Introduction
Cervical cancer is a major cause of female mortality
worldwide, and almost all cases are caused by human papillomavirus
(HPV) infection (1). Cervical
cancer develops in ~500,000 women annually and is responsible for
250,000 deaths (2). The prevalence
of HPV infection among women is known to be highest in those aged
20–24 years (44.8% of prevalence) (3). Most HPV infection resolves
spontaneously. However, cervical cancer develops over a period of
12–15 years when the HPV infection persists (4). Two oncogenic proteins, E6 and E7, of
HPV cause genetic instability and uncontrolled growth of epithelial
cells and can lead to their immortalization (5,6). The
Papanicolau (Pap) smear test has been used for more than 50 years
to detect asymptomatic women with cytological abnormalities
(7), and women with abnormal
cytology are referred for subsequent tests such as colposcopy and
directed biopsy (gold standards).
Screening and prevention are the most critical
factors in reducing the incidence of cervical cancer. Current
cytological screening programs using the Pap test have
significantly reduced the incidence of cervical cancer (7). Despite the success of the Pap test,
its low sensitivity remains a problem, and several auxiliary
techniques have been proposed to increase the fidelity of diagnoses
(8). Ki-67 and p16 have been
identified as molecular biomarkers of cytological status, as their
overexpression is associated with oncogenic potential and cancer
progression (9,10). In addition, mini chromosome
maintenance protein (MCM), cell division cycle protein 6 (CDC 6)
and squamous cell carcinoma antigen (SCC) have been proposed as
useful molecular markers (11–13).
Genomic and proteomic approaches are now the
preferred routes to new diagnostic tools in the cervical cancer
research field (14,15). Biomarkers distinct from proteins and
gene sequences would provide additional approaches to the diagnosis
of cervical cancer. Accumulating evidence indicates that
glycosylation plays a pivotal role in disease progression and that
aberrant glycosylation is a reliable marker of carcinogenesis
(16,17). Increased sialylation and/or
fucosylation of cancer tissues are thought to be associated with
tumorigenicity, invasiveness and metastatic ability (18,19).
Little attention has been paid to changes in the
glycosylation of intracellular proteins in relation to cancer
development. In the present study, we examined such changes for the
first time. Using lectins, we compared the sialylation,
fucosylation and mannosylation levels of cytosolic proteins in
normal and cancer tissues from the human cervix. We showed that the
glycosylation changes in intracellular proteins can provide a
unique means of identifying carcinogenesis.
Materials and methods
Clinical samples
Eleven cervical cancer tissue samples were obtained
from patients who underwent surgical resection for squamous cell
carcinoma or adenocarcinoma at the Ewha Womans University Mokdong
Hospital (Seoul, Korea). Ten cervical normal tissue samples without
neoplastic lesions were obtained from the same source. Pathology,
age, chemotherapy and radiotherapy history, infecting HPV type and
size of the cancer of the women with normal cytology and cervical
cancer are presented when relevant in Table I. All specimens were immediately
frozen and stored at −80°C for subsequent analysis. The procedure
for resection of cervical biopsies was approved by the
Institutional Review Board of Ewha Womans University Mokdong
Hospital.
 | Table IInformation regarding the cervical
biopsies from normal and cancer tissues. |
Table I
Information regarding the cervical
biopsies from normal and cancer tissues.
| Classification | Specimen no. | Age (years) | Pathology | Size of cancer
(cm) | Radiotherapy prior to
resection | Chemotherapy prior to
resection | HPV type
infected |
|---|
| Normal | 1 | 48 | - | - | - | - | Not detected |
| 2 | 48 | - | - | 30 treatments of RT
11 years ago | - | Not detected |
| 3 | 54 | - | - | - | - | Not detected |
| 4 | 43 | - | - | - | - | Not detected |
| 5 | 49 | - | - | - | - | Not detected |
| 6 | 42 | - | - | - | - | Not detected |
| 7 | 50 | - | - | - | - | Not detected |
| 8 | 45 | - | - | - | - | Not detected |
| 9 | 48 | - | - | - | - | Not detected |
| 10 | 47 | - | - | - | - | Not detected |
| Cancer | 1 | 47 | SCC | 2–3 | - | - | HPV 18 |
| 2 | 72 | SCC | 1.7 | - | - | Other type |
| 3 | 71 | SCC | 4 | - | - | HPV 16 |
| 4 | 32 | SCC | 4.5 | - | Taxol,
carboplatin | HPV 58 |
| 5 | 26 | SCC | 3 | - | - | Not determined |
| 6 | 39 | Adenocarcinoma | 2.8 | - | - | HPV 16 |
| 7 | 40 | Adenosquamous cell
carcinoma | 3 | - | - | HPV 16 |
| 8 | 43 | SCC | 2 | - | Cisplatin | HPV 16 |
| 9 | 35 | Neuroendocrine | 5.5 | - | - | Not detected |
| 10 | 93 | SCC | Not determined | - | - | HPV 16 |
| 11 | 48 | SCC | 5 | - | - | HPV 16 |
Preparation of cytoplasmic tissue lysates
from the biopsies and paraffin blocks of the cervix
The cervical biopsies were weighed and mixed with
lysis buffer [100 mM Tris-Cl (Duchefa Biochemie B.V, Haarlem, The
Netherlands) pH 7.4, 150 mM NaCl (Duchefa), 1 mM EDTA-Na (Duchefa)
and 1% Triton X-100 (USB Corp., Cleveland, OH, USA)]. The mixtures
were disrupted with a Dounce homogenizer (Duran Group GmbH, Mainz,
Germany). To obtain the soluble protein fraction, each lysate was
centrifuged at 12,000 × g for 10 min, and the supernatant was
collected. The protein concentration of the supernatant was
determined using a BCA protein assay kit (Pierce, Rockford, IL
USA). To obtain lysates from paraffin blocks, deparaffinization was
carried out as previously described (20). The deparaffinized cervical tissue
was detached and prepared as described above.
SDS-PAGE and lectin blotting of cervical
tissue lysates
SDS-PAGE was performed as previously described
(21), and the fractionated
proteins were visualized with silver staining. Tissue supernatants
were mixed with Laemli’s loading buffer (21), fractionated on a 10% polyacrylamide
gel and transferred to a polyvinylidene difluoride (PVDF) membrane
(ATTO Corp., Tokyo, Japan). The membrane was blocked overnight with
2.5% oxidized bovine serum albumin (oBSA) in TBS-T. oBSA was
prepared as follows. BSA (Bioworld, Dublin, OH, USA) was dissolved
in 10 mM sodium metaperiodate (Sigma, St. Louis, MO, USA),
maintained at 4°C for 1 h and dialyzed against Tris-buffered saline
(TBS) containing 0.1% Tween-20 (TBS-T) for 16 h at 4°C. The PVDF
membrane was reacted with biotinlylated concanavalin A (ConA),
Sambucus nigra lectin (SNA) or Aleuria aurantia
lectin (AAL). All lectins were purchased from Vector Laboratories
Inc. (Burlingame, CA, USA). Proteins bound to the lectins were
detected with HRP-conjugated streptavidin (Pierce) and developed on
X-ray film (Kodak, Rochester, NY, USA) using a chemiluminescence
reagent kit (AbClon, Seoul, Korea). Lectins and streptavidin were
prepared in 0.25% oBSA in TBS-T.
Enzyme-linked lectin assays (ELLA) of the
cervical tissue lysates
ELLA was performed as previously described with
modifications (22). A 96-well
microplate (Greiner Bio-One, Frickenhausen, Germany) was coated
overnight with 5, 2.5 or 1.25 μg of tissue lysate protein per well
at 4°C and blocked with 2.5% oBSA in TBS-T for 4 h at room
temperature (RT). Thereafter, the wells were incubated with
biotinylated ConA, SNA or AAL for 1 h at 37°C followed by
HRP-conjugated streptavidin for 40 min at 37°C. The reactions were
developed with o-phenylenediamine (Sigma) and measured at
492 nm.
Statistical analysis
The statistical significance of differences between
groups was determined by two-tailed Student’s t-tests. P-values
<0.05 were considered to indicate statistically significant
results. Sensitivity and specificity were calculated as follows:
Sensitivity = number of true positives × 100/number of true
positives + number of false negatives; Specificity = number of true
negatives × 100/number of true negatives + number of false
positives. The cut-off value for each assay was set as follows:
(Median value of normal group + median value of cancer
group)/2.
Results
Banding patterns of intracellular
cervical proteins on lectin blots
It is well-known that ConA, SNA and AAL react with
mannoses, α2,6-linked sialic acids and fucoses, respectively
(23,24). Fig.
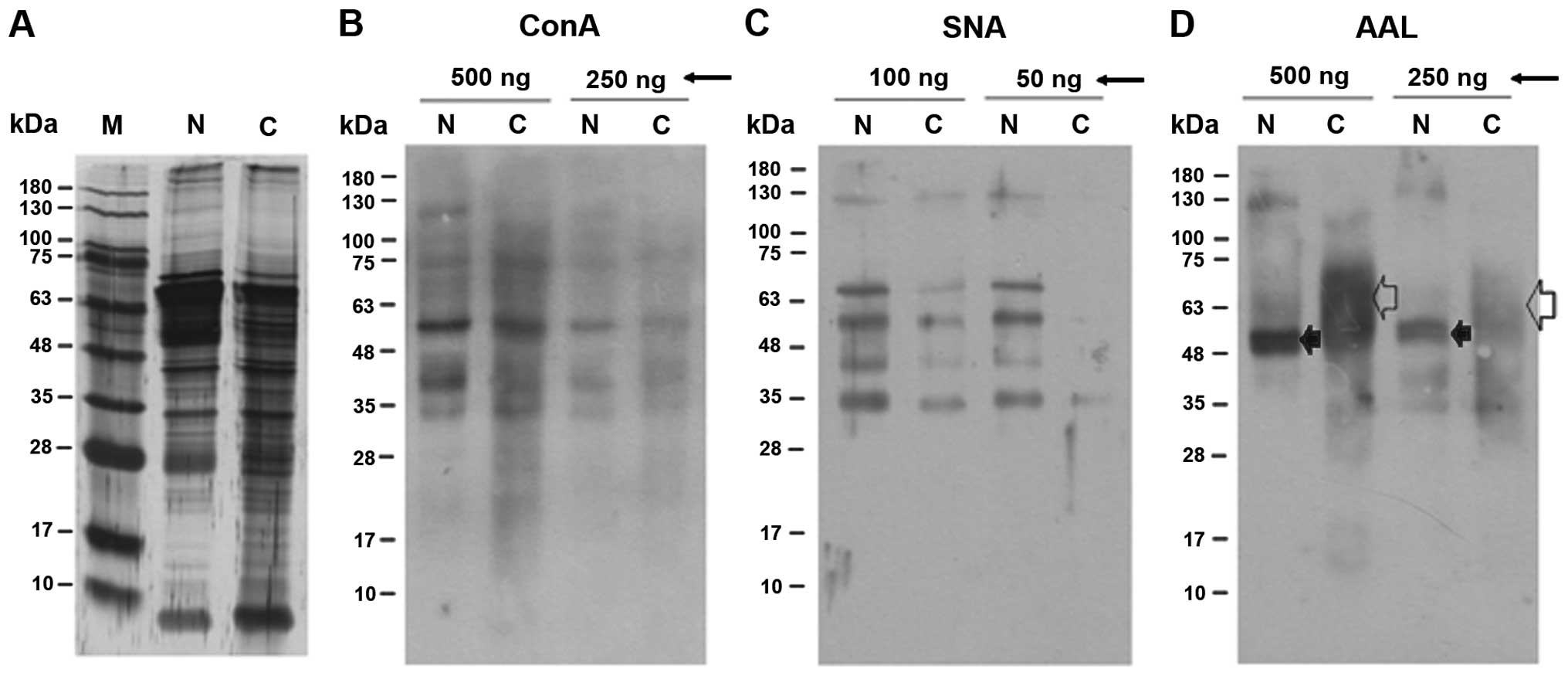
1 shows representative patterns of the cytoplasmic proteins
after SDS-PAGE and visualization by silver staining and lectin
blotting. To obtain representative banding patterns, 10 individual
lysates of normal cervix tissues were combined, as were lysates of
11 cervical cancer tissues (Table
I). Fig. 1A shows that the
protein composition of the cervical cancer tissues was more varied
than that of the normal cervix tissues. The normal and cancer
tissues contained glycoproteins of various molecular masses that
reacted with ConA (Fig. 1B). It is
thought that highly abundant mannosylated proteins are detected
because mannose is the main component of mammalian N-glycans
(25). There was no quantitative
difference between the normal and cancer tissues regarding the
reactivity of their proteins with ConA (Fig. 1B). In contrast, the lysates of the
cancer tissues had markedly reduced reactivity to SNA (Fig. 1C) and the banding pattern after
reaction with AAL was distinct from that of the normal tissues
(Fig. 1D). Concerning AAL binding,
a 48–70 kDa diffuse band was a major band in the cervical cancer
tissue preparation (open arrow) while a tight 50-kDa band was the
major band formed by normal tissues (filled arrow). These results
indicate that there were significant changes in the
α2,6-sialylation and fucosylation of cytosolic glycoproteins in the
cancer tissues.
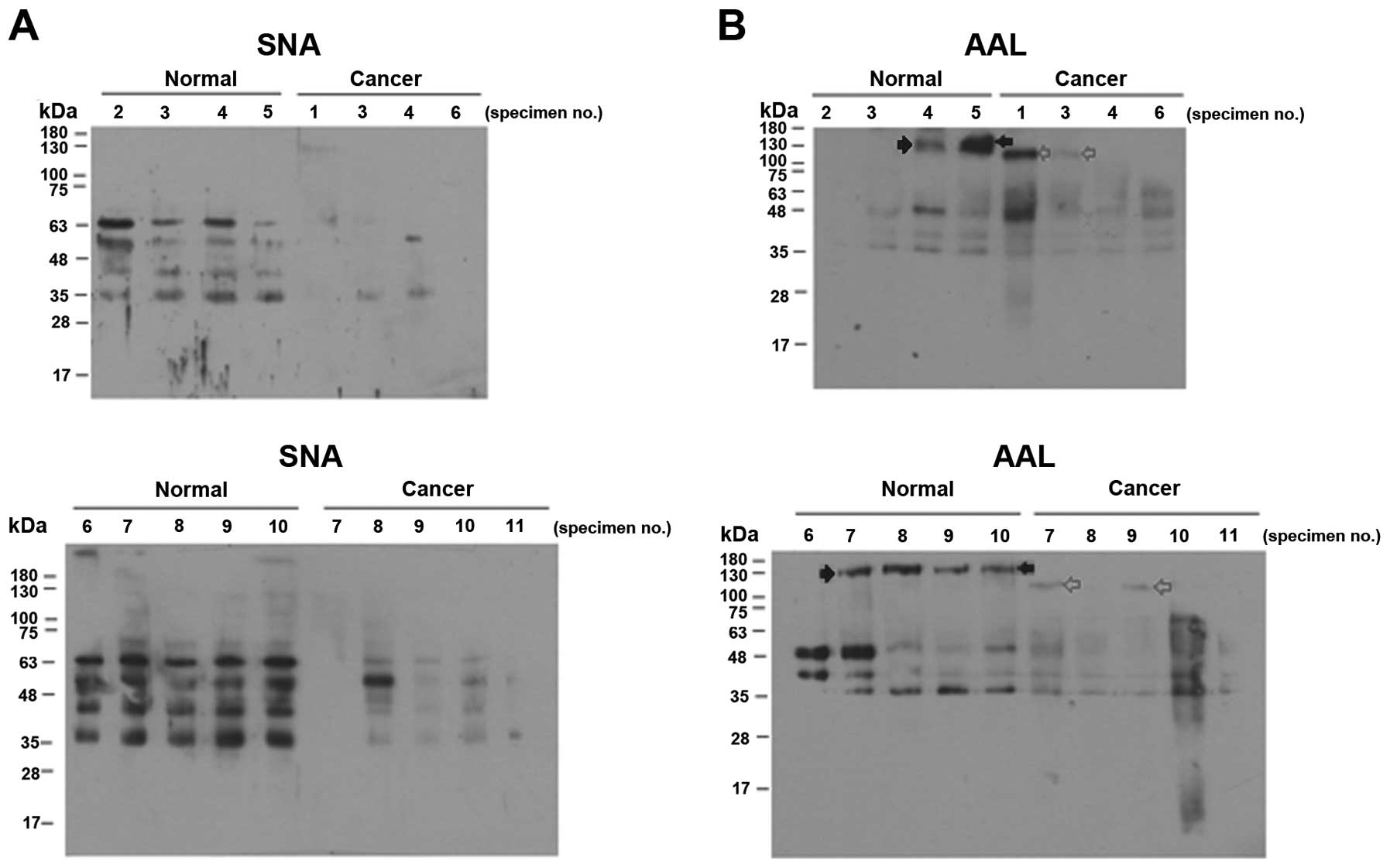
To compare the sialylation and fucosylation levels
of the intracellular proteins in more detail, we analyzed the
reactivities of proteins from individual normal and cancer tissues
to SNA and AAL. Most of the cervical cancer specimens showed
significantly reduced SNA binding (Fig.
2A), and there was little variation between the individual
normal or cancer specimens. The proteins of the individual cancer
tissues also tended to bind less AAL (Fig. 2B). However, the individual samples
of normal and cancer tissues varied considerably with respect to
the banding pattern and band intensities (Fig. 2B).
Reactivities of intracellular proteins of
normal and cancer tissues towards lectins in ELLA
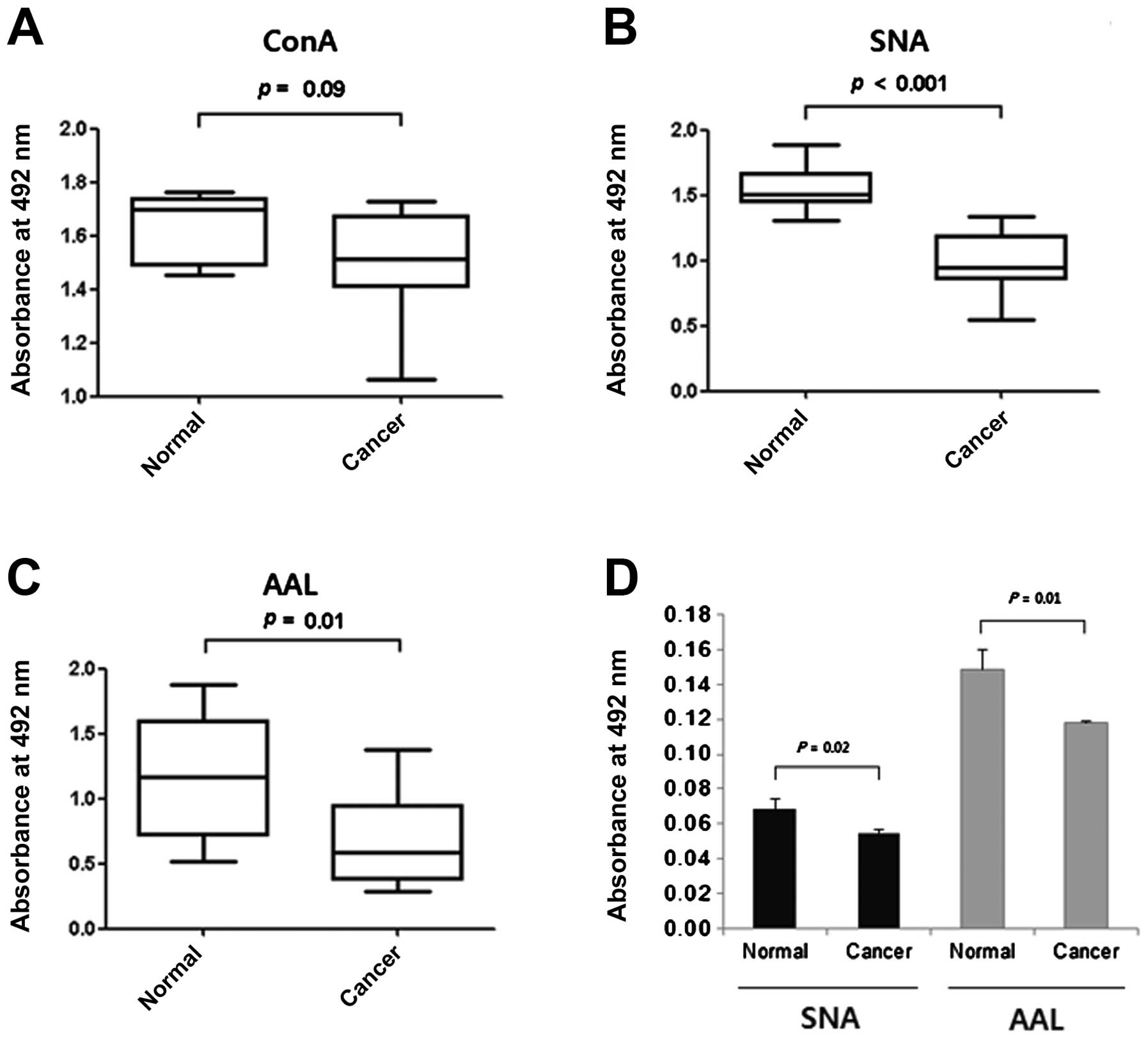
ELLAs were carried out to compare the levels of
mannosylation, sialylation and fucosylation of the cytosolic
proteins in the proteins immobilized in microplate wells. No
difference was noted between the normal and cancer tissues in their
reactivities with ConA (Fig. 3A),
while the proteins of the cancer tissues were less reactive with
SNA and AAL (Fig. 3B and C),
particularly with SNA (P<0.001 for SNA; P=0.01 for AAL). These
reduced reactivities towards SNA were in agreement with the results
of the lectin blotting (Figs. 1C
and 2A). The lesser effect on AAL
binding may have been caused by individual variation in the
fucosylation level (Fig. 2B). We
compared the reactivities for SNA and AAL of proteins recovered
from paraffin blocks. Again the intracellular proteins of cancer
tissues had significantly lower reactivities against SNA and AAL
than those of the normal tissues (Fig.
3D). Thus, our results taken together suggest that the reduced
sialylation and fucosylation of intracellular proteins are a useful
marker for evaluating carcinogenesis in cervical tissue.
Sensitivity and specificity of ELLA using
SNA and AAL
The amounts of cytosolic proteins coating individual
wells were varied to investigate the sensitivity and specificity of
ELLA using SNA and AAL. As shown in Table II, the optimum amount of protein
for detecting reactivity with SNA was 1.25–2.5 μg per well (91–100%
of sensitivity and 82–100% of specificity). The sensitivity and
specificity for ELLA using AAL ranged from 70 to 82% and from 60 to
82%, respectively.
 | Table IIELLA specificity and sensitivity as a
function of the coating amount of cytosolic protein. |
Table II
ELLA specificity and sensitivity as a
function of the coating amount of cytosolic protein.
| Coating amount of
lysate protein | Statistical
parameter | SNA | AAL |
|---|
| 5 μg/well | Sensitivity | 73% | 82% |
| Specificity | 80% | 70% |
| 2.5 μg/well | Sensitivity | 91% | 73% |
| Specificity | 100% | 60% |
| 1.25 μg/well | Sensitivity | 100% | 70% |
| Specificity | 82% | 82% |
Discussion
Glycomics based on genomic and proteomic research is
currently a promising approach to the development of cancer
biomarkers. Numerous studies have shown that aberrant
glycosylations in serum and on the cell surface are a reliable sign
of cancer progression, and these changes in glycosylation are
thought to be associated with the activities of cellular
glycosyltransferases (26). The
glycosylation pathways giving rise to the glycolipids and
glycoproteins secreted in biological fluids have been studied in
detail (25,27). However, the pathways and mechanisms
responsible for the glycosylation of cytosolic protein are less
well understood (28).
Cellular sialylation levels are controlled by
sialyltransferases and sialidases (29,30).
Previous studies have revealed that sialyltransferase expression is
upregulated as cancer progresses (29). There are four types of cellular
sialidases, and their properties and functions are thought to
depend on their particular subcellular location (30). However, their functions still remain
unclear. Recently, it has been proposed that the α2,3- and
α2,6-terminal sialylation levels of cell surface glycoconjugates in
cervical biopsies increase with the grade of squamous
intraepithelial lesions (SILs) (31). In the present study, however, the
reduction in sialylation levels observed in the cytosolic proteins
of cervical cancer tissues was opposite to the increase noted on
the cell surface (31). Therefore,
it appears that the sialylation pathway of cytosolic glycoconjugate
is significantly different from that for cell surface
glycoconjugates.
There is evidence that the intracellular
glycosylation changes occurring during carcinogenesis are
independent of those that occur on the cell surface or in the
blood. Krzeslak et al (32)
observed that the sialylation levels of intracellular proteins in
thyroid lesions, particularly follicular and papillary carcinomas,
were significantly lower than those in non-neoplastic lesions.
However, there have been no subsequent confirmatory studies. In our
cases, we also observed reduced sialylation and fucosylation levels
in the inner part of the cervical cancer tissues. Therefore, it is
evident that there are significant changes in the glycosylation of
intracellular proteins in carcinomas.
Our ELLA results showed that the difference in
fucosylation level in the cytosolic proteins between the normal and
cancer tissues (Fig. 3C) was less
than that of the sialylation level (Fig. 3B). As shown in Fig. 2B, both normal and cancer specimens
showed individual variations in fucosylation levels in the lectin
blots. Therefore, there is an inherent limit to the sensitivity and
specificity of ELLA using AAL with whole tissue supernatants
(Fig. 3C and Table I). However, this limitation may
possibly be overcome by examining the fucosylation level of
specific proteins. As shown in Fig.
2B, a protein band of 150 kDa was noted only in the normal
tissues (filled arrow) while a 120-kDa protein band was noted only
in the cancer tissues (grey arrow). Therefore, increased
specificity and sensitivity may be achieved if the fucosylation of
this specific protein is measured. This type of assay could also be
performed using antibody-based enzyme-linked lectin assays
(22).
False-negatives in conventional Pap tests and in the
liquid-based Pap test have been a major hindrance to accurate
diagnosis for more than five decades. Aproximately 30% of new cases
of cervical cancer are initially interpreted as negative (33,34).
Moreover, many researchers have suggested that the sensitivity of
conventional Pap testing is only ~50%, which means that the Pap
test is not suitable for use as the sole approach to cervical
cancer screening (7). Therefore,
the development of new strategies to complement the cytological
test is a high priority. The ELLA using SNA developed in the
present study showed 90–100% sensitivity and 82–100% specificity
(Table I). Approximately 10–30 μg
of cytosolic protein can be obtained from a Pap smear (data not
shown), and our ELLA system for detecting sialylation requires
1.2–2.5 μg of protein per assay. This indicates that there is
considerable potential for analyzing sialylation levels using
proteins obtained from Pap tests. We anticipate that future
investigation of the combination of the Pap test and our ELLA
system could substantially reduce the proportion of false-negative
results.
Acknowledgements
The present study was supported by a grant of the
Korean Health Technology R&D Project, Ministry of Health and
Welfare, Republic of Korea (HI12C0050).
References
|
1
|
Walboomers JMM, Jacobs MV, Manos MM, et
al: Human papillomavirus is a necessary cause of invasive cervical
cancer worldwide. J Pathol. 189:12–19. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Parkin DM and Bray F: Chapter 2: The
burden of HPV-related cancers. Vaccine. 24(Suppl 3): S11–S25. 2006.
View Article : Google Scholar
|
|
3
|
Steben M and Duarte-Franco E: Human
papillomavirus infection: epidemiology and pathophysiology. Gynecol
Oncol. 107:S2–S5. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Snijders PJ, Steenbergen RD, Heideman DA
and Meijer CJ: HPV-mediated cervical carcinogenesis: concepts and
clinical implications. J Pathol. 208:152–164. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Scheffner M, Romanczuk H, Munger K,
Huibregtse JM, Mietz JA and Howley PM: Functions of human
papillomavirus proteins. Curr Top Microbiol Immunol. 186:83–99.
1994.
|
|
6
|
Arbeit JM, Munger K, Howley PM and Hanahan
D: Progressive squamous epithelial neoplasia in K14-human
papillomavirus type 16 transgenic mice. J Virol. 68:4358–4368.
1994.PubMed/NCBI
|
|
7
|
Jin XW, Sikon A and Yen-Lieberman B:
Cervical cancer screening: Less testing, smarter testing. Cleve
Clin J Med. 78:737–747. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wentzensen N and von Knebel Doeberitz M:
Biomarkers in cervical cancer screening. Dis Markers. 23:315–330.
2007. View Article : Google Scholar
|
|
9
|
Maeda MY, Simoes M, Wakamatsu A, et al:
Relevance of the rates of PCNA, Ki-67 and p53 expression according
to the epithelial compartment in cervical lesions. Pathologica.
93:189–195. 2001.PubMed/NCBI
|
|
10
|
Sano T, Oyama T, Kashiwabara K, Fukuda T
and Nakajima T: Expression status of p16 protein is associated with
human papillomavirus oncogenic potential in cervical and genital
lesions. Am J Pathol. 153:1741–1748. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shin JH, Grabowski B, Kasiviswanathan R,
Bell SD and Kelman Z: Regulation of minichromosome maintenance
helicase activity by Cdc6. J Biol Chem. 278:38059–38067. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cook JG, Park CH, Burke TW, et al:
Analysis of Cdc6 function in the assembly of mammalian
prereplication complexes. Proc Natl Acad Sci USA. 99:1347–1352.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Duk JM, de Bruijn HW, Groenier KH, et al:
Cancer of the uterine cervix: sensitivity and specificity of serum
squamous cell carcinoma antigen determinations. Gynecol Oncol.
39:186–194. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
von Knebel Doeberitz M, Reuschenbach M,
Schmidt D and Bergeron C: Biomarkers for cervical cancer screening:
the role of p16INK4a to highlight transforming HPV
infections. Expert Rev Proteomics. 9:149–163. 2012.PubMed/NCBI
|
|
15
|
Lorincz AT: Screening for cervical cancer:
new alternatives and research. Salud Publica Mex. 45(Suppl 3):
S376–S387. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
An HJ and Lebrilla CB: A glycomics
approach to the discovery of potential cancer biomarkers. Methods
Mol Biol. 600:199–213. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Taylor AD, Hancock WS, Hincapie M,
Taniguchi N and Hanash SM: Towards an integrated proteomic and
glycomic approach to finding cancer biomarkers. Genome Med.
1:572009. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhu J, Wang Y, Yu Y, et al: Aberrant
fucosylation of glycosphingolipids in human hepatocellular
carcinoma tissues. Liver Int. Jul 1–2013. View Article : Google Scholar
|
|
19
|
Passaniti A and Hart GW: Cell surface
sialylation and tumor metastasis. Metastatic potential of B16
melanoma variants correlates with their relative numbers of
specific penultimate oligosaccharide structures. J Biol Chem.
263:7591–7603. 1988.
|
|
20
|
Mouiseddine M, Francois S, Semont A, et
al: Human mesenchymal stem cells home specifically to
radiation-injured tissues in a non-obese diabetes/severe combined
immunodeficiency mouse model. Br J Radiol. 80(Spec No 1): S49–S55.
2007. View Article : Google Scholar
|
|
21
|
Laemmli UK: Cleavage of structural
proteins during the assembly of the head of bacteriophage T4.
Nature. 227:680–685. 1970. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim HJ, Lee SJ and Kim HJ: Antibody-based
enzyme-linked lectin assay (ABELLA) for the sialylated recombinant
human erythropoietin present in culture supernatant. J Pharm Biomed
Anal. 48:716–721. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu AM, Lisowska E, Duk M and Yang ZG:
Lectins as tools in glycoconjugate research. Glycoconjugate J.
26:899–913. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Thompson R, Creavin A, O’Connell M,
O’Connor B and Clarke P: Optimization of the enzyme-linked lectin
assay for enhanced glycoprotein and glycoconjugate analysis. Anal
Biochem. 413:114–122. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hossler P, Khattak SF and Li ZJ: Optimal
and consistent protein glycosylation in mammalian cell culture.
Glycobiology. 19:936–949. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Meany DL and Chan DW: Aberrant
glycosylation associated with enzymes as cancer biomarkers. Clin
Proteomics. 8:72011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Magnoni C, Tenedini E, Ferrari F, et al:
Transcriptional profiles in melanocytes from clinically unaffected
skin distinguish the neoplastic growth pattern in patients with
melanoma. Br J Dermatol. 156:62–71. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Funakoshi Y and Suzuki T: Glycobiology in
the cytosol: the bitter side of a sweet world. Biochim Biophys
Acta. 1790:81–94. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Harduin-Lepers A, Krzewinski-Recchi MA,
Colomb F, Foulquier F, Groux-Degroote S and Delannoy P:
Sialyltransferase functions in cancers. Front Biosci (Elite Ed).
4:499–515. 2012. View
Article : Google Scholar
|
|
30
|
Miyagi T: Aberrant expression of sialidase
and cancer progression. Proc Jpn Acad Ser B Phys Biol Sci.
84:407–418. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lopez-Morales D, Reyes-Leyva J,
Santos-Lopez G, Zenteno E and Vallejo-Ruiz V: Increased expression
of sialic acid in cervical biopsies with squamous intraepithelial
lesions. Diagn Pathol. 5:742010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Krzeslak A, Gaj Z, Pomorski L and Lipinska
A: Sialylation of intracellular proteins of thyroid lesions. Oncol
Rep. 17:1237–1242. 2007.PubMed/NCBI
|
|
33
|
Shingleton HM, Patrick RL, Johnston WW and
Smith RA: The current status of the Papanicolaou smear. CA Cancer J
Clin. 45:305–320. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
ACOG Committee on Practice
Bulletins-Gynecology. ACOG Practice Bulletin no. 109: Cervical
cytology screening. Obstet Gynecol. 114:1409–1420. 2009. View Article : Google Scholar : PubMed/NCBI
|