Introduction
Hepatocellular carcinoma (HCC) is the third leading
cause of cancer-related deaths and the fifth most common cancer
worldwide, particularly in countries with a high prevalence of
chronic hepatitis virus infections (1). Despite recent advances in the
diagnosis and treatment of HCC, it remains a highly lethal disease.
The main reason for mortality in HCC patients is tumor progression
with metastasis (2). Liver fibrosis
is strongly associated with HCC, with 90% of HCC cases arising in
cirrhotic livers, and the presence of liver cirrhosis is the main
risk factor for the development of HCC (3,4).
The liver tumor microenvironment is a complex
mixture of tumoral cells within the extracellular matrix (ECM),
combined with stromal cells and the proteins they secrete.
Together, these elements contribute to the carcinogenic process
(5). Hepatic stellate cells (HSCs),
which were previously known as Ito cells, are pericytes found
within the perisinusoidal space of the liver. During liver injury
due to viral infection or long-term insult of hepatotoxins, HSCs
become activated to myofibroblast-like cells, leading to the
development of hepatic fibrosis and finally, cirrhosis (6,7).
Activated HSCs, belonging to one of the most important stromal cell
types in the liver tumor environment, are infiltrated in the stroma
of HCC and are localized around tumor sinusoids, fibrous septa and
capsules (8,9). They are responsible for the remodeling
and deposition of tumor-associated ECM and altered expression of
growth factors in the tumor environment (10). Several studies have demonstrated
that activated HSCs promote HCC growth and invasiveness. Moreover,
the bidirectional interactions between tumors and HSCs further
enhance metastatic growth in the liver (11,12).
However, the underlying mechanisms by which activated HSCs exert
their oncogenic effects are still not fully understood.
Focal adhesion kinase (FAK), a non-receptor
cytoplasmic protein tyrosine kinase, plays a central role in a
number of cell events including cell proliferation, survival,
migration and invasion (13,14).
Multiple signals, including integrins clustering in response to ECM
and engagement of various growth factor receptors, initiate
autophosphorylation of FAK at tyrosine (Tyr) 397 leading to FAK
activation. Activation of FAK further mediates the induction of
invasive pathways involving signaling to RAC1, JUN N-terminal
kinase (JNK), and matrix metalloproteinases (MMPs) (14,15).
Consistent with the biological functions of FAK, studies have shown
that elevated FAK expression and activity are associated with HCC
metastasis and poor patient prognosis (16,17).
Upregulation of FAK-MMP9 signaling is considered to be one of the
main pathways that promotes HCC cell invasion and metastasis
(18,19).
In the present study, we investigated whether
activated HSCs promote HCC cell migration and invasion via
activating FAK-MMP9 signaling.
Materials and methods
Patients and specimens
Fifty-two fresh tumor samples were collected from
HCC patients who underwent curative resection between 2008 and 2010
at the First Affiliated Hospital of the Medical College, Xi’an
Jiaotong University (Xi’an, China). Resected tumors and
corresponding non-tumor tissue specimens (at least 2 cm away from
the tumors) were immediately cut from the resected liver and fixed
in buffered paraformaldehyde for immunohistochemical study. None of
the patients had received prior radiotherapy or chemotherapy before
the sampling. The clinical data of these patients were obtained
from the medical records and are listed in Table I. The tumor-node-metastasis (TNM)
staging (6th edition), histopathologic Edmonson’s classification,
vascular invasion and the normal tumor-adjacent tissues were all
confirmed by an experienced pathologist who was blinded to the
clinical information. Informed consents were obtained from all
patients recruited into this study. This study was approved by the
Ethics Committee of the First Affiliated Hospital of the College of
Medicine, Xi’an Jiaotong University (Xi’an, China), according to
the Helsinki Declaration of 1975.
 | Table ICorrelation between α-SMA-positive
HSCs and clinicopathologic characteristics in the 52 HCC
patients. |
Table I
Correlation between α-SMA-positive
HSCs and clinicopathologic characteristics in the 52 HCC
patients.
| | α-SMA-positive
HSCs | | |
|---|
| |
| | |
|---|
| Clinicopathologic
parameters | N | + | ++ | +++ | ++++ | R-value | P-value |
|---|
| Age (years) |
| ≤55 | 25 | 1 | 6 | 10 | 8 | −0.117 | 0.410 |
| >55 | 27 | 4 | 7 | 8 | 8 | | |
| Gender |
| Male | 43 | 3 | 13 | 13 | 14 | 0.021 | 0.881 |
| Female | 9 | 2 | 0 | 5 | 2 | | |
| Tumor size (cm) |
| <5 | 16 | 1 | 5 | 4 | 6 | −0.046 | 0.744 |
| ≥5 | 36 | 4 | 8 | 14 | 10 | | |
| No. of tumors |
| Solitary | 47 | 4 | 13 | 16 | 14 | 0.066 | 0.642 |
| Multiple | 5 | 1 | 0 | 2 | 2 | | |
| Edmonson’s
staging |
| I–II | 36 | 5 | 12 | 11 | 8 | 0.397 | 0.004a |
| III–IV | 16 | 0 | 1 | 7 | 8 | | |
| TNM staging |
| I | 35 | 3 | 13 | 12 | 7 | 0.346 | 0.012a |
| II–III | 17 | 2 | 0 | 6 | 9 | | |
| Vascular
invasion |
| Absent | 40 | 5 | 13 | 15 | 7 | 0.525 | <0.001a |
| Present | 12 | 0 | 0 | 3 | 9 | | |
| AFP (ng/ml) |
| ≤20 | 21 | 1 | 4 | 9 | 7 | −0.146 | 0.301 |
| >20 | 31 | 4 | 9 | 9 | 9 | | |
| HBsAg |
| Positive | 40 | 4 | 10 | 14 | 12 | −0.029 | 0.840 |
| Negative | 12 | 1 | 3 | 4 | 4 | | |
Immunohistochemical staining
The sections were dewaxed, rehydrated. Antigen
retrieval was carried out in citrate buffer and the sections were
washed. After neutralization of endogenous peroxidase and blocking
of nonspecific binding, sections were separately incubated
overnight at 4°C with the following primary antibodies: rabbit
anti-phospho-FAK Tyr 397 (p-FAK) (1:100; Abcam, Cambridge, MA,
USA), rabbit anti-MMP9 antibody (1:200; Santa Cruz Biotechnology,
Inc., Santa Cruz, CA, USA) and mouse anti-α-smooth muscle actin
(α-SMA) antibody (1:100; Dako, Carpinteria, CA, USA). Subsequently,
the sections were serially rinsed and incubated with biotinylated
secondary antibodies (Golden Bridge Biotechnology, Beijing, China)
according to the manufacturer’s instructions. The sections were
visualized with diaminobenzidine and counterstained with
hematoxylin. For the negative controls, the primary antibody was
replaced using phosphate-buffered saline (PBS). The degree of
immunohistochemical staining was evaluated independently by 2
observers. The results for p-FAK and MMP9 were semiquantitatively
expressed using an immunohistochemical score combined with the
percentage of liver cells showing specific immunoreactivity
(20). Activated HSCs were
identified by their locations, morphologic features and cytoplasmic
expression of α-SMA. Areas of vessels, Glisson’s capsules, fibrous
septa, and collapsed parenchyma were not assessed (21). The number of activated HSCs was
evaluated semiquantitatively as follows: grade 0, no positive
cells; +, rare positive cells that require careful searching at
high power; ++, scattered positive cells easily identified at
medium power; +++, scattered or clustered positive cells apparent
at low power; and ++++, wide spread positive cells apparent at low
power (22).
Cells and cell culture
The human liver cancer cell lines, Hep3B and HepG2,
were obtained from the American Type Culture Collection (Manassas,
VA, USA). Both cell lines were cultured in complete Dulbecco’s
modified Eagle’s medium (DMEM) (Invitrogen Life Technologies,
Carlsbad, CA, USA) with 10% fetal bovine serum (FBS). Human primary
HSCs were isolated from human resection specimens (ScienCell, San
Diego, CA, USA). The HSCs were cultured on uncoated plastic plates
in DMEM supplemented with 10% FBS and incubated at 37°C in 5%
CO2. In vitro activation of HSCs was gradually
achieved during passages. Activated HSCs were used at passages 6–8
for the following experiments. For preparation of conditioned
medium, HSCs were grown to 60–70% confluency in 100-mm flasks.
Cells were washed 3 times with PBS and then incubated in 10 ml of
DMEM containing 0.2% FBS for 24 h. Conditioned media were
harvested, centrifuged at 1,000 rpm for 10 min to remove cellular
debris, filtered through a 0.22-μm filter and stored at 4°C.
FAK short hairpin RNA (shRNA) vector and
gene transfection
The recombinant plasmid vectors expressing the
double-stranded shRNA targeting FAK or the non-target shRNA control
(NT shRNA) were purchased from Wolsen Biotechnology (Xi’an, China).
Two sets of FAK shRNA vectors, designated as FAK shRNA-1 and FAK
shRNA-2, were tested and proven to be efficient in suppressing FAK
expression in HCC cells. The target sequences used for FAK shRNA
constructs were shRNA-1 (GCCACCTGGGCCAGTATTA) and shRNA-2
(GCGGCCCAGGTTTACTGAA). HCC cells were grown to 60–80% confluence
and transfected with FAK shRNA vectors or the NT shRNA vector using
FuGENE6 Transfection Reagent (Roche Diagnostics, Indianapolis, IN,
USA) for 24, 48 or 72 h, in accordance with the manufacturer’s
protocol. The transfection rate was assessed by counting the number
of positive cells under a fluorescence microscope, which could
reach up to 90%.
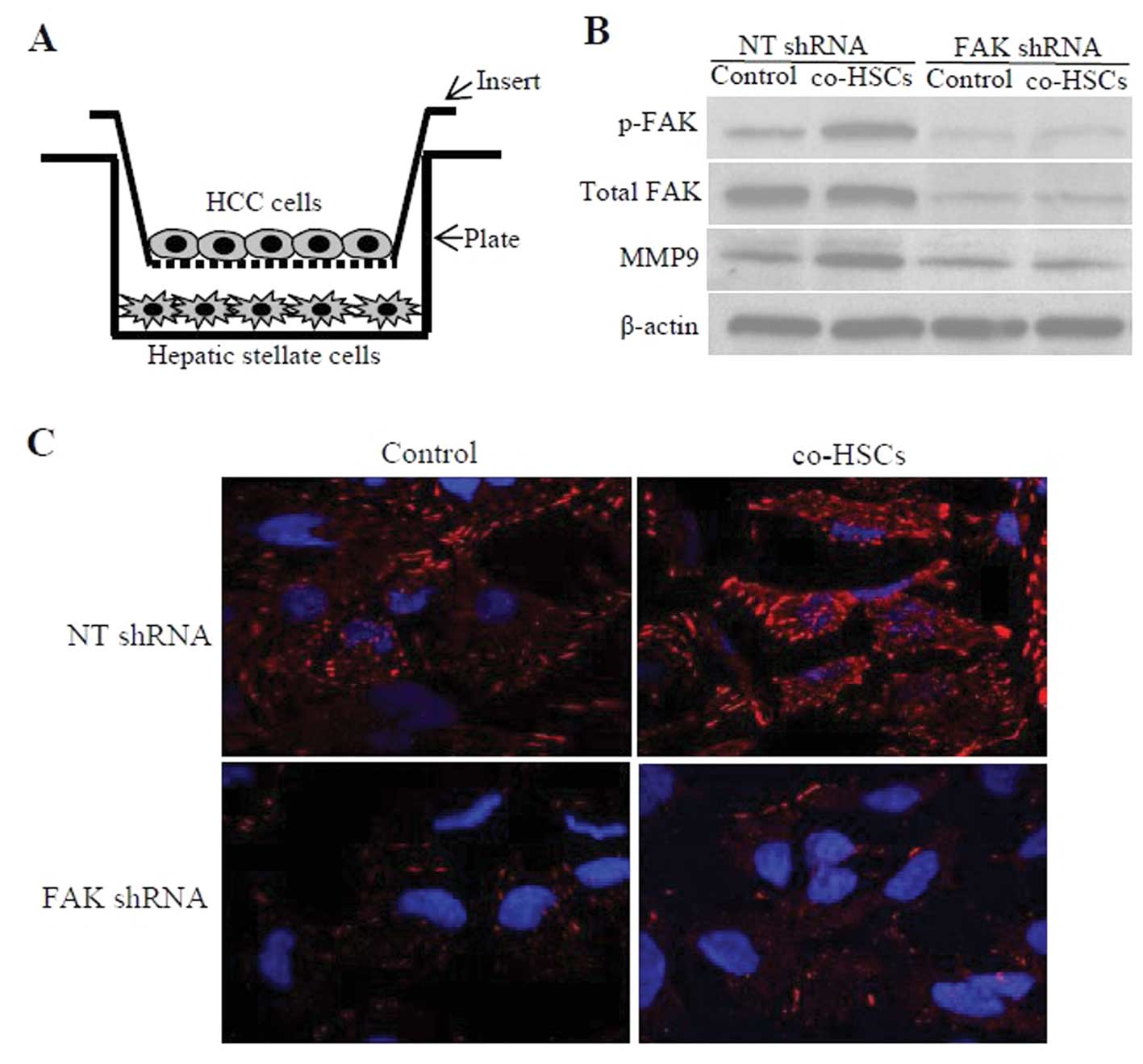
Co-culture experiments
In vitro activated HSCs were cultured in
6-well dishes in medium containing 10% FBS for 12 h at
2×105 cells/well and then maintained in serum-free
medium for 18 h prior to the co-culture experiment. HCC cells
transfected with the FAK shRNA or NT shRNA were cultured on 0.4-μm
pore size cell culture inserts (BD Biosciences, San Jose, CA, USA)
for 12 h at 1×105 cells/well and then maintained in
serum-free medium for 18 h prior to the co-culture experiment.
Inserts were placed in the companion plate for 48 h for the
co-culture experiments (Fig. 2B),
which allowed diffusion of the medium components but prevent cell
migration. Independent culture experiments were carried out at
least in triplicate.
Immunocytochemistry and confocal
microscopy
Cells were grown on glass coverslips for 48 h,
rinsed with Dulbecco’s PBS (D-PBS) at room temperature, and fixed
with 4% paraformaldehyde and permeabilized in 0.2% Triton X-100.
Goat serum (10%) was applied to prevent nonspecific binding.
Subsequently, HCC cells were incubated with the anti-p-FAK antibody
(1:100 for cells) overnight at 4°C. After washing with PBS, the
primary antibodies were labeled by Alexa 594 goat anti-mouse/rabbit
IgG (1:1000; Invitrogen Life Technologies) for 1 h at room
temperature. Then cells were mounted with DAPI and examined by
confocal microscopy. For the negative control, the primary antibody
was replaced using PBS.
Western blot analysis
Total protein was extracted from the HCC cells
exposed to conditioned medium from the activated HSCs or
co-cultured with activated HSCs. The protein concentration was
quantified using a BCA protein assay kit (Pierce Biotechnology,
Inc., Rockford, IL, USA). Equal amounts of proteins (20 μg/lane)
were separated on sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) and transferred to polyvinylidene
fluoride (PVDF) membranes. After blocking for 1 h with 5% BSA and
washed with TBST, the membranes were incubated overnight at 4°C
with a primary antibody against p-FAK (1:500), FAK (1:1,000; Cell
Signalling Technology, Inc., Danvers, MA, USA), MMP9 (1:1,000) and
β-actin (1:1,000; Santa Cruz Biotechnology, Inc.). Subsequently,
the membranes were incubated with goat anti-mouse or goat
anti-rabbit horseradish peroxidase (HRP)-conjugated secondary
antibodies (1:10,000) for 1 h at room temperature. The blots were
then detected by SuperSignal West Pico chemiluminescent substrate
kit (Millipore, Billerica, MA, USA) and exposed to X-ray film.
β-actin was measured to control for equal loading.
Cell migration and invasion assay
The migratory ability of HCC cells exposed to
conditioned medium harvested from activated HSCs was measured by
wound healing assay. HCC cells transfected with FAK shRNA or NT
shRNA were grown to 90–100% confluence in 6-well plates. Cell
monolayers were wounded with a sterile 200-μl pipette tip and then
rinsed with PBS to remove cellular debris. The wounded monolayers
were cultured in conditioned medium or in control medium and
photographed with a phase-contrast microscope at 0, 24 and 48 h.
Cell migration was quantitated by measuring the width of the
wounds. The invasive activity of HCC cells was assessed using
24-well Transwell inserts with a 8-μm pore size coated with
Matrigel (1 mg/ml; BD Biosciences). Forty-eight hours after shRNA
transfection, 2.5×104 HCC cells in serum-free medium
were added into the upper chamber. Conditioned medium from
activated HSCs supplemented with 1% FBS was placed in the lower
chambers as chemoattractants. After a 24-h incubation at 37°C, the
cells remaining on the upper surface of the filter were wiped away
with a cotton swab. The migrated cells, adhering to the lower
surface of the membrane, were fixed with 100% methanol, stained
with crystal violet and counted under a light microscope by
randomly selecting 5 fields/filter. Each experiment was carried out
in triplicate wells and repeated at least 3 times.
Statistical analysis
Statistical analyses were performed with the SPSS
16.0 software. For continuous variables, data are expressed as the
means ± SD. Comparison between groups was carried out using the
Student’s unpaired t-test. The correlations between the number of
activated HSCs and clinicopathological features or p-FAK and MMP9
protein expression were analyzed using Spearman’s rank correlation
coefficient test. Values of P<0.05 were considered to be
statistically significant.
Results
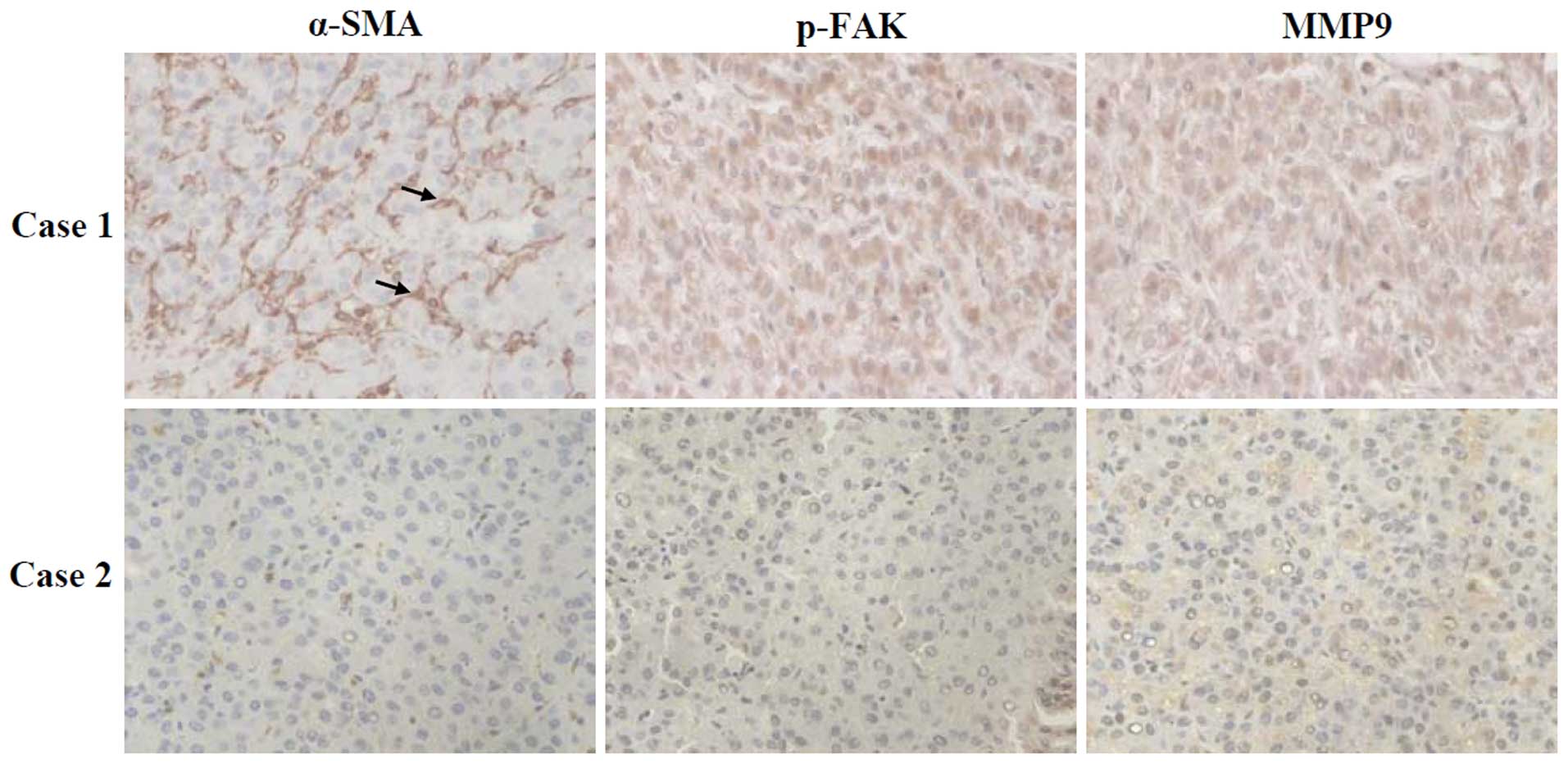
The number of activated HSCs in HCC and
its clinicopathologic significance
The number and distribution of α-SMA-positive
activated HSCs in 52 pairs of HCC tumor and adjacent non-tumorous
tissues were detected by immunohistochemical staining. The
α-SMA-positive activated HSCs were spindle-shaped and were present
within the perisinusoidal space and carcinomatous nodules (Fig. 1, left panels). The number of
activated HSCs identified within hepatocellular carcinoma was more
than that noted in the adjacent non-tumorous tissues. To assess the
significance of activated HSCs in HCC, the correlation between
activated HSCs and the clinicopathological characteristics of the
52 HCC patients was statistically analyzed and the results are
listed in Table I. Patients with a
higher number of activated HSCs in HCC tissues were prone to have
high rates of vascular invasion, advanced TNM staging and poorer
tumor differentiation (P<0.05). However, no significant
correlation was found between the number of activated HSCs and age,
gender, HBV infection, α-fetoprotein (AFP), tumor number and tumor
size.
The number of activated HSCs is
positively correlated with FAK-MMP9 signaling in HCC
To investigate whether the number of activated HSCs
is associated with FAK-MMP9 signaling in HCC, we further determined
the expression of p-FAK and MMP9 by immunohistochemical staining.
The grading of the extent of activated HSCs, p-FAK and MMP9 are
summarized in Table II. Results
showed that p-FAK protein was positively stained in the cytoplasm
of carcinoma cells (Fig. 1, middle
panels), and 53.8% (n=28) of the HCC tumor samples showed positive
staining of p-FAK protein. The number of activated HSCs was
positively correlated with the p-FAK protein expression level in
the HCC tissues (r=0.475, P<0.001), as assessed by Spearman’s
rank correlation coefficient test (Table II). MMP9 protein was diffuse with
moderate or strong staining in the cytoplasm of tumor cells
(Fig. 1, right panels). Positive
staining of MMP9 protein was found in 69.2% (n=36) of the HCC
tissues. The number of activated HSCs was also positively
correlated with MMP9 protein expression level in the HCC tissues
(r=0.446, P<0.001) (Table
II).
 | Table IICorrelation between α-SMA-positive HSCs and p-FAK and MMP9
protein expression in 52 HCC patients. |
Table II
Correlation between α-SMA-positive HSCs and p-FAK and MMP9
protein expression in 52 HCC patients.
| α-SMA-positive HSCs | p-FAK protein | MMP9 protein |
|---|
|
|
|---|
| − | + | ++ | +++ | − | + | ++ | +++ |
|---|
| + | 4 | 0 | 1 | 0 | 4 | 1 | 0 | 0 |
| ++ | 10 | 2 | 0 | 1 | 6 | 7 | 0 | 0 |
| +++ | 8 | 5 | 3 | 2 | 2 | 5 | 8 | 3 |
| ++++ | 2 | 6 | 7 | 1 | 4 | 3 | 7 | 2 |
| R-value | 0.475 | | | | 0.446 | | | |
| P-value | <0.001 | | | | 0.001 | | | |
Activated HSCs induce activation of
FAK-MMP9 signaling in HCC cells
In order to study the interaction between activated
HSCs and HCC cells, a co-culture system was used. Cells were
cultured using 0.4-μm pore size cell culture inserts that separate
both cell populations, which allow diffusion of medium components
but prevent cell migration. Activated HSCs were plated at the
bottom of the well plate, and HCC cells transfected with FAK shRNA
or NT shRNA were plated on the insert (Fig. 2A). Optimal co-culture conditions
were identified in preliminary studies. The most reproducible and
consistent results were obtained at the HCC cells:HSCs ratio of
1:2. HCC cells were transfected with FAK shRNA or NT shRNA, and FAK
mRNA expression levels were measured by real-time PCR at 48 h after
transfection. Cells transfected with FAK shRNA had a >90%
reduction in FAK mRNA levels compared to cells transfected with NT
shRNA (data not shown). Western blotting showed that at 48 h after
co-culture, HCC cells (NT shRNA group) co-cultured with the
activated HSCs exhibited significantly higher levels of p-FAK and
MMP9 expression when compared to these levels in cells cultured in
control medium. However, inhibition of FAK in HCC cells with FAK
shRNA dramatically decreased levels of p-FAK and MMP9 expression,
and abrogated the differences caused by coculture (Fig. 2B). Furthermore, these results were
confirmed by immunocytochemistry performed on HCC cells co-cultured
with activated HSCs (Fig. 2C).
Consistent with the co-culture experiments, conditioned medium from
activated HSCs also significantly increased the expression of p-FAK
and MMP9 in HCC cells.
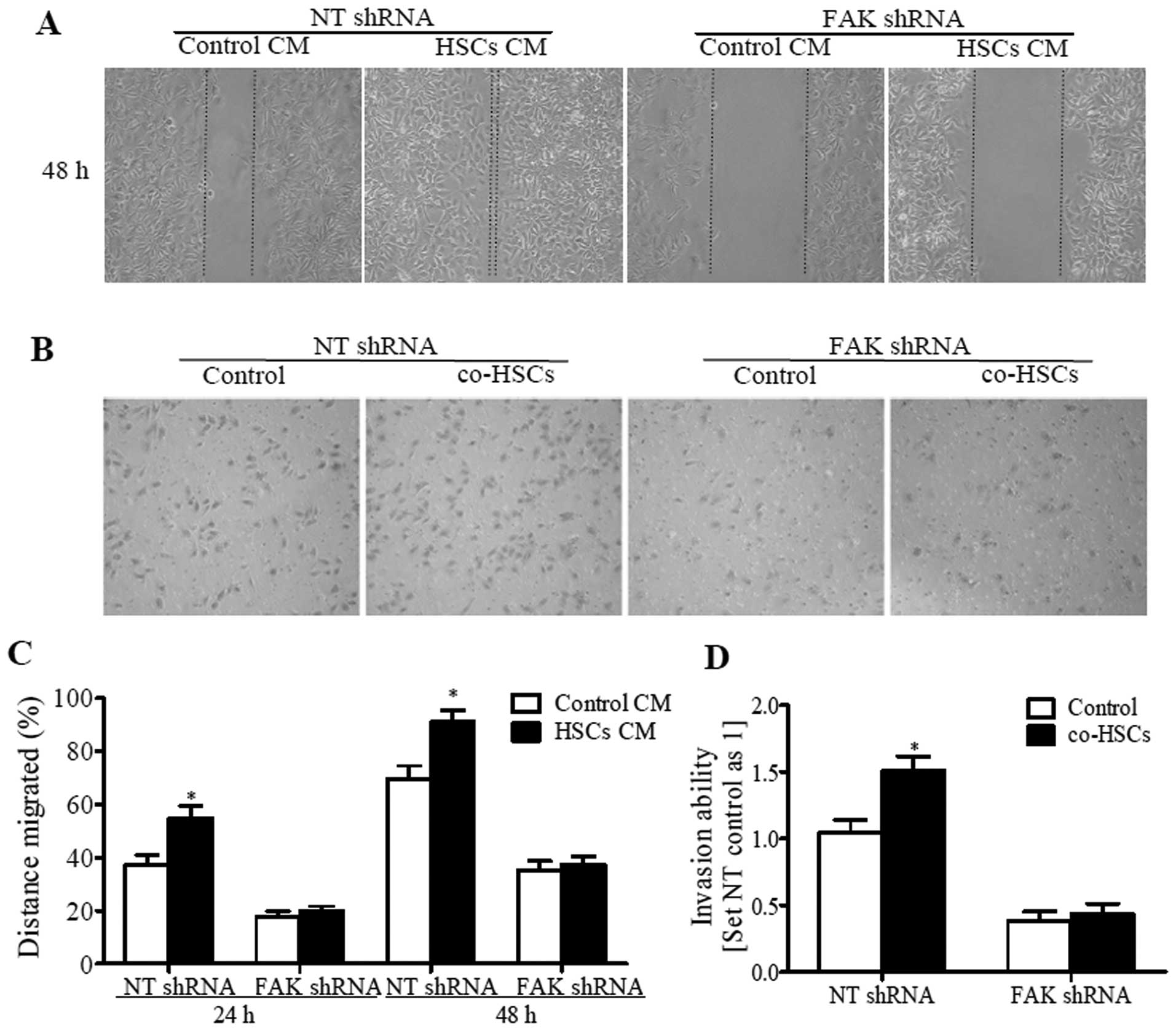
Activated HSCs promote HCC cell migration
and invasion dependent on FAK-MMP9 signaling
Since the activation of FAK-MMP9 signaling was
significantly correlated with the migratory and invasive potential
of HCC cells and is further involved in tumor metastasis (18,19),
we investigated whether activated HSCs promote HCC cell migration
and invasion depending on FAK-MMP9 signaling. The migratory and
invasive abilities of HCC cells exposed to conditioned medium from
activated HSCs were measured by wound healing and Transwell assays.
The results showed that, in comparison with the control medium,
activated HSC-conditioned medium (HSC-CM) significantly enhanced
the migratory and invasive potential of HCC cells (NT shRNA group),
while knockdown of FAK expression in HCC cells with shRNA
diminished the migration and invasion-promoting effect of HSC-CM on
HCC cells. Even stronger migration and invasion was observed when
HCC cells (NT shRNA group) were seeded in the upper part of the
Transwell chamber and activated HSCs were seeded in the bottom part
of the chamber (Fig. 3). Similarly,
suppression of FAK with shRNA in HCC cells decreased cell migration
and invasion, and diminished the difference caused by HSCs. These
results indicate that the migration and invasion-promoting effects
of HSCs on HCC cells depend on FAK-MMP9 signaling.
Discussion
The interaction of tumor cells with the
microenvironment has been recognized to be central for cancer
progression and metastatic colonization (23). Stromal components of the
microenvironment can contribute to several hallmarks of cancer:
sustaining proliferative signaling, evading growth suppressors,
resisting cell death, enabling replicative immortality, inducing
angiogenesis, activating invasion and metastasis, and evading
immune destruction (5). HSCs are
pericytes found within the perisinusoidal space of the liver.
During chronic liver injury, HSCs undergo phenotypic transformation
from the ‘quiescent’ to the ‘activated’ state, and eventually
develop into myofibroblasts (24).
These changes in cell fate of HSCs towards myofibroblasts provide
the cellular basis for the establishment of hepatic fibrosis and
cirrhosis, which are characterized by the vast remodeling of the
ECM and altered expression of growth factors (25). HSCs have been reported to play a
role in the tumoral progression of HCC, since the high density of
peritumoral activated HSCs was found to contribute to early
recurrences and predict poor clinical outcome in HCC after curative
resection (21). In the present
study, we demonstrated a similar finding as previously reported
(26), that α-SMA-positive
activated HSCs are frequently present in carcinomatous nodules, and
the number of activated HSCs in HCC is greater than that in
adjacent non-tumorous tissues. Moreover, a higher number of
activated HSCs in HCC tissues was associated with high rates of
vascular invasion, advanced TNM stage, and poorer tumor
differentiation. These findings, derived from clinic samples,
confirmed the tumor-promoting role of activated HSCs in the
microenvironment and further indicated that activated HSCs may be
related to HCC metastasis. Indeed, studies have shown that
activated HSCs promote HCC migration and invasion, and the
bidirectional interactions between tumors and HSCs enhance
metastatic growth in the liver (11,12).
The underlying mechanisms by which activated HSCs
promote HCC migration and invasion may largely depend on their ECM
remodeling and growth factor regulating function. For example,
previous studies have shown that hepatocyte growth factor (HGF) and
laminin-5 secreted by activated HSCs promote the growth and
invasiveness of HCC cell lines through activating the MEK/ERK
pathway in vitro (11,27,28).
Nevertheless, it is reasonable to suppose that more than one
mechanism occurs. FAK, a widely expressed non-receptor protein
tyrosine kinase has been suggested to play an essential role in
metastasis. FAK is phosphorylated at tyrosine (Tyr) 397 and is
subsequently activated upon both ECM/integrin and growth factor
signaling (14,15,29).
Activation of FAK upregulates MMP9 expression through ERK or PI3K,
thus promoting cell migration and invasion (19,30).
Notably, in the present study we found that the number of activated
HSCs was positively correlated with p-FAK and MMP9 expression
levels in the HCC tissues. This finding indicates that activated
HSCs are associated with FAK-MMP9 signaling in HCC. In order to
study the effects of activated HSCs on HCC cells directly, we
isolated HSCs from resected normal liver tissues, and activated
them in vitro by culture. Consistent with previous studies
(11,27), conditioned medium from activated
HSCs or co-culture with activated HSCs significantly induced the
migration and invasion of HCC cells. Furthermore, HCC cells
co-cultured with activated HSCs or incubated in conditioned medium
exhibited significantly higher levels of p-FAK and MMP9 expression
when compared to cells cultured in control medium, while,
inhibition of FAK in HCC cells with shRNA dramatically decreased
p-FAK and MMP9 expression, and abrogated the differences caused by
co-culture or conditioned medium.
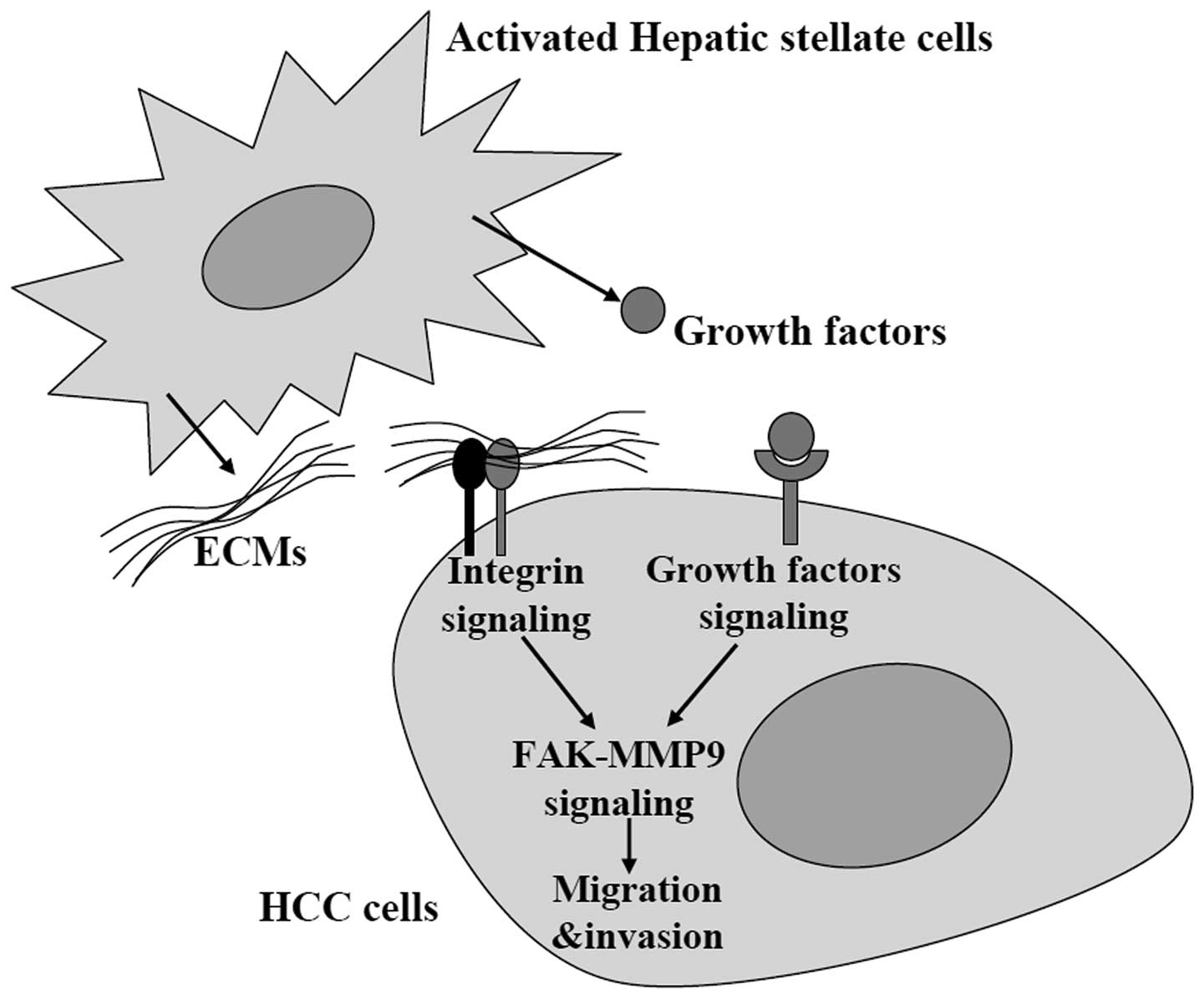
In summary, our study revealed that activated HSCs
promoted HCC cell migration and invasion via activating FAK-MMP9
signaling (Fig. 4). However, we did
not identify which specific ECM components or growth factors
secreted by activated HSCs were responsible for the activation of
FAK-MMP9 signaling in HCC cells. Based on previous studies
(6,11,27),
we speculate that HGF, PDGF, collagen and laminin-5 may be possible
candidates. Their roles in the entire process will be elucidated in
future studies.
Acknowledgements
This study was supported by a grant from the
National Natural Science Foundation of China (no. 81272645).
References
|
1
|
El-Serag HB, Marrero JA, Rudolph L and
Reddy KR: Diagnosis and treatment of hepatocellular carcinoma.
Gastroenterology. 134:1752–1763. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yang JD, Nakamura I and Roberts LR: The
tumor microenvironment in hepatocellular carcinoma: current status
and therapeutic targets. Semin Cancer Biol. 21:35–43. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Farazi PA and DePinho RA: Hepatocellular
carcinoma pathogenesis: from genes to environment. Nat Rev Cancer.
6:674–687. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Seitz HK and Stickel F: Risk factors and
mechanisms of hepatocarcinogenesis with special emphasis on alcohol
and oxidative stress. Biol Chem. 387:349–360. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hernandez-Gea V, Toffanin S, Friedman SL
and Llovet JM: Role of the microenvironment in the pathogenesis and
treatment of hepatocellular carcinoma. Gastroenterology.
144:512–527. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Friedman SL: Hepatic stellate cells:
protean, multifunctional, and enigmatic cells of the liver. Physiol
Rev. 88:125–172. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Friedman SL: Mechanisms of disease:
mechanisms of hepatic fibrosis and therapeutic implications. Nat
Clin Pract Gastroenterol Hepatol. 1:98–105. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Faouzi S, Le Bail B, Neaud V, et al:
Myofibroblasts are responsible for collagen synthesis in the
stromal of human hepatocellular carcinoma: an in vivo and in vitro
study. J Hepatol. 30:275–284. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu SD, Ma YS, Fang Y, Liu LL, Fu D and
Shen XZ: Role of the microenvironment in hepatocellular carcinoma
development and progression. Cancer Treat Rev. 38:218–225. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee JS, Semela D, Iredale J and Shah VH:
Sinusoidal remodeling and angiogenesis: a new function for the
liver-specific pericyte? Hepatology. 45:817–825. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Amann T, Bataille F, Spruss T, et al:
Activated hepatic stellate cells promote tumorigenicity of
hepatocellular carcinoma. Cancer Sci. 100:646–653. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kang N, Gores GJ and Shah VH: Hepatic
stellate cells: partners in crime for liver metastases? Hepatology.
54:707–713. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Siesser PM and Hanks SK: The signaling and
biological implications of FAK overexpression in cancer. Clin
Cancer Res. 12:3233–3237. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
McLean GW, Carragher NO, Avizienyte E,
Evans J, Brunton VG and Frame MC: The role of focal-adhesion kinase
in cancer - a new therapeutic opportunity. Nat Rev Cancer.
5:505–515. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hsia DA, Mitra SK, Hauck CR, et al:
Differential regulation of cell motility and invasion by FAK. J
Cell Biol. 160:753–767. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Itoh S, Maeda T, Shimada M, et al: Role of
expression of focal adhesion kinase in progression of
hepatocellular carcinoma. Clin Cancer Res. 10:2812–2817. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yuan Z, Zheng Q, Fan J, Ai KX, Chen J and
Huang XY: Expression and prognostic significance of focal adhesion
kinase in hepatocellular carcinoma. J Cancer Res Clin Oncol.
136:1489–1496. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen JS, Huang XH, Wang Q, et al: FAK is
involved in invasion and metastasis of hepatocellular carcinoma.
Clin Exp Metastasis. 27:71–82. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jia YL, Shi L, Zhou JN, et al: Epimorphin
promotes human hepatocellular carcinoma invasion and metastasis
through activation of focal adhesion kinase/extracellular
signal-regulated kinase/matrix metalloproteinase-9 axis.
Hepatology. 54:1808–1818. 2011. View Article : Google Scholar
|
|
20
|
Zheng X, Yao Y, Xu Q, Tu K and Liu Q:
Evaluation of glioma-associated oncogene 1 expression and its
correlation with the expression of sonic hedgehog, E-cadherin and
S100a4 in human hepatocellular carcinoma. Mol Med Rep. 3:965–970.
2010.PubMed/NCBI
|
|
21
|
Ju MJ, Qiu SJ, Fan J, et al: Peritumoral
activated hepatic stellate cells predict poor clinical outcome in
hepatocellular carcinoma after curative resection. Am J Clin
Pathol. 131:498–510. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Park YN, Yang CP, Cubukcu O, Thung SN and
Theise ND: Hepatic stellate cell activation in dysplastic nodules:
evidence for an alternate hypothesis concerning human
hepatocarcinogenesis. Liver. 17:271–274. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Witz IP and Levy-Nissenbaum O: The tumor
microenvironment in the post-PAGET era. Cancer Lett. 242:1–10.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Atzori L, Poli G and Perra A: Hepatic
stellate cell: a star cell in the liver. Int J Biochem Cell Biol.
41:1639–1642. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mikula M, Proell V, Fischer AN and
Mikulits W: Activated hepatic stellate cells induce tumor
progression of neoplastic hepatocytes in a TGF-β dependent fashion.
J Cell Physiol. 209:560–567. 2006.PubMed/NCBI
|
|
26
|
Mazzocca A, Dituri F, Lupo L, Quaranta M,
Antonaci S and Giannelli G: Tumor-secreted lysophostatidic acid
accelerates hepatocellular carcinoma progression by promoting
differentiation of peritumoral fibroblasts in myofibroblasts.
Hepatology. 54:920–930. 2011. View Article : Google Scholar
|
|
27
|
Santamato A, Fransvea E, Dituri F, et al:
Hepatic stellate cells stimulate HCC cell migration via laminin-5
production. Clin Sci. 121:159–168. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Neaud V, Faouzi S, Guirouilh J, et al:
Human hepatic myofibroblasts increase invasiveness of
hepatocellular carcinoma cells: evidence for a role of hepatocyte
growth factor. Hepatology. 26:1458–1466. 1997. View Article : Google Scholar
|
|
29
|
Bai X, Wang J, Zhang L, et al:
Prostaglandin E2 receptor EP1-mediated phosphorylation
of focal adhesion kinase enhances cell adhesion and migration in
hepatocellular carcinoma cells. Int J Oncol. 42:1833–1841.
2013.
|
|
30
|
Meng XN, Jin Y, Yu Y, et al:
Characterisation of fibronectin-mediated FAK signalling pathways in
lung cancer cell migration and invasion. Br J Cancer. 101:327–334.
2009. View Article : Google Scholar : PubMed/NCBI
|