibody production is related to aberrant
expression of autoantigens, such as the alteration of expression
level and structural changes of cellular proteins (11,30).
Autoantibodies can be detected in the sera of patients with
autoimmune disease and in many tumors (31). In the present study, the titer of
the autoantibody against Prdx1 in the sera from patients with ESCC
was much higher than that in normal individuals. The positive rate
of the autoantibody to Prdx1 was 13.0% in ESCC, and all the normal
sera were negative when using the mean OD value plus 3 SD of the
NHS group as a cutoff value. Taken together with the results from
western blotting and IIF analysis, our data indicate that Prdx1
induces strong humoral autoimmune responses in some ESCC patients,
suggesting that Prdx1 may be an ESCC-associated autoantigen, and
the autoantibody against Prdx1 can be used as a potential
serological biomarker in the immunodiagnosis of ESCC. The
underlying mechanism of how this protein induces a humoral immune
response in ESCC remains to be investigated.
Acknowledgements
The authors would like to thank Dr Eng M. Tan (The
Scripps Research Institute) for his support. The present study was
supported by grants (SC1CA166016, 5G12RR08124) from the National
Institutes of Health (NIH), and by a grant from the National
Natural Science Foundation of China (30872962, 81172086 and
81372371).
References
|
1
|
Lee SY and Jeoung D: The reverse
proteomics for identification of tumor antigens. J Microbiol
Biotechnol. 17:879–890. 2007.PubMed/NCBI
|
|
2
|
Fernández Madrid F, Tang N, Alansari H,
Karvonen RL and Tomkiel JE: Improved approach to identify
cancer-associated autoantigens. Autoimmun Rev. 4:230–235. 2005.
|
|
3
|
Rhee SG: Cell signaling.
H2O2, a necessary evil for cell signaling.
Science. 312:1882–1883. 2006.PubMed/NCBI
|
|
4
|
Rhee SG, Chae HZ and Kim K:
Peroxiredoxins: a historical overview and speculative preview of
novel mechanisms and emerging concepts in cell signaling. Free
Radic Biol Med. 38:1543–1552. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Neumann CA, Cao J and Manevich Y:
Peroxiredoxin 1 and its role in cell signaling. Cell Cycle.
8:4072–4078. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cao J, Schulte J, Knight A, et al: Prdx1
inhibits tumorigenesis via regulating PTEN/AKT activity. EMBO J.
28:1505–1517. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hoshino I, Matsubara H, Akutsu Y, et al:
Tumor suppressor Prdx1 is a prognostic factor in esophageal
squamous cell carcinoma patients. Oncol Rep. 18:867–871.
2007.PubMed/NCBI
|
|
8
|
Zhang J, Wang K, Zhang J, Liu SS, Dai L
and Zhang JY: Using proteomic approach to identify tumor-associated
proteins as biomarkers in human esophageal squamous cell carcinoma.
J Proteome Res. 10:2863–2872. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim YJ, Lee WS, Ip C, Chae HZ, Park EM and
Park YM: Prx1 suppresses radiation-induced c-Jun NH2-terminal
kinase signaling in lung cancer cells through interaction with the
glutathione S-transferase Pi/c-Jun NH2-terminal kinase complex.
Cancer Res. 66:7136–7142. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim JH, Bogner PN, Baek SH, et al:
Up-regulation of peroxiredoxin 1 in lung cancer and its implication
as a prognostic and therapeutic target. Clin Cancer Res.
14:2326–2333. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chang JW, Lee SH, Jeong JY, et al:
Peroxiredoxin-I is an autoimmunogenic tumor antigen in non-small
cell lung cancer. FEBS Lett. 579:2873–2877. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yonglitthipagon P, Pairojkul C, Chamgramol
Y, et al: Prognostic significance of peroxiredoxin 1 and
ezrin-radixin-moesin-binding phosphoprotein 50 in
cholangiocarcinoma. Hum Pathol. 43:1719–1730. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li J, Yang ZL, Ren X, et al: ILK and PRDX1
are prognostic markers in squamous cell/adenosquamous carcinomas
and adenocarcinoma of gallbladder. Tumour Biol. 34:359–368. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Quan C, Cha EJ, Lee HL, Han KH, Lee KM and
Kim WJ: Enhanced expression of peroxiredoxin I and VI correlates
with development, recurrence and progression of human bladder
cancer. J Urol. 175:1512–1516. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Riddell JR, Bshara W, Moser MT, Spernyak
JA, Foster BA and Gollnick SO: Peroxiredoxin 1 controls prostate
cancer growth through Toll-like receptor 4-dependent regulation of
tumor vasculature. Cancer Res. 71:1637–1646. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chung KH, Lee DH, Kim Y, et al: Proteomic
identification of overexpressed PRDX 1 and its clinical
implications in ovarian carcinoma. J Proteome Res. 9:451–457. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gordis L: Assessing the validity and
reliability of diagnostic and screening tests. Epidemiology.
3:71–94. 1996.
|
|
18
|
Das S, Otani H, Maulik N and Das DK: Redox
regulation of angiotensin II preconditioning of the myocardium
requires MAP kinase signaling. J Mol Cell Cardiol. 41:248–255.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kamata H, Honda S, Maeda S, Chang L,
Hirata H and Karin M: Reactive oxygen species promote TNFα-induced
death and sustained JNK activation by inhibiting MAP kinase
phosphatases. Cell. 120:649–661. 2005.
|
|
20
|
Wen ST and Van Etten RA: The PAG gene
product, a stress-induced protein with antioxidant properties, is
an Abl SH3-binding protein and a physiological inhibitor of c-Abl
tyrosine kinase activity. Genes Dev. 11:2456–2467. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Neumann CA, Krause DS, Carman CV, et al:
Essential role for the peroxiredoxin Prdx1 in erythrocyte
antioxidant defence and tumour suppression. Nature. 424:561–565.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Benhar M, Dalyot I, Engelberg D and
Levitzki A: Enhanced ROS production in oncogenically transformed
cells potentiates c-Jun N-terminal kinase and p38 mitogen-activated
protein kinase activation and sensitization to genotoxic stress.
Mol Cell Biol. 21:6913–6926. 2001. View Article : Google Scholar
|
|
23
|
Barranco-Medina S, Lázaro JJ and Dietz KJ:
The oligomeric conformation of peroxiredoxins links redox state to
function. FEBS Lett. 583:1809–1816. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sirvent A, Benistant C and Roche S:
Cytoplasmic signalling by the c-Abl tyrosine kinase in normal and
cancer cells. Biol Cell. 100:617–631. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mu ZM, Yin XY and Prochownik EV: Pag, a
putative tumor suppressor, interacts with the Myc Box II domain of
c-Myc and selectively alters its biological function and target
gene expression. J Biol Chem. 277:43175–43184. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hoskins ER, Hood BL, Sun M, Krivak TC,
Edwards RP and Conrads TP: Proteomic analysis of ovarian cancer
proximal fluids: validation of elevated peroxiredoxin 1 in patient
peripheral circulation. PLoS One. 6:e250562011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hoshino I, Matsubara H, Hanari N, et al:
Histone deacetylase inhibitor FK228 activates tumor suppressor
Prdx1 with apoptosis induction in esophageal cancer cells. Clin
Cancer Res. 11:7945–7952. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen MF, Keng PC, Shau H, et al:
Inhibition of lung tumor growth and augmentation of
radiosensitivity by decreasing peroxiredoxin I expression. Int J
Radiat Oncol Biol Phys. 64:581–591. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu H, Zhang J, Wang S, et al: Screening
of autoantibodies as potential biomarkers for hepatocellular
carcinoma by using T7 phase display system. Cancer Epidemiol.
36:82–88. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Backes C, Ludwig N, Leidinger P, et al:
Immunogenicity of autoantigens. BMC Genomics. 12:3402011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tan EM and Zhang J: Autoantibodies to
tumor-associated antigens: reporters from the immune system.
Immunol Rev. 222:328–340. 2008. View Article : Google Scholar : PubMed/NCBI
|