Introduction
With recent advances in the development of
molecular-targeted anticancer agents, it is now possible to
identify patients who are likely to respond to treatment and those
who are not. For drugs such as cetuximab and Herceptin,
pre-treatment tests to examine the expression of various marker
molecules have become routine (1,2).
Unfortunately, this type of testing is only available for a small
number of anticancer drugs. For the majority of cancer
chemotherapies, there are no markers to predict response in
individual patients, which adds difficulties in designing protocols
for clinical trials. In the clinical setting, the explanation for
chemotherapy to patients relies mainly on statistical information,
which is often not sufficiently convincing. In our department, in
order to conduct chemotherapies with patient confidence and plan a
precise treatment policy for each patient, we aim at individualized
treatment by testing the sensitivity of tumor cells to
chemotherapeutic agents using the collagen gel droplet-embedded
culture drug sensitive test (CD-DST), a test that simulates
physiological conditions. Although the CD-DST may be sufficient to
evaluate chemotherapeutic agents alone, in our clinical practice we
encounter many patients who are treated with a combination of
chemotherapeutic and immunotherapeutic agents such as biological
response modifiers (BRMs). In these cases, an evaluation method
that reflects the immune capability of patients is needed. For this
purpose, we modified the CD-DST by adding the peripheral blood
mononuclear cells (PBMCs) of patients to the test system and
incubating for long periods (designated immuno-CD-DST). Using this
method, we attempted to evaluate the effect of combination therapy
with BRMs and fluoropyrimidine anticancer agents, and study the
effect of BRMs on intra-tumoral enzymes in the presence of
PBMCs.
Regarding immunotherapeutic agents, we selected to
study protein-bound polysaccharide K (PSK) which is known to be a
BMR. PSK has been reported to enhance the effects of anticancer
drugs, particularly fluoropyrimidine derivatives, against gastric,
colorectal and small cell lung cancer. Currently, PSK is used
clinically in Japan and Taiwan. In Japan, the efficacy of combined
therapy with PSK and fluoropyrimidine derivatives as postoperative
adjuvant therapy for gastric cancer has been reported (3). Clinical trials have also been
conducted on colon, rectal (4) and
lung cancer (5), and PSK was shown
to enhance the effects of fluoropyrimidine derivatives. PSK is a
substance extracted from the fungus Coriolus versicolor, and
is composed of protein and polysaccharide with a β-1,4 glucan
structure. The approximate molecular weight is 100 kDa. Various
mechanisms of the antitumor action of PSK have been reported,
including immunomodulation (enhancing NK cell, cytotoxic T
lymphocyte and lymphokine-activated killer cell activities) and
direct damage to cancer cells (6,7).
Recent study has shown that PSK acts as a TLR2 agonist to induce
immunomodulatory effects (8).
However, there is no report on how PSK enhances the effects of
fluoropyrimidine derivatives, although the mechanisms of action and
metabolic pathways of anticancer agents belonging to the
fluoropyrimidine family have been elucidated. Inside the tumor
cells, 5-fluorouracil (5-FU) is converted to
5′-fluoro-2′-deoxyuridine by thymidine phosphorylase (TP), and is
further converted to 5-fluoro-2′-deoxyuridine 5′-monophosphate by
thymidine kinase, consequently inhibiting the DNA synthesizing
enzyme thymidylate synthase (TS). Moreover, orotate phosphoribosyl
transferase (OPRT) converts 5-FU to 5-fluoro-uridine monophosphate,
thereby inhibiting DNA and RNA syntheses. On the other hand,
dihydropyrimidine dehydrogenase (DPD) is known to metabolize 5-FU
to the inactive form dihydrofluorouracil. Therefore, the lower the
activity of DPD, the higher is the activity of 5-FU.
In the present study, we examined whether the
addition of PBMCs to the CD-DST alters the antiproliferative effect
of PSK combined with 5-FU or 5′-DFUR against tumor cells.
Furthermore, focusing on the possible mechanism of how PSK enhances
the effect of fluoropyrimidines, we also examined the effect of PSK
on the expression of the metabolic enzymes of fluoropyrimidines
(DPD, TP, TS and OPRT). The present study reports for the first
time the possibility that inhibition of DPD expression by PSK may
be a mechanism by which PSK enhances the effect of
fluoropyrimidines, as shown experimentally using the
immune-CD-DST.
Materials and methods
Materials
5-FU was purchased from Kyowa Hakko Kogyo, Co., Ltd.
(Tokyo, Japan). 5′-DFUR was a gift from Chugai Pharmaceutical Co.,
Ltd. (Tokyo, Japan). PSK was manufactured at Kureha Corp. (Tokyo,
Japan) and dissolved in Dulbecco's phosphate-buffered saline
(Gibco-BRL, Grand Island, NY, USA). In each experiment, freshly
prepared PSK solution was used. Human gastric cancer cell lines
(GCIY and MKN45) and human colon cancer cell lines (HCT116 and
WiDr) were obtained from ATCC (Manassas, VA, USA). Cells were
maintained in RPMI-1640 medium (Gibco-BRL) supplemented with 10%
fetal bovine serum (FBS) (Life Technologies, Milan, Italy) and
non-essential amino acids (Sigma, St. Louis, MO, USA). Recombinant
IFN-α and TNFα were purchased from Humanzyme Inc. (Chicago, IL,
USA) and TRAIL from PeproTech (Rocky Hill, NJ, USA).
Peripheral blood mononuclear cells
Blood donors were selected from healthy volunteers
registered at the Department of Surgery, Shiga University of
Medical Science, and Biomedical Research Laboratories, Kureha Corp.
All donors provided written informed consent. Lymphoprep
(Axis-Shield, Oslo, Norway) was used to isolate PBMCs from
peripheral blood.
Tumor cell proliferation assay
Tumor cell proliferation assay was conducted using
the CD-DST as previously described (9). Briefly, HCT-116 cells were cultured in
RPMI-1640 medium supplemented with 10% FBS (10% FBS-RPMI) at 37°C
in a 5% CO2 atmosphere. Using a Collagen Gel Culture kit
(Nitta Gelatin Inc., Osaka, Japan), each cell line was mixed in
molten collagen at a final density of 2×105 cells/ml.
After solidification, the collagen gel-embedded cells were overlaid
with 10% FBS-RPMI containing 5-FU (0.3 μg/ml) or 5′-DFUR (3 μg/ml),
PSK (0, 100 or 300 μg/ml) and PBMCs (none or 4 times the number of
cancer cells) and cultured stationarily at 37°C in 5%
CO2 atmosphere for 144 h. At the end of the incubation,
Neutral red was added to each well at a final concentration of 50
μg/ml, and colonies of HCT-116 cells in the collagen gel droplet
were stained for 2 h. The cells were then fixed with 10%
neutral-buffered formalin. Images of the stained gel were acquired
with a video microscope (VH-5910; Keyence, Osaka, Japan), and the
optical density was measured, which indicated the cell
proliferation rate. Sensitivity was expressed as T/C (%), where T
is the image optical density of the treated group and C is that of
the control.
Collagen gel culture
Experiment 1. Expression of
fluoropyrimidine metabolic enzymes in tumor cells
Cells were pre-cultured in 10% FBS-RPMI at 37°C in a
5% CO2 atmosphere. Using a Collagen Gel Culture kit,
each cell line was mixed with molten collagen at a final density of
2×105 cells/ml, and then spread on a culture dish (ϕ=100
mm; Asahi Glass Co., Ltd., Tokyo, Japan). After solidification, the
collagen gel-embedded cells were overlaid with 10% FBS-RPMI
containing PSK (0, 100 or 300 μg/ml) and PBMCs (none or 4 times the
number of cancer cells) and cultured stationarily at 37°C in a 5%
CO2 atmosphere for 144 h. At the end of the incubation,
the culture was treated with 1% collagenase (Sigma) for 15 min at
37°C and cells were recovered. Total RNA was extracted from the
cells using RNeasy Mini kit (Qiagen Inc., Valencia, CA, USA).
Messenger RNA expression of fluoropyrimidine metabolic enzymes was
measured quantitatively by real-time PCR.
Experiment 2. Effect of addition of
cytokines on DPD expression
Using a Collagen Gel Culture kit, cells were
embedded in collagen gel at a final density of 8×105
cells/ml. After solidification, the collagen gel-embedded cells
were overlaid with 10% FBS-RPMI containing various human
recombinant cytokines and incubated at 37°C in a 5% CO2
atmosphere for 72 h. At the end of the incubation, collagenase was
added to recover the cells from the collagen gel, and total RNA was
extracted using FastPure RNA kit (Takara Bio Inc., Shiga, Japan).
Messenger RNA expression of cytokines was quantitatively measured
by real-time PCR.
Real-time polymerase chain reaction
From the total RNA, cDNA was synthesized using the
PrimeScript RT reagent kit (Takara Bio). Using the cDNA as
template, real-time PCR was performed with SYBR Premix Ex Taq II
(Takara Bio). The quantity of expression of each gene was
normalized to that of GUSB, GAPDH or β-actin gene. The gene
expression level was expressed relative to the expression level of
the control (=1). The primers used are shown in Table I. The primer for GUSB (HA067813) was
purchased from Takara Bio.
 | Table ISequences of the primers used in the
present study. |
Table I
Sequences of the primers used in the
present study.
| Name | Forward | Reverse |
|---|
| DPD |
5′-aggacgcaaggagggtttg-3′ |
5′-gtccgccgagtccttactga-3′ |
| TP |
5′-agctggagtctattcctggatt-3′ |
5′-ggctgcatataggattccgtc-3′ |
| TS |
5′-cacactttgggagatgcaca-3′ |
5′-ctttgaaagcaccctaaacagccat-3′ |
| OPRT |
5′-tcctgggcagatctagtaaatgc-3′ |
5′-tgctcctcagccattctaacc-3′ |
| IFN-α |
5′-gatctgcctcaaacccacag-3′ |
5′-ctggttgccaaactcctcct-3′ |
| TRAIL |
5′-caagtcaagtggcaactccg-3′ |
5′-gtgagctgctactctctgag-3′ |
| GAPDH |
5′-atggggaaggtgaaggtcg-3′ |
5′-ggggtcattgatggcaacaata-3′ |
| β-actin |
5′-catccgcaaagacctgtacg-3′ |
5′-gatcttcattgtgctgggtgc-3′ |
Culture of the peripheral blood
mononuclear cells
The PBMCs were isolated from 5 healthy volunteers
and adjusted to a density of 1×106 cells/ml in 10%
FBS-RPMI. The cells were incubated at 37°C in a 5% CO2
atmosphere for 24 h. In the PSK-treated group, PBMCs were suspended
in 10% FBS-RPMI containing PSK (100 μg/ml) and cultured in the same
manner. At the end of the culture, the cells were recovered by
centrifugation, and total RNA was extracted using the FastPure RNA
kit (Takara Bio Inc.).
Results
PSK augments the antiproliferative effect
of 5-FU and 5′-DFUR in the presence of PBMCs
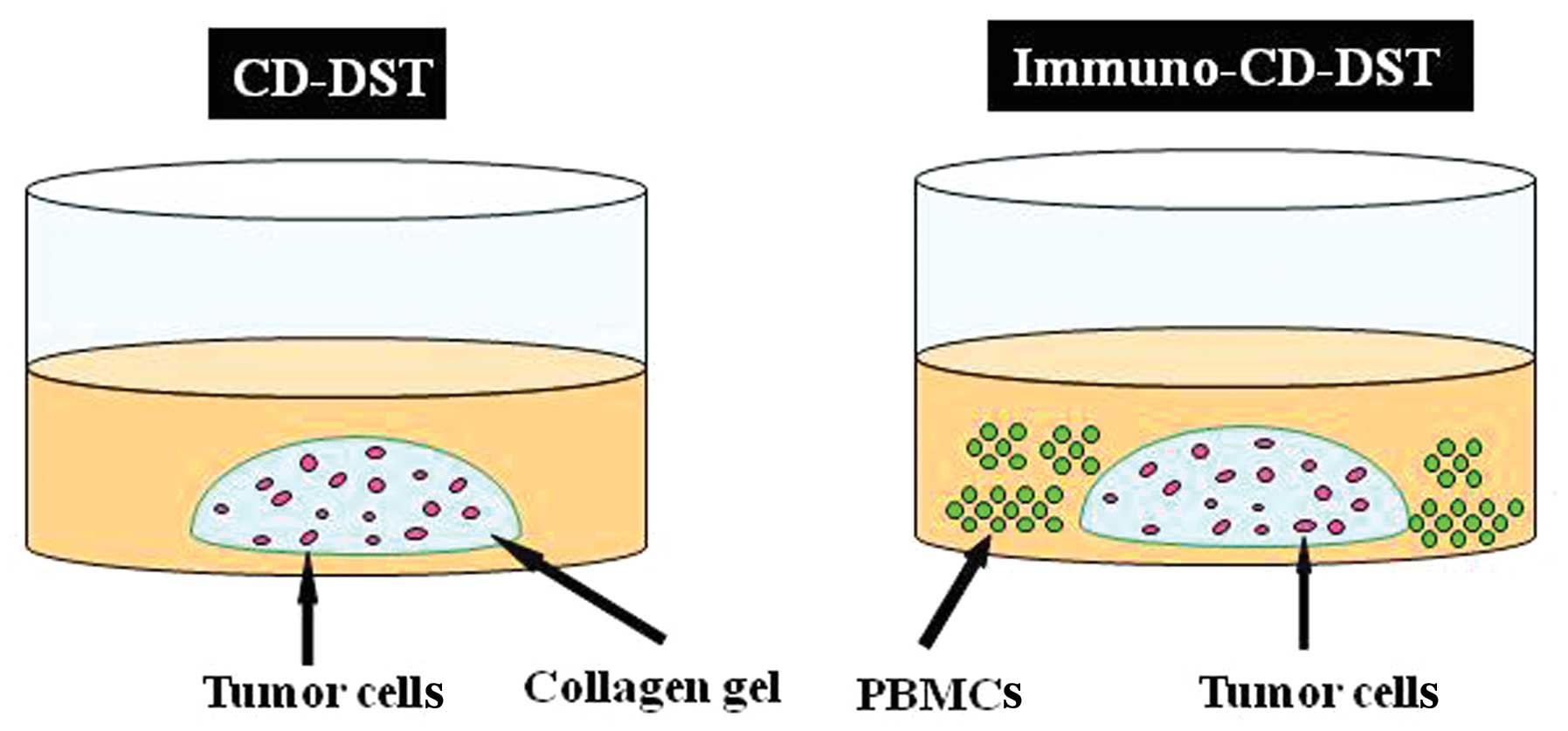
We modified the CD-DST that we developed for
evaluating the sensitivity of tumors to chemotherapeutic agents
(9), by adding PBMCs to the culture
system (immuno-CD-DST) (Fig. 1).
Using this system, we examined whether addition of PSK, which is
known to have an immunomodulatory activity, affects the antitumor
effect. The concentrations of 5-FU and 5′-DFUR were the same as
used in our previous study using the CD-DST (9). We embedded the human colon cancer cell
line HCT116 in collagen, and cultured the collagen gel-embedded
cells with PSK and 5-FU or 5′-DFUR in the presence or absence of
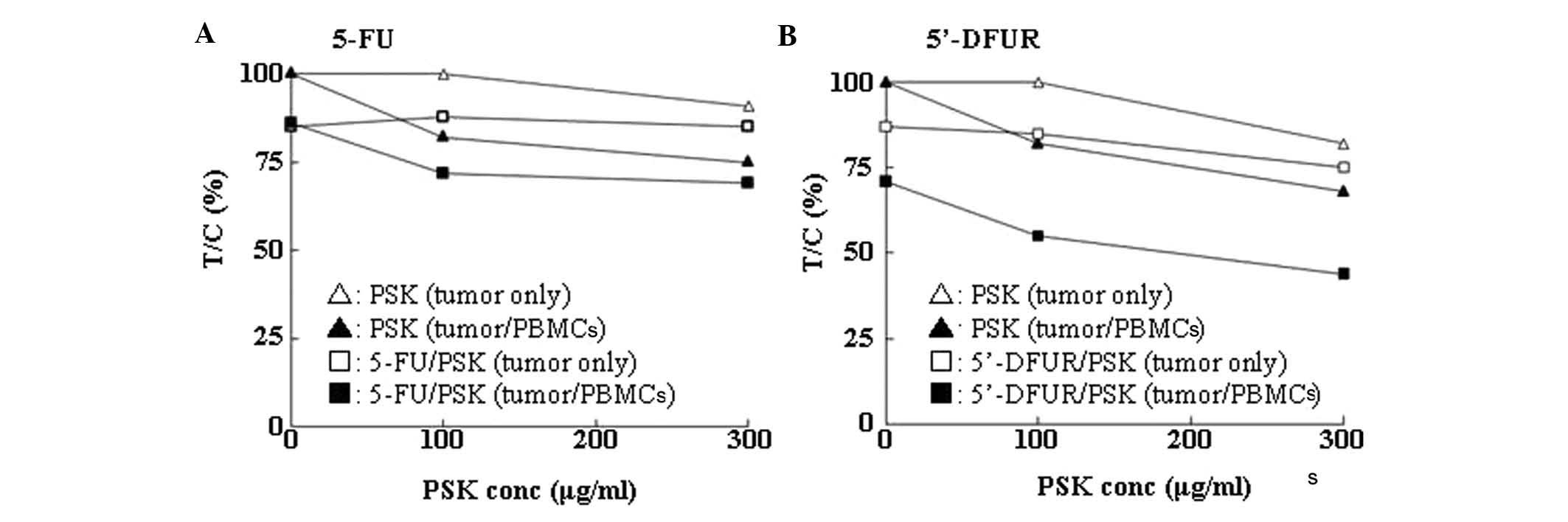
PMBCs for 144 h. The addition of PSK suppressed cell proliferation
both in the presence and in the absence of PBMCs, but the
suppression was stronger in the presence of PMBCs. When 5-FU
together with PSK were added, the antiproliferative effect was
enhanced both in the presence and in the absence of PBMCs, but the
effect was the strongest in the presence of PMBCs (Fig. 2A). Likewise, 5′-DFUR showed similar
results (Fig. 2B).
PSK reduces DPD mRNA expression in
gastrointestinal cells
Based on finding that the antiproliferative effect
of fluoropyrimidine derivatives is the strongest with the addition
of PSK in the presence of PMBCs, we next examined the effects of
PMBCs and PSK on the expression of enzymes (DPD, TS, TP and OPRT)
associated with the metabolism of fluoropyrimidine derivatives.
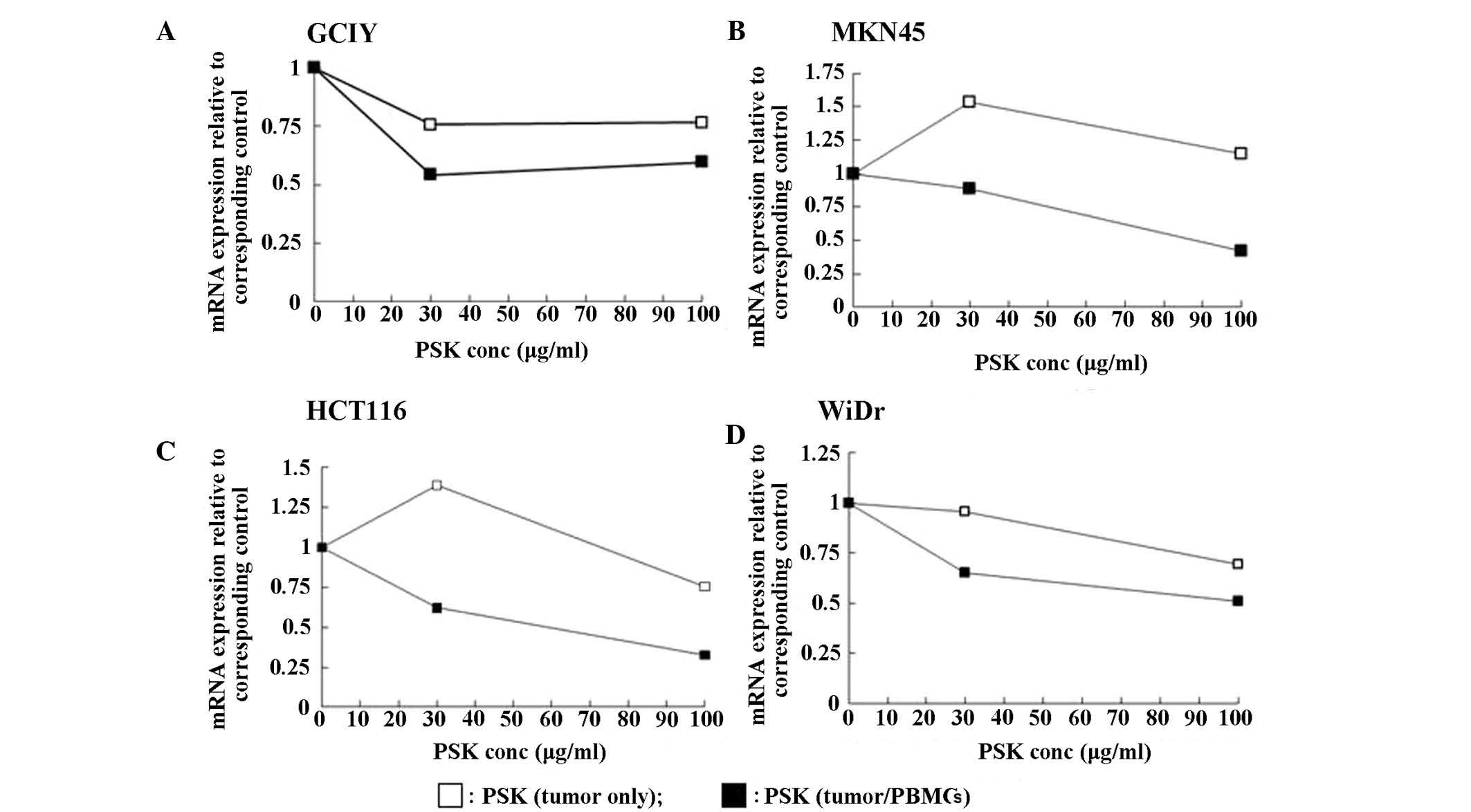
When collagen gel-embedded gastric cancer cell lines (GCIY and
MKN45) and colon cancer cell lines (HCT116 and WiDr) were incubated
with PSK, no marked changes in DPD mRNA expression were observed.
In contrast, when the collagen gel-embedded cancer cells were
incubated with PSK in the presence of PBMCs, DPD mRNA expression
was reduced in both the gastric and colon cancer cell lines
(Fig. 3). Although no consistent
changes in RNA expression of the other fluoropyrimidine metabolic
enzymes TS, TP and OPRT, were observed by the addition of PSK and
PBMCs, TS expression in HCT-116 cells and TP expression in GCIY
cells were strongly suppressed (data not shown).
Since the PSK-induced suppression of DPD mRNA
expression was consistently lower in the presence of PBMCs than in
the absence of PBMCs in all the cell lines examined, we further
examined the effect of PSK on DPD expression.
IFN-α and TRAIL reduce DPD mRNA
expression in GCIY and HCT-116 cells
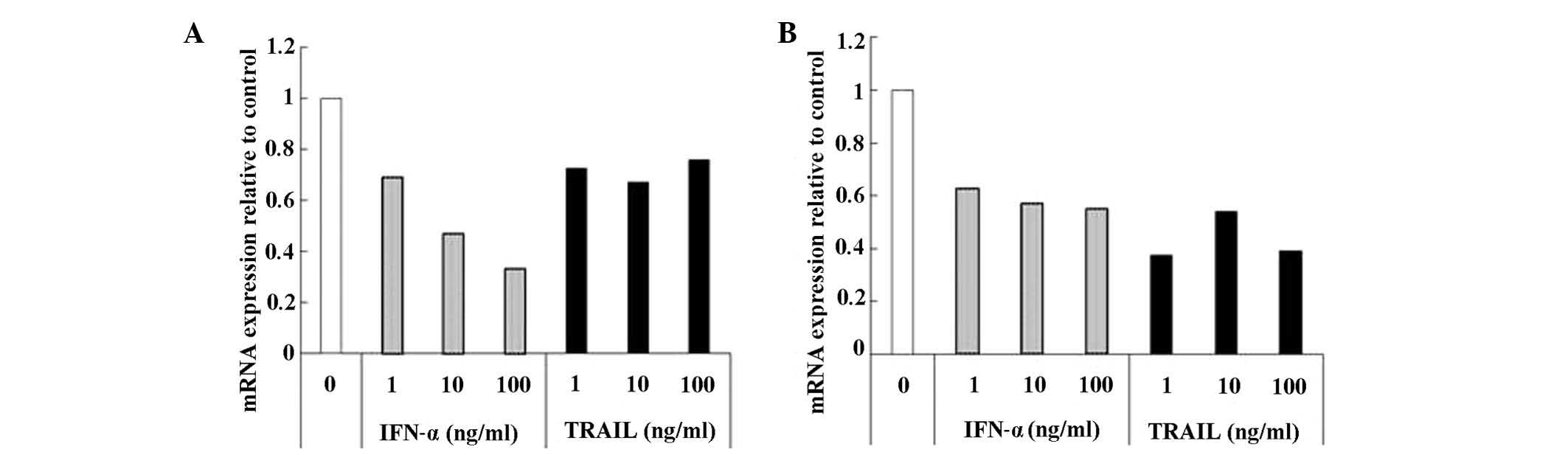
IFN-α and TRAIL have been reported to suppress DPD
expression in cells (10,11). Using CD-DST, we examined whether
addition of these cytokines suppresses DPD expression in GCIY and
HCT-116 cell cultures. In both cell lines, DPD expression was
reduced by incubation with IFN-α or TRAIL (Fig. 4A and B). Since TRAIL belongs to the
TNF-α family, we also examined the effect of TNF-α on DPD
expression but observed no changes in DPD mRNA expression (data not
shown).
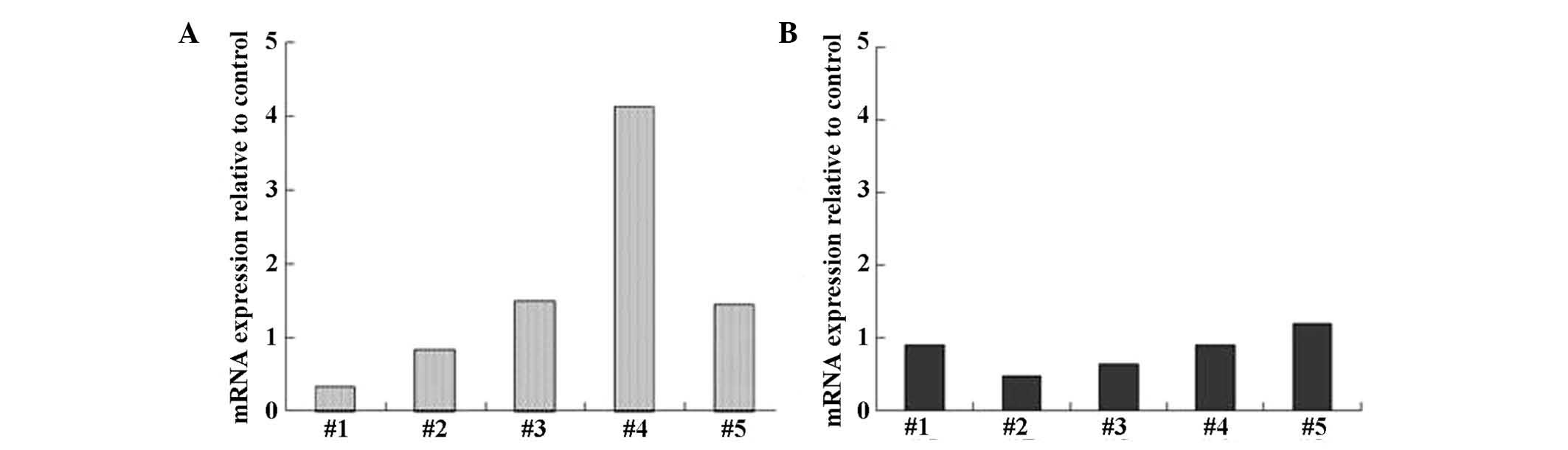
PSK possibly induces IFN-α mRNA
expression in PBMCs
Next, we examined whether the expression of IFN-α
and TRAIL is induced in PBMCs obtained from healthy volunteers when
cultured in the presence or absence of PSK. IFN-α mRNA expression
in PBMCs was increased by 4-fold in 1 of 5 subjects, and by 1.4- to
1.5-fold in 2 of 5 subjects (Fig.
5A). In contrast, TRAIL expression in PBMCs was reduced by 0.5-
to 0.6-fold in 2 of 5 subjects, with no marked changes (Fig. 5B).
Discussion
In the present study, we developed an experimental
culture model by adding human PBMCs in suspension to collagen gel
droplet-embedded cancer cells (immuno-CD-DST), and used this model
to examine whether PSK affects the anti-proliferative activity of
the fluoropyrimidine derivatives, 5-FU and 5′-DFUR. Our results
indicated that PSK augmented the effects of both agents.
Furthermore, when various cancer cells (human gastric cancer cell
lines GCIY and MKN45, and human colon cancer cell lines HCT116 and
WiDr) were co-cultured with PBMCs, the addition of PSK reduced DPD
mRNA expression. Using hepatocellular carcinoma cells, Oie et
al(10) demonstrated that IFN-α
inhibited DPD mRNA expression. Moreover, Mizutani et
al(11) reported that TRAIL
inhibited DPD mRNA expression in renal cell carcinoma. In contrast,
Miyazaki et al(12) reported
no inhibition of DPD mRNA expression when human colon cancer cell
lines were incubated with IFN-α and TRAIL. Using the CD-DST system,
we cultured GCIY and HCT116 cells with IFN-α or TRAIL and observed
decreased DPD mRNA expression. The mechanism by which IFN-α reduces
DPD mRNA expression is unknown, but it is important due to its
association with increased activity of fluoropyrimidine anticancer
agents.
PSK has immunostimulatory actions and has been
reported to induce the production of various chemokines and
cytokines including TNF-α, IL-2 and IL-8 (8,13,14).
Kitani et al(15) reported
that PSK enhanced polyinosinic:polycytidylic acid (poly
I:C)-induced IFN production. When we incubated PBMCs obtained from
healthy subjects with PSK, increased IFN-α mRNA expression was
observed in 3 of the 5 subjects examined. This result suggests that
PSK-induced IFN-α expression in PBMCs may contribute to decreased
DPD expression in tumor cells. In order to verify this hypothesis,
more studies such as concerning the IFN-α protein expression in
PBMCs are necessary, and these have to be conducted using a large
number of subject to allow statistical analysis. The present study
focused on DPD and further studies are ongoing to elucidate the
role of IFN-α.
Since human cancer cells were examined in the
present study, we used human PBMCs in the test system. Due to the
limited number of PBMCs available, we were not able to test
multiple samples in each experiment. We, thus, repeated the same
experiment at least once to examine whether the results
consistently showed the same tendency. As individual differences
inevitably exist among PBMCs, as expected, there was variability in
the data obtained. However, the same tendency of PSK-induced
suppression of DPD mRNA expression in the presence of PBMCs was
observed with all 4 cancer cell lines, suggesting a high
possibility that PSK inhibits DPD mRNA expression. For TS and TP,
reduced expression was observed in some cell lines but not in all.
Further studies are required.
Recently, PSK has been reported to be an agonist of
TLR2 (8) and TLR4 (16). In general, IFN-α production is
considered to be triggered by TRL3, 7 and 9 (17). Recent studies have reported that
IFN-α is induced also by TLR2 (18,19),
suggesting a possibility that PSK induces IFN-α production via
TLR2. On the other hand, PSK had no effect on TRAIL expression.
There are no reports of the effects of cytokines other than IFN-α
and TRAIL on DPD expression. The question of how various cytokines
affect fluoropyrimidine metabolic enzymes is of great interest.
The clinical effect of combined 5-FU and IFN-α
therapy remains controversial (20,21).
In the study of Nagano et al(20), patients with resectable
hepatocellular carcinoma (HCC) and portal vein invasion (Vp3)
treated with IFN-α/5-FU as postoperative adjuvant therapy had more
favorable 1- and 3-year disease-free and overall survival compared
to patients not treated with IFN-α/5-FU. On the other hand, Nagano
et al(20) pointed out
controversial results that IFN-α alone and IFN-α/doxorubicin
combination had no effect on the treatment of HCC, and IFN-α/5-FU
combined chemotherapy yielded a marked effect compared to 5-FU
monotherapy. Their report suggests that it is possible that IFN-α
exerts some influence on the antitumor effect of fluoropyrimidines.
One possible mechanism may be the suppression of DPD expression
resulting in enhancement of the antitumor effect of
fluoropyrimidines.
In randomized control trials on gastric cancer
(3) and colorectal cancer (4), PSK when used with oral 5-FU or
tegafur/uracil improved the 5-year disease-free survival compared
to the monotherapy of each agent. Apart from the immunomodulatory
effect, PSK has been reported to ameliorate an immunosuppressive
state and to act directly on tumors inducing apoptosis and
suppressing proliferation (6,7),
suggesting that these complementary functions are associated with
the beneficial effect observed in combination therapy. The present
study results suggest that the cytokines induced by PSK act on the
metabolic enzymes of fluoropyrimidines resulting in enhancement of
the antitumor effect, proposing a novel mechanism of action of PSK
that has not been reported hitherto. Combination of PSK and
fluoropyrimidine derivatives is very useful as a therapeutic
regimen that augments the effects of chemotherapy.
In conclusion, using the novel immuno-CD-DST to
co-culture collagen gel-embedded tumor cells with suspended human
PBMCs, we examined how PSK affects the antitumor effect of 5-FU and
5′-DFUR against gastrointestinal tumor cells. PSK enhanced the
antitumor proliferative effects of 5-FU and 5′-DFUR. In studies
concerning the effect of PSK on fluoropyrimidine metabolic enzymes,
PSK was found to lower DPD mRNA expression. Further experiments
suggest that IFN-α may inhibit DPD gene expression in tumor cells,
and PSK may induce IFN-α production by PBMCs. The results obtained
from the immuno-CD-DST propose the possibility that PSK acts on
immunocompetent cells and induces cytokines that inhibit DPD gene
expression to augment the antitumor effect of fluoropyrimidine
derivatives. Immuno-CD-DST is potentially useful as an evaluation
tool for drugs with immune-mediated effects. Further validation of
this system is warranted.
Acknowledgements
The present study was conducted at the Shiga
University of Medical Science, Shiga, Japan and Kureha Corp.,
Tokyo, Japan.
Abbreviations:
|
PSK
|
protein-bound polysaccharide K
|
|
CD-DST
|
collagen gel droplet-embedded culture
drug sensitivity test
|
|
PBMCs
|
peripheral blood mononuclear cells
|
|
5-FU
|
5-fluorouracil
|
|
5′-DFUR
|
5′-deoxy-5-fluorouridine
|
|
DPD
|
dihydropyrimidine dehydrogenase
|
|
TS
|
thymidylate synthase
|
|
TP
|
thymidine phosphorylase
|
|
OPRT
|
orotate phosphoribosyl transferase
|
References
|
1
|
Prenen H, Tejpar S and Cutsem EV: New
strategy for treatment of KRAS mutant metastatic colorectal cancer.
Clin Cancer Res. 16:2921–2926. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chang HR: Trastuzumab-based neoadjuvant
therapy in patients with HER2-positive breast cancer. Cancer.
116:2856–2867. 2010. View Article : Google Scholar
|
|
3
|
Nakazato H, Koike A, Saji S, Ogawa N and
Sakamoto J: Efficacy of immunochemotherapy as adjuvant treatment
after curative resection of gastric cancer. Lancet. 343:1122–1126.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ohwada S, Ikeya T, Yokomori T, Kusaba T,
Roppongi T, Takahashi T, Nakamura S, Kakinuma S, Iwazaki S,
Ishikawa H, Kawate S, Nakajima T and Morishita Y: Adjuvant
immunochemotherapy with oral tegafur/uracil plus PSK in patients
with stage II or III colorectal cancer: a randomized controlled
study. Br J Cancer. 90:1003–1010. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Konno K, Motomiya M, Oizumi K, Sato M,
Yamamoto F, Tamiya K, Hasuike T, Yokosawa A, Uchiyama T, Ogawa N
and Nakai Y: Effects of protein-bound polysaccharide preparation
(PSK) in small cell carcinoma of the lung. Lung Cancer. 28:19–28.
1988.
|
|
6
|
Tsukagoshi S, Hashimoto Y, Fujii G,
Kobayashi H, Nomoto K and Orita K: Krestin (PSK). Cancer Treat Rev.
11:131–155. 1984. View Article : Google Scholar
|
|
7
|
Maehara Y, Tsujitani S, Saeki H, Oki E,
Yoshinaga K, Emi Y, Morita M, Kohnoe S, Kakeji Y, Yano T and Baba
H: Biological mechanism and clinical effect of protein-bound
polysaccharide K (KRESTIN®): review of development and
future perspectives. Surg Today. 42:8–28. 2012. View Article : Google Scholar
|
|
8
|
Lu H, Yang Y, Gad E, Wenner CA, Chang A,
Larson ER, Dang Y, Martzen M, Standish LJ and Disis ML:
Polysaccharide krestin is a novel TLR2 agonist that mediates
inhibition of tumor growth via stimulation of CD8 T cells and NK
cells. Clin Cancer Res. 17:67–76. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Okumura K, Mekata E, Shiomi H, Naitoh H,
Abe H, Endo Y, Kurumi Y and Tani T: Expression level of thymidylate
synthase mRNA reflects 5-fluorouracil sensitivity with low dose and
long duration in primary colorectal cancer. Cancer Chemother
Pharmacol. 61:587–594. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Oie S, Ono M, Fukushima H, Hosoi F, Yano
H, Maruyama Y, Kojiro M, Terada T, Hirano K, Kuwano M and Yamada Y:
Alteration of dihydropyrimidine dehydrogenase expression by IFN-α
affects the antiproliferative effects of 5-fluorouracil in human
hepatocellular carcinoma cells. Mol Cancer Ther. 6:2310–2318.
2007.PubMed/NCBI
|
|
11
|
Mizutani Y, Nakanishi H, Yoshida O,
Fukushima M, Bonavida B and Miki T: Potentiation of the sensitivity
of renal cell carcinoma cells to TRAIL-mediated apoptosis by
subtoxic concentrations of 5-fluorouracil. Eur J Cancer.
38:167–176. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Miyazaki K, Shibahara T, Sato D, Uchida K,
Suzuki H, Matsui H, Yanaka A, Nakahara A and Matsuzaki Y: Influence
of chemotherapeutic agents and cytokines on the expression of
5-fluorouracil-associated enzymes in human colon cancer cell lines.
J Gastroenterol. 41:140–150. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hirose K, Zachariae C, Oppenheim J and
Matsusima K: Induction of gene expression and production of
immunomodulating cytokines by PSK in human peripheral blood
mononuclear cells. Lymphokine Res. 94:475–483. 1990.PubMed/NCBI
|
|
14
|
Asai H, Iijima H, Matsunaga K, Oguchi Y,
Katsuno H and Maeda K: Protein-bound polysaccharide K augments IL-2
production from murine mesenteric lymph node CD4+ T
cells by modulating T cell receptor signaling. Cancer Immunol
Immunother. 57:1647–1655. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kitani H, Trsuru S, Oka M and Zinnaka Y:
Effect of immunomodulator PSK on the production of interferon in
tumor-bearing mice. J Natl Def Med Coll. 6:30–35. 1981.
|
|
16
|
Price LA, Wenner CA, Sloper DT, Slaton JW
and Novack JP: Role of toll-like receptor 4 in TNF-alpha secretion
by murine macrophages in response to polysaccharide Krestin, a
Trametes versicolor mushroom extract. Fitoterapia.
81:914–919. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Trinchieri G: Type I interferon: friend or
foe? J Exp Med. 207:2053–2063. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lijeroos M, Vuolteenaho R, Rounioja S,
Henriques-Normark B, Hallman M and Ojaniemi M: Bacterial ligand of
TLR2 signals Stat activation via induction of IRF1/2 and
interferon-α production. Cell Signal. 20:1873–1881. 2008.PubMed/NCBI
|
|
19
|
Barbalat R, Lau L, Locksley RM and Barton
GM: Toll-like receptor 2 on inflammatory monocytes induces type I
interferon in response to viral but not bacterial ligands. Nat
Immunol. 10:1200–1207. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nagano H, Sakon M, Eguchi H, Kondo M,
Yamamoto T, Ota H, Nakamura M, Wada H, Damdinsuren B, Marubashi S,
Miyamoto A, Takeda Y, Dono K, Umeshita K, Nakamori S and Monden M:
Hepatic resection followed by IFN-α and 5-FU for advanced
hepatocellular carcinoma with tumor thrombus in the major portal
branch. Hepatogastroenterology. 54:172–179. 2007.
|
|
21
|
Kornmann M, Staib L, Wiegel T, Kreuser ED,
Kron M, Baumann W, Henne-Bruns D and Link KH: Adjuvant
chemoradiotherapy of advanced resectable rectal cancer: results of
a randomized trial comparing modulation of 5-fluorouracil with
folic acid or with interferon-α. Br J Cancer. 103:1163–1172.
2010.
|