Introduction
Pancreatic cancer is one of the most aggressive
types of cancer. Although accounting for only 3% of all cancers,
pancreatic cancer is the fourth most frequent cause of
cancer-related mortality, with a median survival of 6 months and a
5-year survival rate under 5% (1).
Many clinical trials have tested new therapeutic agents in patients
with pancreatic cancer, but the median survival has remained
virtually unchanged (2,3). New agents and combination agents are,
therefore, needed to treat patients with pancreatic cancer.
Apoptosis is a cellular process that maintains the
normal homeostasis of eukaryotic cells, with abnormally controlled
apoptosis being one of the primary causes of cancer development and
progression (4). Most anticancer
agents induce apoptosis by activating the intrinsic or extrinsic
cell death pathway. Activation of the extrinsic pathway is
triggered by the ligation of a death ligand to its receptor on
plasma membranes, leading to the formation of the death-inducing
signaling complex (DISC) and the activation of apoptosis proteases
(5).
The tumor necrosis factor-related apoptosis-inducing
ligand (TRAIL) induces cell death by signaling through TRAIL
receptors on the plasma membrane, including TRAIL-receptor 1 (DR4)
and TRAIL-receptor 2 (DR5) (6,7). TRAIL
is a cytokine with selective cell death-inducing activity in
malignant cancer cells, but with minimal toxicity towards normal
cells (8). Due to its
cancer-specific cell death-inducing activity, several clinical
trials have been initiated to test the anticancer activities of
recombinant human TRAIL protein and antibodies that bind to the
TRAIL receptor, but most primary tumors and cancer cell lines were
found to be resistant to TRAIL-induced apoptosis (9). These include various human pancreatic
cancer cell lines, highlighting the need to understand their
mechanisms of resistance (10–14).
Among the various mechanisms by which cells are
resistant to TRAIL are abnormalities in TRAIL receptors (15–18),
cell death signaling (19–22) and the assembly of death-inducing
signaling complexes (23,24). Many chemical agents have been
reported to enhance TRAIL toxicity in cancer cells. In particular,
protein synthesis inhibitors, including cycloheximide and emetine,
are thought to sensitize cancer cells to TRAIL (25–28).
These protein synthesis inhibitors are thought to sensitize cells
to TRAIL by targeting certain cellular pathways, including the
c-Jun N-terminal kinase in prostate cancer cells (27). However, these effects are only
partly understood.
In this study, we show that emetine hydrochloride
strongly sensitizes pancreatic cancer cells to TRAIL-induced
apoptosis by downregulating myeloid cell leukemia sequence-1
(Mcl-1) protein. We also found that Mcl-1 activity was required
during hypoxia-induced resistance of pancreatic cancer cells to
TRAIL, suggesting that Mcl-1 is the main regulator of TRAIL-induced
apoptosis under both normoxic and hypoxic conditions.
Materials and methods
Cell culture and materials
Human pancreatic AsPC-1 and BxPC-3 cancer cells were
maintained in RPMI-1640 medium, and PANC-1 cells were maintained in
Dulbecco’s modified Eagle’s medium (DMEM). All media were
supplemented with 10% fetal bovine serum (FBS; Life Technologies,
Grand Island, NY, USA), 2 mM L-glutamine and
penicillin-streptomycin (100 U/ml). Emetine hydrochloride was from
Sigma (St. Louis, MO, USA). Recombinant human TRAIL, Lipofectamine
2000 and Lipofectamine RNAiMAX reagents were purchased from Life
Technologies and CellTiter-Glo ATP measuring luminescence assay
solution was from Promega (Madison, WI, USA).
Compound screening
AsPC-1 cells were recovered from culture plates and
seeded in 96-well plates at a density of 5×103
cells/well in 50 μl medium. The plates were incubated for 20 h at
37°C in a cell culture incubator. Using an automatic liquid handler
(Perkin-Elmer model AJM8M01), 25 μl of culture medium containing
the test compound was added to each well in columns 2 to 11 to
achieve final concentrations of 5 μM, while 25 μl of the culture
medium was added to each well in columns 1 and 12. After 2 h, 25 μl
of TRAIL-containing medium was added to each well, yielding a final
TRAIL concentration of 50 ng/ml, except for the wells in column 1,
to which 25 μl of culture medium was added. After 24 h, the ATP
reactive luminescence value of each well was measured using a
cellular ATP content assay (CellTiter-Glo) and an EnVision
Multilabel reader (Perkin-Elmer). Raw values were transferred to
Prism Statistics software to calculate relative cell survival.
Using this system, we screened a library of 1,200 bioactive drugs
(Prestwick-1200TM).
Immunoblotting
Cell lysates were prepared by adding lysis buffer
(50 mM Tris-Cl, pH 7.4, 1% Igepal, 300 mM NaCl, 2 mM
MgCl2, 2 mM Na3VO4, 5 mM
β-glycerophosphate, protease inhibitor mixture and phosphatase
inhibitor mixture) to collected cells. After determining the sample
protein concentration by bicinchoninic acid assay, 40 μg of the
cell extract was separated by sodium dodecyl sulfate-polyacrylamide
gel electrophoresis (SDS-PAGE) and transferred to membranes, which
were incubated with specific antibodies diluted at 1:1,000. Primary
antibodies for immunoblotting included antibodies to DR5 (CS-8074),
caspase-3 (CS-9665), Bid (CS-2002), PARP (CS-9532), Bcl-2
(CS-2872), Bcl-XL (CS-2764) and Mcl-1 (CS-5453), (all
from Cell Signaling Technology, Danvers, MA, USA); and antibodies
to DR4 (cat #7863; Santa Cruz Biotechnology, Santa Cruz, CA, USA)
and α-tubulin (T9026; Sigma). After thorough washing, the membranes
were incubated with horseradish peroxidase-conjugated secondary
antibody (Bio-Rad Laboratories) and the enhanced chemiluminescent
substrate (SuperSignal West Pico-Pierce).
Cell viability analysis
Cell viability was evaluated using a cellular ATP
content-based luminescence assay (CellTiter-Glo assay; Promega),
flow cytometric analysis or trypan blue dye exclusion assay. For
the ATP assays, cells were seeded in 96-well plates at a density of
5×103 cells/well in 50 μl of culture medium. The cells
were pretreated for 1 h with 25 μl of the emetine-containing
medium, at the indicated concentrations, followed by incubation for
24 h with the indicated concentrations of recombinant human TRAIL
in 25 μl of medium. To each well, 10–20 μl assay solution was added
and luminescence was evaluated. Raw values were analyzed using
GraphPad Prism software (Graphpad Software Inc., La Jolla, CA, USA)
to calculate relative cell survival rate.
For the flow cytometric analysis, 2×105
cells were placed in each well of a 12-well plate, pre-incubated
with 2.5 μM emetine chloride and treated with TRAIL, as described
above. After 24 h, the recovered cells were washed with cold
phosphate-buffered saline (PBS) and resuspended in 500 μl Annexin V
reaction buffer containing Annexin V-FITC (50 μg/ml). The
percentage of apoptotic cells was evaluated by flow cytometry,
using a FACSCalibur instrument (BD Biosciences, San Jose, CA,
USA).
siRNA transfection and cell viability
assay after Mcl-1 knockdown
Mcl-1 siRNA (5′-aaguaucacagacguucuctt-3′) and
non-silencing siRNA (5′-acgugacacguucggagaauu-3′) were synthesized
by Genolution Pharmaceuticals, Inc. (Seoul, Korea) and transfected
into pancreatic cancer cells using RNAiMax Reagent™ (Life
Technologies). In brief, 30 pmol of siRNA in 5 μl of transfection
reagent was added to cells in 0.5 ml culture medium and incubated
for 2 days. The cells were treated with TRAIL for 24 h, and cell
viability was assessed using trypan blue exclusion assays. Gene
knockdown was confirmed by western blotting.
Cell viability assay under hypoxic
conditions
PANC-1 cells were seeded into 96-well plates in a
normoxic incubator. The plates were transferred to an Xvivo hypoxia
incubator (model Step Two; BioSpherix Ltd., Lacona, NY, USA) and
pre-subjected to hypoxia for 6 h. After treatment with emetine and
TRAIL, as described above, the cells were further incubated for 24
h under hypoxic conditions. Cell viability was measured using the
ATP contents assay (CellTiter-Glo).
Results
Screening of compounds sensitizing AsPC-1
cells to TRAIL
To assess the sensitivity of human pancreatic cancer
cells to TRAIL, we first analyzed the viability of the cells after
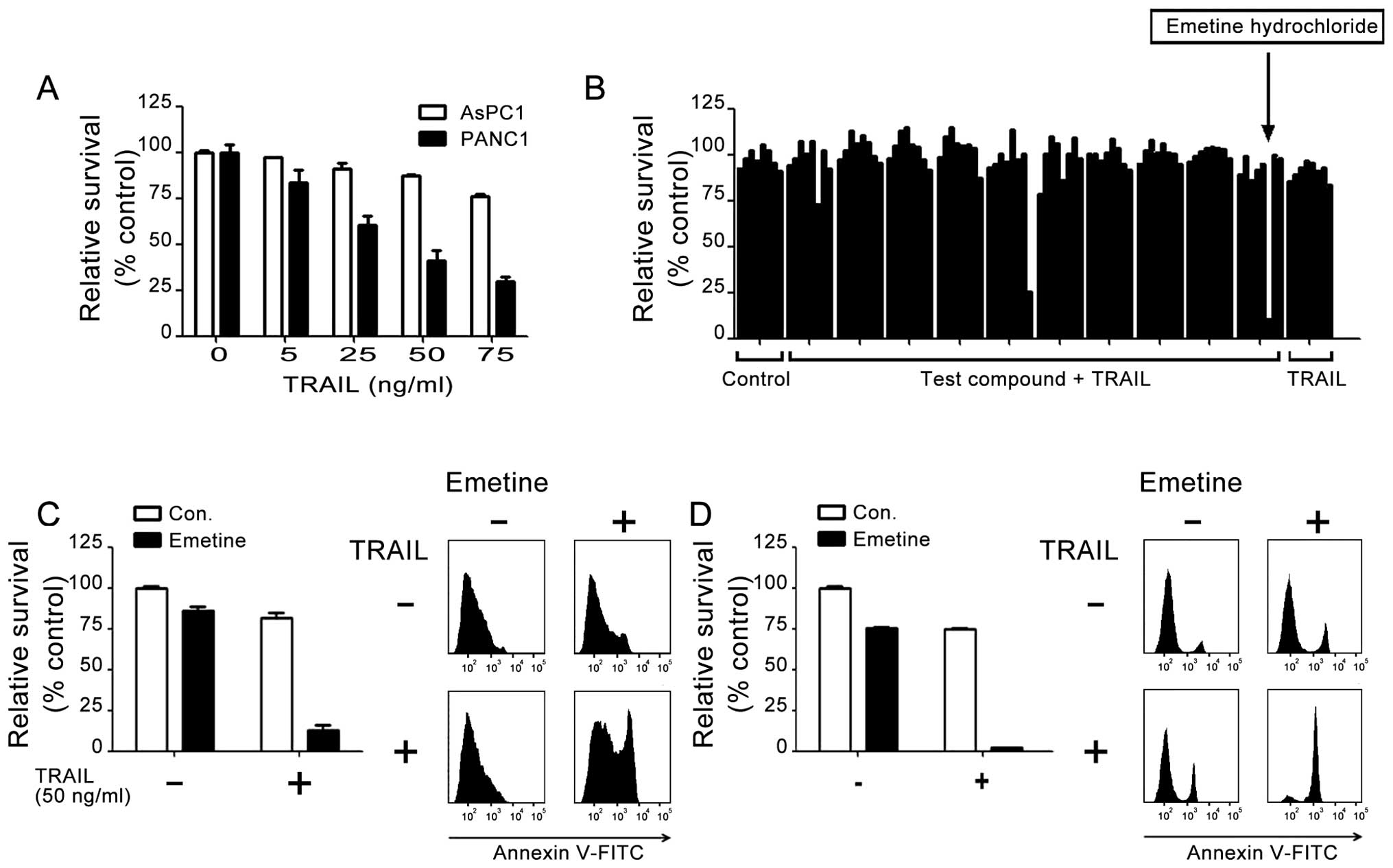
exposure to recombinant TRAIL protein. PANC-1 cells showed
dose-dependent cell death when treated with TRAIL (Fig. 1A), whereas AsPC-1 cells were
resistant to TRAIL. At a concentration of 75 ng/ml, TRAIL induced
the death of 71% of PANC-1 cells relative to the control (Fig. 1A), while >70% of AsPC-1 cells
remained viable.
To characterize the mechanism by which AsPC-1 cells
are resistant to TRAIL, we screened a library of bioactive small
molecules and marketed drugs (Prestwick-1200™) (Fig. 1B). In brief, these cells were
pretreated with 5 μM compound for 1 h, followed by treatment with a
subtoxic dose of TRAIL (50 ng/ml). A primary ‘hit’ was defined as a
compound that, when combined with TRAIL, induced >50% of cell
death, relative to the control treatment. This screening identified
8 compounds (Table I) that
reproducibly sensitized cells to TRAIL and had minimal self
cytotoxicity, including emetine hydrochloride (Fig. 1B).
 | Table ISensitizers of TRAIL in AsPC-1 cells
(% relative viability are shown). |
Table I
Sensitizers of TRAIL in AsPC-1 cells
(% relative viability are shown).
| Function | − TRAIL | + TRAIL | Ref. |
|---|
| Control | | 100 | 82 | |
| Daunorubicin | DNA intercalating
agent | 81 | 13 | (46) |
| Doxorubicin | DNA intercalating
agent | 83 | 11 | (47) |
| Vorinostat | Histone deacetylase
inhibitor | 89 | 21 | (48) |
| Topotecan | Topoisomerase II
inhibitor | 82 | 11 | (49) |
| Docetaxel | Microtubule
stabilizer | 89 | 47 | (50) |
| CGP74514A | Cyclin-dependent
kinase 1 inhibitor | 94 | 9 | |
| Emetine | Protein synthesis
inhibitor | 82 | 7 | |
| Brefeldin A | Golgi complex
transport inhibitor | 80 | 39 | |
We next compared the ability of emetine to sensitize
AsPC-1 and BxPC-3 cells to TRAIL. ATP assays of relative cell
viability and flow cytometric analysis showed that treatment of
these cells with 2.5 μM emetine hydrochloride and TRAIL (50 ng/ml)
reduced the viability of both cell lines to <20% (Fig. 1C and D). By contrast, treatment with
TRAIL or emetine alone showed minimal effects in both assays.
Emetine pretreatment downregulates
Mcl-1
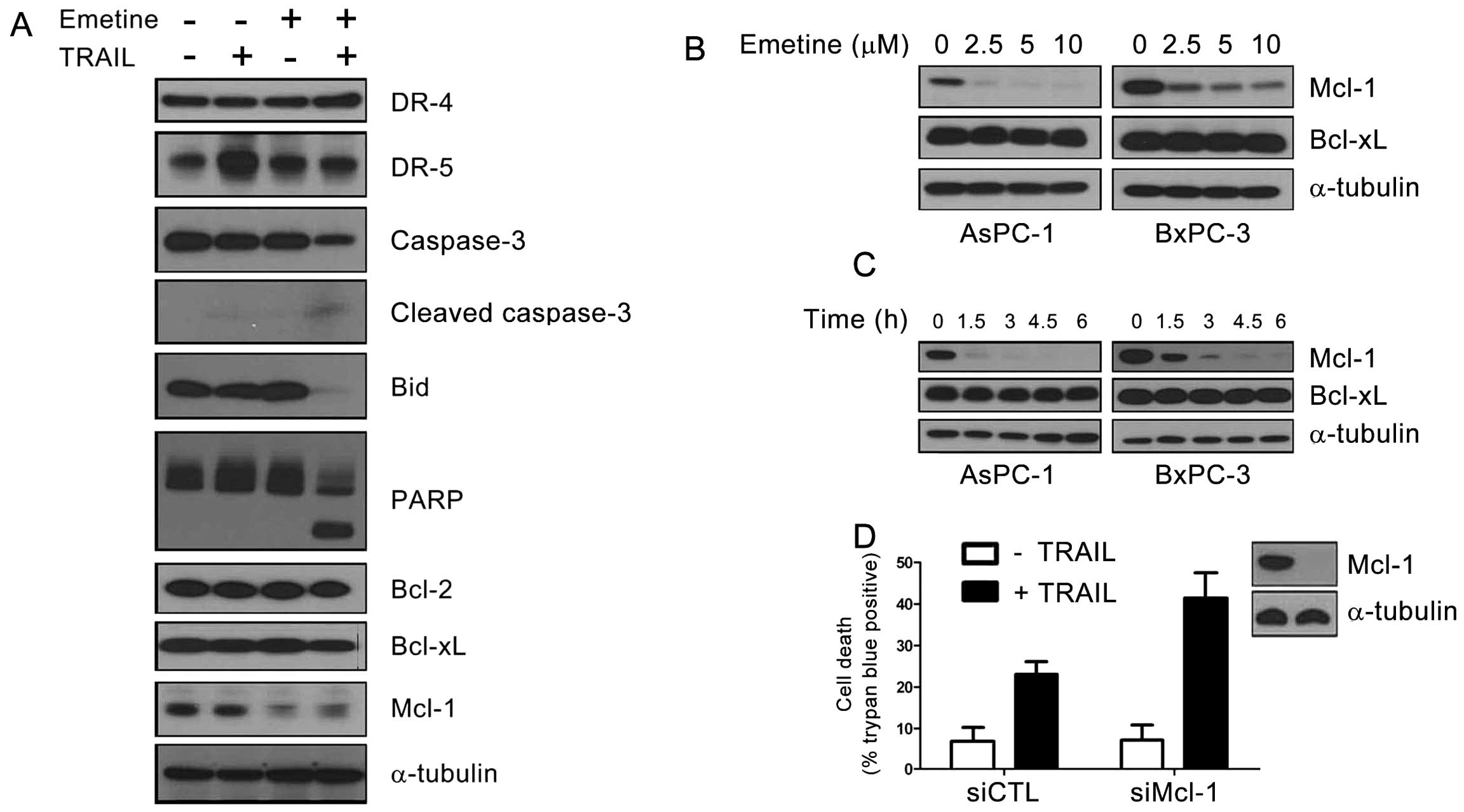
To characterize the mechanism by which emetine
enhances TRAIL-induced cell death, we attempted to identify
TRAIL-associated cell death proteins modulated by emetine in AsPC-1
cells. Western blot analysis showed that the levels of expression
of DR4, Bcl-2 and Bcl-XL were not altered by emetine or
TRAIL (Fig. 2). DR5 expression was
upregulated by TRAIL, but this upregulation was abolished by
emetine. However, the expression level of Mcl-1 protein was
strongly reduced by emetine treatment, with or without TRAIL
(Fig. 2A).
To determine whether Mcl-1 is downregulated in
response to emetine in different pancreatic cancer cells, we
examined the dose- and time-dependent effects of preincubation with
emetine, followed by incubation with TRAIL, on Mcl-1 expression in
AsPC-1 and BxPC-3 cells. We found that emetine downregulated Mcl-1
expression in both cancer cell lines in a dose- and time-dependent
manner (Fig. 2B and C). In both
cell lines, 2.5 μM emetine reduced Mcl-1 expression to <50%.
Moreover, after treatment with 2.5 μM of emetine, 1.5 h was
required for Mcl-1 expression in AsPC-1 and 3 h for BxPC-3 cells to
reach a minimum level (Fig.
2C).
Sensitization effect of emetine is
specific to TRAIL
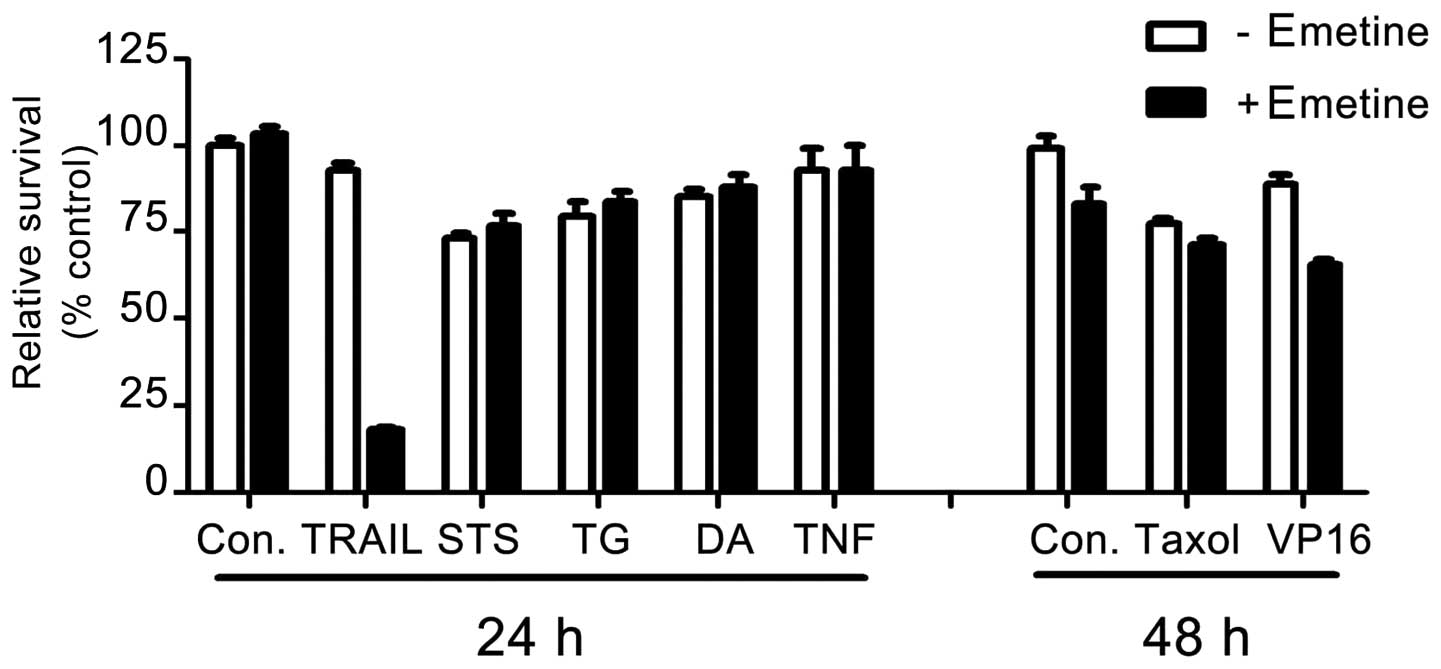
We next assessed whether the ability of emetine to
enhance cell death is specific to TRAIL signaling, or whether
emetine can sensitize cells to a broad spectrum of cell death
signaling, either intrinsic or extrinsic. Briefly, to test its
involvement in intrinsic cell death signaling, AsPC-1 cells were
pre-incubated in the presence or absence of 2.5 μM emetine,
followed by treatment with staurosporine (STS), a pan kinase
inhibitor; thapsigargin (TG), a SERCA inhibitor that induces ER
stress; daunorubicin (DA), a DNA damaging agent; taxol, a mitosis
inhibitor; or VP16, a DNA damaging agent. To test its involvement
in extrinsic cell death signaling, cells pre-incubated with emetine
were incubated with tumor necrosis factor α (TNF-α) or with TRAIL
as a control. We found that emetine sensitized AsPC-1 cells only to
TRAIL, not to any of these other agents (Fig. 3) implying that the cell death
sensitization effect of emetine is a specific event for
TRAIL-induced apoptotic signaling.
Emetine-induced Mcl-1 downregulation
sensitizes pancreatic cancer cells to TRAIL under hypoxic
conditions
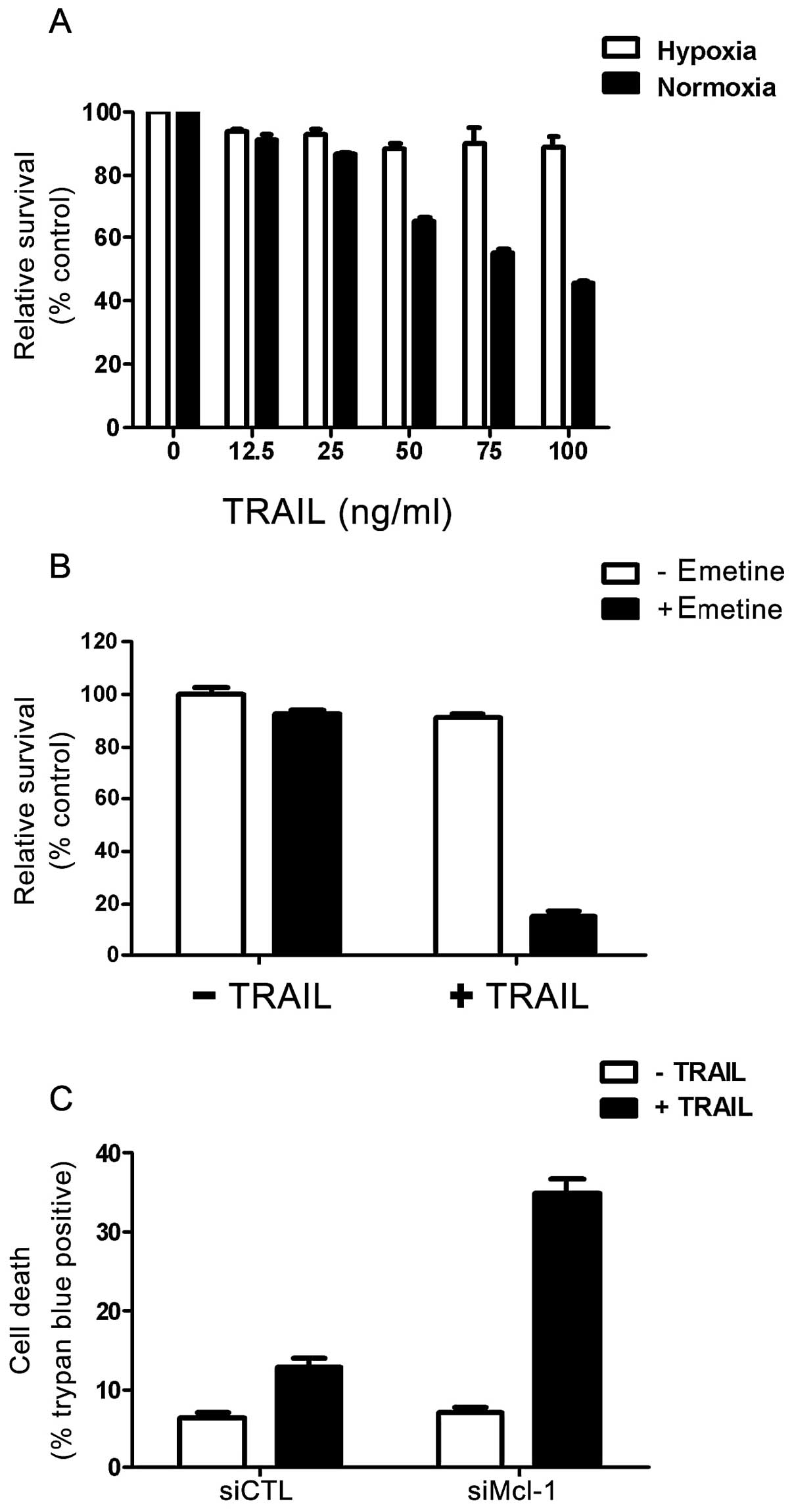
Tumor hypoxia is a situation in which primary tumor
cells are deprived of their oxygen supply. Desmoplasia, a
characteristic of pancreatic cancers, has been reported to reduce
blood supply and induce hypoxia. In general, hypoxic tumor cells
are resistant to radiotherapy and chemotherapy.
To assess the effect of a hypoxic environment on
TRAIL-induced pancreatic cancer cell death, we tested whether
hypoxia affects emetine-mediated sensitivity to TRAIL. We found
that TRAIL induced PANC-1 cell death in a dose-dependent manner,
with 100 ng/ml of TRAIL reducing PANC-1 cell viability to 45%, as
measured by the ATP content assay (Fig.
1A and 4A). Under hypoxic
conditions, the same concentration of TRAIL had little effect on
cell viability, suggesting that hypoxia is associated with the
mechanism of pancreatic cancer cell resistance to TRAIL.
We subsequently assessed whether hypoxia-induced
desensitization to TRAIL can be reversed by emetine. Importantly,
pretreatment of emetine significantly increased the sensitivity of
PANC-1 cells to TRAIL under a hypoxic condition (Fig. 4B). To determine whether this
increased sensitivity was mediated by downregulation of Mcl-1, we
knocked down Mcl-1 by treating PANC-1 cells with Mcl-1-specific
siRNA and measured the cell viability following treatment with
TRAIL (Fig. 4C). The cells showed
marked sensitivity to TRAIL treatment, suggesting that Mcl-1 is a
critical mediator of TRAIL-induced cell death in pancreatic cancer
cells under hypoxic conditions.
Discussion
The ultimate goal of oncology research is the
development of anticancer therapeutic regimes with little toxicity
to normal cells. TRAIL has a potent therapeutic window as a
tumor-specific agent (7,29,30),
but many primary tumors have been found to be resistant to TRAIL
(31,32). The development of TRAIL as a potent
anticancer therapeutic agent requires understanding of the
mechanism by which cells become resistant.
Inhibition of protein synthesis by cycloheximide and
emetine has been found to effectively sensitize cancer cells to
extrinsic cell death pathways. For example, TNF-α itself does not
induce cell death in various cell lines, due to anti-apoptotic
proteins that inhibit activation of the cell death pathway
triggered by TNF-α. Chemical inhibition of the synthesis of these
anti-apoptotic proteins has been found to sensitize these cells to
extrinsic cell death inducers, including TNF-α Fas ligand and
TRAIL. However, the main regulatory proteins targeted by
cycloheximide and emetine in their inhibition of protein synthesis
have not yet been clearly identified.
Emetine is a natural alkaloid derived from
Psychotria ipecacuanha that strongly inhibits the synthesis
of biomolecules. While cycloheximide is limited to in vitro
research, emetine has been widely used as an anti-amoebiasis drug
since the early 1900s (33).
Although the severe side-effects of emetine led to its replacement
by metronidazole (34), several
studies suggest that emetine may have anticancer drug activity
(35–38). Emetine was found to induce apoptosis
in U937 lymphoma cells (36),
A549-S lung cancer cells (37),
Jurkat T cell leukemia cells (39),
HL-60 promyelocytic leukemia cells (40) and rat hepatocytes (38). The cytotoxicity of emetine towards
cancer cells is due to its ability to inhibit protein synthesis in
eukaryotic cell ribosomes and to interact with DNA (35). In addition, recent reports have
shown that emetine regulates the level of expression of
apoptosis-associated genes.
Emetine has been found to control alternative
splicing of Bcl-x in cancer cells (41), downregulating anti-apoptotic
Bcl-XL mRNA and upregulating pro-apoptotic Bcl-xS mRNA,
and finally significantly reducing the Bcl-XL/Bcl-xS
ratio in MCF-7 breast cancer, PC-3 prostate cancer, C33A cervical
cancer and A549 lung cancer cell lines (41). Emetine has also been found to
upregulate the pro-apoptotic genes caspase-9, death associated
protein 6 (Daxx), granzyme B, caspase-8 and fas receptor (TNFRSF6)
in leukemia cells and to downregulate the anti-apoptotic genes
Bcl-2 and EGFR in Jurkat cells (42,43).
Our findings indicate that emetine sensitizes
pancreatic cancer cells to TRAIL-induced apoptosis by
downregulating the expression of Mcl-1 protein (Figs. 1 and 2). The effect of emetine was highly
specific to TRAIL since emetine did not have any effect on other
cell death-inducing agents involved in intrinsic and extrinsic
apoptosis signaling (Fig. 3). Our
knockdown approaches involving the targeting of Mcl-1 also strongly
support the specificity of emetine to TRAIL as Mcl-1 knockdown
effectively sensitized AsPC-1 cells to TRAIL. These results also
indicate that Mcl-1 is the main regulator of pancreatic cancer cell
resistance to TRAIL-induced apoptosis.
Significantly, only Mcl-1, but not other
apoptosis-related proteins, was downregulated in pancreatic cancer
cells by short-term exposure to emetine (Fig. 2). This result can be explained by
the fact that Mcl-1 is a fragile protein which is readily degraded
by the ubiquitin proteasome system with a very short half life
(44). Additionally, we tested
whether emetine can enhance ubiquitination of Mcl-1. However, we
found that emetine did not specifically increase the ubiquitination
of Mcl-1 (data not shown).
One characteristic of pancreatic cancer is the
desmoplastic reaction with strong hypoxia which limits cancer drug
delivery (45). Therefore, we also
assessed whether emetine-induced Mcl-1 downregulation effectively
sensitizes pancreatic cancer cells to TRAIL under hypoxic
conditions (Fig. 4). Although
PANC-1 cells are normally TRAIL-sensitive, they became resistant to
TRAIL-induced apoptosis under hypoxic conditions (Fig. 4A). We found, however, that emetine
re-sensitized these PANC-1 cells to TRAIL under hypoxic conditions
in an Mcl-1-dependent manner (Fig. 4B
and C). These findings, therefore, suggest that: i) Mcl-1 is a
critical regulator of TRAIL resistance in pancreatic cancer cells,
under both normoxic and hypoxic conditions; and that ii) treatment
with emetine is a promising method of downregulating Mcl-1 under
hypoxic conditions and sensitizing cells to TRAIL. Emetine
sensitization of cancer cells to TRAIL via Mcl-1 downregulation may
be limited to pancreatic cancer cells, a possibility that requires
further investigation.
In summary, we found that emetine sensitizes
pancreatic cancer cells, which were initially resistant to TRAIL,
to TRAIL-induced apoptosis. Although emetine was previously shown
to sensitize cells by inhibiting protein synthesis, we demonstrated
in the present study, for the first time, that the downregulation
of the expression of Mcl-1 protein by emetine is a key factor in
sensitizing pancreatic cancer cells to TRAIL. Our findings also
indicate that Mcl-1 downregulation may be a promising target for
sensitizing pancreatic cancers to TRAIL.
Acknowledgements
The present study was supported, in part, by the
International Research & Development Program of the National
Research Foundation of Korea (NRF), which is funded by the Ministry
of Education, Science and Technology (MEST) of Korea (grant no.
2012K1A3A0704533 to I.K.K.) and was supported, in part, by a
general research grant of the National Research Foundation of Korea
(NRF-2013R1A1A1007693 to K.W.K.) and by a grant (2013-512) from the
Asan Institute for Life Science, Seoul, Korea.
References
|
1
|
Schneider G, Siveke JT, Eckel F and Schmid
RM: Pancreatic cancer: basic and clinical aspects.
Gastroenterology. 128:1606–1625. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wong HH and Lemoine NR: Pancreatic cancer:
molecular pathogenesis and new therapeutic targets. Nat Rev
Gastroenterol Hepatol. 6:412–422. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hamacher R, Schmid RM, Saur D and
Schneider G: Apoptotic pathways in pancreatic ductal
adenocarcinoma. Mol Cancer. 7:642008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Favaloro B, Allocati N, Graziano V, Di
Ilio C and De Laurenzi V: Role of apoptosis in disease. Aging
(Albany NY). 4:330–349. 2012.
|
|
5
|
Lavrik I, Golks A and Krammer PH: Death
receptor signaling. J Cell Sci. 118:265–267. 2005. View Article : Google Scholar
|
|
6
|
Pan G, O’Rourke K, Chinnaiyan AM, et al:
The receptor for the cytotoxic ligand TRAIL. Science. 276:111–113.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ashkenazi A and Dixit VM: Apoptosis
control by death and decoy receptors. Curr Opin Cell Biol.
11:255–260. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pitti RM, Marsters SA, Ruppert S, Donahue
CJ, Moore A and Ashkenazi A: Induction of apoptosis by Apo-2
ligand, a new member of the tumor necrosis factor cytokine family.
J Biol Chem. 271:12687–12690. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bellail AC, Qi L, Mulligan P, Chhabra V
and Hao C: TRAIL agonists on clinical trials for cancer therapy:
the promises and the challenges. Rev Recent Clin Trials. 4:34–41.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Van Geelen CM, de Vries EG and de Jong S:
Lessons from TRAIL-resistance mechanisms in colorectal cancer
cells: paving the road to patient-tailored therapy. Drug Resist
Updat. 7:345–358. 2004.PubMed/NCBI
|
|
11
|
Bhojani MS, Rossu BD and Rehemtulla A:
TRAIL and anti-tumor responses. Cancer Biol Ther. 2:S71–S78. 2003.
View Article : Google Scholar
|
|
12
|
Koschny R, Walczak H and Ganten TM: The
promise of TRAIL - potential and risks of a novel anticancer
therapy. J Mol Med (Berl). 85:923–935. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang P, Zhang J, Bellail A, et al:
Inhibition of RIP and c-FLIP enhances TRAIL-induced apoptosis in
pancreatic cancer cells. Cell Signal. 19:2237–2246. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hylander BL, Pitoniak R, Penetrante RB, et
al: The anti-tumor effect of Apo2L/TRAIL on patient pancreatic
adenocarcinomas grown as xenografts in SCID mice. J Transl Med.
3:222005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang Y and Zhang B: TRAIL resistance of
breast cancer cells is associated with constitutive endocytosis of
death receptors 4 and 5. Mol Cancer Res. 6:1861–1871. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fisher MJ, Virmani AK, Wu L, et al:
Nucleotide substitution in the ectodomain of trail receptor DR4 is
associated with lung cancer and head and neck cancer. Clin Cancer
Res. 7:1688–1697. 2001.PubMed/NCBI
|
|
17
|
Kim K, Fisher MJ, Xu SQ and el-Deiry WS:
Molecular determinants of response to TRAIL in killing of normal
and cancer cells. Clin Cancer Res. 6:335–346. 2000.PubMed/NCBI
|
|
18
|
Shin MS, Kim HS, Lee SH, et al: Mutations
of tumor necrosis factor-related apoptosis-inducing ligand receptor
1 (TRAIL-R1) and receptor 2 (TRAIL-R2) genes in metastatic breast
cancers. Cancer Res. 61:4942–4946. 2001.
|
|
19
|
Nakshatri H, Rice SE and Bhat-Nakshatri P:
Antitumor agent parthenolide reverses resistance of breast cancer
cells to tumor necrosis factor-related apoptosis-inducing ligand
through sustained activation of c-Jun N-terminal kinase. Oncogene.
23:7330–7344. 2004. View Article : Google Scholar
|
|
20
|
Ryu BK, Lee MG, Chi SG, Kim YW and Park
JH: Increased expression of cFLIP(L) in colonic adenocarcinoma. J
Pathol. 194:15–19. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Okano H, Shiraki K, Inoue H, et al:
Cellular FLICE/caspase-8-inhibitory protein as a principal
regulator of cell death and survival in human hepatocellular
carcinoma. Lab Invest. 83:1033–1043. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fulda S, Meyer E and Debatin KM:
Inhibition of TRAIL-induced apoptosis by Bcl-2 overexpression.
Oncogene. 21:2283–2294. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Eggert A, Grotzer MA, Zuzak TJ, et al:
Resistance to tumor necrosis factor-related apoptosis-inducing
ligand (TRAIL)-induced apoptosis in neuroblastoma cells correlates
with a loss of caspase-8 expression. Cancer Res. 61:1314–1319.
2001.
|
|
24
|
Daniel PT, Wieder T, Sturm I and
Schulze-Osthoff K: The kiss of death: promises and failures of
death receptors and ligands in cancer therapy. Leukemia.
15:1022–1032. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gasparian ME, Chernyak BV, Dolgikh DA, et
al: Generation of new TRAIL mutants DR5-A and DR5-B with improved
selectivity to death receptor 5. Apoptosis. 14:778–787. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gasparian ME, Ostapchenko VG, Yagolovich
AV, et al: Overexpression and refolding of thioredoxin/TRAIL fusion
from inclusion bodies and further purification of TRAIL after
cleavage by enteropeptidase. Biotechnol Lett. 29:1567–1573. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sah NK, Munshi A, Kurland JF, McDonnell
TJ, Su B and Meyn RE: Translation inhibitors sensitize prostate
cancer cells to apoptosis induced by tumor necrosis factor-related
apoptosis-inducing ligand (TRAIL) by activating c-Jun N-terminal
kinase. J Biol Chem. 278:20593–20602. 2003. View Article : Google Scholar
|
|
28
|
Hellwig CT, Kohler BF, Lehtivarjo AK, et
al: Real time analysis of tumor necrosis factor-related
apoptosis-inducing ligand/cycloheximide-induced caspase activities
during apoptosis initiation. J Biol Chem. 283:21676–21685. 2008.
View Article : Google Scholar
|
|
29
|
Walczak H, Miller RE, Ariail K, et al:
Tumoricidal activity of tumor necrosis factor-related
apoptosis-inducing ligand in vivo. Nat Med. 5:157–163. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ashkenazi A, Pai RC, Fong S, et al: Safety
and antitumor activity of recombinant soluble Apo2 ligand. J Clin
Invest. 104:155–162. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mahalingam D, Szegezdi E, Keane M, de Jong
S and Samali A: TRAIL receptor signalling and modulation: are we on
the right TRAIL? Cancer Treat Rev. 35:280–288. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Smyth MJ, Takeda K, Hayakawa Y, Peschon
JJ, van den Brink MR and Yagita H: Nature’s TRAIL - on a path to
cancer immunotherapy. Immunity. 18:1–6. 2003.
|
|
33
|
Lambert AC: The treatment of amoebic
dysentery with emetine and bismuth iodide. Br Med J. 1:116–118.
1918. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mackey-Lawrence NM and Petri WA Jr:
Amoebic dysentery. Clin Evid (Online). 2011:pii 0918. 2011.
|
|
35
|
Grollman AP: Structural basis for
inhibition of protein synthesis by emetine and cycloheximide based
on an analogy between ipecac alkaloids and glutarimide antibiotics.
Proc Natl Acad Sci USA. 56:1867–1874. 1966. View Article : Google Scholar
|
|
36
|
Bicknell GR, Snowden RT and Cohen GM:
Formation of high molecular mass DNA fragments is a marker of
apoptosis in the human leukaemic cell line, U937. J Cell Sci.
107:2483–2489. 1994.PubMed/NCBI
|
|
37
|
Watanabe N, Iwamoto T, Dickinson DA, Iles
KE and Forman HJ: Activation of the mitochondrial caspase cascade
in the absence of protein synthesis does not require c-Jun
N-terminal kinase. Arch Biochem Biophys. 405:231–240. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Meijerman I, Blom WM, de Bont HJ, Mulder
GJ and Nagelkerke JF: Induction of apoptosis and changes in nuclear
G-actin are mediated by different pathways: the effect of
inhibitors of protein and RNA synthesis in isolated rat
hepatocytes. Toxicol Appl Pharmacol. 156:46–55. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Moller M, Weiss J and Wink M: Reduction of
cytotoxicity of the alkaloid emetine through P-glycoprotein
(MDR1/ABCB1) in human Caco-2 cells and leukemia cell lines. Planta
Med. 72:1121–1126. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rosenkranz V and Wink M: Alkaloids induce
programmed cell death in bloodstream forms of trypanosomes
(Trypanosoma b. brucei). Molecules. 13:2462–2473.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Boon-Unge K, Yu Q, Zou T, Zhou A,
Govitrapong P and Zhou J: Emetine regulates the alternative
splicing of Bcl-x through a protein phosphatase 1-dependent
mechanism. Chem Biol. 14:1386–1392. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Moller M, Herzer K, Wenger T, Herr I and
Wink M: The alkaloid emetine as a promising agent for the induction
and enhancement of drug-induced apoptosis in leukemia cells. Oncol
Rep. 18:737–744. 2007.PubMed/NCBI
|
|
43
|
Pan D, Boon-Unge K, Govitrapong P and Zhou
J: Emetine regulates the alternative splicing of caspase 9 in tumor
cells. Oncol Lett. 2:1309–1312. 2011.PubMed/NCBI
|
|
44
|
Zhong Q, Gao W, Du F and Wang X:
Mule/ARF-BP1, a BH3-only E3 ubiquitin ligase, catalyzes the
polyubiquitination of Mcl-1 and regulates apoptosis. Cell.
121:1085–1095. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Koong AC, Mehta VK, Le QT, et al:
Pancreatic tumors show high levels of hypoxia. Int J Radiat Oncol
Biol Phys. 48:919–922. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Oh B, Park S, Pak JH and Kim I:
Downregulation of Mcl-1 by daunorubicin pretreatment reverses
resistance of breast cancer cells to TNF-related apoptosis-inducing
ligand. Biochem Biophys Res Commun. 422:42–47. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kelly MM, Hoel BD and Voelkel-Johnson C:
Doxorubicin pretreatment sensitizes prostate cancer cell lines to
TRAIL induced apoptosis which correlates with the loss of c-FLIP
expression. Cancer Biol Ther. 1:520–527. 2002. View Article : Google Scholar
|
|
48
|
Kim DR, Park MY, Lee CS, et al:
Combination of vorinostat and adenovirus-TRAIL exhibits a
synergistic antitumor effect by increasing transduction and
transcription of TRAIL in lung cancer cells. Cancer Gene Ther.
18:467–477. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ciusani E, Croci D, Gelati M, et al: In
vitro effects of topotecan and ionizing radiation on
TRAIL/Apo2L-mediated apoptosis in malignant glioma. J Neurooncol.
71:19–25. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Yoo J, Park SS and Lee YJ: Pretreatment of
docetaxel enhances TRAIL-mediated apoptosis in prostate cancer
cells. J Cell Biochem. 104:1636–1646. 2008. View Article : Google Scholar : PubMed/NCBI
|