Introduction
In recent years, targeted therapies in the treatment
of lung cancer have been implemented. Aside from thyrosine kinase
inhibitors targeting EGFR and EML4-ALK, such as gefitinib,
erlotinib and crizotinib as well as monoclonal antibodies against
VEGFR (bevacizumab), specific therapies using cellular immunity
have also been established or are in clinical examination (1–3). It is
known that T cell mediated immune responses can play a crucial role
in tumor defense; tumor cells can be efficiently killed by specific
T cells of the immune system (4). T
cell activating antibodies against CTLA-4 (ipilimumab) and against
the inhibitory T cell receptor programmed death-1 (PD-1) showed
promising results against lung cancer in clinical testing (5,6).
Active immunotherapies induce a specific immune response by
recognizing epitopes being expressed in the tumor cell surface. Due
to their association with tumor cells, these epitopes are called
tumor-associated antigens (TAAs). TAAs are exclusively expressed
and/or overexpressed in tumor cells, and are therefore useful to
induce a tumor-specific immune response without harming healthy
organs lacking TAA-expression (7–9). In
the past years, several TAAs in hematological and oncological
neoplasms have been identified. These TAAs are proteins or
receptors playing an important role in cell cycle, proliferation
and differentiation (10,11).
The primary objective of active cancer immunotherapy
is the induction of targeted cell death by activated specific
CD8+ cytotoxic T cells (CTLs) (12,13).
These activated CTLs recognize tumor cells that display the
corresponding peptide derived from TAAs by MHC class I molecule on
their surface which leads to a differentiation and expansion of
CTLs and finally results in induction of lyses of the peptide
presenting cells by the release of cytotoxins including perforin,
granzymes and granulysin (14–16).
In these processes, interferon γ (IFNγ) plays a crucial role in
promoting various immunoregulatory effects.
Previously, peptide vaccines against different TAAs
such as MAGE-A3 and hTERT have been tested in lung cancer patients
and phase III trials are ongoing (17–19).
Tumor vaccination seems to be a promising strategy especially in
situations of reduced lung tumor load, i.e. in maintenance therapy
after induction chemotherapy or for patients with a low tumor load
in further treatment lines (2,20).
Aside from single peptide vaccination, peptide mixtures may be a
potent strategy inducing cancer-defending immunity. It was
demonstrated that immune responses to multipeptide vaccine IMA901
were associated with prolonged overall survival in patients with
advanced renal cell cancer (21).
Therefore, it is important to identify TAA-derived peptides
inducing CTL response in lung cancer that are potential candidates
for developing potent single or polyvalent vaccines.
Materials and methods
Blood samples and preparation
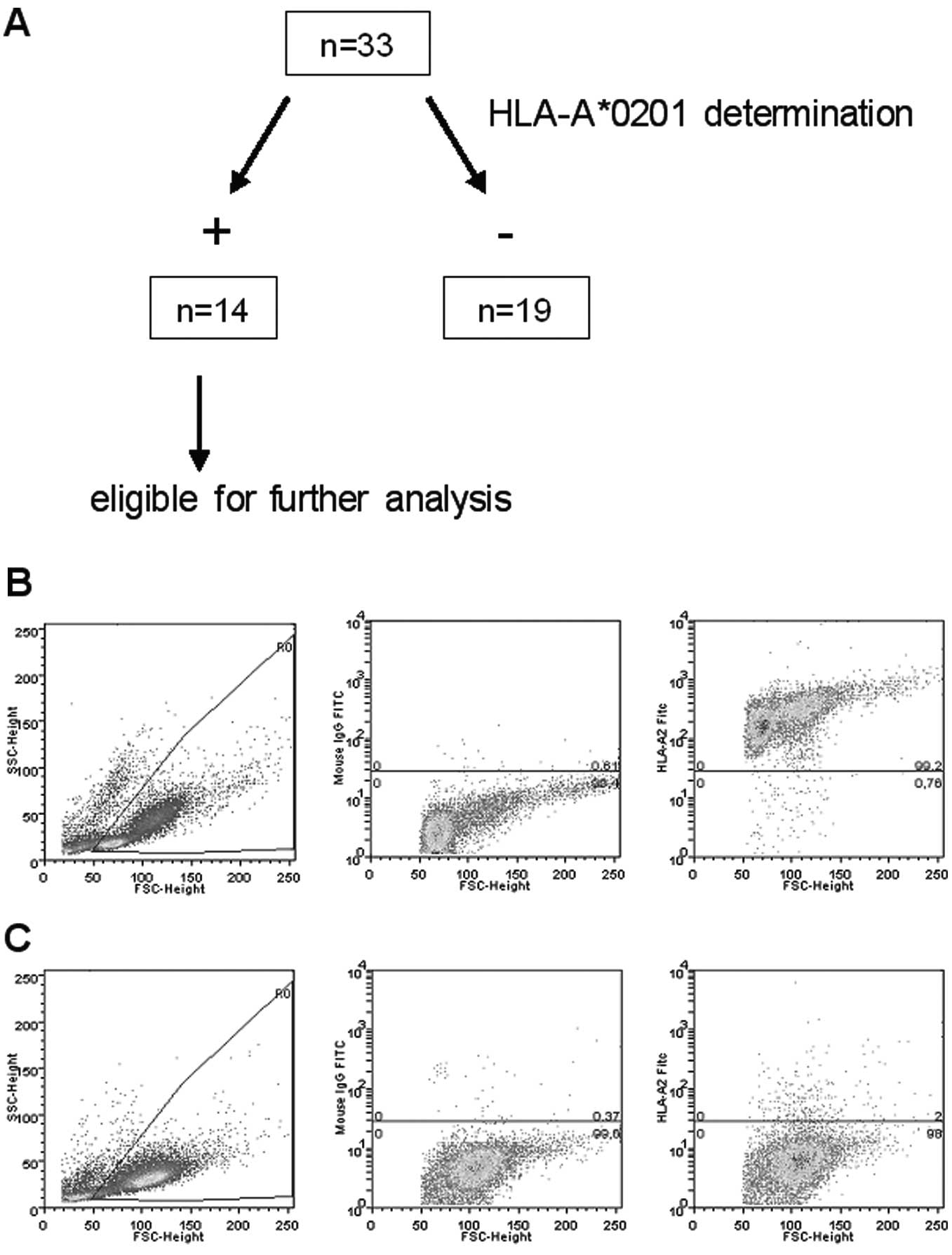
A total of 33 patients were screened for
HLA-A*0201 expression (see below) and subsequently
analyses were performed in 14 HLA-A*0201-positive
patients with diagnosed lung cancer, either non-small cell lung
cancer (NSCLC; n=12; 8 adenocarcinoma and 3 squamous cell
carcinoma, 1 sarcomatoid carcinoma) or small cell lung cancer
(SCLC, n=2). Clinical characteristics of analyzed patients
including age, tumor stage at time of diagnosis, histology, first
line therapy and response to first-line therapy are shown in
Table I. Additionally, mutation
status concerning EGFR and EML4-ALK (if available) is shown. All
patients underwent treatment at our Department of Internal
Medicine. For blood analyses, an ethics proposal was filed and
accepted by the Ethics Committee of the University of Ulm
(application no. 09/10).
 | Table IClinical characteristics of analyzed
patients. |
Table I
Clinical characteristics of analyzed
patients.
| Patient | Age (years) |
Diagnosis/stagea | Histology | EGFR/EML4-ALK | Therapyb | Responsec |
|---|
| 1 | 57 | NSCLC/IV | Squamous-cell | WT/neg. |
Cisplatin/gemcitabine + radiaton | PD |
| 2 | 60 | NSCLC/IV | Adeno | WT/neg. |
Cisplatin/pemetrexed/bevacizumab
maintenance bevacizumab | SD |
| 3 | 67 | NSCLC/IIIA | Adeno | WT/neg. | Neoadjuvant
radiochemotherapy | PD |
| 4 | 44 | NSCLC/IV | Adeno | WT/n.a. | No therapy | PD |
| 5 | 49 | NSCLC/IV | Adeno | WT/n.a. |
Carboplatin/pemetrexed | PD |
| 6 | 64 | NSCLC/IB | Adeno | n.a. | Tumor
resection | CR |
| 7 | 62 | SCLC/ED | | n.a. |
Cisplatin/etoposide | PR |
| 8 | 78 | NSCLC/IV | Sarcomatoid | WT/n.a. | Not known | PD |
| 9 | 54 | NSCLC/IA | Squamous-cell | n.a. | Tumor
resection | CR |
| 10 | 52 | NSCLC/IV | Squamous-cell | n.a. | Tumor
resection | PD |
| 11 | 79 | NSCLC/IV | Adeno | WT/n.a. |
Carboplatin/docetaxel | PD |
| 12 | 61 | NSCLC/IV | Adeno | WT/n.a. |
Cisplatin/vinorelbine | PD |
| 13 | 80 | SCLC/ED | | n.a. | Topotecan | PR |
| 14 | 65 | NSCLC/IV | Adeno | Mut./neg. |
Carboplatin/docetaxel/erlotinib | SD |
Pre-treatment peripheral blood [anti-coagulated with
ethylenediaminetetraacetic acid (EDTA)] was obtained at date of
diagnosis and in the course of following visitations during tumor
therapy. For analyses described in these experiments we used blood
samples from the date of diagnosis.
Ficoll density gradient centrifugation peripheral
blood mononuclear cells (PBMCs) were resuspended in fetal calf
serum (FCS; 20%) and 10% DMSO (1×107 cells/sample) and
cryopreserved as previously described (22).
HLA-typing by flow cytometry
All peptides used in our experiments were
HLA-A*0201 restricted. To identify HLA-A*0201
positive cells, flow cytometry was used (Fig. 1). Cells were incubated with an FITC
labeled monoclonal anti-HLA-A*0201 antibody (BD,
Heidelberg, Germany). As positive and negative control, the T2 and
the K562 cell lines were used, respectively. After incubation at
4°C for 20 min in the dark and after washing twice, stained cells
were analyzed by flow cytometry as previously described (23). From 33 screened patients, 14 were
eligible for further experiments.
Peptides used in experiments
The peptides used in our experiments were derived
from the well known TAAs RHAMM, PRAME, WT1, from hTERT, survivin,
MAGE-A3, G250, HER2, Aurora kinase (Aura) A and B. Except for the
peptides derived from Aurora kinases (Aura A1 and Aura B1) all
peptides are well known (11,24–26).
The entire amino acid sequences of Aura A1 and Aura B1 were
screened for HLA-A*0201 binding T cell epitopes using
the algorithms of the SYFPEITHI (www.syfpeithi.de),
the Rankpep (http://imed.med.ucm.es/tools) and the HLA-Bind
(www.bimas.cit.nih.gov) software programs. For the
novel antigens, >10 HLA-A*0201-binding peptides were
predicted and these Aurora kinase-derived peptides were tested in
20 healthy volunteers and 14 lung cancer patients at primary
diagnosis. The peptides were purchased from GL Biochem Ltd.,
Shanghai, China and Thermo Fisher Scientific, Ulm, Germany.
The sequences of the peptides used in our
experiments are shown in Table II.
Peptides from cytomegalovirus and influenza virus were used as
positive controls due to their endemic occurrence (Table II).
 | Table IIPeptides used for of specific T cell
response against different TAAs. |
Table II
Peptides used for of specific T cell
response against different TAAs.
| Peptide | Sequence | Position |
|---|
| PRAME-P3 | ALYVDSLFFL | 300–309 |
| RHAMM-R3 | ILSLELMKL | 165–173 |
| G250 | QLLLSLLLL | 24–32 |
| WT1 | RMFPNAPYL | 126–134 |
| Aura A1 | TLCGTLDYL | 288–296 |
| Aura B1 | KIADFGWSV | 215–223 |
| Survivin | ELTLGEFLKL | 95–104 |
| hTERT | ILAKFLHWL | 540–548 |
| HER2-2 | RLLQETELV | 689–697 |
| MAGE-A3_01 | FLWGPRALV | 271–279 |
| MAGE-A3_02 | KVAELVHFL | 112–120 |
| CMV |
NLVPMVATV |
65–73 |
| IMP |
GILGFVFTL |
58–66 |
Mixed lymphocyte peptide culture
(MLPC)
PBMCs from patients and healthy volunteers were
selected by magnetic beads through a magnetic-activated
cell-sorting (MACS) column (Miltenyi Biotec, Bergisch Gladbach,
Germany). CD8− antigen presenting cells (APCs) were
irradiated with 30 Gy and pulsed for 2 h with either a TAA-derived
peptide or a control peptide at a concentration of 20 μg/ml. For
all peptides, as well as for the control peptides, the same
conditions and reagents were used as previously described (27).
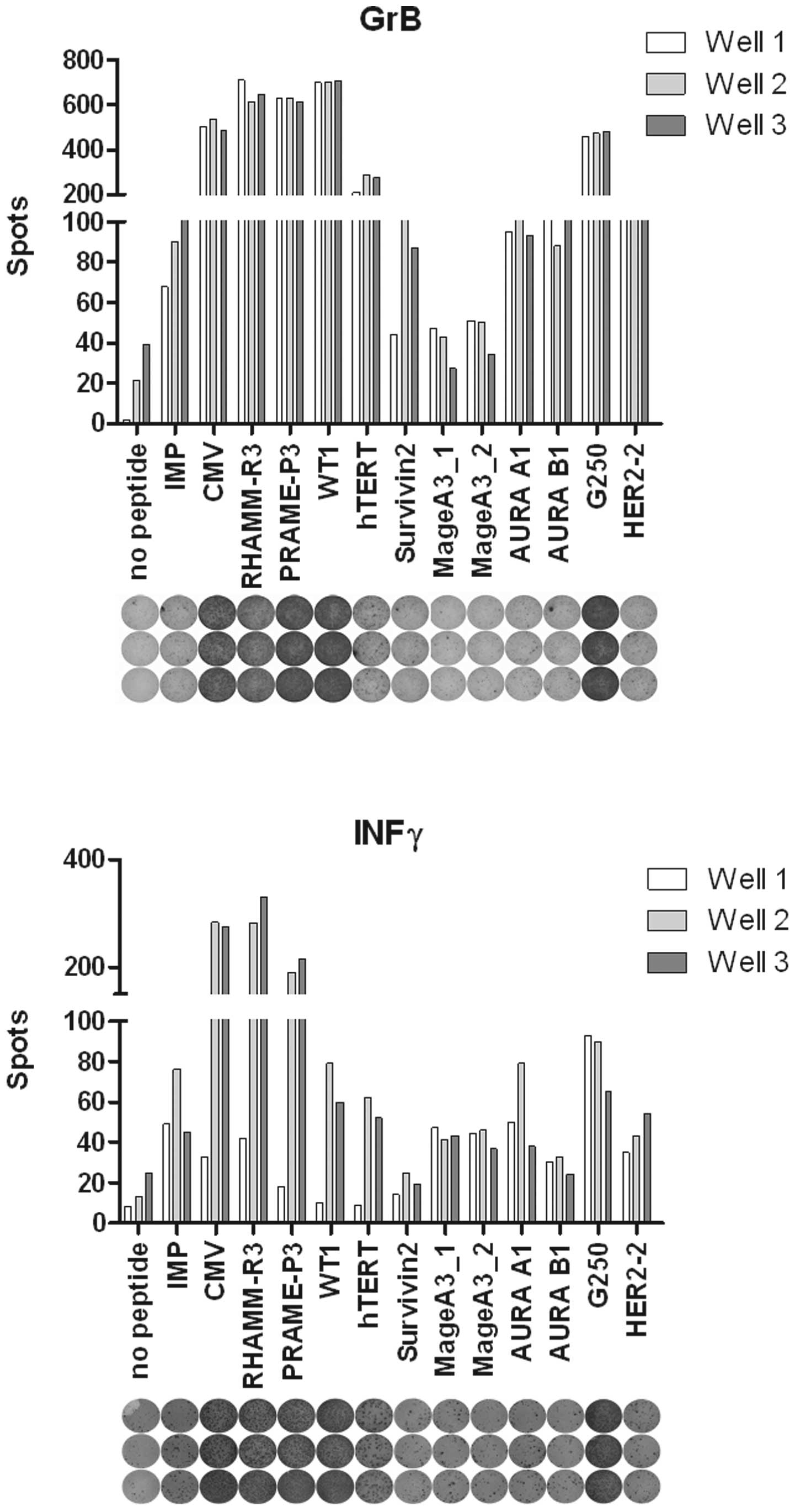
ELISpot analysis
ELISpots were performed ex vivo on cultured
patient cells and the T2 cell line as APCs. Briefly, the irradiated
CD8− fraction of autologous patient PBMCs pulsed with
peptide was used as APCs for the CD8+ fraction in MLPC.
After 8 days of MLPC culture, the T2-cell line pulsed with peptide
was used as APCs in the ELISpot. IFNγ and GrB ELISpot assays were
performed as previously described (22,27).
Fig. 2 shows an exemplary ELISpot
detecting specific CTL response of an analyzed lung cancer
patient.
Results
Specific T cell response to different
TAAs
Due to the HLA restriction of the peptide used in
our analyses, 14 (42%) of 33 screened patients were eligible for
further experiments after determining HLA-A*0201
positivity by FACS analysis (Fig.
1).
Due to the restriction of patient material, not all
peptides could be analyzed for each patient (HER2, n=10; G250,
n=12; hTERT; survivin; G250; MAGE-A3 and AURA A1/B1, n=13 each;
Table II). Using interferon γ
(IFNγ) and granzyme B (GrB) ELISpot assays, specific cytotoxic T
cell responses against at least one of these TAAs could be detected
in 13/14 patients (93%). In one patient diagnosed as
adenocarcinoma, no specific immune responses against any TAAs could
be detected (data not shown). Furthermore, 11/14 (79%) patients
exhibited T cell responses against at least 2 TAAs (data not
shown). The most frequent specific CTL responses were detected
against G250 (IFNγ 58/GrB, 58%) and PRAME (57/43%) followed by
hTERT (31/23%) and RHAMM (29/29%). Lower frequency was measured
against the 2 MAGE-A3 peptides (23/8% and 31/31%, respectively),
survivin (23/23%) and WT1 (15/7%; Table III). Specific T cell responses
were also detected against novel peptides from the Aurora kinases A
and B. These peptides, Aura A1 and Aura B1, showed specific T cell
responses in 36/29% and 29/43% of patients, respectively.
 | Table IIISpecific T cell responses measured by
IFNγ and GrB ELISpot. |
Table III
Specific T cell responses measured by
IFNγ and GrB ELISpot.
| Peptide | Sequence | IFNγ (%) | GrB (%) |
|---|
| PRAME-P3 | ALYVDSLFFL | 8/14 (57) | 6/14 (43) |
| RHAMM-R3 | ILSLELMKL | 4/14 (29) | 4/14 (29) |
| G250 | QLLLSLLLL | 7/12 (58) | 7/12 (58) |
| WT1 | RMFPNAPYL | 2/14 (15) | 1/14 (7) |
| Aura A1 | TLCGTLDYL | 4/13 (31) | 3/13 (23) |
| Aura B1 | KIADFGWSV | 4/13 (31) | 5/13 (38) |
| Survivin | ELTLGEFLKL | 3/13 (23) | 3/13 (23) |
| hTERT | ILAKFLHWL | 4/13 (31) | 3/13 (23) |
| HER2-2 | RLLQETELV | 5/10 (50) | 0/10 (0) |
| MAGE-A3_01 | FLWGPRALV | 3/13 (23) | 1/13 (8) |
| MAGE-A3_02 | KVAELVHFL | 4/13 (31) | 4/13 (31) |
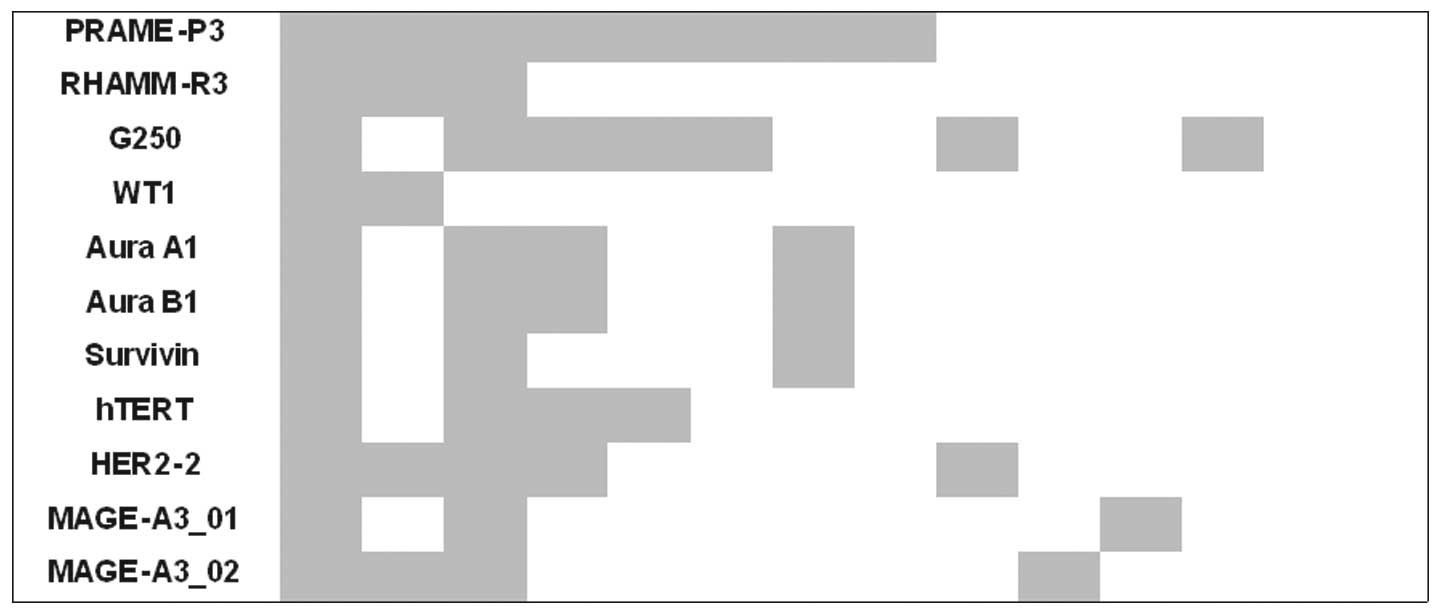
Regarding identification of simultaneous CTL
response, IFNγ ELISpot showed that all patients with specific T
cell response for epitopes of RHAMM and hTERT also had a CTL
response against PRAME. Second, in all patient samples with CTL
response against Aura A1, a reaction against the peptide Aura B1
was also detected (Fig. 3).
Discussion
In the treatment of lung cancer, significant
developments have been made towards new therapeutic strategies. One
promising option is the use of tumor vaccination. In our study, we
demonstrated that peptides derived from different TAAs induce
specific CTLs in a high percentage of patients with advanced lung
cancer. It is necessary to identify novel peptides that can be used
for targeted immunotherapy.
In most of the patients analyzed, specific CTL
responses were detected. The methods that we used and the read out
of parameters are well established. For both IFNγ and GrB ELISpot
results were mostly similar. Different results were detected only
for HER2-2 with a trend to significance (p=0.06). GrB ELISpot
directly measures release of a cytolytic protein of these activated
specific CTLs, while IFNγ ELISpot detects the key cytokine in the
complex process of T cell response. One reason for the different
results in both ELISpots regarding the HER2 peptide may be that
activation with this peptide does lead to a specific T cell
response but not to a lytic activity of CTL.
The most frequent specific CTL response was detected
for peptides derived from the known TAAs G250/CAIX and PRAME. The
carbonic anhydrase IX, G250/CAIX, is a well known TAA, particularly
in solid tumors such as renal cell carcinoma. Specific T cell
response of CD8+ T cells after activating PBMCs with the
G250/CAIX derived peptide could be seen in patients with squamous
cell cancer of head and neck. In 3/4 patients, specific CTL
response was detected by GrB ELISpot, 1/7 by IFNγ ELISpot
respectively, (23). In lung
cancer, there are different expression rates ranging from 75 to 95%
(28,29). For renal cancer, clinical trials
have been carried out using a monoclonal antibody against G250
(30,31); however, peptide vaccinations are not
yet in clinical testing. Our data indicate that this peptide
derived from G250/CAIX could be a useful target for tumor
vaccination in lung cancer.
Second, the preferentially expressed antigen in
melanoma (PRAME) derived peptide P3 induced a high CTL activity in
the examined lung cancer patients which was confirmed in ELISpot
assays. PRAME plays a crucial role in the inhibition of cell
differentiation and apoptosis by repressing retinoic receptor
signaling and showed a correlation with poor prognosis in high
stage neuroblastomas (32,33). In previous experiments by our group,
the peptide PRAME-P3 induced a specific CTL response in 60% of AML
patients (n=10) (11). PRAME is
expressed in several tumor entities, including lung cancer in up to
50% (34), making it a notable
target for immunotherapeutic therapies. There is an ongoing phase I
trial testing a new anticancer vaccine for patients with
PRAME-positive NSCLC after tumor surgery (30).
Receptor for hyaluronic acid-mediated motility
(RHAMM, CD168) has been defined as a leukemia-associated antigen
and various RHAMM-derived HLA-A2-restricted peptides were tested
for their immunogenicity by our group. In previous experiments, we
generated RHAMM-R3-specific cytotoxic T cells of the peripheral
blood from 7 of 13 AML patients. These cells were able to lyse
RHAMM-R3 pulsed T2 cells and patient dendritic cells and were even
able to lyse untreated AML blasts. It was also shown that RHAMM-R3
is a naturally processed epitope presented on HLA A2. Therefore, a
phase I vaccination trial was initiated in which patients developed
specific CTL response after 4 vaccinations (22). Similar results were obtained in
patients with chronic lymphocytic leukemia and tumors of the
head/neck region (35).
Since there are no clear data regarding
overexpression of RHAMM in lung cancer, further analyses must be
conducted.
Aside from these frequent responses against
generally well known TAAs, we also analyzed specific T cell
response against TAAs that are well known especially in lung
cancer, such as hTERT and MAGE-A3. Human telomerase reverse
transcriptase (hTERT) is the catalytic subunit of the human
telomerase. Data have shown that it is overexpressed in lung cancer
in up to 85% of lung cancer patients (36). Studies have also demonstrated that
hTERT overexpression in lung cancer correlates with poor prognosis
(37,38). Trials evaluating the immunological
and clinical response of a peptide vaccination with the optimized
peptide telomerase reverse transcriptase p572Y (TERT572Y) showed
that CTL response was detected in 76.2% of 21 patients after first
vaccination and in 90.9% of 11 patients after the sixth
vaccination. An early immunological response correlated with
prolonged time to progression and overall survival (17). In our in vitro experiments, a
specific CTL response was detected in 31/23%. In contrast to our
results, Bolonaki et al(17)
could not measure any specific T cell response by IFNγ ELISpot or
pentamer staining before the first vaccination of lung cancer
patients. Analysis of more patients will provide further
information on the immunogenicity of hTERT peptides. Furthermore, a
comparison between CTL response and hTERT expression in tumor
tissue is warranted.
Melanoma-associated antigen 3 (MAGE-A3) is expressed
in up to 55% of the NSCLC. The rate of expression is correlated
with the stage of the disease and a poor prognosis (39). We examined specific T cell response
for the peptides MAGE-A3_01 and MAGE-A3_02 and detected IFNγ
production against MAGE-A3_01 in 23% and against MAGE-A3_02 in 31%
of the analyzed patients. It could be demonstrated that specific T
cells against MAGE-A3_01 generated from healthy volunteers
recognize lung carcinoma cells (40). In previous investigations, 182 NSCLC
patients with MAGE-A3 positive tumors demonstrated a positive trend
for activity of MAGE-A3 protein vaccine with a relative improvement
of DFI and DFS (41). Based on
these data, an ongoing randomized phase III trial, MAGRIT,
investigates the efficacy of MAGE-A3 tumor vaccination agents in
preventing cancer relapse, when administered after tumor resection
in patients with MAGE-A3-positive stages IB, II, and IIIA NSCLC
(19).
Additionally, we analyzed T cell responses against
novel peptides. In our experiments, we used the Aurora kinase
derived peptides Aura A1 and B1 which also induced specific CTL
response at a lower level (30%).
To date, 3 Aurora kinases are identified in mammals.
Aurora kinases are serine/threonine kinases which play an essential
role in cell mitosis and cell division. Aurora kinase A (Aura A) is
localized in the centromers of interphase cells and is essential
for formation of mitotic spindle. An ectopic expression may lead to
malignant transformation of tissue. Its overexpression has been
detected in several tumor tissues, including lung cancer. It could
be demonstrated that its overexpression correlates with different
histological subtypes of lung cancer, but does not indicate poorer
prognosis (42). Furthermore, there
are hints that an overexpression of Aura A is associated with drug
resistance of tumor cells (43).
Aurora kinase B (Aura B) is known as the chromosomal passenger
protein and is localized in centromers of the prophase to the
metaphase-anaphase transition. It is then localized to the midzone
spindle during telophase and thereafter to midbody during
cytokinesis. Aura B is overexpressed in lung cancer and an
excessive overexpression correlates with poorer prognosis (44). Their frequent expression in lung
cancer but also their ability to induce a specific immune response
indicates that Aurora kinases could be novel targets in vaccination
strategies.
Comparing immune responses against the different
peptides could show a simultaneous CTL response in several cases.
Regarding IFNγ production, in all patient samples which showed an
IFNγ production against RHAMM and hTERT, response against PRAME
could also be measured. One explanation for this could be a
frequently occurring co-expression of different antigens in lung
cancer, which has not yet been described. Recent published data
verified a common co-expression of PRAME and MAGE-A3 in tumor
tissue of lung cancer patients, which may have a poor prognostic
value in some tumor stages (45).
Further analysis of more lung cancer patient samples will provide
insight into further epitopes leading to the discovery of usable
peptides for polyvalent immunization with stronger efficacy
comparing single peptide vaccinations.
Additionally, we observed a simultaneous occurrence
of CTL response for the two Aurora kinase peptides, Aura A1 and
Aura B1. Immunohistochemical analyses of a working group displayed
a co-expression of both Aurora kinases in 33 patients with advanced
lung cancer (46). A frequent
co-expression in lung cancer could make them feasible targets for
multipeptide vaccination as well. Furthermore, both Aurora kinases
share a significant homology in their catalytic domain (47). An immunogenic peptide, which is
found in both kinases, could enlarge the attack surface of a
vaccination strategy.
In conclusion, specific T cell responses against
several TAAs could be detected for the antigens hTERT, PRAME, G250
and RHAMM in high frequency in patients with lung cancer as well as
in lower frequency against epitopes from other antigens. Moreover,
novel immunogenic targets such as Aurora kinases A and B have been
identified as immunogenic targets in lung cancer. Therefore,
several antigen structures are appropriate candidates for
immunotargeted approaches in lung cancer patients. Further analysis
concerning the relationship between specific CTL response against
different tumor antigen peptides and histology, clinical status of
the patients and response to therapy, is mandatory. Furthermore,
comparison of TAA expression in tumor tissue and specific cytotoxic
T cell response will lead to a better understanding of the disease
in an immunological manner and may extract patients that are
appropriate candidates for tumor vaccination.
Acknowledgements
This study was supported by generous grants from the
German José Carreras Leukemia Foundation (DJCLS A 10/01), the
German Research Foundation (DFG GR2676/3-1) and the Else
Kröner-Fresenius-Stiftung (2011-A63).
References
|
1
|
Sandler A, Gray R, Perry MC, et al:
Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell
lung cancer. N Engl J Med. 355:2542–2550. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cappuzzo F, Ciuleanu T, Stelmakh L, et al:
Erlotinib as maintenance treatment in advanced non-small-cell lung
cancer: a multicentre, randomised, placebo-controlled phase 3
study. Lancet Oncol. 11:521–529. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kwak EL, Bang YJ, Camidge DR, et al:
Anaplastic lymphoma kinase inhibition in non-small-cell lung
cancer. N Engl J Med. 363:1693–1703. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Raez LE, Fein S and Podack ER: Lung cancer
immunotherapy. Clin Med Res. 3:221–228. 2005. View Article : Google Scholar
|
|
5
|
Lynch TJ, Bondarenko I, Luft A, et al:
Ipilimumab in combination with paclitaxel and carboplatin as
first-line treatment in stage IIIB/IV non-small-cell lung cancer:
results from a randomized, double-blind, multicenter phase II
study. J Clin Oncol. 30:2046–2054. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Topalian SL, Hodi FS, Brahmer JR, et al:
Safety, activity, and immune correlates of anti-PD-1 antibody in
cancer. N Engl J Med. 366:2443–2454. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Marigo I, Dolcetti L, Serafini P,
Zanovello P and Bronte V: Tumor-induced tolerance and immune
suppression by myeloid derived suppressor cells. Immunol Rev.
222:162–179. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pardoll D: Does the immune system see
tumors as foreign or self? Annu Rev Immunol. 21:807–839. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Novellino L, Castelli C and Parmiani G: A
listing of human tumor antigens recognized by T cells: March 2004
update. Cancer Immunol Immunother. 54:187–207. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Greiner J, Bullinger L, Guinn BA, Döhner H
and Schmitt M: Leukemia-associated antigens are critical for the
proliferation of acute myeloid leukemia cells. Clin Cancer Res.
14:7161–7166. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Greiner J, Schmitt M, Li L, et al:
Expression of tumor-associated antigens in acute myeloid leukemia:
implications for specific immunotherapeutic approaches. Blood.
108:4109–4117. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ribas A, Butterfield LH, Glaspy JA and
Economou JS: Current developments in cancer vaccines and cellular
immunotherapy. J Clin Oncol. 21:2415–2432. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rosenberg SA: Development of effective
immunotherapy for the treatment of patients with cancer. J Am Coll
Surg. 198:685–696. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Banchereau J and Steinman RM: Dendritic
cells and the control of immunity. Nature. 392:245–252. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kelly RJ, Gulley JL and Giaccone G:
Targeting the immune system in non-small-cell lung cancer: bridging
the gap between promising concept and therapeutic reality. Clin
Lung Cancer. 11:228–237. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gridelli C, Rossi A, Maione P, Ferrara ML,
Castaldo V and Sacco PC: Vaccines for the treatment of non-small
cell lung cancer: a renewed anticancer strategy. Oncologist.
14:909–920. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bolonaki I, Kotsakis A, Papadimitraki E,
et al: Vaccination of patients with advanced non-small-cell lung
cancer with an optimized cryptic human telomerase reverse
transcriptase peptide. J Clin Oncol. 25:2727–2734. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Peled N, Oton AB, Hirsch FR and Bunn P:
MAGE A3 antigen-specific cancer immunotherapeutic. Immunotherapy.
1:19–25. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tyagi P and Mirakhur B: MAGRIT: the
largest-ever phase III lung cancer trial aims to establish a novel
tumor-specific approach to therapy. Clin Lung Cancer. 10:371–374.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Eaton KD and Martins RG: Maintenance
chemotherapy in non-small cell lung cancer. J Natl Compr Canc Netw.
8:815–821. 2010.PubMed/NCBI
|
|
21
|
Walter S, Weinschenk T, Stenzl A, et al:
Multipeptide immune response to cancer vaccine IMA901 after
single-dose cyclophosphamide associates with longer patient
survival. Nat Med. 18:1254–1261. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schmitt M, Schmitt A, Rojewski MT, et al:
RHAMM-R3 peptide vaccination in patients with acute myeloid
leukemia, myelodysplastic syndrome, and multiple myeloma elicits
immunologic and clinical responses. Blood. 111:1357–1365. 2008.
View Article : Google Scholar
|
|
23
|
Greiner J, Li L, Ringhoffer M, et al:
Identification and characterization of epitopes of the receptor for
hyaluronic acid-mediated motility (RHAMM/CD168) recognized by
CD8+ T cells of HLA-A2-positive patients with acute
myeloid leukemia. Blood. 106:938–945. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Greiner J, Dohner H and Schmitt M: Cancer
vaccines for patients with acute myeloid leukemia - definition of
leukemia-associated antigens and current clinical protocols
targeting these antigens. Haematologica. 91:1653–1661. 2006.
|
|
25
|
Bellantuono I, Gao L, Parry S, et al: Two
distinct HLA-A0201-presented epitopes of the Wilms tumor antigen 1
can function as targets for leukemia-reactive CTL. Blood.
100:3835–3837. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Parkhurst MR, Riley JP, Igarashi T, Li Y,
Robbins PF and Rosenberg SA: Immunization of patients with the
hTERT:540–548 peptide induces peptide-reactive T lymphocytes that
do not recognize tumors endogenously expressing telomerase. Clin
Cancer Res. 10:4688–4698. 2004.
|
|
27
|
Greiner J, Schmitt A, Giannopoulos K, et
al: High-dose RHAMM-R3 peptide vaccination for patients with acute
myeloid leukemia, myelodysplastic syndrome and multiple myeloma.
Haematologica. 95:1191–1197. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Swinson DE, Jones JL, Richardson D, et al:
Carbonic anhydrase IX expression, a novel surrogate marker of tumor
hypoxia, is associated with a poor prognosis in non-small-cell lung
cancer. J Clin Oncol. 21:473–482. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Vermylen P, Roufosse C, Burny A, et al:
Carbonic anhydrase IX antigen differentiates between preneoplastic
malignant lesions in non-small cell lung carcinoma. Eur Respir J.
14:806–811. 1999. View Article : Google Scholar
|
|
30
|
ClinicalTrials.gov. http://www.clinicaltrials.gov/.
H-Cg. NCT00003102.
|
|
31
|
Siebels M, Rohrmann K, Oberneder R, et al:
A clinical phase I/II trial with the monoclonal antibody cG250
(RENCAREX®) and interferon-alpha-2a in metastatic renal
cell carcinoma patients. World J Urol. 29:121–126. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Epping MT, Wang L, Edel MJ, Carlée L,
Hernandez M and Bernards R: The human tumor antigen PRAME is a
dominant repressor of retinoic acid receptor signaling. Cell.
122:835–847. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Oberthuer A, Hero B, Spitz R, Berthold F
and Fischer M: The tumor-associated antigen PRAME is universally
expressed in high-stage neuroblastoma and associated with poor
outcome. Clin Cancer Res. 10:4307–4313. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kessler JH, Beekman NJ, Bres-Vloemans SA,
et al: Efficient identification of novel
HLA-A*0201-presented cytotoxic T lymphocyte epitopes in
the widely expressed tumor antigen PRAME by proteasome-mediated
digestion analysis. J Exp Med. 193:73–88. 2001.PubMed/NCBI
|
|
35
|
Schmitt A, Barth TF, Beyer E, et al: The
tumor antigens RHAMM and G250/CAIX are expressed in head and neck
squamous cell carcinomas and elicit specific CD8+ T cell
responses. Int J Oncol. 34:629–639. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Albanell J, Lonardo F, Rusch V, et al:
High telomerase activity in primary lung cancers: association with
increased cell proliferation rates and advanced pathologic stage. J
Natl Cancer Inst. 89:1609–1615. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang L, Soria JC, Kemp BL, Liu DD, Mao L
and Khuri FR: hTERT expression is a prognostic factor of survival
in patients with stage I non-small cell lung cancer. Clin Cancer
Res. 8:2883–2889. 2002.PubMed/NCBI
|
|
38
|
Fujita Y, Fujikane T, Fujiuchi S, et al:
The diagnostic and prognostic relevance of human telomerase reverse
transcriptase mRNA expression detected in situ in patients with
nonsmall cell lung carcinoma. Cancer. 98:1008–1013. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gure AO, Chua R, Williamson B, et al:
Cancer-testis genes are coordinately expressed and are markers of
poor outcome in non-small cell lung cancer. Clin Cancer Res.
11:8055–8062. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Eifuku R, Takenoyama M, Yoshino I, et al:
Analysis of MAGE-3 derived synthetic peptide as a human lung cancer
antigen recognized by cytotoxic T lymphocytes. Int J Clin Oncol.
6:34–39. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Vansteenkiste J, Zielinski M, Linder A,
Dahabre J, Esteban E, Malinowski W, Jassem J, Passlick B, Lehmann F
and Brichard VG: Final results of a multi-center, double-blind,
randomized, placebo-controlled phase II study to assess the
efficacy of MAGE-A3 immunotherapeutic as adjuvant therapy in stage
IB/II non-small cell lung cancer (NSCLC). J Clin Oncol.
25:75542007.
|
|
42
|
Lo Iacono M, Monica V, Saviozzi S, et al:
Aurora Kinase A expression is associated with lung cancer
histological-subtypes and with tumor de-differentiation. J Transl
Med. 9:1002011.PubMed/NCBI
|
|
43
|
Anand S, Penrhyn-Lowe S and Venkitaraman
AR: AURORA-A amplification overrides the mitotic spindle assembly
checkpoint, inducing resistance to Taxol. Cancer Cell. 3:51–62.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Smith SL, Bowers NL, Betticher DC, et al:
Overexpression of aurora B kinase (AURKB) in primary non-small cell
lung carcinoma is frequent, generally driven from one allele, and
correlates with the level of genetic instability. Br J Cancer.
93:719–729. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Linder A, Budihardjo-Welim H, Velehorschi
W, Coche T, Gruselle O, D'Agostino D, Louahed J, Lehmann F and
Brichard VG; Lungenklinik Hemer, Hemer Nordrhein-Westfalen,
Germany; Praxis für Pathologie Hemer, Hemer, Germany;
GlaxoSmithKline Biologicals, Rixensart, Belgium. Prognostic value
of MAGE-A3 and PRAME gene expression in non-small-cell lung cancer
(NSCLC). J Clin Oncol. 30:70562012.
|
|
46
|
Gautschi O, Heighway J, Mack PC, Purnell
PR, Lara PN Jr and Gandara DR: Aurora kinases as anticancer drug
targets. Clin Cancer Res. 14:1639–1648. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Fu J, Bian M, Jiang Q and Zhang C: Roles
of Aurora kinases in mitosis and tumorigenesis. Mol Cancer Res.
5:1–10. 2007. View Article : Google Scholar : PubMed/NCBI
|