Introduction
Acute lymphoblastic leukemia (ALL) is the most
common childhood malignancy, accounting for ~25% of cancers in
children <15 years of age; the peak onset occurs at 2–5 years of
age (1,2). The incidence of childhood ALL has
increased yearly since the middle of the last century, and
30/1,000,000 children are diagnosed with ALL annually in China
(3). Most ALLs in children arise
from a B-cell precursor (BCP) (~85%), whereas the remaining ~15% of
cases are accounted for by T-cell acute lymphoblastic leukemias
(T-ALLs) (4). The pathogenesis of
ALL results from the interplay of multiple environmental and
inherited factors (5). Furthermore,
alterations affecting genes that encode transcriptional regulators
of B-cell development and maturation are potentially important
contributors to the pathogenesis of B-cell precursor acute
lymphoblastic leukemia (BCP-ALL) (6).
One important group of transcription factors in
hematopoietic lineages is the IKAROS family. IKAROS family genes
encode a group of zinc-finger DNA-binding proteins essential in
normal lymphocyte development (7–9).
AIOLOS is an IKAROS family member that was first described in
committed lymphoid progenitors, and is strongly upregulated as
these progenitors become restricted into T- and B-lymphoid pathways
(8). AIOLOS is an important
regulator of B-cell differentiation, proliferation and maturation
to an effector state (10). In
addition, AIOLOS null mutation in mice causes B-cell
hyperproliferation, elevated serum antibody levels, auto-antibody
formation and development of lymphomas demonstrating tumor
suppressor function (10).
Deregulated AIOLOS expression has been associated with adult B-cell
ALL and chronic lymphocytic leukemia (CLL) in human patients
(11–13) and aberrant AIOLOS expression levels
have been reported in lymphoma (14). However, the function of AIOLOS in
childhood BCP-ALL is not fully understood.
BCP-ALL is a malignancy characterized by progressive
accumulation of immature clonal B-cell precursors in the bone
marrow (BM). Based on the function of AIOLOS in B-cell development,
we sought to promote differentiation and maturation of BCP-ALL
cells by delivering AIOLOS into leukemia cells. To this end, we
employed a lentiviral system to stably overexpress the AIOLOS gene
in Nalm-6 cells, a BCP-ALL cell line. Subsequently, we examined the
effects of AIOLOS overexpression on proliferation, apoptosis and
cell cycle distribution of Nalm-6 cells in vitro. The
results demonstrated that lentivirus-mediated overexpression of
AIOLOS suppresses cell apoptosis and arrests the cell cycle at the
G0/G1 phase, which possibly contributes to growth inhibition of
Nalm-6 cells to some extent. Furthermore, these changes are
possibly associated with downregulation of BAX and cyclin D3
(CCND3).
Materials and methods
Cell lines
Five leukemia cell lines were used in the present
study. Two BCP-ALL cell lines (Ball-1 and Nalm-6), two T-cell
leukemia cell lines (Jurkat and Molt-4), and chronic myeloblastic
leukemia cell line K-562 were purchased from the American Type
Culture Collection (ATCC; Manassas, VA, USA). These cell lines were
cultured in standard culture medium [RPMI-1640 (Gibco, Grand
Island, NY, USA) containing 10% fetal bovine serum (FBS; Gibco) and
1% penicillin-streptomycin (Gibco)] at 37°C in 5% CO2 in
air.
Lentiviral vector construction, virus
production and transfection
For construction and identification of the AIOLOS
expression plasmid, an AIOLOS insert was isolated by polymerase
chain reaction (PCR) amplification from a complementary DNA (cDNA)
library (Shanghai GeneChem, Shanghai, China) with two pairs of
restriction primers. PCR products were sequenced (ABI Prism 3100
DNA Sequencer; Applied Biosystems, Foster City, CA, USA) and
confirmed to contain the entire AIOLOS coding sequence. The insert
was then cloned into the pWPT-PURO-GFP plasmid (Telebio Biomedical
Co., Ltd., Shanghai, China), which was co-transfected with
packaging plasmids (Telebio Biomedical Co., Ltd.) into HEK293T
cells. Viral supernatant was harvested, filtered and concentrated
by centrifugation. Lenti-Mock was constructed similarly, but
without the AIOLOS insert. The viral concentrate was diluted in
polybrene (5 μg/ml; Sigma, St. Louis, MO, USA) to infect Nalm-6
cells at multiplicities of infection (MOI) of 100. A successful
transduction was confirmed by visualizing enhanced green
fluorescent protein (EGFP; included in the pWPT-PURO-GFP vector)
after 4 days. Cells were maintained and allowed to grow for another
3–5 days, and then AIOLOS expression level was confirmed by
quantitative reverse transcription-polymerase chain reaction
(qRT-PCR) and western blot analysis. Virus-infected cells were
selected with 8 μg/ml puromycin (Invitrogen, Carlsbad, CA, USA).
The antibiotic-resistant clones were pooled and used for subsequent
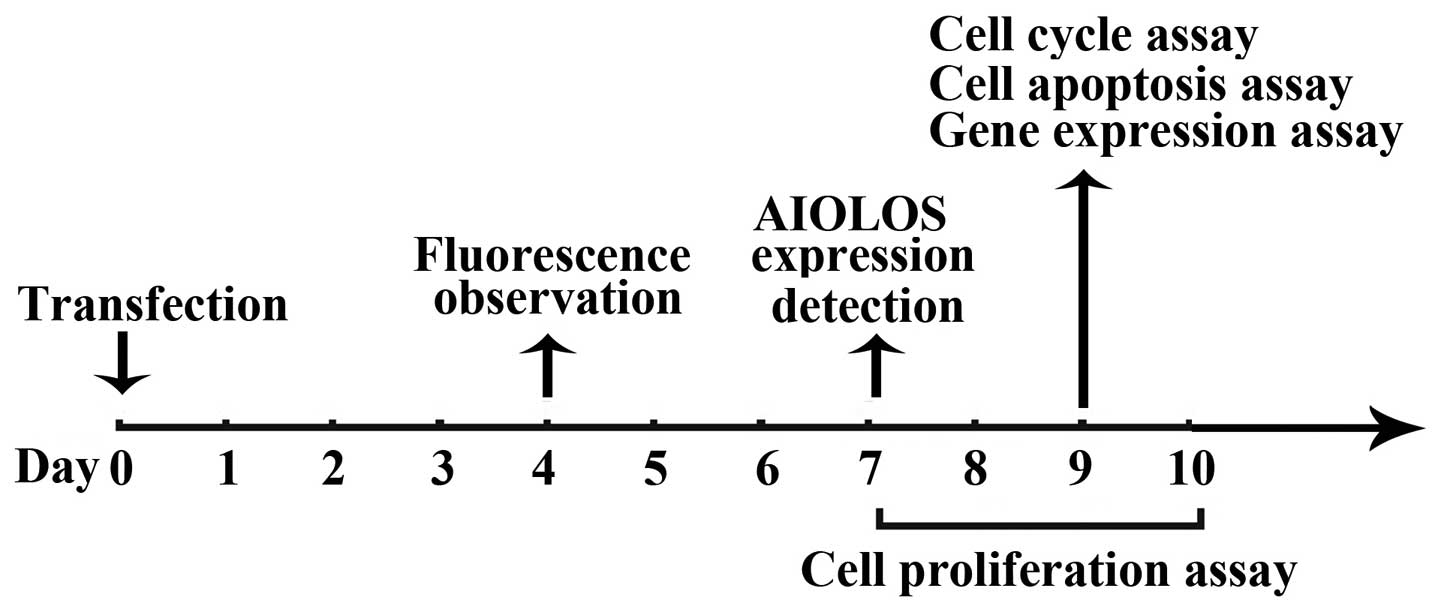
assays (Fig. 1).
Nalm-6 cells were divided into three groups:
untransfected control (UT), Lenti-Mock and AIOLOS-transfected
(Lenti-AIOLOS) group.
Isolation of RNA and qRT-PCR
analysis
Total RNA was purified from each cell line or each
group of Nalm-6 cells using TRIzol (Invitrogen) as per the
manufacturer’s protocol. We ensured that equal amounts of RNA from
each subject were used. Expression levels of AIOLOS and other
related genes were analyzed by qRT-PCR. Total RNA was reverse
transcribed to cDNA with the Omniscript cDNA synthesis kit (Qiagen,
Hamburg, Germany) according to the manufacturer’s instructions.
Approximately 0.2 μg of total RNA was reverse transcribed in a
20-μl reaction mixture containing the following components: 1X RT
buffer, deoxynucleotide triphosphate mix (5 mM each), RNase
inhibitor (10 U/μl RNaseOut; Invitrogen), and 4 units Omniscript
RT. Samples were incubated at 37°C for 60 min. The resulting cDNA
was stored at −80°C prior to qRT-PCR.
All reagents and primers were obtained from Bioasi
Co., Ltd., Shanghai, China. β-actin was validated as an internal
control. Each gene expression relative to β-actin was determined
using the 2−ΔCT method, where ΔCT = (CTtarget
gene − CTβ-actin). qRT-PCR conditions were: 95°C
for 4 min, 94°C for 15 sec, and 60°C for 1 min, for a total of 40
cycles. qRT-PCR was performed using an ABI 7500 PCR system (Applied
Biosystems, Foster City, CA, USA) and SYBR-Green I dye (Toyobo,
Osaka, Japan). Successful amplification was defined by the presence
of a single dissociation peak on the thermal melting curve. Data
were analyzed with the Sequence detection software 1.4 (Applied
Biosystems). Results are expressed as the normalized fold
expression for each gene. Reported data are representative of at
least three independent experiments. The primers used are listed in
Table I.
 | Table IPrimer sequences (5′-3′) used for
qRT-PCR. |
Table I
Primer sequences (5′-3′) used for
qRT-PCR.
| Gene | Primer sequences | Product length
(bp) |
|---|
| β-actin | F:
5′-GGACATCCGCAAAGACCTGTA-3′
R: 5′-GCATCCTGTCGGCAATGC-3′ | 80 |
| AIOLOS | F:
5′-GCCCTTCAAGTGTTTCACCAA-3′
R: 5′-GCCTTTCCAGCCAGACAAATAT-3′ | 90 |
| BCL-2 | F:
5′-GCTGGGAGAACAGGGTACGA-3′
R: 5′-CCTCTGCGACAGCTTATAATGGA-3′ | 80 |
| BAX | F:
5′-CTTGTTGCCCAGGCTTGAGT-3′
R: 5′-GCAGGAGAATCGCTTGAACCT-3′ | 81 |
| CCND3 | F:
5′-GAGGTGCAATCCTCTCCTCG-3′
R: 5′-TCACATACCTCCTCGTCAGGT-3′ | 87 |
| C-MYC | F:
5′-TCTCCGTCCTCGGATTCTCT-3′
R: 5′-TTCCTCCTCAGAGTCGCTGC-3′ | 85 |
| P27 | F:
5′-TCCGGCTAACTCTGAGGACA-3′
R: 5′-GAAGAATCGTCGGTTGCAGG-3′ | 81 |
| IKZF1 | F:
5′-AGAAGCCACACTGGAGAACG-3′
R: 5′-GCAGAGGTGGCATTTGAAGG-3′ | 83 |
| NF-κB | F:
5′-TCCATATTTGGGAAGGCCTGA-3′
R: 5′-GGTATGGGCCATCTGCTGT-3′ | 89 |
Protein extraction and western blot
analysis
Leukemia cells were harvested and the expression
levels of total AIOLOS, P27, BCL-2, CCND3 and nuclear NF-κB were
analyzed. Equal amounts of proteins were separated by sodium
dodecyl sulfate-polyacrylamide gel electrophoresis and then
transferred to polyvinylidene difluoride membranes. Membranes were
incubated with antibodies against AIOLOS, P27, BCL-2, CCND3, NF-κB
or GAPDH (Abcam Inc., Cambridge, MA, USA) at 4°C overnight.
Antibody binding was assessed by incubation with horseradish
peroxidase-conjugated secondary antibodies (Beyotime Institute of
Biotechnology, Shanghai, China). Chemiluminescence was detected
using an ECL Plus immunoblotting detection system (Beyotime
Institute of Biotechnology).
Cell proliferation assay
For cell proliferation assay, Nalm-6 cells of the
UT, Lenti-Mock and Lenti-AIOLOS groups were plated onto a 6-well
culture plate at a density of 5×105 cells/well on day 7
after transfection. Viable cells were counted on days 8, 9 and 10,
respectively. Each time-point was counted in triplicate. Results
are given as the fold-change relative to the initial cell numbers
(5×105), which were set at fold-change = 1. Growth
curves were plotted with the means of each experiment, and error
bars represent the standard errors of means (SEM).
Cell cycle analysis
A total of 1×106 cells from each group
were washed three times and resuspended in ~50 μl PBS. Resuspended
cells were added into the tube containing 1 ml of ice cold 70%
ethanol dropwise while vortexing at medium speed. The tubes were
frozen at −20°C for 3 h prior to staining. Subsequently, cells were
washed and treated with 200 μl of Muse™ Cell Cycle reagent
(Millipore Corp., Bedford, MA, USA) according to the manufacturer’s
protocol. After 30 min of incubation at room temperature in the
dark, cell suspension samples were transferred into 1.5 ml
microcentrifuge tubes and analyzed using the Muse™ Cell Analyzer.
Results are expressed as percentage of cells in each cell cycle
phase and error bars represent SEM.
Cell apoptosis assay
Nalm-6 cell apoptosis was assayed using the Muse™
Annexin V and Dead Cell kit (Millipore) according to the user’s
guide. A total of 1×105 cells from each group were
collected by centrifugation (2,000 rpm, 5 min) and washed with PBS.
Cells were resuspended in PBS with 1% bovine serum albumin and 1%
FBS, mixed with the Muse™ Annexin V and Dead Cell reagent, and then
incubated for 20 min at room temperature in the dark. Assay results
were measured using the Muse™ Cell Analyzer. Results are expressed
as percentage of apoptotic cells and error bars represent SEM.
Statistical analysis
All experiments were performed three times and the
differences between the experimental and control cells were
analyzed by the Student’s t-test. The data are presented as means ±
SEM of three independent experiments and P<0.05 was considered
to indicate a statistically significant result.
Results
AIOLOS is differentially expressed in
five leukemia cell lines
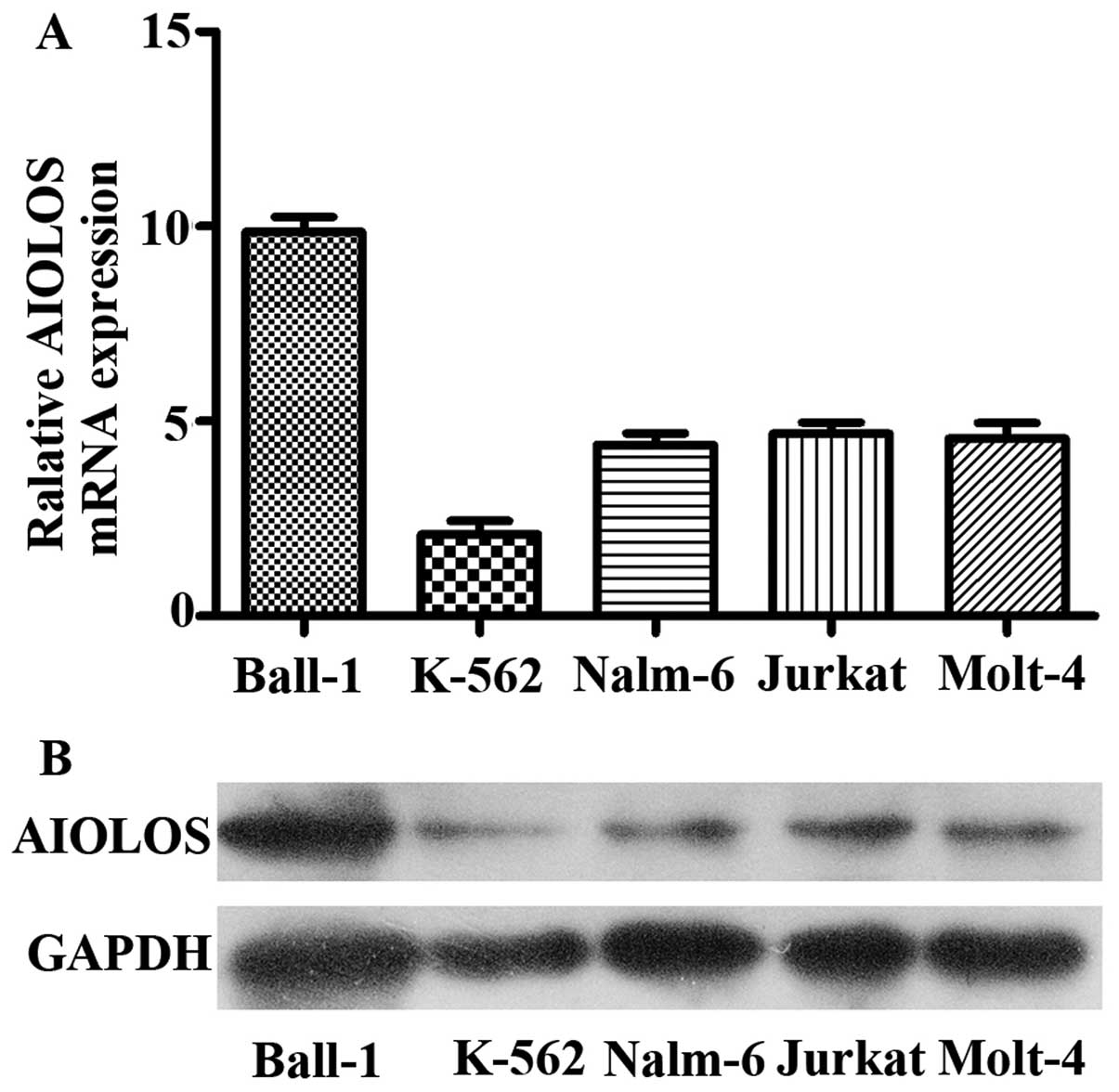
AIOLOS expression level was quantified by qRT-PCR
and western blot analysis in five human leukemia cell lines. As
indicated in Fig. 2A, although
expression levels varied, AIOLOS mRNA was expressed in each cell
line. Among the cell lines, Ball-1 expressed the highest level of
AIOLOS, whereas K-562 expressed the lowest. Nalm-6, Jurkat and
Molt-4 exhibited moderate expression levels of AIOLOS. Furthermore,
we performed western blot analysis to verify the level of AIOLOS
protein in the five cell lines. The results were consistent with
those of qRT-PCR (Fig. 2B). BCP-ALL
is the main form of ALL in children, therefore Nalm-6 cells, a
BCP-ALL cell line, were chosen for a series of functional
experiments.
Identification of recombinant plasmid
pWPT-PURO-GFP-AIOLOS and overexpression of AIOLOS by stable
transfection in Nalm-6 cells
To upregulate AIOLOS expression in Nalm-6 cells, the
entire AIOLOS coding sequence was cloned into a lentivirus vector
pWPT-PURO-GFP. The resulting construct was verified by DNA
sequencing. The detected sequence was identical to the known AIOLOS
sequence in GenBank (NM_012481.4). Western blot analysis revealed a
58-kDa band in the cell extracts, which was the expected size of an
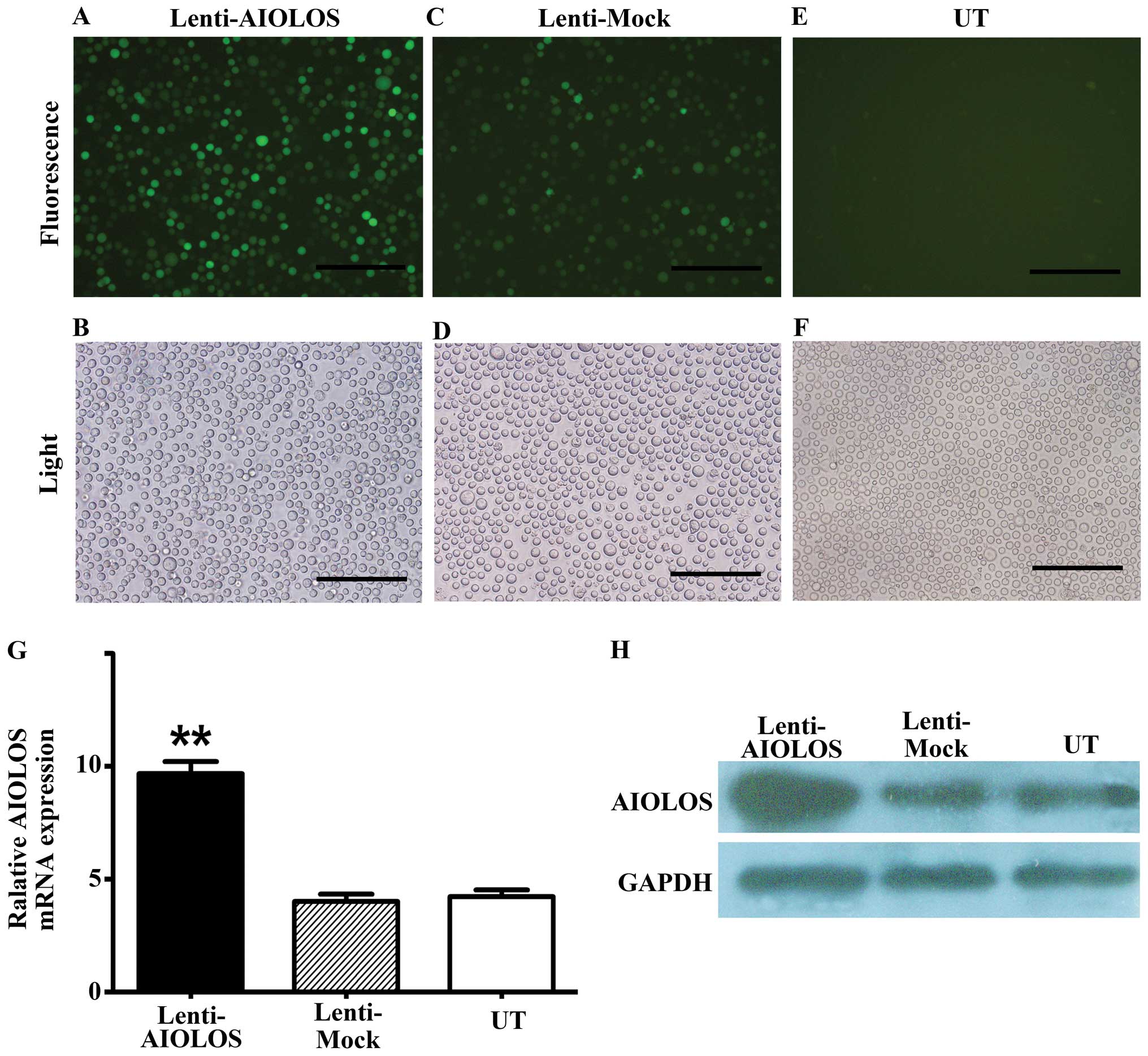
AIOLOS protein (Fig. 3H).
Nalm-6 cells were infected with lentivirus vector
pWPT-PURO-GFP-AIOLOS. As control, Nalm-6 cells were either infected
with a lentivirus vector expressing GFP or not. At precisely 96 h
after infection of Nalm-6 cells, the infection efficiency was
detected using a fluorescence microscope. A large number of cells
emitted bright green fluorescence, which represented high infection
efficiency (Fig. 3A–F). Cells were
maintained and allowed to grow for 3–5 days. AIOLOS mRNA and
protein expression levels in Nalm-6 cells of the three groups were
determined by qRT-PCR and western blot assays on day 7 after
infection. qRT-PCR demonstrated that the expression level of
AIOLOS mRNA in Nalm-6 cells of the Lenti-AIOLOS group
markedly increased compared with that of the Lenti-Mock group and
UT group, which was consistent with the increase in AIOLOS protein
expression (Fig. 3G and H). No
significant difference between cells of the Lenti-Mock group and
the UT group was observed. These results indicated that the stable
transfection of pWPT-PURO-GFP-AIOLOS upregulated AIOLOS expression
in Nalm-6 cells.
Overexpression of AIOLOS suppresses the
proliferation of Nalm-6 cells in vitro
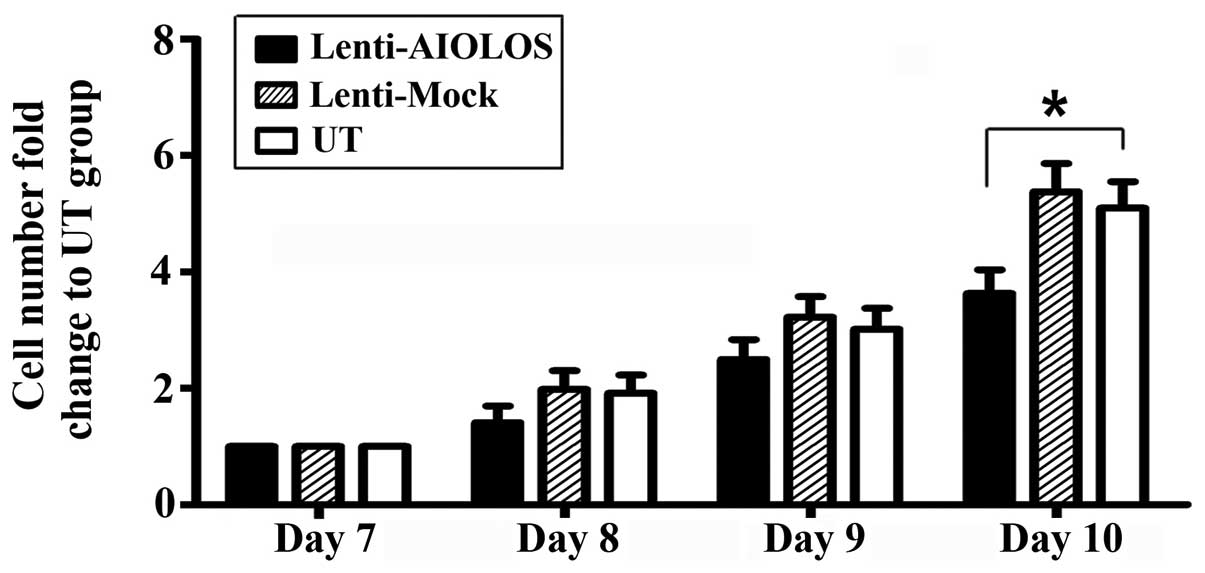
To assess the effect of AIOLOS overexpression on
cell proliferation of Nalm-6 cells, cell numbers were counted on
days 8, 9 and 10 after transfection. As shown in Fig. 4, proliferation of Nalm-6 cells in
the Lenti-AIOLOS group was reduced by 16% on day 8 compared with
that in the UT group cells (P>0.05). The reduction peaked at 29%
on day 10 (P<0.05). The Lenti-Mock group exhibited similar
growth ability as the UT group (P>0.05). The results indicated
that overexpression of AIOLOS suppressed the growth of Nalm-6 cells
to some degree.
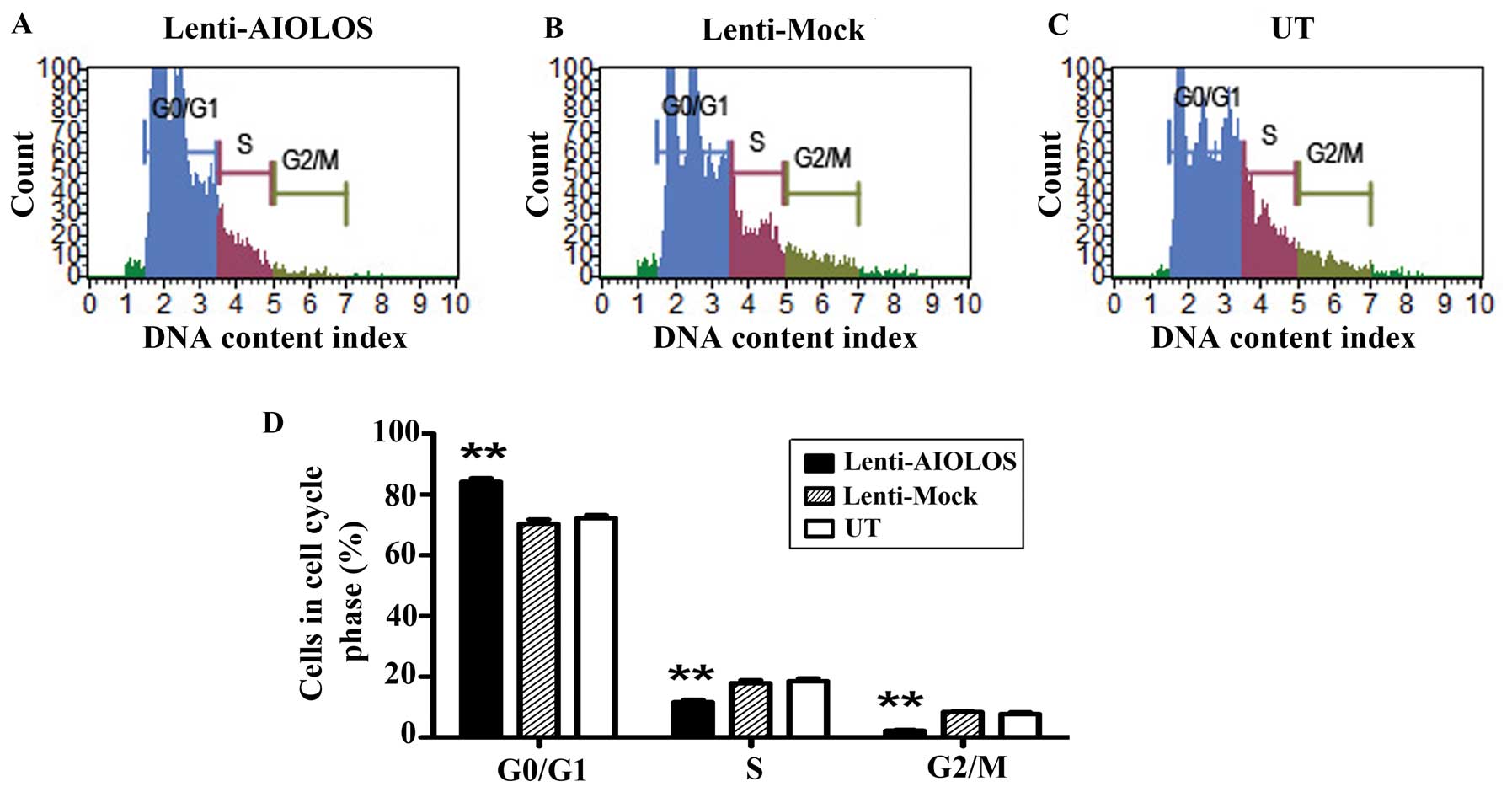
Upregulation of AIOLOS arrests Nalm-6
cells at the G0/G1 phase
The different proliferation rates of Nalm-6 cells
infected with Lenti-AIOLOS vs. control Nalm-6 cells may partly be
due to the differences in cell cycle regulation. Therefore, the
cell cycle of Nalm-6 cells in the Lenti-AIOLOS, Lenti-Mock and UT
groups were characterized by fluorescence-activated cell sorting
(FACS) analysis on day 9 after transfection. As shown in Fig. 5, no significant difference between
the Lenti-Mock and Nalm-6 cells was observed (P>0.05). However,
the percentage of Nalm-6 cells in G0/G1 phase increased from 70.4
(UT) to 84.1% (Lenti-AIOLOS) (P<0.01), and the S-phase cells
decreased from 20.3 (UT) to 11.7% (Lenti-AIOLOS) (P<0.01). The
difference between Nalm-6 cells of the Lenti-AIOLOS group and the
UT group in G2/M phase was significant (2.2 vs. 7.3%; P<0.01).
The data indicated that upregulation of AIOLOS expression arrested
Nalm-6 cells at the G0/G1 phase, which possibly contributed to the
growth inhibition of Nalm-6 cells.
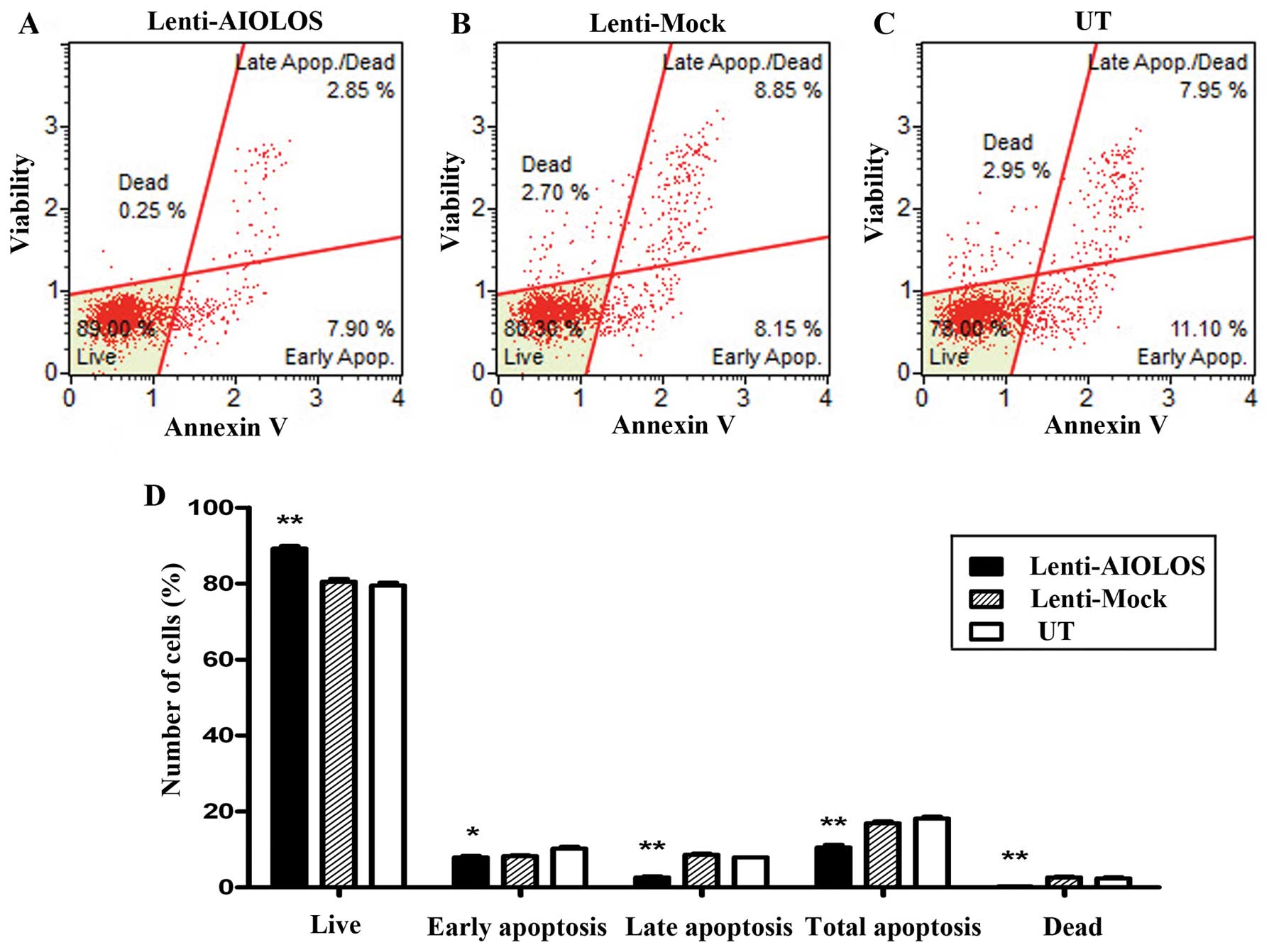
Overexpression of AIOLOS suppresses cell
apoptosis of Nalm-6 cells
To determine whether AIOLOS overexpression results
in apoptosis in Nalm-6 cells, we used a Muse™ Annexin V and Dead
Cell kit to measure the changes in cell apoptosis on day 9. As
shown in Fig. 6, total apoptotic
cells were significantly decreased in AIOLOS-transfected Nalm-6
cells (10.75%) compared with those in the Lenti-Mock (17.00%) or UT
groups (19.05%) (P<0.01). In particular, the difference between
AIOLOS-transfected Nalm-6 cells and UT Nalm-6 cells in the
percentage of early apoptotic cells was minimal (7.90 vs. 11.10%;
P<0.05), whereas the difference between the cell groups in the
percentage of late apoptotic cells was significant (2.85 vs. 7.95%;
P<0.01). These data suggest that overexpression of AIOLOS
suppresses cell apoptosis in Nalm-6 cells.
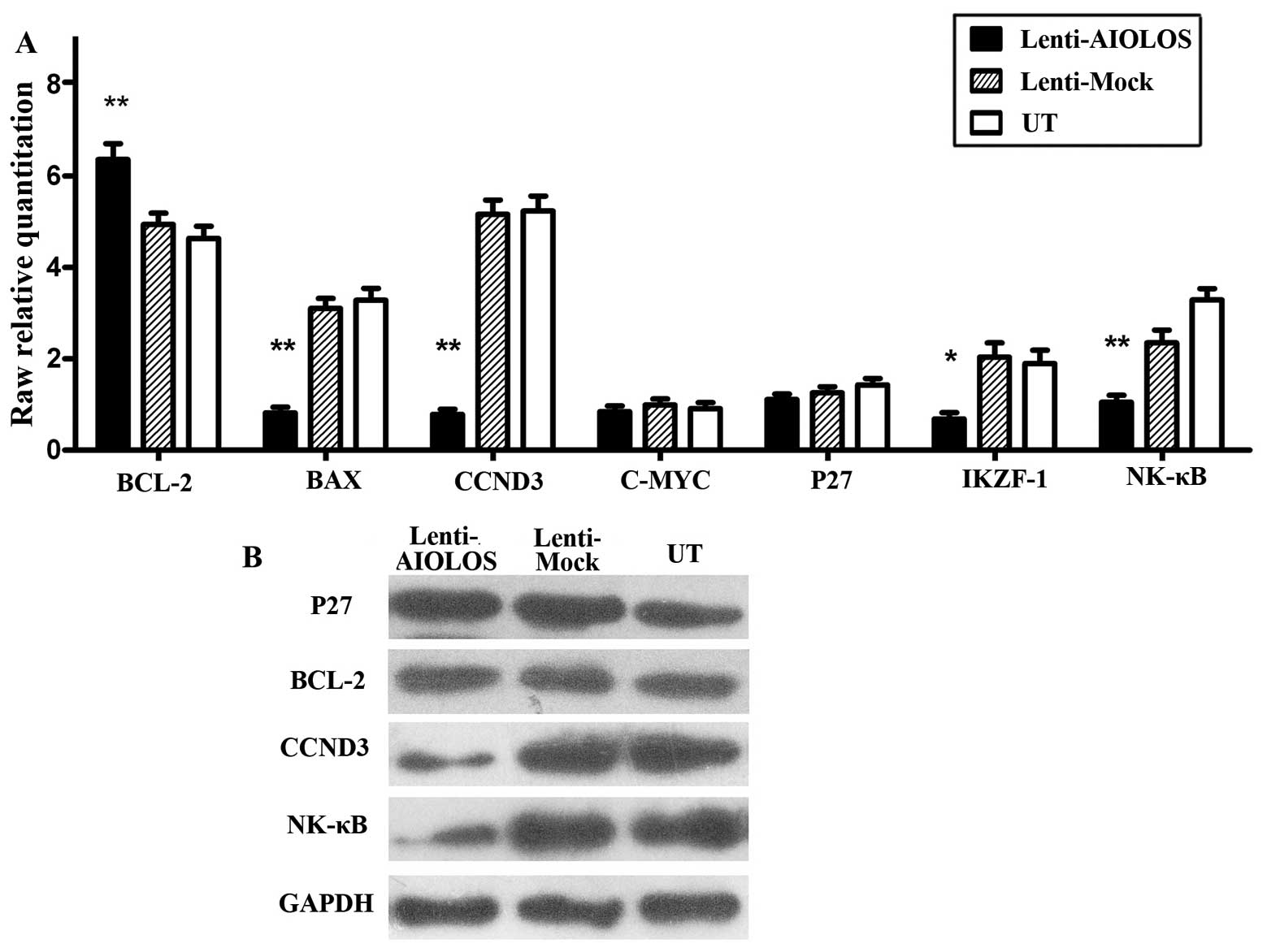
Effect of AIOLOS on the expression levels
of apoptosis and cell cycle-related genes in Nalm-6 cells
To further explore the underlying mechanism in the
above-mentioned changes of biological behaviors, we examined the
expression of genes associated with apoptosis and cell cycle in
response to AIOLOS overexpression by qRT-PCR (Fig. 7A) and western blot analysis
(Fig. 7B). We also examined the
expression level of IKZF1, another IKAROS family member.
qRT-PCR showed that the levels of pro-apoptotic gene BAX
(P<0.01), and cell-cycle-associated gene CCND3
(P<0.01), were markedly reduced in Nalm-6 cells of the
Lenti-AIOLOS group compared with that in the control groups, while
the expression level of anti-apoptotic gene BCL-2
(P<0.05) was increased. However, minimal change was observed in
the expression of C-MYC (P>0.05) and P27
(P>0.05). In addition, downregulation of IKZF1
(P<0.05) and NF-κB (P<0.01) was confirmed in the
Lenti-AIOLOS group compared with the control groups. Consistent
with qRT-PCR, decreased expressions of CCND3 and NF-κB, as well as
steady expression level of P27, were observed by western blot
analysis. No significant change in the expression of BCL-2 protein
was observed, which was inconsistent with qRT-PCR results.
Discussion
The AIOLOS transcription factor IKZF3, encoded by
the gene IKZF3 located on chromosome 17q12, is a member of the
IKAROS family of zinc-finger proteins, and is important in the
control of mature B-lymphocyte differentiation and proliferation.
Thus, to better understand the function of AIOLOS in the
pathogenesis of BCP-ALL, we chose the BCP-ALL cell line Nalm-6 for
a series of functional studies.
At least 16 different isoforms of AIOLOS resulting
from alternate splicing have different cellular localizations and
the ability to change the localization of other IKAROS members
(15). Moreover, the AIO1
transcript represents more than 80% of the AIOLOS isoforms in
B-cells (16). To mimic the
isoforms and cellular localizations of AIOLOS in B-cells, we
constructed a plasmid pWPT-PURO-GFP-AIOLOS containing the entire
AIOLOS coding sequence and performed lentivirus-mediated
transduction in Nalm-6 cells to create a stable transfection cell
line. Our results indicated that Nalm-6 cells were successfully
transduced with lentivirus and AIOLOS was successfully
overexpressed in Nalm-6 cells.
Previous studies have shown that loss of AIOLOS in
mice results in increased pre-B and immature B cells precursors
(10). In the present study, we
showed that overexpression of AIOLOS could suppress the
proliferation of Nalm-6 in vitro. To explore the potential
mechanisms underlying the action of AIOLOS in Nalm-6 cell growth,
cell cycling was characterized by FACS analysis. Data indicated
that upregulation of AIOLOS expression arrested Lenti-AIOLOS cell
cycling at the G0/G1 phase. This result supports the hypothesis
that AIOLOS inhibits Nalm-6 cell proliferation through cell cycle
regulation. Proliferation of pre-B cells requires both Ccnd3 and
c-Myc (17,18). Recent studies have demonstrated that
cell cycle arrest in pre-B cells is associated with downregulation
of c-Myc and Ccnd3 and induction of p27 in mice (19). We then investigated whether
overexpression of AIOLOS in Nalm-6 cells could affect these cell
cycle-associated genes. However, we only found downregulation of
CCND3, whereas C-MYC and P27 expressions showed minimal change. We
attributed this conflict to two possible reasons; first, AIOLOS
directly represses C-MYC, which in turn leads to upregulation of
P27 and downregulation of CCND3 (19). This process follows a time sequence.
Therefore, C-MYC and P27 returned to their original expression
levels in our experiment. Second, unlike in mice, AIOLOS could
possibly arrest cell cycle in pre-B cells via other pathways in
humans.
AIOLOS reportedly controls T cell death by
regulating the expression and localization of the anti-apoptotic
molecule Bcl-2 (20), suggesting
the possibility that evasion of apoptotic cell death is a common
mechanism through which IKAROS family proteins participate in
leukemogenesis. In the present study, we confirmed that
overexpression of AIOLOS in Nalm-6 cell inhibited cell apoptosis,
consistent with previous research demonstrating that disruption of
AIOLOS causes B cells to be more prone to apoptosis (21). Previous studies have found that the
lack of AIOLOS accelerates premature B cell apoptosis mediated by
BCR signaling through elevation in cytochrome c release
(22). In the present study, we
investigated whether the disruption of apoptosis-related genes
BCL-2 and BAX contributed to apoptosis inhibition of Nalm-6 cells
by AIOLOS overexpression. Although BCL-2 protein showed minimal
change in western blot analysis, BCL-2 mRNA was significantly
upregulated by qRT-PCR. This inconsistency is possibly caused by
complicated post-transcriptional regulations. These results
indicated that increased level of BCL-2 and decreased level of BAX
is a possible reason for the decreased apoptosis in Nalm-6 cells.
However, further research is required to explore the complete
mechanism.
IKAROS is a master regulator during the early stages
of lymphocyte ontogeny and differentiation (9,23).
NF-κB is widely recognized as a key positive regulator of cancer
cell proliferation and survival via its ability to
transcriptionally activate many pro-survival and anti-apoptotic
genes, such as XIAP, Bcl-2, Bcl-Xl, IκBα, cIAP-1, cIAP-2 and
survivin (24). Previous studies
showing that IKAROS and NF-κB have the potential to stimulate
AIOLOS expression suggest that these transcription factors are
possible upstream AIOLOS effectors (25). Thus, the expression levels of IKAROS
and NF-κB mRNA were detected by qRT-PCR, and NF-κB protein was
detected by western blot analysis. Notably, both IKAROS and NF-κB
expression decreased following AIOLOS overexpression. We speculate
that since IKAROS and NF-κB could stimulate AIOLOS expression,
highly expressed levels of AIOLOS could exhibit feedback inhibition
to IKAROS and NF-κB for maintenance of homeostasis.
In summary, the present study explored the function
of the transcription factor AIOLOS in biological behaviors in human
BCP-ALL cell line for the first time, which will provide foundation
for further research on the pathogenesis of BCP-ALL. Our results
indicated that upregulation of AIOLOS expression in Nalm-6 cells
inhibited cell proliferation, suppressed cell apoptosis and
arrested cell cycle at the G0/G1 phase. The present study
emphasized the hypothesis that disruption of AIOLOS may be critical
for BCP-ALL pathogenesis. However, the mechanism through which
AIOLOS interacts with other proliferation and apoptosis regulators
is poorly understood. Characterizing these potential genetic
interactions will be of future interest.
Acknowledgements
The present study was supported by grants from the
Shandong Province Natural Science Foundation (ZR2011HM007 and
2013GSF11812) and the Innovation Fund Project of Shandong
University (2012ZD023).
References
|
1
|
Eden T: Aetiology of childhood leukaemia.
Cancer Treat Rev. 36:286–297. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kaatsch P: Epidemiology of childhood
cancer. Cancer Treat Rev. 36:277–285. 2010. View Article : Google Scholar
|
|
3
|
Howard SC, Metzger ML, Wilimas JA,
Quintana Y, Pui CH, Robison LL and Ribeiro RC: Childhood cancer
epidemiology in low-income countries. Cancer. 112:461–472. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pui CH, Campana D and Evans WE: Childhood
acute lymphoblastic leukaemia - current status and future
perspectives. Lancet Oncol. 2:597–607. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Belson M, Kingsley B and Holmes A: Risk
factors for acute leukemia in children: a review. Environ Health
Perspect. 115:138–145. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cobaleda C and Sanchez-Garcia I: B-cell
acute lymphoblastic leukaemia: towards understanding its cellular
origin. Bioessays. 31:600–609. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kelley CM, Ikeda T, Koipally J, Avitahl N,
Wu L, Georgopoulos K and Morgan BA: Helios, a novel dimerization
partner of Ikaros expressed in the earliest hematopoietic
progenitors. Curr Biol. 8:508–515. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Morgan B, Sun L, Avitahl N, Andrikopoulos
K, Ikeda T, Gonzales E, Wu P, Neben S and Georgopoulos K: Aiolos, a
lymphoid restricted transcription factor that interacts with Ikaros
to regulate lymphocyte differentiation. EMBO J. 16:2004–2013. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Georgopoulos K, Winandy S and Avitahl N:
The role of the Ikaros gene in lymphocyte development and
homeostasis. Ann Rev Immunol. 15:155–176. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang JH, Avitahl N, Cariappa A, Friedrich
C, Ikeda T, Renold A, Andrikopoulos K, Liang L, Pillai S, Morgan BA
and Georgopoulos K: Aiolos regulates B cell activation and
maturation to effector state. Immunity. 9:543–553. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nakase K, Ishimaru F, Avitahl N, Dansako
H, Matsuo K, Fujii K, Sezaki N, Nakayama H, Yano T, Fukuda S,
Imajoh K, Takeuchi M, Miyata A, Hara M, Yasukawa M, Takahashi I,
Taguchi H, Matsue K, Nakao S, Niho Y, Takenaka K, Shinagawa K,
Ikeda K, Niiya K and Harada M: Dominant negative isoform of the
Ikaros gene in patients with adult B-cell acute lymphoblastic
leukemia. Cancer Res. 60:4062–4065. 2000.PubMed/NCBI
|
|
12
|
Nuckel H, Frey UH, Sellmann L, Collins CH,
Duhrsen U and Siffert W: The IKZF3 (Aiolos) transcription factor is
highly upregulated and inversely correlated with clinical
progression in chronic lymphocytic leukaemia. Br J Haematol.
144:268–270. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Billot K, Soeur J, Chereau F, Arrouss I,
Merle-Beral H, Huang ME, Mazier D, Baud V and Rebollo A:
Deregulation of Aiolos expression in chronic lymphocytic leukemia
is associated with epigenetic modifications. Blood. 117:1917–1927.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Antica M, Cicin-Sain L, Kapitanovic S,
Matulic M, Dzebro S and Dominis M: Aberrant Ikaros, Aiolos, and
Helios expression in Hodgkin and non-Hodgkin lymphoma. Blood.
111:3296–3297. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Caballero R, Setien F, Lopez-Serra L,
Boix-Chornet M, Fraga MF, Ropero S, Megias D, Alaminos M,
Sanchez-Tapia EM, Montoya MC, Esteller M, Gonzalez-Sarmiento R and
Ballestar E: Combinatorial effects of splice variants modulate
function of Aiolos. J Cell Sci. 120:2619–2630. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Duhamel M, Arrouss I, Merle-Beral H and
Rebollo A: The Aiolos transcription factor is up-regulated in
chronic lymphocytic leukemia. Blood. 111:3225–3228. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cooper AB, Sawai CM, Sicinska E, Powers
SE, Sicinski P, Clark MR and Aifantis I: A unique function for
cyclin D3 in early B cell development. Nat Immunol. 7:489–497.
2006. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Habib T, Park H, Tsang M, de Alboran IM,
Nicks A, Wilson L, Knoepfler PS, Andrews S, Rawlings DJ, Eisenman
RN and Iritani BM: Myc stimulates B lymphocyte differentiation and
amplifies calcium signaling. J Cell Biol. 179:717–731. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ma S, Pathak S, Mandal M, Trinh L, Clark
MR and Lu R: Ikaros and Aiolos inhibit pre-B-cell proliferation by
directly suppressing c-Myc expression. Mol Cell Biol. 30:4149–4158.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Romero F, Martinez AC, Camonis J and
Rebollo A: Aiolos transcription factor controls cell death in T
cells by regulating Bcl-2 expression and its cellular localization.
EMBO J. 18:3419–3430. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Narvi E, Nera KP, Terho P, Mustonen L,
Granberg J and Lassila O: Aiolos controls gene conversion and cell
death in DT40 B cells. Scand J Immunol. 65:503–513. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kikuchi H, Yamashita K, Nakayama M,
Toyonaga K, Tsuneyoshi I, Takasaki M and Nakayama T: Lacking of
Aiolos accelerates pre-mature B cell apoptosis mediated by BCR
signaling through elevation in cytochrome c release. Biochim
Biophys Acta. 1793:1304–1314. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Georgopoulos K, Bigby M, Wang JH, Molnar
A, Wu P, Winandy S and Sharpe A: The Ikaros gene is required for
the development of all lymphoid lineages. Cell. 79:143–156. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sethi G, Ahn KS and Aggarwal BB: Targeting
nuclear factor-kappa B activation pathway by thymoquinone: role in
suppression of antiapoptotic gene products and enhancement of
apoptosis. Mol Cancer Res. 6:1059–1070. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ghadiri A, Duhamel M, Fleischer A, Reimann
A, Dessauge F and Rebollo A: Critical function of Ikaros in
controlling Aiolos gene expression. FEBS Lett. 581:1605–1616. 2007.
View Article : Google Scholar : PubMed/NCBI
|