Introduction
Hepatocellular carcinoma (HCC) is one of the most
common malignancies worldwide and its treatment may be complicated
by underlying cirrhosis, multiple lesions, the invasion of vital
structures within the porta hepatis, or the involvement of organs
outside the liver (1–3). Surgical procedures such as hepatic
resection or liver transplantation represent radical treatment
modalities for HCC patients. Local ablation can substitute for
resection in patients at early stages for whom surgical therapies
are not suitable (4,5). For intermediate-advanced HCC, there
are many potential therapeutic options, but their effects are
limited due to the cancer’s highly variable biologic behavior and
morphology. Recent investigations have highlighted the utility of
combination molecular targeted therapy (6,7).
However, the high incidence of tumor recurrence and metastasis
remains a major problem that contributes to the high mortality
rate, which indicates that the therapeutic strategies against HCC
requires further investigation (8,9).
Transarterial chemoembolization (TACE) is widely
used as a palliative treatment for patients with primary or
recurrent HCC and has shown encouraging results in terms of
survival (10,11). The rationale for transarterial
embolization (TAE) is based on occluding the blood flow to the
tumor and inducing tumor necrosis while preserving adequate liver
function. It is generally accepted that TAE rarely achieves total
necrosis of the targeted liver tumor and the behavior of more
aggressive residual tumors could detract from the merit of TAE.
Multiple and diverse mechanisms for this effect have been proposed,
and there are considerable data indicating that elevated expression
of hypoxia-inducible factor-1α (HIF-1α) is induced after TACE and
is associated with poor prognosis in HCC patients (12). Recent studies have further
demonstrated that local hypoxia induced by embolization, through
activation of the HIF-1α transcription factor and the resulting
increased expression of vascular endothelial growth factor (VEGF),
represents an important driving force in tumor progression
(13,14). Currently, increasing evidence
suggests that TACE for HCC may accelerate the development of
invasion and metastasis (14,15).
Generally, the loss of cell-to-cell contacts and the gain of motile
and invasive abilities are essential prerequisites for metastasis
(16). These phenotypic changes in
tumor cells, designated as the epithelial to mesenchymal transition
(EMT), have been described in different types of carcinoma cells
including HCC (17,18). EMT, which is a physiological process
that also occurs during embryological development, is characterized
by loss of the epithelial marker E-cadherin and increased
expression of mesenchymal markers, such as N-cadherin and vimentin.
In adult organisms, EMT can be involved in tissue repair such as
wound healing and the development of diseases such as chronic
inflammation and carcinoma progression (19–21).
In recent years, EMT has been regarded as a critical step in tumor
invasion and metastasis (22,23).
Furthermore, accumulating evidence indicates that local hypoxia may
be related to the invasiveness and metastatic potential of HCC
after embolization, although the altered metastatic potential of
residual cancer after TACE is not completely understood.
Therefore, we designed the present study to
investigate whether TAE could enhance the metastatic potential of
residual HCC and explore the relationship between EMT and TAE in
vivo and in vitro.
Materials and methods
Cell culture
The Buffalo rat hepatoma cell line McA-RH7777 was
obtained from the American Type Culture Collection (no. CRL1601;
ATCC, Manassas, VA, USA). Transfection of McA-RH7777 cells with a
lentiviral gene-transfer vector encoding the green fluorescent
protein (GFP) sequence (constructed by GeneChem, Shanghai, China)
resulted in dramatic GFP expression, which was confirmed by
sequencing. The following experiments were performed with sorted
GFP+ cells, and cells were cultivated in Dulbecco’s
modified Eagle’s medium (DMEM; Gibco-Life Technologies, Inc.,
Carlsbad, CA, USA) containing 10% heat-inactivated fetal bovine
serum (FBS; Gibco), 100 U/ml penicillin G and 100 mg/ml
streptomycin at 37°C in a humidified incubator with 5%
CO2.
Chemically induced hypoxia and cell
growth in vitro
Hypoxic conditions were achieved by exposing
normoxic cells to cobalt chloride (CoCl2, 100 μM/l),
which is known to activate hypoxia-dependent pathways under normal
oxygen levels by stabilizing HIF-1α in a number of cell lines
(24). Cells
(2×103/well) were seeded in triplicate in 96-well
microplates and incubated under normoxic or hypoxic conditions.
After 24 and 48 h, cell proliferation was determined in triplicate
using the Cell Counting kit-8 assay (CCK-8; Dojindo Laboratories,
Tokyo, Japan). The results were expressed as the corrected
absorbance (A570 nm), which represented the actual
absorbance of each well recorded at 570 nm.
Cell invasion assays
Cell invasion was assessed using 24-well BioCoat
Matrigel Invasion Chambers (Corning, Cambridge, UK). A total of
1×105 cells was plated onto Matrigel-coated filters, and
750 μl DMEM containing 10% FBS was added to the lower chamber of
each well. After 48 h under hypoxic or normoxic culture conditions,
cells that had reached the underside of the membrane were stained
with Giemsa (Sigma). Invading cells in 3 adjacent microscope fields
for each membrane were counted at ×200 magnification under a
microscope (Nikon, Tokyo, Japan).
Experimental animals
Buffalo rats (8-weeks old, initially weighing
201.67±3.77 g, ranging from 181 to 222 g) were obtained from
Charles River Laboratories (Davis, CA, USA). All animals were
maintained in the specific pathogen-free facility of the Animal
Experimental Center of Fudan University and were provided with
rodent chow and tap water ad libitum. Studies were conducted
in accordance with the animal care policy of the Fudan University
and the Animal Research Committee.
Establishment of the rat tumor model
GFP+ McA-RH7777 cells (1×106)
were injected subcutaneously into the right hindlimbs of Buffalo
rats. The subcutaneous tumors were removed when they reached ~1 cm
in length (~1 month after injection) and were then minced into
small cubes of ~2×2×2 mm3. Pieces of this tumor tissue
were then transplanted into the left lobe of the livers of 20 male
Buffalo rats to induce HCC. Tumor implantation was performed using
a modification of the technique previously described (25).
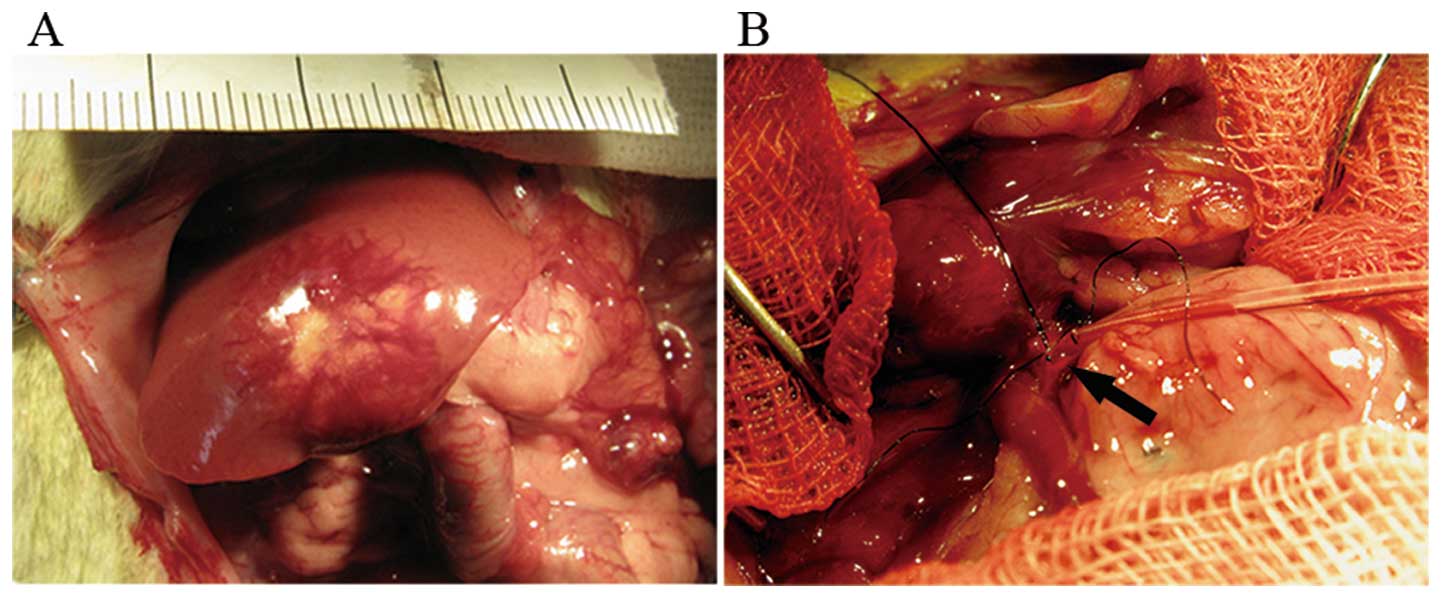
Interventional procedures
Two weeks after orthotopic implantation, a second
laparotomy (following the 2nd MRI) was performed for TAE therapy.
Twenty rats were randomly assigned to either the TAE group or the
sham group. A silastic catheter (Dow Corning, Midland, MI, USA)
with an inner diameter of 0.28 mm and an outer diameter 0.61 mm was
used for catheterization. Using a binocular operative microscope
(SMZ1500; Nikon), the catheter was inserted in a retrograde manner
into the gastroduodenal artery and pushed forward to the opening of
the common hepatic artery (Fig. 1)
(26). Then embolization was
performed as follow. In the TAE group, the rats received an
intra-arterial injection of iodized oil (0.2 ml/kg), which had been
mixed with double the volume of saline. In the sham group, rats
received an equivalent amount of saline via intra-arterial
injection (final volume, 0.6 ml/kg). The entire injection process
was observed under the microscope to ensure that the embolic agent
was delivered into the liver. The iodized oil was washed into the
proper hepatic artery via the blood flow from the common hepatic
artery. The gastroduodenal artery was then fixed with a silk suture
and the abdominal wall was closed. The animals were kept under
standard conditions after treatment. None of the animals died
during tumor cell implantation or surgery, and we observed no
fistulas or injuries to the bile ducts, vessels or other
surrounding structures during treatment.
Tumor growth and lung metastasis
analysis
To assess tumor growth, magnetic resonance imaging
(MRI) were performed by 3.0 T Magnetom (Avanto Trio, Siemens,
Germany) with small animal body coil (Siemens). All animals
underwent a T2-weighted imaging (T2WI) every
7 days after inoculation with a multi-slice acquisition providing
complete coverage of the entire liver. T2WI is acquired
using TSE BLADE technique, 70 mm field of view (FOV), 1.5 mm slice
thickness, 192 imaging acquisition matrix, repetition and echo time
(TR/TE) = 3500/103 ms and total examination time, 4 min 5 sec. The
volume of the tumors was calculated as follows: (L ×
S2)/2, where L is the longest diameter and S is the
shortest diameter. All animals were euthanized 2 weeks after TAE,
and their livers and lungs were harvested to evaluate molecular
changes and distant metastasis. Lung metastases were visualized
using fluorescence stereomicroscopy (Leica Microsystems Imaging
Solutions, Cambridge, UK). Part of tumor tissues were frozen in
liquid nitrogen for western blot analysis, while other parts were
fixed in 10% formalin, embedded in paraffin and sectioned (3 μm)
for histopathological studies.
Western blot assays
The concentration of proteins extracted from cell
specimens (treatment with or without CoCl2) and tumor
tissues was determined using the BCA Protein Assay kit (Beyotime
Institute of Biotechnology, Shanghai, China). Then, the expression
of HIF-1α, E-cadherin, N-cadherin and vimentin (monoclonal
antibodies; Bioworld Technology) was determined by western blotting
according to the manufacturer’s instructions. The density of each
band was measured and compared to that of the internal control,
GAPDH.
Immunohistochemistry and hematoxylin and
eosin (H&E) staining
Tissue sections were used for H&E staining and
immunostaining of HIF-1α, E-cadherin, N-cadherin and vimentin.
Three fields (x400 magnification) from each slide were counted to
determine the frequency of nuclear or cytoplasmic staining. The
expression of each target protein was assessed according to the
percentage of immunoreactive cells within the region of neoplastic
cells. All slides were evaluated by 2 pathologists in a blinded
manner under a light microscope and divergent results were resolved
by discussion.
Statistical analysis
The cell invasion and proliferation assays were
analyzed using the Student’s t-test. Tumor volume, lung metastasis,
western blot assays and immunohistochemistry staining were analyzed
using t-tests or Wilcoxon rank-sum tests. Statistical analysis was
performed with SPSS (Statistical Package for the Social Sciences)
15.0 for Windows (SPSS Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant result.
Results
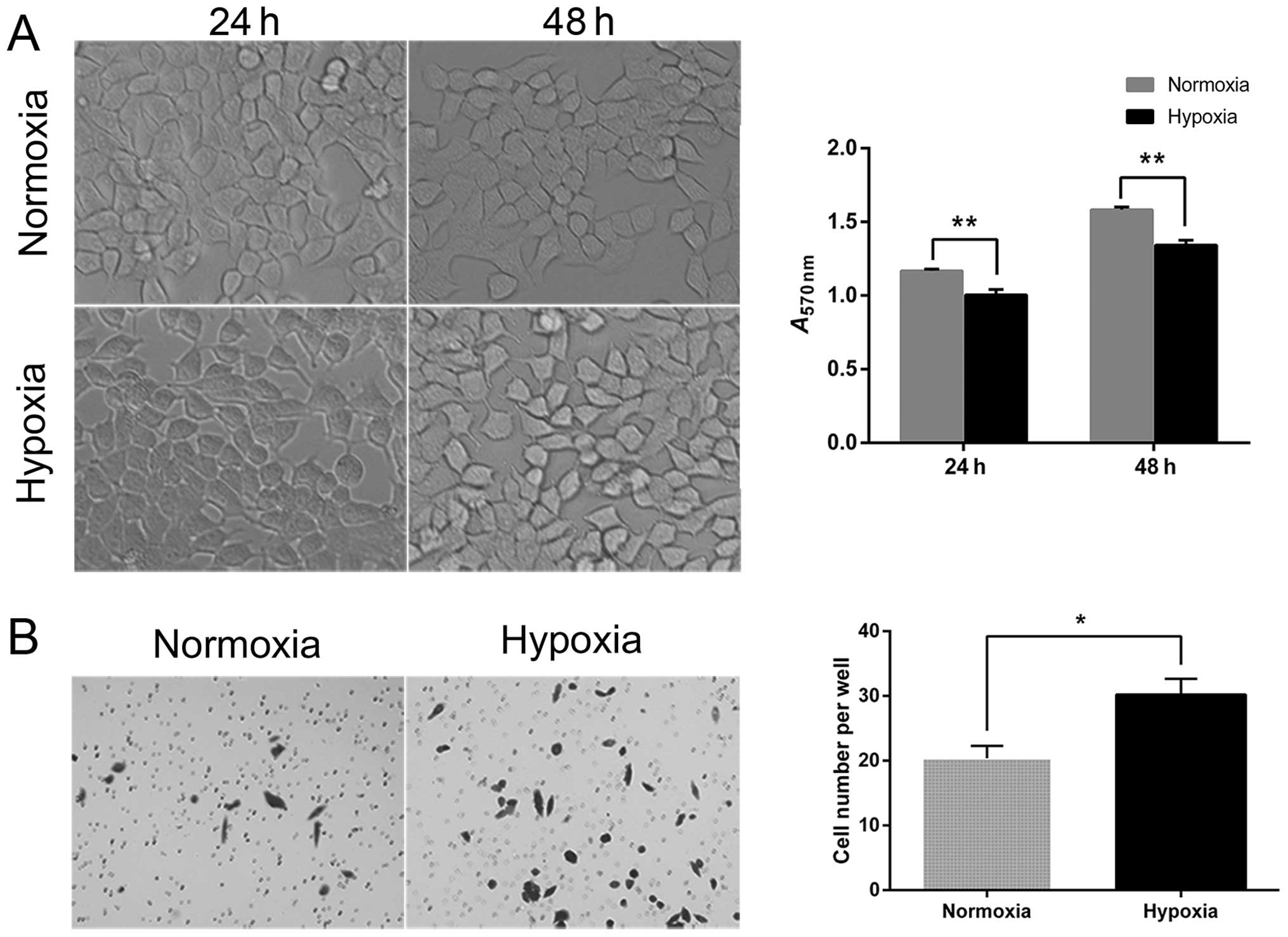
Hypoxia promotes the invasiveness and
increases the expression of EMT markers in McA-RH7777 cells
The analysis of tumor cell proliferation at 24 and
48 h after hypoxia induced by CoCl2 showed that hypoxia
inhibited cell proliferation in comparison to the normoxic
controls. In addition, the morphology of hypoxic cells was altered
from a typical epithelial orbicular-ovate appearance to a
tenuous/irregular shape with formation of pseudopodia,
demonstrating reduced cell-cell adhesion (Fig. 2A). The results of the Transwell
assay showed that the number of hypoxic tumor cells that invaded
through the Matrigel was significantly higher than the
corresponding number of normoxic cells (30.2±2.46 vs. 20.4±1.89;
P=0.013) (Fig. 2B).
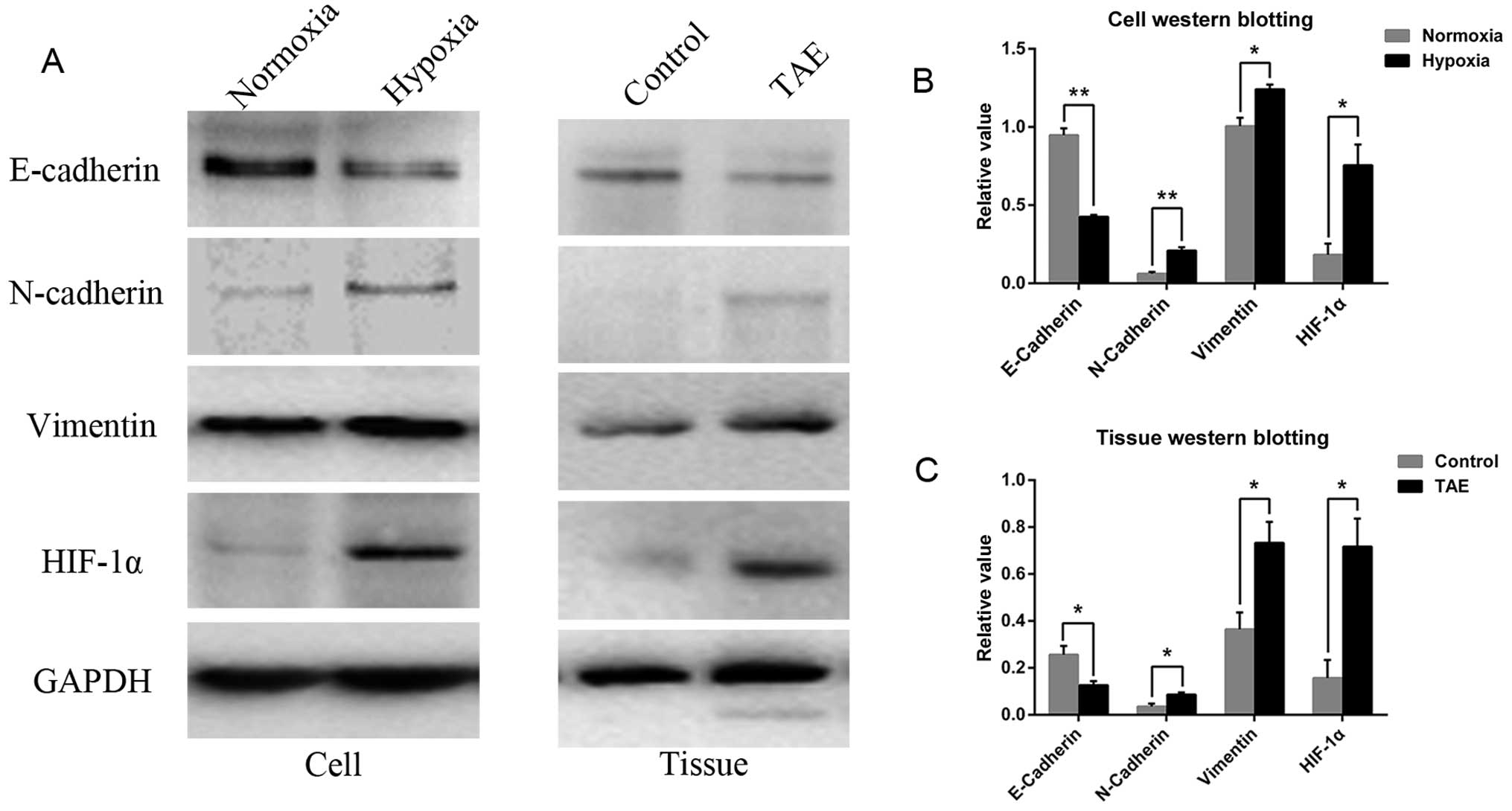
Immunoblotting demonstrated that hypoxia was
successfully induced with CoCl2, as indicated by the
increase in HIF-1α expression, which was significantly higher in
the hypoxic as compared to the normoxic group (Fig. 3A and B). Accompanying morphological
changes, the western blot analysis demonstrated reduced expression
of the epithelial cell marker E-cadherin in hypoxic cells relative
to normoxic cells. Furthermore, the level of the mesenchymal cell
markers vimentin and N-cadherin was increased (Fig. 3A and B). This phenomenon was related
to EMT, which is characterized by the loss of E-cadherin expression
and the simultaneous upregulation of N-cadherin, which results in
the ability of cells to transmigrate from basement membranes into
stromal tissues.
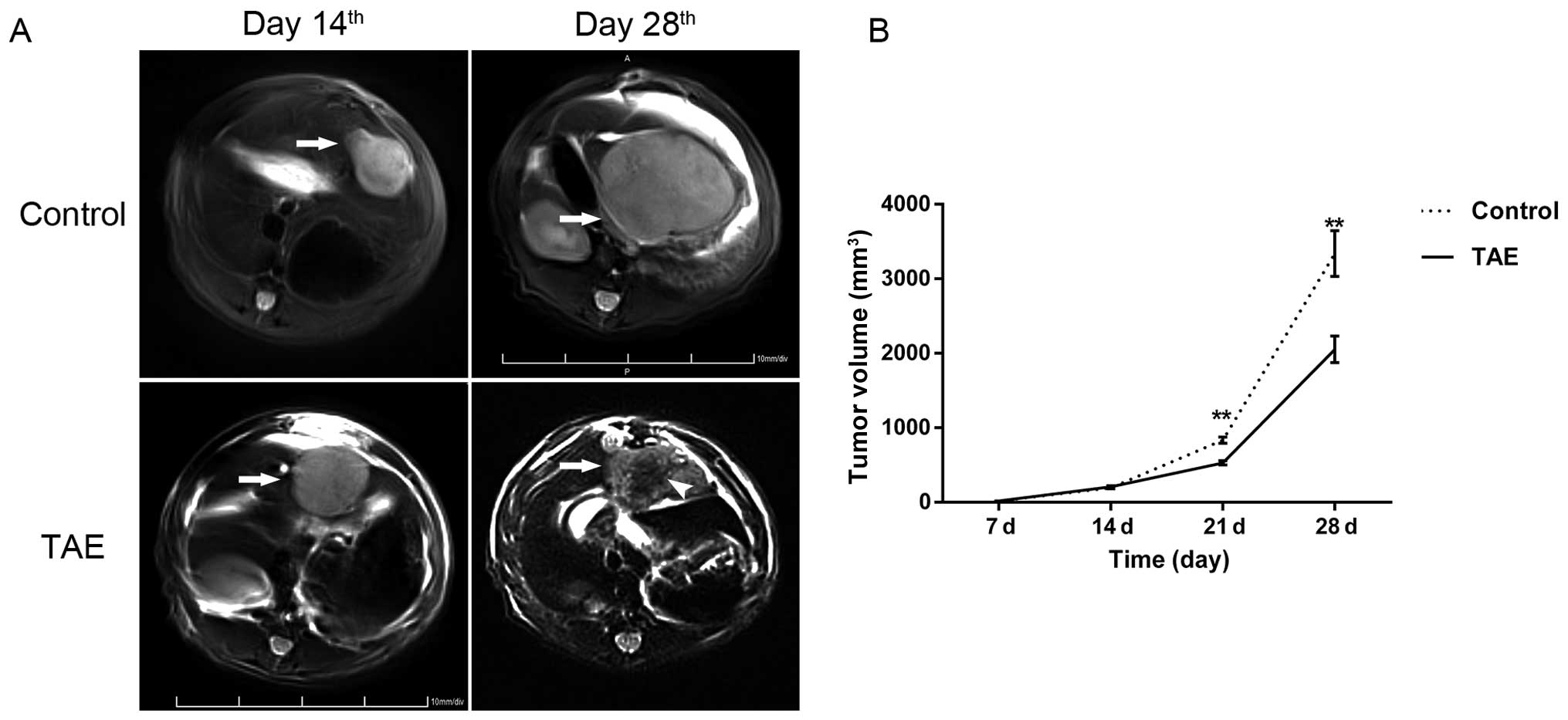
The growth characteristics and imaging
properties of the hepatoma model
Tumor implantation led to the outgrowth of solitary
liver tumors in all 20 animals. None of the animals died during
implantation or during the postoperative period. Seven days after
orthotopic liver tumor implantation, the orthotopic liver tumors
were so small that it was difficult to assess the tumors using MRI
in most cases. Two weeks after implantation, the liver tumors
reached ~1 cm in diameter (8.15±0.13 mm) and showed high signals on
T2WI imaging (Fig. 4A).
The tumors were sharply demarcated from the surrounding normal
hepatic parenchyma with an incomplete capsule. On T2WI
imaging, the necrotic tissue was observed as an area of lower
intensity in the center. No significant difference was found in
pretreatment tumor size between the TAE-treated and control groups
(208.56±13.62 mm3 vs. 193.88±12.32 mm3;
P=0.435).
TAE inhibits tumor growth but
significantly promotes metastases
TAE was performed successfully in all 20 animals,
and no technical complications occurred during the experimental
period. Two weeks after TAE, the tumor volume of the TAE group was
2,052.20±178.37 mm3, which was smaller than that of
sham-operated controls (3,339.50±306.18 mm3; P=0.002)
(Fig. 4). However, the lung
metastatic rate of the TAE group was the same as the sham-operated
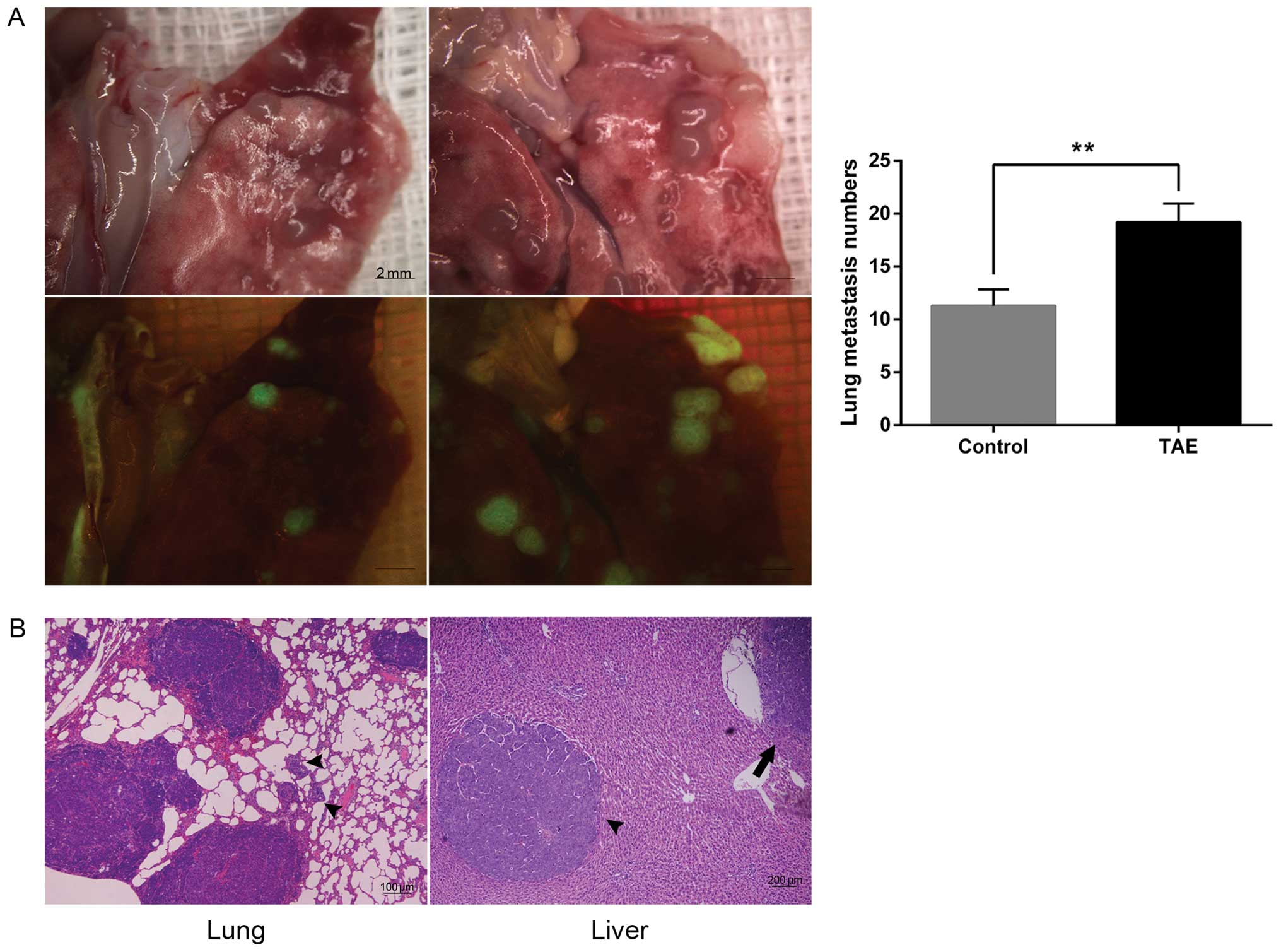
group (100%, 6/6). Additionally, the TAE group showed more lung
metastatic nodules in comparison to the sham-operated group
(19.20±1.76 vs. 11.30±1.54; P=0.003) by GFP fluorescence (Fig. 5A). Furthermore, the proinvasive
consequences of TAE could be observed in animals bearing McA-RH7777
homografts, and microscopic intrahepatic dissemination was also
present in the TAE group (Fig.
5B).
TAE induces changes consistent with EMT
in HCC
After TAE, the increase in HIF-1α expression and
induction of EMT were demonstrated by immunoblotting, characterized
by the loss of E-cadherin and upregulation of N-cadherin and
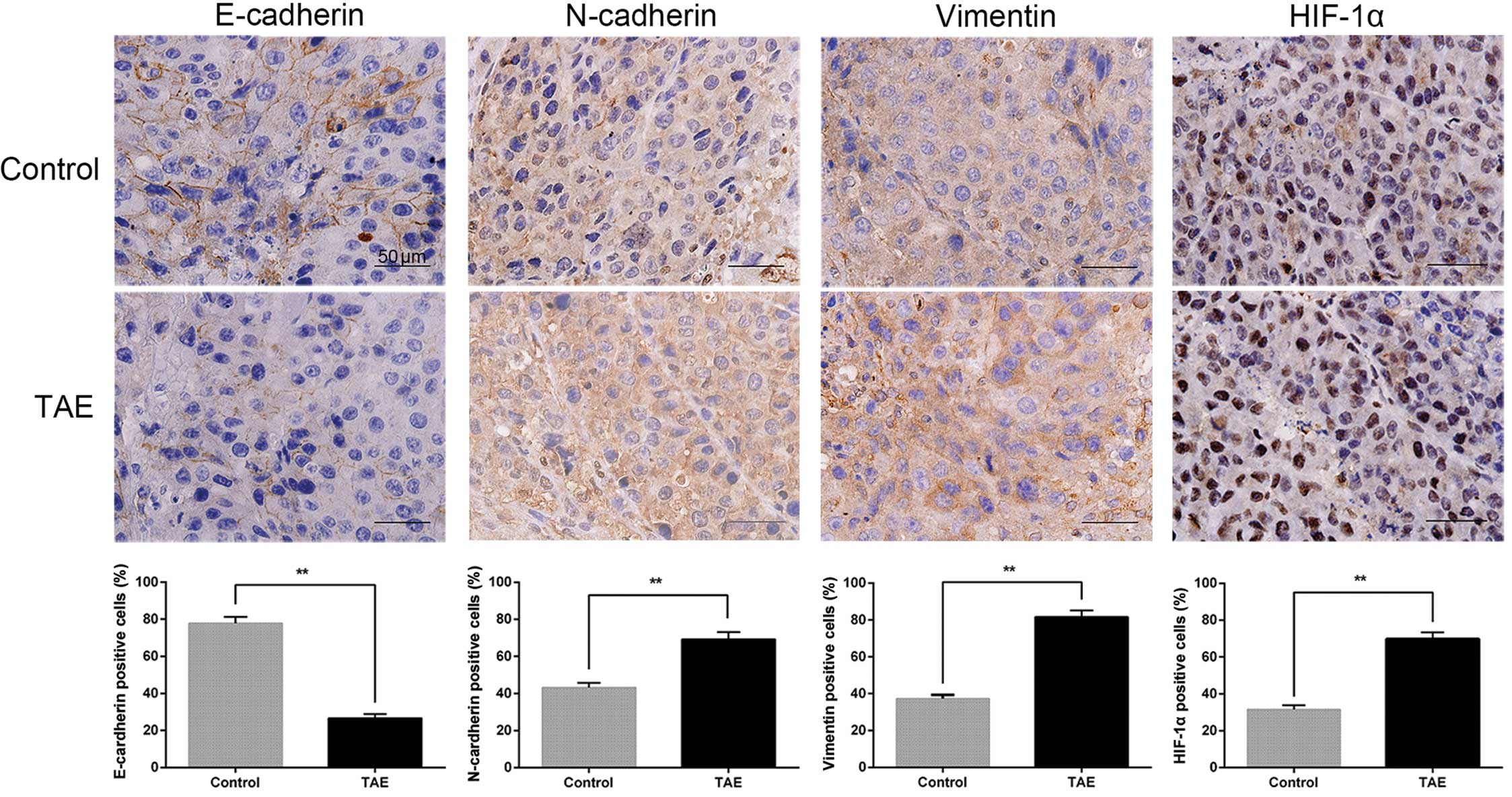
vimentin in the tumor tissues of TAE-treated rats (Fig. 3A and C). Immunostaining showed that
E-cadherin-positive cells in sham-operated mice were localized in
the tumor areas and showed a membranous distribution. Furthermore,
this analysis also revealed a trend towards enhanced expression of
HIF-1α, which showed a nuclear distribution in TAE-treated rats in
comparison to the corresponding sham-operated controls, as well as
increased expression of N-cadherin and vimentin (Fig. 6).
Discussion
The aim of TAE for liver tumors is the induction of
ischemic/hypoxic tumor necrosis through embolization of the
tumor-feeding vessel. Considering patients’ reserved liver function
and the complexity of the blood supply for liver tumors,
embolization of the tumor-feeding vessel is always incomplete and
tumor necrosis can be partial, which allows residual tumor to
survive and adapt to a microenvironment of hypoxia. Moreover, the
merits of TACE or TAE for HCC are disputable because of the
possibility of increasing the likelihood of metastasis, and some
findings have ascribed the failure of TAE to embolization-induced
hypoxia (27). In the present
study, we used McA-RH7777 hepatoma-bearing rats to mimic incomplete
embolization and investigate the influence of hypoxia after TAE on
the invasiveness and metastatic potential of residual
carcinoma.
Hypoxia presents fundamental implications in many
cancers. In fact, part of the tumor is almost always hypoxic, which
increases as tumors grow rapidly, especially in the tumor core due
to insufficient vascularization. The consequences of hypoxia for
tumor cells vary according to the extent and duration of hypoxia.
Severe hypoxia causes cell death, whereas on the other hand, mild
hypoxia can induce a series of adaptive changes, such as
angiogenesis (28). The
transcription factor HIF-1, an oxygen-sensitive transcriptional
activator, plays an important role in antagonizing apoptosis and
mediating adaptive cell responses to hypoxia following TAE. HIF-1
is a heterodimer that consists of an oxygen-regulated HIF-1α
subunit and a constitutively expressed HIF-1β subunit. In normoxia,
the HIF-1α proteins are rapidly degraded, whereas in hypoxia,
HIF-1α becomes stabilized and free to form dimers with HIF-1β, and
the HIF-1 complex then translocates to the nucleus, where it
regulates the transcription of genes involved many key biological
functions (29). Moreover, studies
on tumor angiogenesis, cell survival/apoptosis, invasion,
metastasis and adhesion have revealed a critical role for HIF-1α in
these processes (30,31). Because of the limitations of TACE in
comparison to radical resection, a protective response against
ischemic/hypoxic injury arises in the residual viable tumor tissue.
Significant evidence indicates that HIF-1α serves as a prognostic
factor for tumor recurrence in human and murine HCC and that high
expression of HIF-1α at the edge of a tumor may correlate with
migration and invasion (32).
Consistent with previous studies, we showed that HIF-1α was
overexpressed as a result of hypoxia generated by the TAE procedure
in vivo. Furthermore, we investigated the effect of hypoxia
induced by CoCl2in vitro, and the increase in
HIF-1α protein demonstrated the successful induction of a hypoxic
microenvironment, analogous to that resulting from TAE.
Our findings further demonstrated that hypoxia,
mediated by HIF-1α, led to increased motility and invasiveness of
residual viable tumor tissue. The acquisition of motile and
invasive abilities represents a critical step in metastasis, and
the escape of carcinoma cells from the primary tumor generally
occurs along with the loss of cell-to-cell contacts and the
degradation of the extracellular matrix (ECM), a process referred
to as EMT. In fact, EMT is the key process that drives cancer
metastasis and is characterized by loss of the epithelial marker
E-cadherin; increased expression of the mesenchymal markers
vimentin, N-cadherin and ICAM-1; and enhanced metastatic potential
of tumor cells (22,33). Our results showed that hypoxia
stimulated the transformation of HCC cells from a typical
epithelial phenotype to a spindle-shaped mesenchymal phenotype,
accompanied by the loss of E-cadherin and upregulation of
N-cadherin and vimentin. These observations imply that HIF-1α may
play a role in EMT. After the initiation of hypoxia, tumor cells
that have undergone EMT are endowed with the ability to
transmigrate across basement membranes and stromal tissues as well
as to intravasate and pass into the circulatory system. These
changes increase the likelihood of recurrence and metastasis, and
accordingly, the number of lung metastases in the TAE group was
significantly higher than that observed in the sham group.
In contrast to other studies that have focused on
the therapeutic effects of TAE on primary tumor growth, with less
attention on metastasis, we illustrated the significance of
incomplete TAE on tumor growth and invasiveness in an orthotopic
tumor implantation rat model. Although the growth of allografts was
inhibited after TAE, the residual tumors were more invasive, and
this result supports the relationship between intratumoral hypoxia
and tumor cell EMT. Following the introduction of incomplete TAE,
our results indicated that the non-selective embolization of blood
flow could elicit an adaptive response in hepatic tumor cells,
involving an augmented invasive ability, malignant phenotype,
increased intrahepatic dissemination and even distant
metastasis.
In general, metastasis, rather than growth of the
primary tumor, represents the major cause of cancer-related
mortality (34). Although TACE has
been established as the standard of care for patients who meet the
criteria for the intermediate stage of the BCLC staging system
(1), high metastasis and recurrence
rates following TACE as well as other treatments drive physicians
to exploit combined strategies. Given that VEGF expression and
VEGF-receptor activation are markedly increased in HCC after TACE
(35,36), and these observations are widely
accepted as indicative of tumor degradation and metastasis,
anti-angiogenic or anti-VEGF agents have been administered in
combination with embolization (37). Recent investigations have also
demonstrated encouraging results in terms of survival following
combined therapy consisting of TACE and sorafenib (38). However, angiogenesis may, at least
in part, account for the failure of embolization. Alternatively,
hypoxia may induce EMT, and this process is known to convert
adherent epithelial cells into migratory cells that invade the ECM
and has consistently been associated with tumor metastasis
(33,39). Notably, embolization of the hepatic
artery using lipiodol induced EMT in hepatic tumor cells in the
present study, and this process was linked to the hypoxic response.
Thus, our results seem to be comparable to those of previous in
vivo studies of EMT in hypoxic ovarian, breast, renal and
hepatic tumor cells. However, we also demonstrated that EMT
contributed to the depravation of HCC after TAE, which has only
rarely been reported, and these findings may provide important
evidence concerning the clinical application of TACE for HCC
patients.
There were several limitations of the present study.
First, because tumor metastasis is influenced by numerous molecular
mechanisms and microcirculation factors and because the sample
sizes were limited in the present study, the contribution of
hypoxia to EMT warrants further investigation and should be studied
in combination with chemotherapeutic agents. Second, we did not
assess the correlation between HIF-1α protein and EMT at different
time points after TAE, although it has been shown that HIF-1α
activity changes dynamically and that VEGF expression and the
microvessel density are significantly increased in liver tumors 2–3
days after TAE (35). Thus, the
degree of EMT may vary at different time-points, and further study
with optimized time points of analysis is warranted. Third,
experimental rats such as those used in the present study can
develop severe liver dysfunction, and because the effect of
embolization was limited using non-selective catheterization, we
did not evaluate the degree of tumor necrosis after TAE.
In conclusion, we have shown that HIF-1α protein is
increased in liver tumors after TAE as a result of the local
hypoxia induced by the procedure and that this process is involved
in the activation of EMT in the residual viable tumor. Similarly,
hypoxia after TACE may increase the invasiveness and metastatic
potential of residual HCC tumors in clinical settings, and
combination therapy with targeted molecular agents against EMT
induced by hypoxia may augment the therapeutic effects of TACE.
Acknowledgements
We thank Zuxing Kan (MD Anderson Cancer Center,
Houston, TX, USA) for technical support and executive assistance.
The present study was supported in part by the grants from the
National Natural Sciences Foundation of China (no. 81171432).
References
|
1
|
EASL-EORTC clinical practice guidelines:
management of hepatocellular carcinoma. J Hepatol. 56:908–943.
2012.PubMed/NCBI
|
|
2
|
El-Serag HB: Hepatocellular carcinoma. N
Engl J Med. 365:1118–1127. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Forner A, Llovet JM and Bruix J:
Hepatocellular carcinoma. Lancet. 379:1245–1255. 2012. View Article : Google Scholar
|
|
4
|
Hasegawa K, Makuuchi M, Takayama T, et al:
Surgical resection vs. percutaneous ablation for hepatocellular
carcinoma: a preliminary report of the Japanese nationwide survey.
J Hepatol. 49:589–594. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen MS, Li JQ, Zheng Y, et al: A
prospective randomized trial comparing percutaneous local ablative
therapy and partial hepatectomy for small hepatocellular carcinoma.
Ann Surg. 243:321–328. 2006. View Article : Google Scholar
|
|
6
|
Llovet JM, Ricci S, Mazzaferro V, et al:
Sorafenib in advanced hepatocellular carcinoma. N Engl J Med.
359:378–390. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fernandez M, Semela D, Bruix J, Colle I,
Pinzani M and Bosch J: Angiogenesis in liver disease. J Hepatol.
50:604–620. 2009. View Article : Google Scholar
|
|
8
|
Llovet JM, Schwartz M and Mazzaferro V:
Resection and liver transplantation for hepatocellular carcinoma.
Semin Liver Dis. 25:181–200. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sala M, Fuster J, Llovet JM, et al: High
pathological risk of recurrence after surgical resection for
hepatocellular carcinoma: an indication for salvage liver
transplantation. Liver Transpl. 10:1294–1300. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Llovet JM and Bruix J: Systematic review
of randomized trials for unresectable hepatocellular carcinoma:
chemoembolization improves survival. Hepatology. 37:429–442. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lo CM, Ngan H, Tso WK, et al: Randomized
controlled trial of transarterial lipiodol chemoembolization for
unresectable hepatocellular carcinoma. Hepatology. 35:1164–1171.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Virmani S, Rhee TK, Ryu RK, et al:
Comparison of hypoxia-inducible factor-1α expression before and
after transcatheter arterial embolization in rabbit VX2 liver
tumors. J Vasc Interv Radiol. 19:1483–1489. 2008.
|
|
13
|
Wang B, Xu H, Gao ZQ, Ning HF, Sun YQ and
Cao GW: Increased expression of vascular endothelial growth factor
in hepatocellular carcinoma after transcatheter arterial
chemoembolization. Acta Radiol. 49:523–529. 2008. View Article : Google Scholar
|
|
14
|
Sergio A, Cristofori C, Cardin R, et al:
Transcatheter arterial chemoembolization (TACE) in hepatocellular
carcinoma (HCC): the role of angiogenesis and invasiveness. Am J
Gastroenterol. 103:914–921. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shim JH, Park JW, Kim JH, et al:
Association between increment of serum VEGF level and prognosis
after transcatheter arterial chemoembolization in hepatocellular
carcinoma patients. Cancer Sci. 99:2037–2044. 2008.
|
|
16
|
Friedl P and Wolf K: Tumour-cell invasion
and migration: diversity and escape mechanisms. Nat Rev Cancer.
3:362–374. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Thiery JP and Sleeman JP: Complex networks
orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell
Biol. 7:131–142. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Polyak K and Weinberg RA: Transitions
between epithelial and mesenchymal states: acquisition of malignant
and stem cell traits. Nat Rev Cancer. 9:265–273. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
van Zijl F, Zulehner G, Petz M, et al:
Epithelial-mesenchymal transition in hepatocellular carcinoma.
Future Oncol. 5:1169–1179. 2009.
|
|
20
|
Choi SS and Diehl AM:
Epithelial-to-mesenchymal transitions in the liver. Hepatology.
50:2007–2013. 2009. View Article : Google Scholar
|
|
21
|
Copple BL: Hypoxia stimulates hepatocyte
epithelial to mesenchymal transition by hypoxia-inducible factor
and transforming growth factor-β-dependent mechanisms. Liver Int.
30:669–682. 2010.PubMed/NCBI
|
|
22
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
De Larco JE, Wuertz BR, Manivel JC and
Furcht LT: Progression and enhancement of metastatic potential
after exposure of tumor cells to chemotherapeutic agents. Cancer
Res. 61:2857–2861. 2001.PubMed/NCBI
|
|
24
|
Ankoma-Sey V, Wang Y and Dai Z: Hypoxic
stimulation of vascular endothelial growth factor expression in
activated rat hepatic stellate cells. Hepatology. 31:141–148. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kan Z, Phongkitkarun S, Kobayashi S, et
al: Functional CT for quantifying tumor perfusion in antiangiogenic
therapy in a rat model. Radiology. 237:151–158. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Maataoui A, Qian J, Mack MG, et al: Liver
metastases in rats: chemoembolization combined with interstitial
laser ablation for treatment. Radiology. 237:479–484. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ramsey DE, Kernagis LY, Soulen MC and
Geschwind JF: Chemoembolization of hepatocellular carcinoma. J Vasc
Interv Radiol. 13:S211–S221. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Harris AL: Hypoxia - a key regulatory
factor in tumour growth. Nat Rev Cancer. 2:38–47. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ivan M, Kondo K, Yang H, et al: HIFα
targeted for VHL-mediated destruction by proline hydroxylation:
implications for O2 sensing. Science. 292:464–468.
2001.
|
|
30
|
Brahimi-Horn MC, Chiche J and Pouyssegur
J: Hypoxia and cancer. J Mol Med (Berl). 85:1301–1307. 2007.
View Article : Google Scholar
|
|
31
|
Sendoel A, Kohler I, Fellmann C, Lowe SW
and Hengartner MO: HIF-1 antagonizes p53-mediated apoptosis through
a secreted neuronal tyrosinase. Nature. 465:577–583. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Daskalow K, Rohwer N, Raskopf E, et al:
Role of hypoxia-inducible transcription factor 1α for progression
and chemosensitivity of murine hepatocellular carcinoma. J Mol Med
(Berl). 88:817–827. 2010.
|
|
33
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gupta GP and Massague J: Cancer
metastasis: building a framework. Cell. 127:679–695. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gupta S, Kobayashi S, Phongkitkarun S,
Broemeling LD and Kan Z: Effect of transcatheter hepatic arterial
embolization on angiogenesis in an animal model. Invest Radiol.
41:516–521. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liang B, Zheng CS, Feng GS, et al:
Correlation of hypoxia-inducible factor 1α with angiogenesis in
liver tumors after transcatheter arterial embolization in an animal
model. Cardiovasc Intervent Radiol. 33:806–812. 2010.
|
|
37
|
Maataoui A, Qian J, Vossoughi D, et al:
Transarterial chemoembolization alone and in combination with other
therapies: a comparative study in an animal HCC model. Eur Radiol.
15:127–133. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Abou-Alfa GK: TACE and sorafenib: a good
marriage? J Clin Oncol. 29:3949–3952. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Acloque H, Adams MS, Fishwick K,
Bronner-Fraser M and Nieto MA: Epithelial-mesenchymal transitions:
the importance of changing cell state in development and disease. J
Clin Invest. 119:1438–1449. 2009. View
Article : Google Scholar : PubMed/NCBI
|