Introduction
microRNAs (miRNAs) are a class of non-coding RNAs
~22 nucleotides in length that hybridize to mRNAs and cause either
translation repression or mRNA cleavage (1,2), and
which were first discovered by Lee et al during an
investigation of the gene lin-14 in Caenorhabditis elegans
(C. elegans) development in the early 1990s (3). miRNAs are recognized as a distinct
class of biological post-transcriptional regulators involved in
most biological processes including differentiation, apoptosis,
proliferation, the immune response and development (4–10).
miRNAs are found in animals, plants and fungi and are usually
transcribed by RNA polymerase II (1). We recently demonstrated that exogenous
plant MIR168a cross-kingdomly targets mammalian LDLRAP1, suggesting
that miRNAs could represent a novel group of universal modulators
that mediate animal-plant interactions at the molecular level
(11). Also, miRNAs play crucial
roles in disease progression including cancer, and they may act as
either tumor suppressors or oncogenes depending on the biological
functions of their target genes (5). A wide variety of pathways are affected
by miRNAs through regulating gene expression at the
post-transcriptional level.
Lung cancer (LC) is the leading cause of
cancer-related mortality worldwide and is commonly induced by
long-term exposure to tobacco smoke (12). The present pathologic staging on the
basis of morphology is inadequate to predict outcome for patient
treatment. The emergence of molecular target therapy has improved
the management of LC patients according to differentially expressed
molecules in several subtypes. The identification and
classification of LC-miRNA-related pathways for the analysis of
several molecular subtypes of LC (KRAS, EGFR, ALK, BRAF, PIK3CA,
MET, HER2, MEK1 and NRAS) raises the question of whether these
approaches can be used to characterize and treat human LC.
Identifying the molecular causes of LC represented a major
breakthrough in the history of medicine, moving the discipline from
pattern recognition and therapeutic strategies based on syndromic
pathophysiology to molecular mechanism and evidence-based therapies
derived from clinical trials designed on the basis of molecular
mechanism (13). Recent therapeutic
advances include the use of epidermal growth factor receptor
tyrosine kinase inhibitors (EGFR-TKIs) including gefitinib and
erlotinib (14). These drugs would
be most effective with patient selection on the basis of target
expression. miRNAs are thought to be grossly dysregulated in LCs
and may also serve as oncogenes or tumor suppressors (15). Thus, miRNAs can be applied to
sub-classify non-small cell LC (NSCLC) accounting for 80% of LC
cases (16). For NSCLC, in
particular, previous results have indicated that miRNA expression
patterns could be proposed biomarkers used for diagnosis, prognosis
and personalized therapy (17–19).
In the present study, we summarized 8 LC-miRNAs
which are associated with three subtypes of LC from previous
studies. Protein class, molecular function, biological process and
canonical pathways involved by the targets of each LC-miRNA as well
as the 5 main canonical pathways all participated with certain
LC-miRNAs, and were identified and analyzed, which may offer
significant treatment insight into the clinical therapy of LC.
Materials and methods
miRNA target predictions
TargetScan (http://www.targetscan.org) was used to predict
biological targets of miRNAs by searching for the presence of
conserved 8mer and 7mer sites that match the seed region of each
LC-miRNA (20) and generate lists
of predicted gene targets of each miRNA. The list of targeted genes
was input into PANTHER (Protein ANalysis THrough Evolutionary
Relationships, http://www.pantherdb.org/) classification system
designed to classify proteins in order to facilitate
high-throughput analysis (21). The
web-based functional annotation tool Database for Annotation,
Visualization and Integrated Discovery (DAVID) v6.7(http://david.abcc.ncifcrf.gov/tools.jsp)
has key components for disease analysis, Gene Ontology analysis and
pathway analysis (22).
Signaling pathway mapping
The signaling pathways and processes were explored
using the systems biology tool KEGG Mapper (http://www.genome.jp/kegg/tool/map_pathway2.html)
which is a collection of tools for KEGG mapping: KEGG pathway
mapping, BRITE mapping and MODULE mapping (23). The KEGG database consists of the 16
main databases (systems information, KEGG PATHWAY, KEGG BRITE, KEGG
MODULE, KEGG DISEASE, KEGG DRUG and KEGG ENVIRON; genomic
information, KEGG ORTHOLOGY, KEGG GENOME, KEGG GENES, KEGG SSDB and
KEGG; chemical information, KEGG COMPOUND, KEGG GLYCAN, KEGG
REACTION, KEGG RPAIR, KEGG RCLASS and KEGG ENZYME).
Results and Discussion
LC-associated miRNAs
Based on previous experimental data, 8 miRNAs were
summarized as LC-miRNAs within three molecular subtypes (Table I). These LC-miRNA deregulations
could drive tumorigenesis, through the roles LC-miRNAs can adopt as
tumor suppressors or oncogenes in LC.
 | Table IPredictions of each LC-miRNA
target. |
Table I
Predictions of each LC-miRNA
target.
| LC-miRNA | Molecular subtype
of LC | Expression in
LC | No. of predicted
target genes |
|---|
| let-7 family
(let-7a-i) | KRAS | Downregulation | 372 |
| miR-7 | EGFR | Downregulation | 444 |
| miR-17 | MEK | Upregulation | 468 |
| miR-21 | EGFR | Upregulation | 164 |
| miR-96 | KRAS | Upregulation | 434 |
| miR-125a-5p | EGFR | Downregulation | 339 |
| miR-128b | EGFR | Downregulation | 1,039 |
| miR-145 | EGFR | Downregulation | 731 |
The let-7 family (let-7a to i), a conserved
anti-oncomir set, is identified as a post-transcriptional
gatekeeper during the cell proliferation process. The genomic
regions located at let-7 family are usually eliminated in LC
(24). Univariate analysis showed
that downregulation of let-7 gene expression in NSCLC patients is
correlated with poor prognosis (25,26).
Cell cycle arrest and cell death were caused by the expression of
let-7g in K-Ras (G12D)-expressing murine LC cells and its
overexpression clearly inhibited growth of both murine and human
non-small cell lung tumors in tumor xenografts (27).
miR-7, a potential tumor suppressor in various types
of human cancer, is frequently downregulated in LC. miR-7
overexpression not only inhibited NSCLC cell proliferation, but
also caused cell apoptosis and decreased tumorigenicity (28). LC is under EGFR-mediated oncogenesis
(29), and miR-7 regulates the EGFR
pathway through attenuating the activation of Akt and ERK (30) and modulates EGFR oncogenic addiction
(31).
From a large-scale miRNome analysis on LC, some
miRNAs have been found with well characterized cancer association,
which include miR-17 and miR-21 (32). Occasional amplification of the
miR-17–92 cluster locus has been reported in human LC (33). miR-17, belonging to the miR-17–92
cluster, is known to act as oncogenes in multiple malignancies.
Expression of miR-17 promotes cell proliferation, suppresses
apoptosis of cancer cells and induces tumor angiogenesis (34).
Cancer and other diseases result in the upregulation
of the miR-21 expression, which regulates many target genes
associated with cellular survival, apoptosis and cell invasiveness.
miR-21, a specific oncomir, is elevated under EGFR signaling
stimulated conditions, especially in the context of EGFR-activating
mutations, suggesting that miR-21 is related to lung carcinogenesis
in never smokers (35). The miR-21
overexpression induced by diseases is reported to be a valuable
approach for the new therapeutic strategies.
The high expression of miR-96 is found in tumor and
serum, which may be considered a potential novel biomarker for the
diagnosis and prognosis of LC, and the increase of miR-96
expression was also related with the overall poor survival in
patients with LC (36). Although
evidence indicates that miR-96 decreased pancreatic cancer cell
invasion and migration and slowed tumor growth in a manner
associated with KRAS downregulation (37), miR-96 appears to be a potential
oncomir in LC.
The miR-125 family has been implicated in a variety
of carcinomas as a tumor-suppressor. In LC, miR-125a-5p was
reported to be stimulated by EGFR and served as a metastatic
suppressor for the purpose of restraining tube formation and tumor
formation (38). Jiang et
al(39) stated that miR-125a-5p
suppresses proliferation and induces apoptosis via a p53-dependent
pathway in LC cells.
Admittedly, miRNA-128b is located on chromosome 3p
and a putative regulator of EGFR and was proven to be correlated
with response to targeted EGFR inhibition (40). Particularly, miR-128b
loss-of-heterozygosity is continually identified in NSCLC patients
and is closely associated with clinical response and survival after
gefitinib treatment.
miR-145 is a tumor suppressor in the carcinogenesis
of lung adenocarcinoma (41).
miR-145 has been shown to suppress NSCLC cell proliferation by
targeting c-Myc (42), lung
adenocarcinoma-initiating cell proliferation by targeting OCT4
(43), cell proliferation of human
lung adenocarcinoma by targeting EGFR and NUDT1 (44) and cell invasion and metastasis by
directly targeting mucin 1 (45).
Thus, the let-7 family (let-7a to i), miR-7,
miR-125a-5p, miR-128b and miR-145 are classified as anti-oncomirs
or tumor suppressors, while miR-17, miR-21 and miR-96 are oncomirs
or OG (oncogenes). Identifying miRNAs as regulators of oncogenes or
tumor suppressors could have far-reaching implications for LC
patients, including improving patient selection for targeted
agents, development of novel therapeutics or early biomarkers of
disease.
Predictions and protein classifications
of LC-miRNA targets
Many target genes are regulated by a single miRNA.
Therefore, to advance the understanding of the integrated functions
of miRNAs, all its targets should be analyzed. As shown in Table I, each LC-miRNA or miRNA family has
the ability to target between 164 and 1,039 mRNAs of predicted
genes, and, in addition, multiple binding sites were observed in
the 3′UTRs of the mRNAs for a unique miRNA. A total of 3,081 unique
targeted genes, regulated by these 8 identified LC-miRNAs, have
been found. Of all the targeted genes, 2,436 items are from targets
of anti-oncomirs, thus, these genes are more likely upregulated in
LC cells. Consequently, 994 downregulated targeted genes have been
discovered from that of oncomiRs. Moreover, 13 genes (ALX4,
CD69, CPEB3, DCUN1D3, DUSP8,
FAM126B, FGD4, GAB1, NR2C2,
SNTB2, SOCS6, STAT3 and ZNF217) were
found in the targets of 2 oncomirs, and 421 genes were identified
as targets of at least two anti-oncomiRs in LC in which 5 genes
(HIPK2, MBD2, NFIB, SP1 and
SRGAP2) seemed to be common targets of 4 anti-oncomirs.
Those common target genes could be potential targets for drug
discovery.
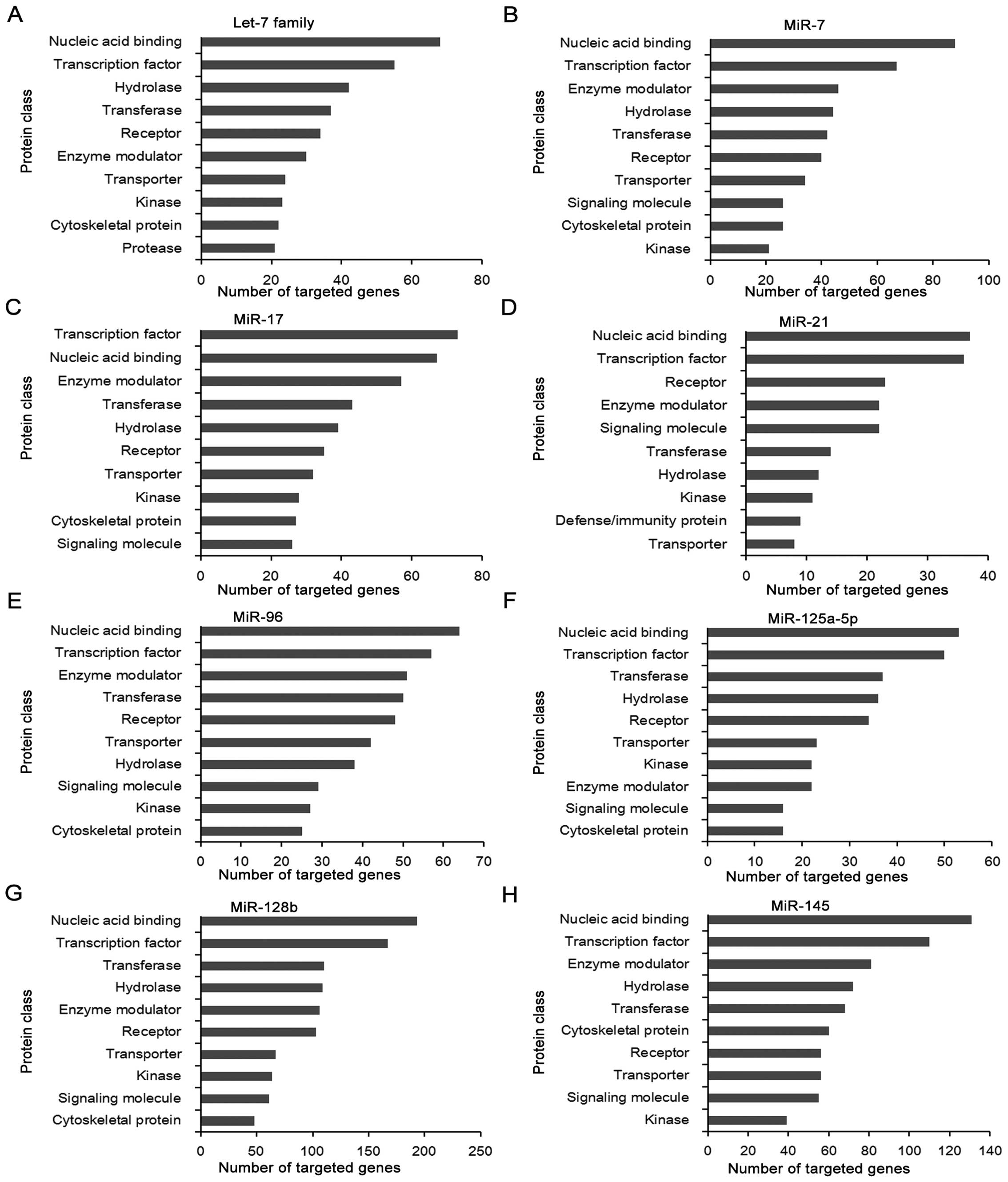
Fig. 1 shows the
protein class of potential targets of each LC-miRNA. The largest
number of genes is targeted by miR-128b, whereas miR-21 targets the
minimum amount of genes. Their target genes of various LC-miRNAs
with different seed region sequences belong to similar protein
classes, including transcription factor, nucleic acid binding,
enzyme modulator, kinase, cytoskeletal protein, transferase and
hydrolase. In agreement with our previous studies, nucleic acid
binding and transcription factor are the most important protein
classes of targeted genes of LC-miRNAs (5,46).
Nucleic acid binding is described as interacting selectively and
non-covalently with any nucleic acid, which consists of base
pairing, regulatory region nucleic acid binding, DNA binding, RNA
binding, DNA/RNA hybrid binding and translation regulator activity
with nucleic acid binding. These targets function as helicase,
transcription factor, transmembrane receptor protein kinase,
isomerase via nucleic acid binding during mRNA processing, DNA
replication, cell cycle, tissue development and gamete generation.
Of note, transcription factors control the activity of a gene by
determining whether the gene’s DNA is transcribed into mRNA and are
detected in all living organisms. The number of transcription
factors found within an organism increases with genome size, and
larger genomes tend to have more transcription factors per gene
(47). Transcription factors are
vital for the normal development of an organism, as well as for
routine cellular functions and response to disease. Recently, Kim
et al(48) applied mass
spectrometry to identify and quantify LC-related transcription
factors by integrating previously reported genomic, transcriptomic,
and proteomic data in various cell lines, and they found 14
differentially expressed transcription factors, such as STAT1 and
SMAD4, which tuned genes associated with drug resistance and cell
differentiation-related processes.
Molecular function, biological process
and signaling pathway analysis of targets related to each
LC-miRNA
To further clarify the functions of LC-miRNAs,
molecular function, biological process and signaling pathway were
analyzed and concluded. The top five items for these analyses of
LC-miRNAs are listed in Tables II
and III. Consistent with the
aforementioned results in protein classification, over-represented
molecular function and biological process of target genes are
involved in transcription and binding, which are the dominant
functions in the molecular regulation of mammals. Markedly, the
targets for LC-miRNAs were most prominently predicted to function
in pathways in cancer and MAPK signaling pathway shown in Table III, indicating that these miRNAs
may regulate carcinogenesis mainly through the very two pathways.
Due to the relevance of LC and multiple signaling pathway,
endocytosis, regulation of actin cytoskeleton, p53 signaling
pathway, mTOR signaling pathway and focal adhesion were also
confirmed in LC as previously reported (5).
 | Table IIMolecular function and biological
process analysis of each LC-miRNA. |
Table II
Molecular function and biological
process analysis of each LC-miRNA.
| LC-miRNA | Molecular function
and biological process | % Regulated by
LC-miRNAs | P-value |
|---|
| let-7 family | Regulation of
transcription | 20.6 | 2.80E-04 |
| Transcription | 14.4 | 5.20E-02 |
| Regulation of
transcription, DNA-dependent | 13.7 | 7.90E-03 |
| Regulation of RNA
metabolic process | 13.7 | 1.20E-02 |
| Phosphorus
metabolic process | 10.2 | 9.40E-05 |
| miR-7 | Transcription
factor activity | 8.0 | 7.80E-03 |
| Enzyme binding | 6.2 | 1.10E-05 |
| Ion binding | 28.2 | 2.80E-02 |
| Metal ion
binding | 27.7 | 2.40E-02 |
| Cation binding | 27.7 | 3.10E-02 |
| miR-17 | Transition metal
ion binding | 22.1 | 2.90E-04 |
| Zinc ion
binding | 18.2 | 1.60E-03 |
| Ion binding | 31.6 | 8.60E-04 |
| Metal ion
binding | 31.4 | 4.20E-04 |
| Cation binding | 31.4 | 6.50E-04 |
| miR-21 | Transition metal
ion binding | 18.8 | 2.80E-03 |
| DNA binding | 17.3 | 3.50E-05 |
| Ion binding | 29.4 | 1.00E-01 |
| DNA binding | 19.0 | 4.00E-02 |
| Transcription
regulator activity | 17.8 | 3.00E-04 |
| miR-96 | Transition metal
ion binding | 19.8 | 4.20E-02 |
| DNA binding | 18.2 | 6.40E-03 |
| Nucleotide
binding | 16.0 | 2.90E-02 |
| Purine nucleotide
binding | 13.7 | 4.70E-02 |
| Purine
ribonucleotide binding | 12.7 | 8.30E-02 |
| miR-125a-5p | Transition metal
ion binding | 20.1 | 1.10E-02 |
| DNA binding | 18.0 | 3.30E-03 |
| Cation binding | 31.3 | 5.10E-05 |
| Ion binding | 31.3 | 9.80E-05 |
| Metal ion
binding | 30.7 | 1.00E-04 |
| miR-128b | Ion binding | 28.2 | 2.40E-04 |
| Metal ion
binding | 27.9 | 9.50E-05 |
| Cation binding | 27.9 | 2.00E-04 |
| Transition metal
ion binding | 18.8 | 2.80E-03 |
| DNA binding | 17.3 | 3.50E-05 |
| miR-145 | DNA binding | 16.4 | 2.40E-02 |
| Zinc ion
binding | 15.8 | 4.90E-02 |
| Transcription
regulator activity | 11.8 | 5.30E-03 |
| Transcription
factor activity | 8.0 | 7.80E-03 |
| Enzyme binding | 6.2 | 1.10E-05 |
 | Table IIICanonical pathway analysis of each
LC-miRNA. |
Table III
Canonical pathway analysis of each
LC-miRNA.
| LC-miRNA | Canonical
pathways | % Regulated by
LC-miRNAs | P-value |
|---|
| let-7 family | Pathways in
cancer | 4.2 | 9.00E-04 |
| MAPK signaling
pathway | 3.7 | 9.60E-04 |
| p53 signaling
pathway | 2.0 | 4.30E-04 |
| mTOR signaling
pathway | 1.2 | 2.10E-02 |
| miR-7 | Pathways in
cancer | 3.2 | 6.80E-02 |
| Focal adhesion | 3.0 | 4.40E-03 |
| Endocytosis | 2.8 | 6.30E-03 |
| Regulation of actin
cytoskeleton | 2.5 | 4.30E-02 |
| Neurotrophin
signaling pathway | 2.1 | 1.20E-02 |
| Ubiquitin mediated
proteolysis | 2.1 | 2.10E-02 |
| miR-17 | Pathways in
cancer | 2.8 | 1.60E-05 |
| MAPK signaling
pathway | 2.7 | 1.90E-08 |
| Endocytosis | 2.2 | 8.00E-10 |
| Regulation of actin
cytoskeleton | 1.8 | 3.70E-04 |
| Focal adhesion | 1.8 | 1.40E-04 |
| miR-21 | MAPK signaling
pathway | 6.7 | 1.20E-04 |
| Pathways in
cancer | 5.5 | 9.50E-03 |
| Cytokine-cytokine
receptor interaction | 4.9 | 9.50E-03 |
| Jak-STAT signaling
pathway | 4.3 | 2.80E-03 |
| Pancreatic
cancer | 3.7 | 4.80E-04 |
| Chemokine signaling
pathway | 3.7 | 2.80E-02 |
| Regulation of actin
cytoskeleton | 3.7 | 4.70E-02 |
| miR-96 | Calcium signaling
pathway | 2.6 | 4.20E-03 |
| Endocytosis | 2.6 | 5.70E-03 |
| Focal adhesion | 2.1 | 6.60E-02 |
| Gap junction | 1.9 | 2.80E-03 |
| GnRH signaling
pathway | 1.7 | 1.80E-02 |
| Melanogenesis | 1.7 | 1.90E-02 |
| miR-125a | MAPK signaling
pathway | 2.7 | 6.10E-02 |
| Axon guidance | 1.8 | 5.40E-02 |
| TGF-β signaling
pathway | 1.5 | 4.90E-02 |
| Biosynthesis of
unsaturated fatty acids | 0.9 | 4.70E-02 |
| miR-128b | Pathways in
cancer | 3.2 | 1.00E-03 |
| MAPK signaling
pathway | 2.9 | 6.80E-04 |
| Focal adhesion | 2.1 | 7.10E-03 |
| Neurotrophin
signaling pathway | 1.9 | 1.30E-04 |
| Regulation of actin
cytoskeleton | 1.9 | 4.90E-02 |
| miR-145 | MAPK signaling
pathway | 3.9 | 1.40E-05 |
| Axon guidance | 3.1 | 5.80E-08 |
| Endocytosis | 3.1 | 2.20E-05 |
| Pathways in
cancer | 3.1 | 3.30E-02 |
| Focal adhesion | 2.6 | 1.70E-03 |
Pathway mapping of LC-miRNA targets
To sum up the pathway implicated in all targets of 8
LC-miRNAs, 3,081 unique targets were used for further pathway
analysis. As shown in Table IV,
PI3K-Akt signaling pathway, pathways in cancer, MAPK signaling
pathway, HTLV-I infection and focal adhesion were the top five
pathways regulated by 8 LC-miRNAs. These pathways have been
demonstrated to play essential roles in morphological changes,
intercellular communication and invasion of cancer.
 | Table IVTop five pathways regulated by all 8
LC-miRNAs. |
Table IV
Top five pathways regulated by all 8
LC-miRNAs.
| Pathway DB | Name | Hits | Total | Percent (%) |
|---|
| KEGG | PI3K-Akt signaling
pathway - Homo sapiens | 94 | 336 | 27.97 |
| Pathways in cancer
- Homo sapiens | 91 | 343 | 26.53 |
| MAPK signaling
pathway - Homo sapiens | 87 | 284 | 35.08 |
| HTLV-I infection-
Homo sapiens | 68 | 198 | 34.34 |
| Focal adhesion -
Homo sapiens | 64 | 207 | 30.92 |
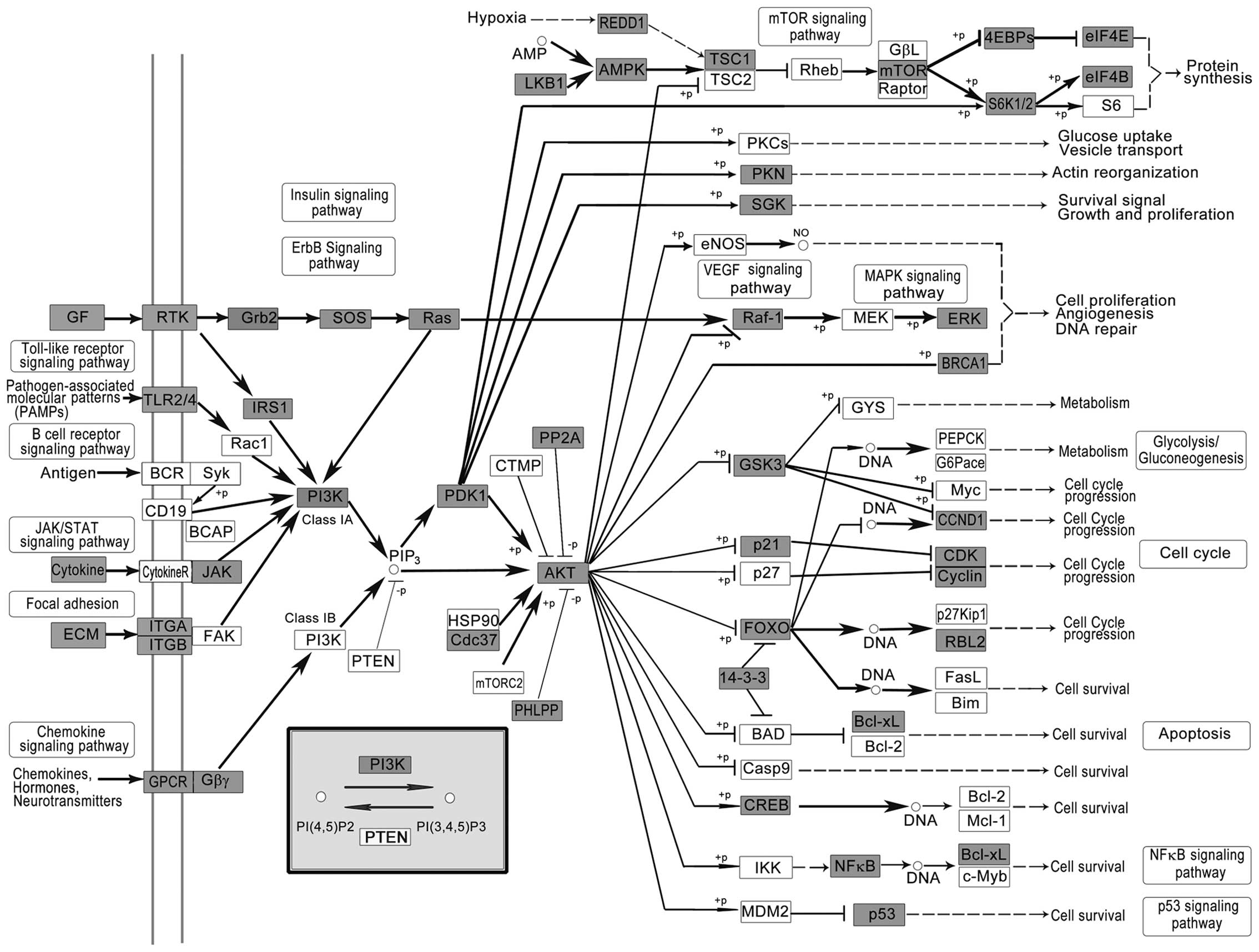
Phosphatidylinositol-3-kinase (PI3K)-Akt signaling
pathway (Fig. 2), a pathway thought
to be specific to EGFR/ERBB family receptors, has been reported to
change frequently in diverse human cancer (49). PI3K phosphorylates 3 position of the
inositol ring of PI(4,5)P2, to generate PI(3,4,5) P3 (50). Previous studies have demonstrated
that numerous components of the PI3K-AKT pathway rather than any
other pathway in human cancer, crucial to many aspects of cell
growth and survival, are altered by amplification, mutation and
translocation frequently, with resultant activation of the pathway.
Also, the PI3K/AKT pathway is targeted for LC drug discovery
(51).
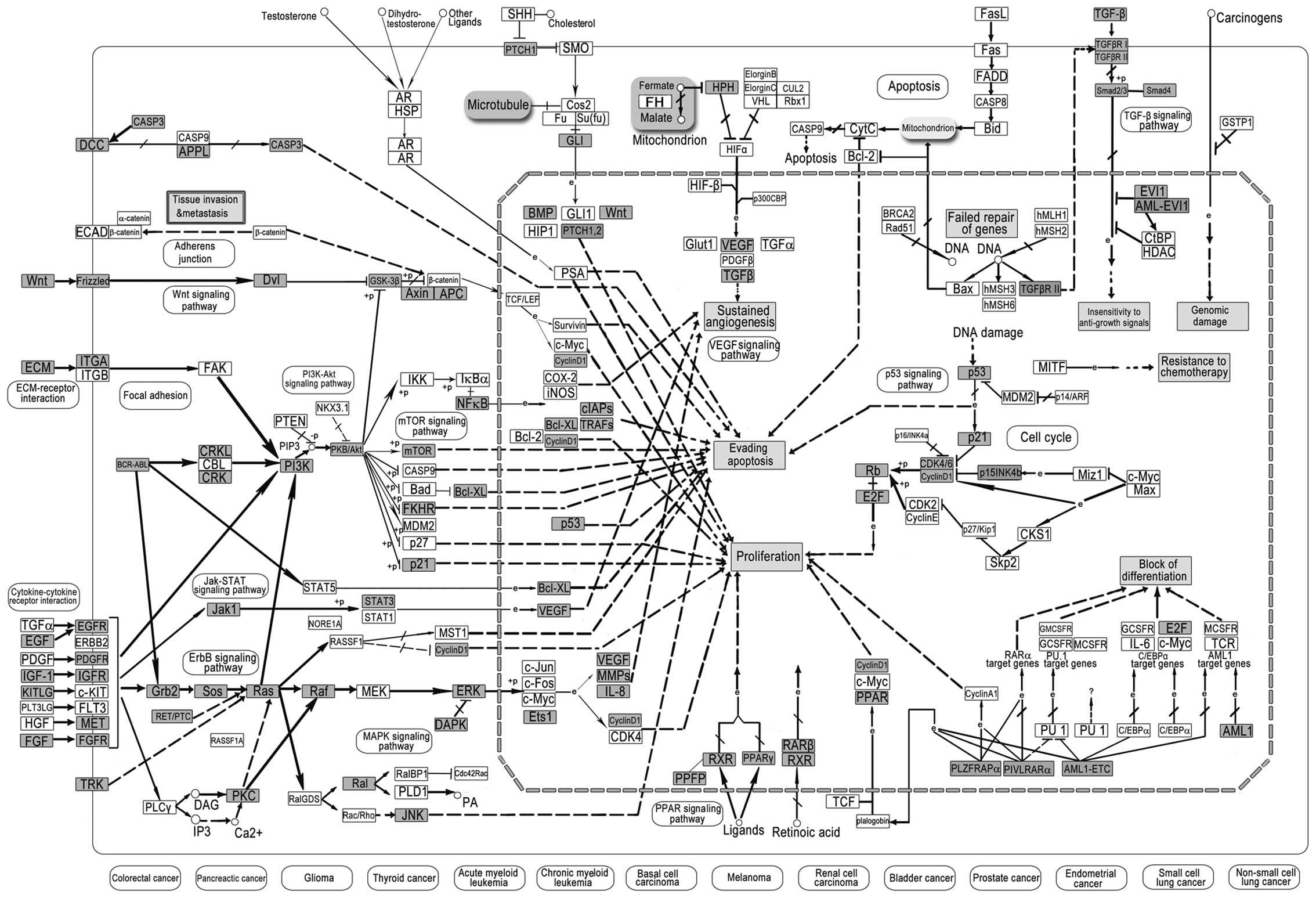
Pathway in cancer is a comprehensive biological
signaling pathway related to tissue invasion and metastasis,
genomic damage, insensitivity to anti-growth signals, resistance to
chemotherapy, evading apoptosis, sustained angiogenesis,
proliferation and block of differentiation, which also links to
diversified cancers, including colorectal, pancreatic, thyroid,
bladder, prostate, endometrial and LC. Many targets of LC-miRNAs
were found in pathways in cancer (Fig.
3), and these significant findings in LC may lead to customized
therapy based on targeting specific genes. The signaling pathway
could offer valuable roadmaps for drug discovery and therapy.
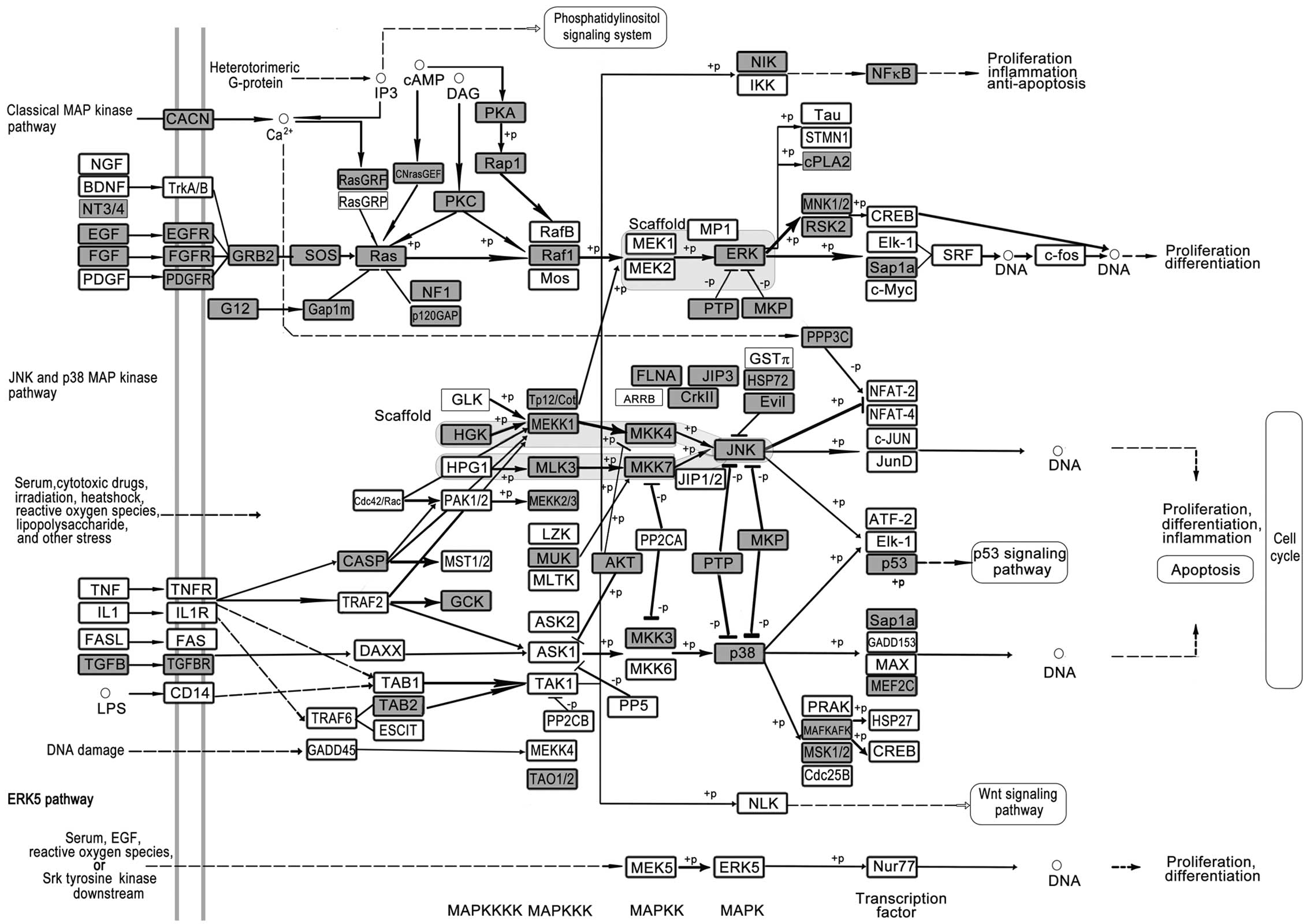
Mitogen-activated protein kinase (MAPK) cascade
activated by peptide growth factors, cytokines, hormones, and
various cellular stressors is a highly conserved module involved in
various cellular functions, including cell proliferation,
differentiation and migration (Fig.
4). Mammals express at least three distinctly regulated groups
of MAPKs, extracellular signal-regulated kinase (ERK), p38 and
c-Jun NH2-terminal kinase and each of these enzymes exists in
several isoforms. The pathway has been suggested to function in
several steps of tumorigenesis including cancer cell proliferation,
migration and invasion. Lung metastasis was found to be
substantially delayed in MEKK1 knockout mice (52). Mutations in EGFR activating the
pathway occur frequently in LC (53). Experimental mouse models help to
elucidate the mechanism of how these MAPKs control cancer
development, and appear to provide new strategies for the design of
improved therapeutic approaches (54). Small-molecule inhibitors designed to
target various steps of this pathway have entered clinical trials
(55).
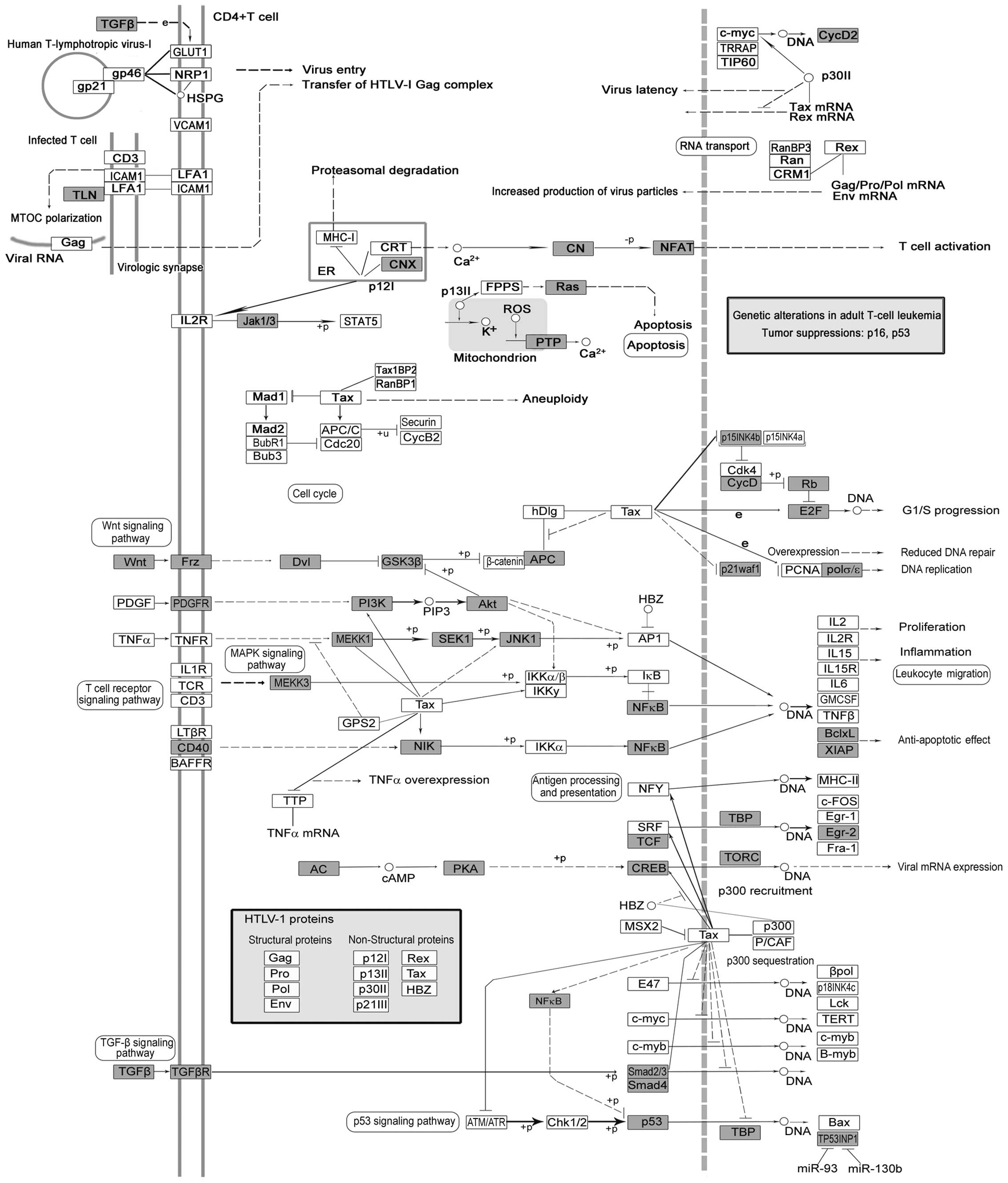
Human T-lymphotropic virus type 1 (HTLV-1) is a
pathogenic retrovirus associated with adult T-cell
leukemia/lymphoma. Tax encoded by HTLV-I has been implicated in
oncogenesis, which is a transcriptional co-factor that disturbs
anti-apoptosis or cell proliferation (56). Although the infection rate of HTLV-1
may not be associated with increased risk of cancer, the Wnt, TGFβ,
Ras, NF-κB, p53 in HTLV-1 infection pathways may play pivotal roles
in lung carcinogenesis (Fig.
5).
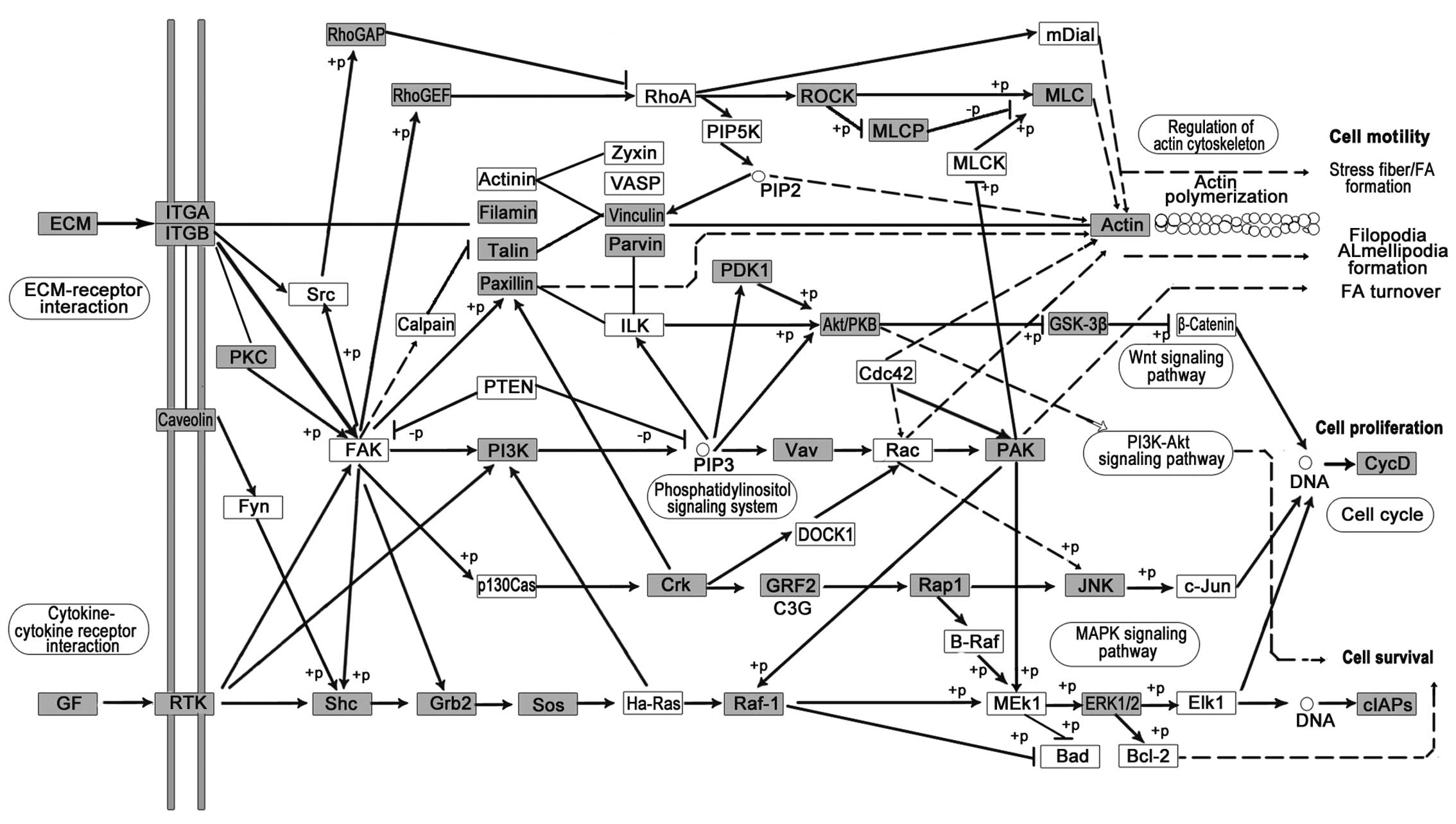
Focal adhesion (Fig.
6), directly connected with cell mobility, proliferation and
survival, was altered in LC, possibly due to the development of
malignant LC, which is associated with perturbations in these
processes. Inducible expression of an inhibitory focal adhesion
kinase (FAK) protein and FAK-related non-kinase (FRNK) suppressed
the growth of primary tumors and blocked metastasis formation in
the lungs. Lung metastasis formation was nearly completely avoided
when FAK-related non-kinase (FRNK) was already expressed prior to
tumor cell injection; nevertheless, FRNK expression after injection
did not influence lung metastasis formation (57).
We found specific pathways from the Top 10 pathways
of each subtype of LC (Table V).
The regulation of actin cytoskeleton pathway is specific to EGFR
sub-type LC (EGFR-LC); cholinergic synapse and calcium signaling
pathway belong to KRAS-LC; ubiquitin mediated proteolysis and
cytokine-cytokine receptor interaction are unique to MEK-LC.
Regulation of actin cytoskeleton pathway is often stimulated in
invasive and metastatic LC cells as malignant LC cells utilize
their intrinsic migratory ability to invade adjacent tissues and
the vasculature and ultimately to metastasize (58). In particular, a subunit of Arp 2/3
complex is observed in malignant human LC and correlated with poor
patient outcome which is in accordance with our results (59). Cholinergic synapse and calcium
signaling pathways were closely related to LC since both muscarinic
cholinergic receptor 3 (mAChR3) and the nicotinic cholinergic
receptor (nAChR) are expressed in small cell LC (SCLC). A number of
LC studies have focused on nAChR due to the close relationship
between tobacco and LC. Specifically, nicotine stimulates LC
carcinogenesis, proliferation and angiogenesis and also inhibits LC
apoptosis induced by chemotherapeutic drugs through the nAChR
(60). It is reported that the
mAChR3 antagonist could be developed as a beneficial therapeutic
approach for SCLC patients, notably those with a comorbidity of
chronic obstructive pulmonary disease (61). Ubiquitin-mediated proteolysis can
maintain protein homeostasis and is critical in regulating
cancer-related cellular processes, and inhibitors of the proteasome
and their molecular mechanisms for targeting substrate-specific E3
ligases that are likely to yield a new class of therapeutics that
will serve as anti-LC drugs due to multitude of E3s and their
specific substrate recognition (62). Cytokine-cytokine receptor
interaction is important in cell-cell communications, whereas
abnormal expression of cytokines or cytokine receptors could
certainly contribute to LC.
 | Table VSpecific pathways of each subtype of
LC with KEGG mapper. |
Table V
Specific pathways of each subtype of
LC with KEGG mapper.
| Subtype of LC | Specific
pathways | Hits | Percent (%) |
|---|
| EGFR | Regulation of actin
cytoskeleton - Homo sapiens | 50 | 23 |
| KRAS | Cholinergic synapse
- Homo sapiens | 13 | 32 |
| KRAS | Calcium signaling
pathway - Homo sapiens | 14 | 8 |
| MEK | Ubiquitin mediated
proteolysis - Homo sapiens | 9 | 6 |
| MEK | Cytokine-cytokine
receptor interaction - Homo sapiens | 9 | 3 |
In the present study, we used theoretical gene
target identification and pathway mapping to create a biological
framework by which to test the relevance of LC-miRNAs in LC
induction. The recognition of LC-miRNA-related pathways, with the
ability to regulate a complex pathological process in three
molecular subtypes of LC, can be improved using bioinformatic
techniques followed by experimental validation.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (31000323, 31070672,
81250044), the Natural Science Foundation of Jiangsu Province
(BK20131272), the Specialized Research Fund for the Doctoral
Program of Higher Education of China (20100091120023) and the
Fundamental Research Funds for the Central Universities
(1095020823).
References
|
1
|
Bartel DP: MicroRNAs: genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
He L and Hannon GJ: MicroRNAs: small RNAs
with a big role in gene regulation. Nat Rev Genet. 5:522–531. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lee RC, Feinbaum RL and Ambros V: The
C. elegans heterochronic gene lin-4 encodes small RNAs with
antisense complementarity to lin-14. Cell. 75:843–854. 1993.
|
|
4
|
Lim LP, Lau NC, Garrett-Engele P, et al:
Microarray analysis shows that some microRNAs downregulate large
numbers of target mRNAs. Nature. 433:769–773. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang H, Zhang H, Zhu L, Wang J, Zhang C
and Li D: Pathway analysis of cancer-associated microRNA targets.
Int J Oncol. 41:2213–2226. 2012.PubMed/NCBI
|
|
6
|
Zhu D, Pan C, Li L, et al:
MicroRNA-17/20a/106a modulate macrophage inflammatory responses
through targeting signal-regulatory protein alpha. J Allergy Clin
Immunol. 132:426–436.e8. 2013. View Article : Google Scholar
|
|
7
|
Brennecke J, Hipfner DR, Stark A, Russell
RB and Cohen SM: bantam encodes a developmentally regulated
microRNA that controls cell proliferation and regulates the
proapoptotic gene hid in Drosophila. Cell. 113:25–36.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cuellar TL and McManus MT: MicroRNAs and
endocrine biology. J Endocrinol. 187:327–332. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Poy MN, Eliasson L, Krutzfeldt J, et al: A
pancreatic islet-specific microRNA regulates insulin secretion.
Nature. 432:226–230. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen CZ, Li L, Lodish HF and Bartel DP:
MicroRNAs modulate hematopoietic lineage differentiation. Science.
303:83–86. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang L, Hou D, Chen X, et al: Exogenous
plant MIR168a specifically targets mammalian LDLRAP1: evidence of
cross-kingdom regulation by microRNA. Cell Res. 22:107–126. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar
|
|
13
|
Loscalzo J, Kohane I and Barabasi AL:
Human disease classification in the postgenomic era: a complex
systems approach to human pathobiology. Mol Syst Biol. 3:1242007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nie W, Tang L, Zhang H, et al: Structural
analysis of the EGFR TK domain and potential implications for EGFR
targeted therapy. Int J Oncol. 40:1763–1769. 2012.PubMed/NCBI
|
|
15
|
Croce CM: Causes and consequences of
microRNA dysregulation in cancer. Nat Rev Genet. 10:704–714. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bishop JA, Benjamin H, Cholakh H, Chajut
A, Clark DP and Westra WH: Accurate classification of non-small
cell lung carcinoma using a novel microRNA-based approach. Clin
Cancer Res. 16:610–619. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Patnaik SK, Kannisto E, Knudsen S and
Yendamuri S: Evaluation of microRNA expression profiles that may
predict recurrence of localized stage I non-small cell lung cancer
after surgical resection. Cancer Res. 70:36–45. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Raponi M, Dossey L, Jatkoe T, et al:
MicroRNA classifiers for predicting prognosis of squamous cell lung
cancer. Cancer Res. 69:5776–5783. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lin PY, Yu SL and Yang PC: MicroRNA in
lung cancer. Br J Cancer. 103:1144–1148. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lewis BP, Burge CB and Bartel DP:
Conserved seed pairing, often flanked by adenosines, indicates that
thousands of human genes are microRNA targets. Cell. 120:15–20.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mi H, Muruganujan A and Thomas PD: PANTHER
in 2013: modeling the evolution of gene function, and other gene
attributes, in the context of phylogenetic trees. Nucleic Acids
Res. 41:D377–D386. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huang da W, Sherman BT, Tan Q, et al: The
DAVID Gene Functional Classification Tool: a novel biological
module-centric algorithm to functionally analyze large gene lists.
Genome Biol. 8:R1832007.PubMed/NCBI
|
|
23
|
Kanehisa M, Araki M, Goto S, et al: KEGG
for linking genomes to life and the environment. Nucleic Acids Res.
36:D480–D484. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Calin GA, Sevignani C, Dumitru CD, et al:
Human microRNA genes are frequently located at fragile sites and
genomic regions involved in cancers. Proc Natl Acad Sci USA.
101:2999–3004. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Takamizawa J, Konishi H, Yanagisawa K, et
al: Reduced expression of the let-7 microRNAs in human lung cancers
in association with shortened postoperative survival. Cancer Res.
64:3753–3756. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yanaihara N, Caplen N, Bowman E, et al:
Unique microRNA molecular profiles in lung cancer diagnosis and
prognosis. Cancer Cell. 9:189–198. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kumar MS, Erkeland SJ, Pester RE, et al:
Suppression of non-small cell lung tumor development by the let-7
microRNA family. Proc Natl Acad Sci USA. 105:3903–3908. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xiong S, Zheng Y, Jiang P, Liu R, Liu X
and Chu Y: MicroRNA-7 inhibits the growth of human non-small cell
lung cancer A549 cells through targeting BCL-2. Int J Biol Sci.
7:805–814. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wong KK: Searching for a magic bullet in
NSCLC: the role of epidermal growth factor receptor mutations and
tyrosine kinase inhibitors. Lung Cancer. 60(Suppl 2): S10–S18.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Webster RJ, Giles KM, Price KJ, Zhang PM,
Mattick JS and Leedman PJ: Regulation of epidermal growth factor
receptor signaling in human cancer cells by microRNA-7. J Biol
Chem. 284:5731–5741. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chou YT, Lin HH, Lien YC, et al: EGFR
promotes lung tumorigenesis by activating miR-7 through a
Ras/ERK/Myc pathway that targets the Ets2 transcriptional repressor
ERF. Cancer Res. 70:8822–8831. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Volinia S, Calin GA, Liu CG, et al: A
microRNA expression signature of human solid tumors defines cancer
gene targets. Proc Natl Acad Sci USA. 103:2257–2261. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hayashita Y, Osada H, Tatematsu Y, et al:
A polycistronic microRNA cluster, miR-17–92, is overexpressed in
human lung cancers and enhances cell proliferation. Cancer Res.
65:9628–9632. 2005.
|
|
34
|
Mendell JT: miRiad roles for the miR-17–92
cluster in development and disease. Cell. 133:217–222.
2008.PubMed/NCBI
|
|
35
|
Seike M, Goto A, Okano T, et al: MiR-21 is
an EGFR-regulated anti-apoptotic factor in lung cancer in
never-smokers. Proc Natl Acad Sci USA. 106:12085–12090. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhu W, Liu X, He J, Chen D, Hunag Y and
Zhang YK: Overexpression of members of the microRNA-183 family is a
risk factor for lung cancer: a case control study. BMC Cancer.
11:3932011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yu S, Lu Z, Liu C, et al: miRNA-96
suppresses KRAS and functions as a tumor suppressor gene in
pancreatic cancer. Cancer Res. 70:6015–6025. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang G, Mao W, Zheng S and Ye J: Epidermal
growth factor receptor-regulated miR-125a-5p - a metastatic
inhibitor of lung cancer. FEBS J. 276:5571–5578. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jiang L, Huang Q, Chang J, Wang E and Qiu
X: MicroRNA HSA-miR-125a-5p induces apoptosis by activating p53 in
lung cancer cells. Exp Lung Res. 37:387–398. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Weiss GJ, Bemis LT, Nakajima E, et al:
EGFR regulation by microRNA in lung cancer: correlation with
clinical response and survival to gefitinib and EGFR expression in
cell lines. Ann Oncol. 19:1053–1059. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cho WCS, Chow ASC and Au JSK: Restoration
of tumour suppressor hsa-miR-145 inhibits cancer cell growth in
lung adenocarcinoma patients with epidermal growth factor receptor
mutation. Eur J Cancer. 45:2197–2206. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chen Z, Zeng H, Guo Y, et al: miRNA-145
inhibits non-small cell lung cancer cell proliferation by targeting
c-Myc. J Exp Clin Cancer Res. 29:1512010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yin R, Zhang S, Wu Y, et al: microRNA-145
suppresses lung adenocarcinoma-initiating cell proliferation by
targeting OCT4. Oncol Rep. 25:1747–1754. 2011.PubMed/NCBI
|
|
44
|
Cho WCS, Chow ASC and Au JSK: MiR-145
inhibits cell proliferation of human lung adenocarcinoma by
targeting EGFR and NUDT1. RNA Biol. 8:125–131. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sachdeva M and Mo YY: MicroRNA-145
suppresses cell invasion and metastasis by directly targeting mucin
1. Cancer Res. 70:378–387. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yang H, Zhang H, Zhu L, Zhang C and Li D:
Identification and characterization of microRNAs in macaca
fascicularis by EST analysis. Comp Funct Genomics.
2012:9576072012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
van Nimwegen E: Scaling laws in the
functional content of genomes. Trends Genet. 19:479–484.
2003.PubMed/NCBI
|
|
48
|
Kim JS, Lee Y, Lee MY, et al: Multiple
reaction monitoring of multiple low-abundance transcription factors
in whole lung cancer cell lysates. J Proteome Res. 12:2582–2596.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Engelman JA, Zejnullahu K, Mitsudomi T, et
al: MET amplification leads to gefitinib resistance in lung cancer
by activating ERBB3 signaling. Science. 316:1039–1043. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Carpenter CL and Cantley LC:
Phosphoinositide kinases. Curr Opin Cell Biol. 8:153–158. 1996.
View Article : Google Scholar
|
|
51
|
Hennessy BT, Smith DL, Ram PT, Lu Y and
Mills GB: Exploiting the PI3K/AKT pathway for cancer drug
discovery. Nat Rev Drug Discov. 4:988–1004. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Cuevas BD, Winter-Vann AM, Johnson NL and
Johnson GL: MEKK1 controls matrix degradation and tumor cell
dissemination during metastasis of polyoma middle-T driven mammary
cancer. Oncogene. 25:4998–5010. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Hynes NE and MacDonald G: ErbB receptors
and signaling pathways in cancer. Curr Opin Cell Biol. 21:177–184.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wagner EF and Nebreda AR: Signal
integration by JNK and p38 MAPK pathways in cancer development. Nat
Rev Cancer. 9:537–549. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Sebolt-Leopold JS and Herrera R: Targeting
the mitogen-activated protein kinase cascade to treat cancer. Nat
Rev Cancer. 4:937–947. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Gatza ML, Watt JC and Marriott SJ:
Cellular transformation by the HTLV-I Tax protein, a
jack-of-all-trades. Oncogene. 22:5141–5149. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
van Nimwegen MJ, Verkoeijen S, van Buren
L, Burg D and van de Water B: Requirement for focal adhesion kinase
in the early phase of mammary adenocarcinoma lung metastasis
formation. Cancer Res. 65:4698–4706. 2005.PubMed/NCBI
|
|
58
|
Yamaguchi H and Condeelis J: Regulation of
the actin cytoskeleton in cancer cell migration and invasion.
Biochim Biophys Acta. 1773:642–652. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Semba S, Iwaya K, Matsubayashi J, et al:
Coexpression of actin-related protein 2 and Wiskott-Aldrich
syndrome family verproline-homologous protein 2 in adenocarcinoma
of the lung. Clin Cancer Res. 12:2449–2454. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Dasgupta P, Kinkade R, Joshi B, Decook C,
Haura E and Chellappan S: Nicotine inhibits apoptosis induced by
chemotherapeutic drugs by up-regulating XIAP and survivin. Proc
Natl Acad Sci USA. 103:6332–6337. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Zhang S, Togo S, Minakata K, Gu T, Ohashi
R, Tajima K, Murakami A, Iwakami S, Zhang J, Xie C and Takahashi K:
Distinct roles of cholinergic receptors in small cell lung cancer
cells. Anticancer Res. 30:97–106. 2010.PubMed/NCBI
|
|
62
|
Burger AM and Seth AK: The
ubiquitin-mediated protein degradation pathway in cancer:
therapeutic implications. Eur J Cancer. 40:2217–2229. 2004.
View Article : Google Scholar : PubMed/NCBI
|