Introduction
In spite of continuous progress in the therapy of
acute lymphoblastic leukemia (ALL), relapses still occur in up to
20% of children and most adults with ALL (1–4).
Moreover, the outcome of relapsed ALL patients is extremely poor
(3,5,6). To
improve the survival of ALL patients, it is critical to identify
new molecular biomarkers such as BCR/ABL which are involved in the
regulation of the malignant biological behavior of leukemia cells
and are valuable in the prognosis and therapy of leukemia
patients.
Ikaros is a lymphoid transcription factor that was
identified as a hematological tumor suppressor (7,8). Only
Ikaros isoforms that contain at least three DNA-binding zinc
fingers possess functional activity, such as Ik1, Ik2 and Ik3.
Isoforms lacking two or more zinc-finger domains cannot bind DNA
and impair the function of Ikaros proteins in a dominant-negative
manner (9). Dominant-negative
Ikaros isoform 6, (Ik6), with a deletion of coding exons 3 through
6, is the most common and strongest transcriptional repressor in
the Ikaros family (10–12). Ample evidence indicates that
overexpression of Ik6 is associated with a poor prognosis of ALL
patients (13–15). A recent study showed that the
prognosis of Ph-negative patients with Ik6 was close to that of
Ph-positive patients (16).
The clinical data available to date suggest that Ik6
should be evaluated as a prognostic marker for newly diagnosed ALL
patients and may be involved in leukemogenesis. However, there are
few studies concerning the role of Ik6 in the therapy of ALL. In
the present study, in order to ascertain whether Ik6 is a marker of
chemotherapeutic efficacy and is a potential therapeutic target, we
investigated changes in Ik6 expression during treatment of ALL
patients and assessed the effects in vitro of Ik6 expression
on cell proliferation, cell cycle, chemosensitivity to vincristine
(VCR), daunorubicin (DNR) and L-asparaginase (L-Asp) and invasion
in ALL cell lines.
Materials and methods
Patients and samples
The 25 patients included in the present study were
diagnosed with Ik6-positive B lineage ALL between January 2009 and
January 2012 and were treated at Wuhan Union Hospital, in
accordance with the CCLG-ALL-2008 Protocol (Children’s Cancer and
Leukemia Group). All samples were bone marrow aspirates and were
obtained following informed consent in strict accordance with the
Declaration of Helsinki. The endpoint of the present study was
January 2013, and the expression of Ik6 was measured at the point
of initial diagnosis, end of induction therapy, at complete
remission and at relapse.
Cell culture
Sup-B15 and Nalm-6, human B-cell precursor leukemia
cell lines, were used for the study. Both cell lines were purchased
from the American Type Culture Collection (ATCC) (Rockville, MD,
USA) and cultured in RPMI-1640 medium supplemented with 10% fetal
bovine serum (FBS) (both from Gibco-BRL, Carlsbad, CA, USA) at 37°C
in a humidified atmosphere with 5% CO2.
Overexpression or silencing of Ik6 in
acute lymphoblastic leukemia cell lines
Nalm-6 cells stably overexpressing Ik6 were obtained
as follows. The complete Ik6 coding sequence was amplified by PCR
from Sup-B15 cells and cloned into the lentiviral expression vector
pHR-SIN-CSIGW. The vector pHR-CSIGW-Ik6 and the viral packaging
system (containing an optimized mixture of two packaging plasmids,
pMD2.G and psPAX2) were co-transfected into 293T cells to produce
competent lentivirus. The viral supernatant was harvested at 48 h
post-transfection and was used to infect Nalm-6 cells. The
pHR-CSIGW-mock vector was also packaged and used as a negative
control. For transfection, 1×106 Nalm-6 cells were
collected on day 2 and resuspended in 1 ml complete medium
(RPMI-1640 medium supplemented with 10% FBS). Cells were
transfected with pHR-CSIGW-Ik6 or pHR-CSIGW-mock at a multiplicity
of infection (MOI) of 50 for 8 h. Then half of the above medium was
replaced with 1 ml fresh medium. Thereafter, cells were cultured
for another 64 h and analyzed for expression of GFP by flow
cytometry. The expression of Ik6 protein was further confirmed by
western blotting.
Sup-B15 cells with stably silenced Ik6 were obtained
through a similar procedure. Firstly, we designed several small
interfering RNAs (siRNAs) and screened the most effective one. The
target sequence for Ik6 was 5′-GCTACGAGAAGG AGAACGA-3′ and the
negative control sequence was 5′-TTC TCCGAACTGTCACGT-3′. Then, the
small hairpin RNA (shRNA) was cloned into the self-inactivating
lentiviral vector (GeneChem, Shanghai, China) containing a
CMV-driven GFP reporter and a U6 promoter upstream of the cloning
sites (AgeI and EcoRI).
Real-time RT-PCR assay
Total cellular RNA was extracted from cells using
TRIzol reagent and converted to single-stranded cDNA using the
Toyobo kit. Real-time PCR amplification was performed using the
SYBR-Green Master Mix (Toyobo, Japan) and the StepOnePlus™
Real-Time PCR System (Bio-Rad, Hercules, CA, USA). The primers for
Ik6, vascular endothelial growth factor (VEGF), vascular
endothelial growth factor receptor (Flt-1), placenta growth factor
fragment (PlGF), angiogenin-1 (Ang-1), angiogenin-2 (Ang-2), matrix
metalloproteinase-2 (MMP-2) and matrix metalloproteinase-9 (MMP-9)
are shown in Table I. Cycling
conditions were 95°C for 30 sec followed by 40 cycles of 95°C for
10 sec and 60°C for 35 sec. The relative levels of mRNA expression
were quantified by comparison with the internal control (GAPDH).
All the samples were performed in triplicate, and the results were
analyzed using the 2−ΔΔCt method.
 | Table ISequences of primers used in real-time
PCR. |
Table I
Sequences of primers used in real-time
PCR.
| Gene | Primer sequences |
|---|
| Ik6 | F |
ccctgtaagcgatactccag |
| R | ttgtcccccacgact |
| VEGF | F |
atcttcaagccatcctgtgtgc |
| R |
gctcaccgcctcggcttgt |
| Flt-1 | F |
atcattccgaagcaaggtgtg |
| R |
aaacccatttggcacatctgt |
| PlGF | F |
cacttccccctgttcttctgaa |
| R |
caagcaaatggcaaagtgtga |
| Ang-1 | F |
ttcctttcctttgctttcctc |
| R |
ctgcagagcgtttgtgttgt |
| Ang-2 | F |
aacatcccagtccacctgag |
| R |
ggtcttgctttggtccgtta |
| MMP-2 | F |
ggaagcatcaatcggactg |
| R |
gggcgggagaaagtagca |
| MMP-9 | F |
cccacttactttggaaacg |
| R |
gaagatgaatggaaatacgc |
| GAPDH | F |
cctccaaggagtaagacccc |
| R |
aggggtctacatggcaactg |
Western blotting
Cells were lysed using a nuclear and cytoplasmic
protein extraction kit (Beyotime, China) according to the
manufacturer’s instructions. The extracts were centrifuged at
12,000 rpm for 15 min at 4°C, and the supernatant was collected. A
BCA protein assay kit (Pierce, Rockford, IL, USA) was used to
determine the protein concentrations. Samples were resolved in 10%
SDS-PAGE and then transferred onto nitrocellulose membranes
(Bio-Rad). After being blocked with 5% skim milk in Tris-buffered
saline with 0.1% Tween-20, proteins were detected by respective
antibodies using an ECL kit (Pierce) and exposed to X-ray film.
Blots were visualized with a western blotting detection system
(Bio-Rad).
Proliferation assay
To investigate the effects of the alteration in
expression Ik6 on leukemia cells, exponentially growing cells
(2×104/well) were seeded in quintuplicate in 96-well
plates. After seeding for 1, 2, 3 and 4 days, the quantity of
viable cells was determined. Ten microliters of CCK-8 Cell Counting
Reagent (Dojindo Molecular Technologies, Inc., Japan) was added
directly to each well. The plates were sequentially incubated for 4
h at 37°C, and the WST-8 formazan product was measured at 490 nm
using a microplate reader (Tecan Sunrise, Switzerland). To
investigate the effects of the alteration in Ik6 expression
combined with chemotherapeutics on leukemia cells, cells were
incubated with culture medium containing various concentrations of
the chemotherapeutics [2.5 to 50 ng/ml for vincristine (VCR); 2.5
to 50 ng/ml for daunorubicin (DNR) and 0.1 to 2.5 IU/ml for
L-asparaginase (L-Asp)], respectively. After allowing cells to grow
for 24 h at 37°C, the viable cell population in each well was
reflected by the OD values. Then the fraction of surviving cells
was calculated and the IC50 was determined by nonlinear
regression analysis using SPSS 11.5 software.
Cell cycle analysis
The cells (106 cells) were collected and
fixed with ice-cold 70% ethanol overnight at 4°C. After washing
with PBS and resuspension, fixed cells were treated with 50 μg/ml
RNase A (Amresco Inc., Solon, OH, USA) for 15 min at 37°C, and then
incubated with 5 μg/ml propidium iodide (Sigma Chemical Co., St.
Louis, MO, USA) for 30 min at room temperature in the dark. The
cell cycle distribution was detected by flow cytometry
(Becton-Dickinson, Franklin Lakes, NJ, USA).
Analysis of apoptosis
The apoptosis detection kit was from KeyGen Biotech
(Nanjing, China). Cells were incubated with culture medium
containing final concentrations of 5.0 ng/ml VCR, 0.5 ng/ml DNR and
0.1 IU/ml L-Asp, respectively, for 24 h. Treated cells were stained
with propidium iodide and Annexin V-FITC for 15 min according to
the manufacturer’s instructions. The stained cells were subjected
to flow cytometric analysis.
Cell migration and invasion assays
Cell migration was evaluated using an uncoated
Transwell assay. Cells (2×105) were suspended in 200 μl
of serum-free RPMI-1640 medium and placed in the upper chambers of
the Transwell plate (Corning, Cambridge, MA, USA). RPMI medium plus
10% FBS (250 μl) and NIH3T3-conditioned medium (250 μl) were added
to the lower chambers. Plates were incubated at 37°C for 8 h. The
cells of the lower compartments were counted, and the rate of
migration was expressed as a percentage of the total number of
cells added to each well. The cell invasion assay was similar to
the migration assay but a Matrigel-coated Transwell was used.
Statistical analysis
Data are presented as means ± SD. Comparisons
between groups were carried out by the Student’s t-test with
software SPSS 11.5. Differences were considered to be statistical
significant at P<0.05.
Results
Expression of Ik6 during
chemotherapy
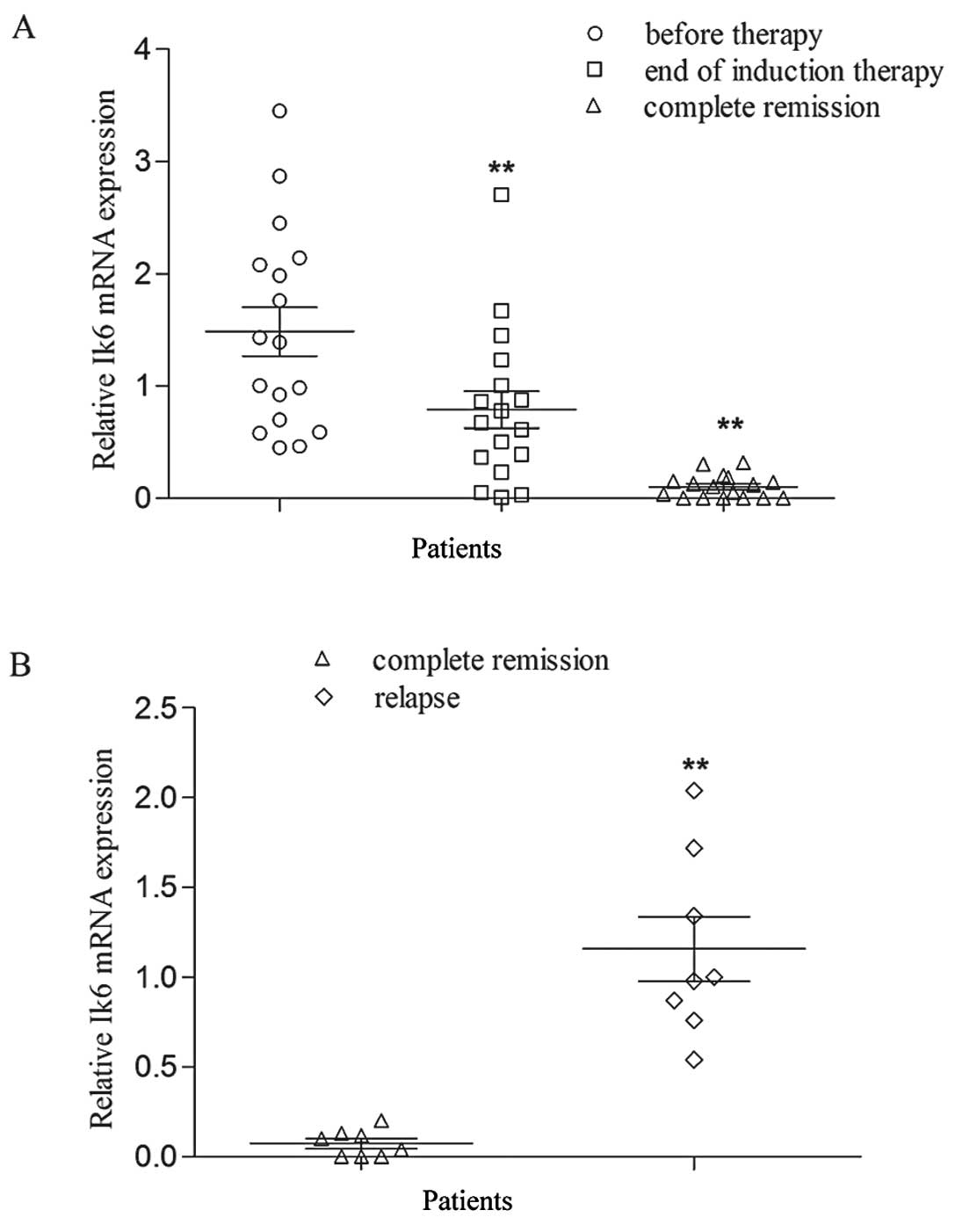
In the 25 patients with Ik6-positive expression, 8
responded poorly to chemotherapy. Seventeen patients achieved
complete remission but 8 patients of these 17 patients later
suffered from relapse. After induction chemotherapy, the Ik6
expression was significantly downregulated compared with that
before treatment (P<0.01). However, in the children with
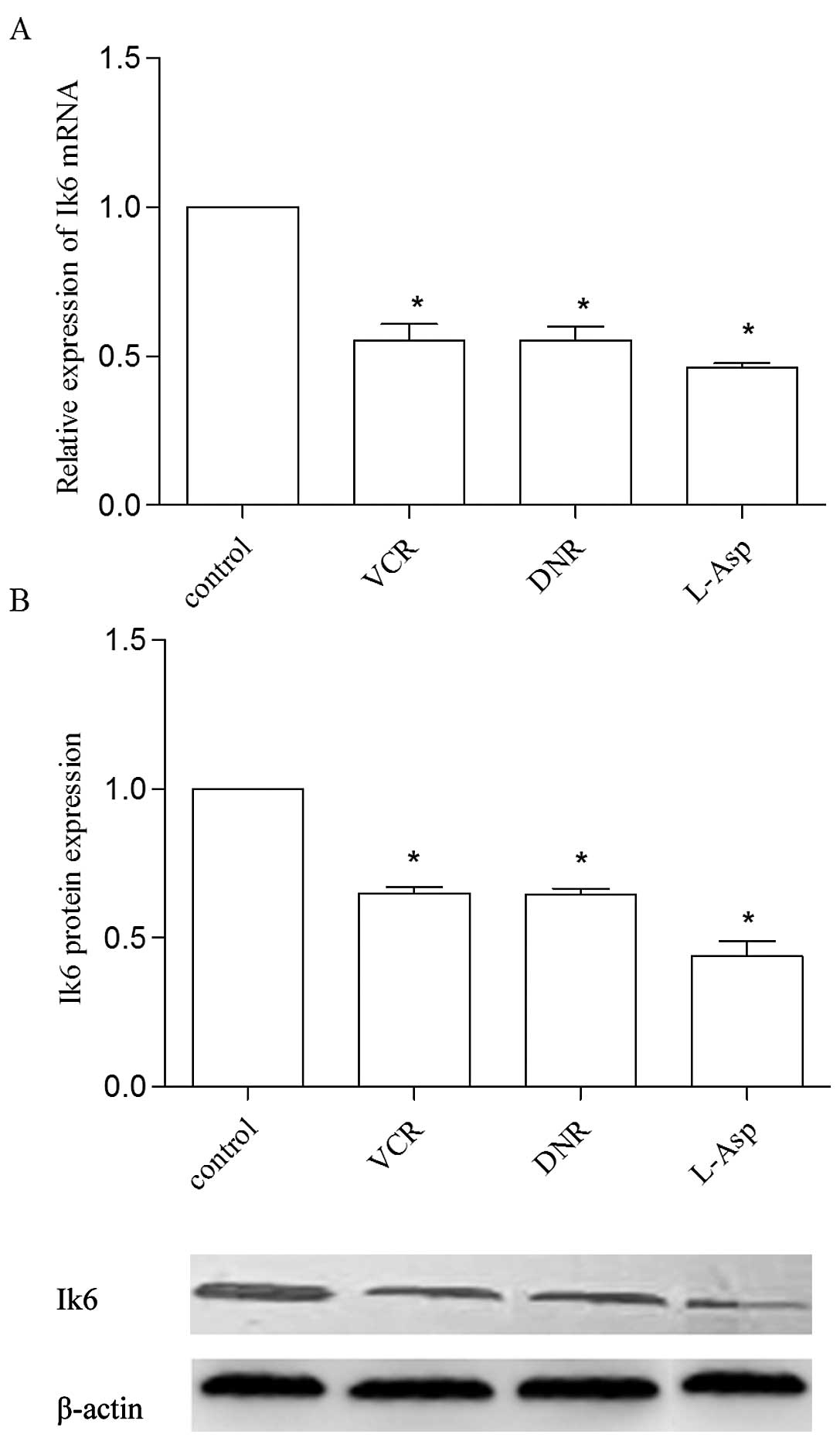
relapse, Ik6 expression was again increased (P<0.01) (Fig. 1). We also determined the alteration
in Ik6 expression in the Sup-B15 cell line after co-culture with
different chemotherapeutics. A similar result was found in that
VCR, DNR and L-Asp treatment markedly decreased the Ik6 mRNA and
protein expression (P<0.05) (Fig.
2).
Overexpression of Ik6 in Nalm-6 cells
enhances cell proliferation and alters cell cycle distribution
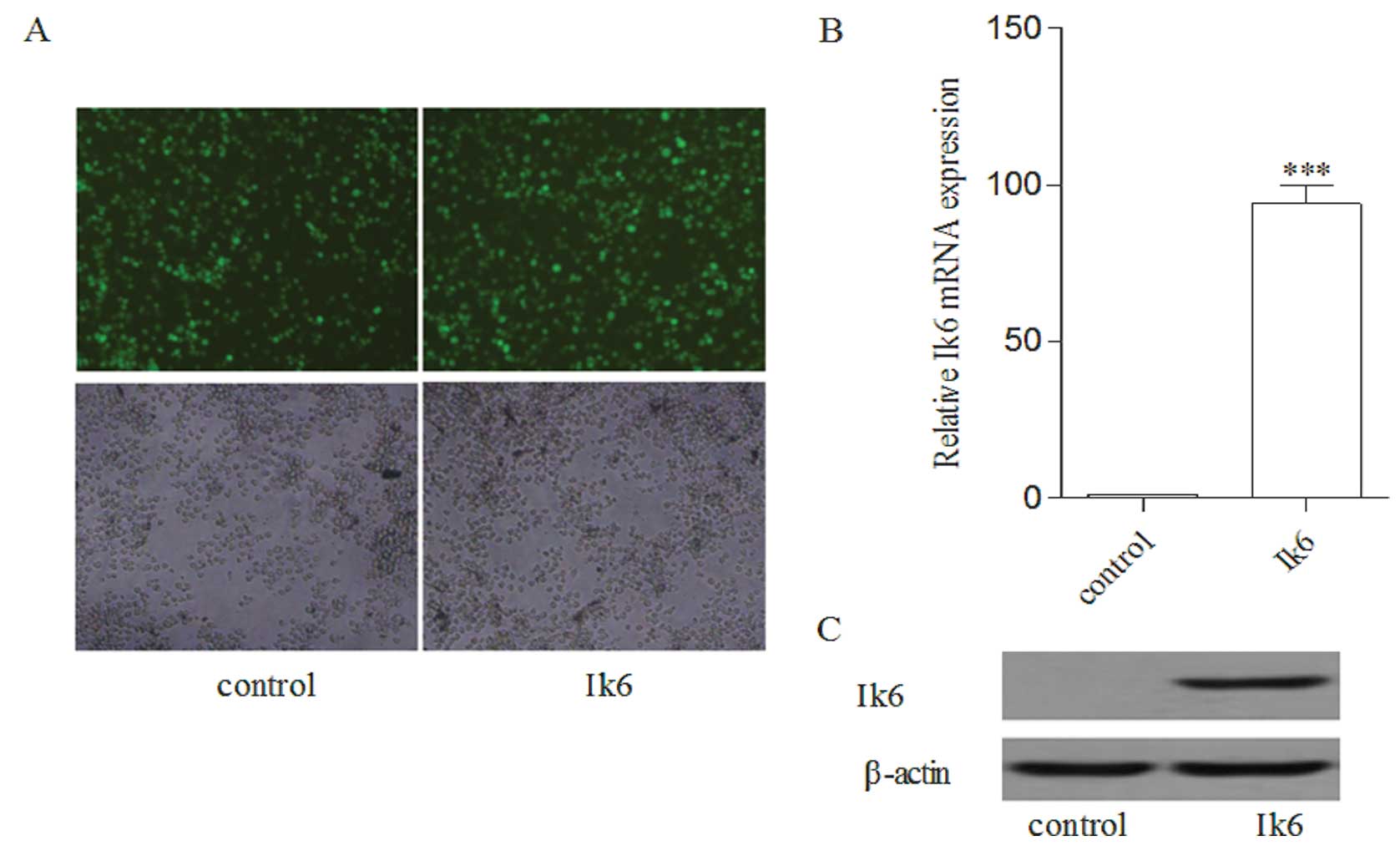
Nalm-6 cells expressed GFP after being transfected
with Ik6 or with the control, and GFP fluorescence was observed in
almost 95% of the cells (Fig. 3A).
Real-time PCR and immunoblotting showed a generally higher level of
Ik6 mRNA and protein expression in Nalm-6 cells following Ik6
overexpression (Fig. 3B and C).
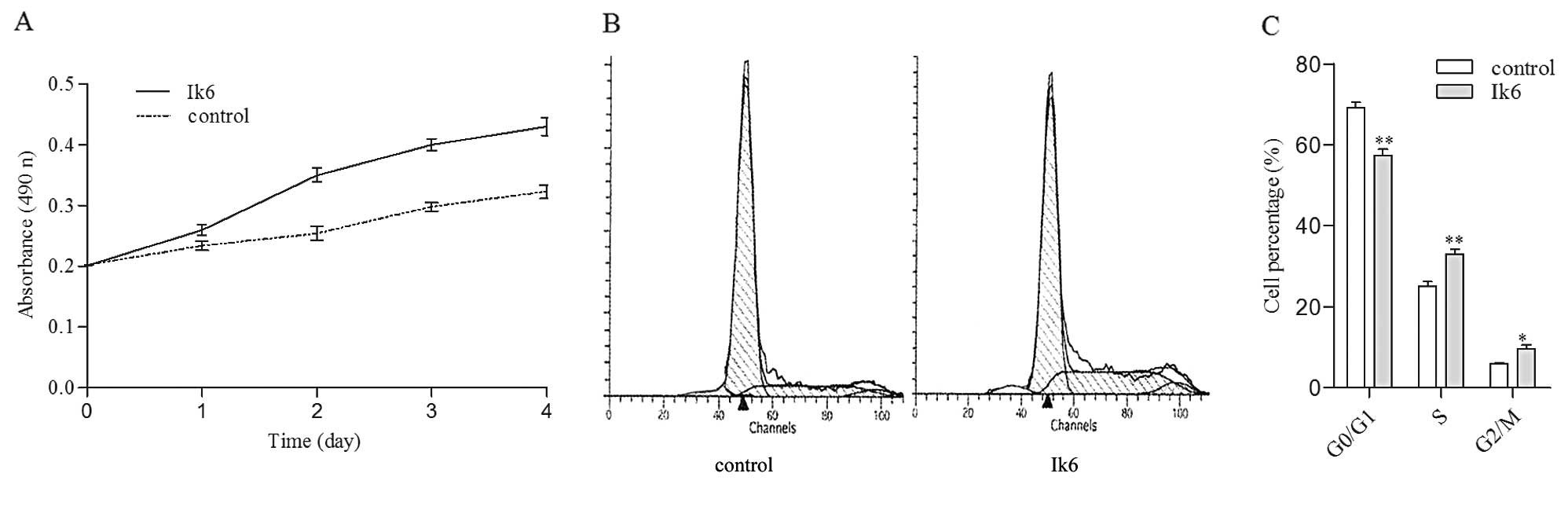
After transfection with Ik6, Nalm-6/Ik6 cells
exhibited increased cell proliferation compared with the Nalm-6
cells lacking the protein (Fig. 4A,
P<0.05, n=5). Cell cycle results showed that Ik6-expressing
Nalm-6 cells exhibited a decreased proportion of cells in the
static phase (G0/G1) and an increased proportion in the synthetic
(S) and mitotic phases (G2/M) of the cell cycle (Fig. 4B and C). Therefore, expression of
Ik6 in Nalm-6 cells promoted cell cycle progression from the G0/G1
phase to the S and G2/M phase.
Overexpression of Ik6 in Nalm-6 cells
decreases sensitivity to chemotherapeutics through an
anti-apoptotic effect
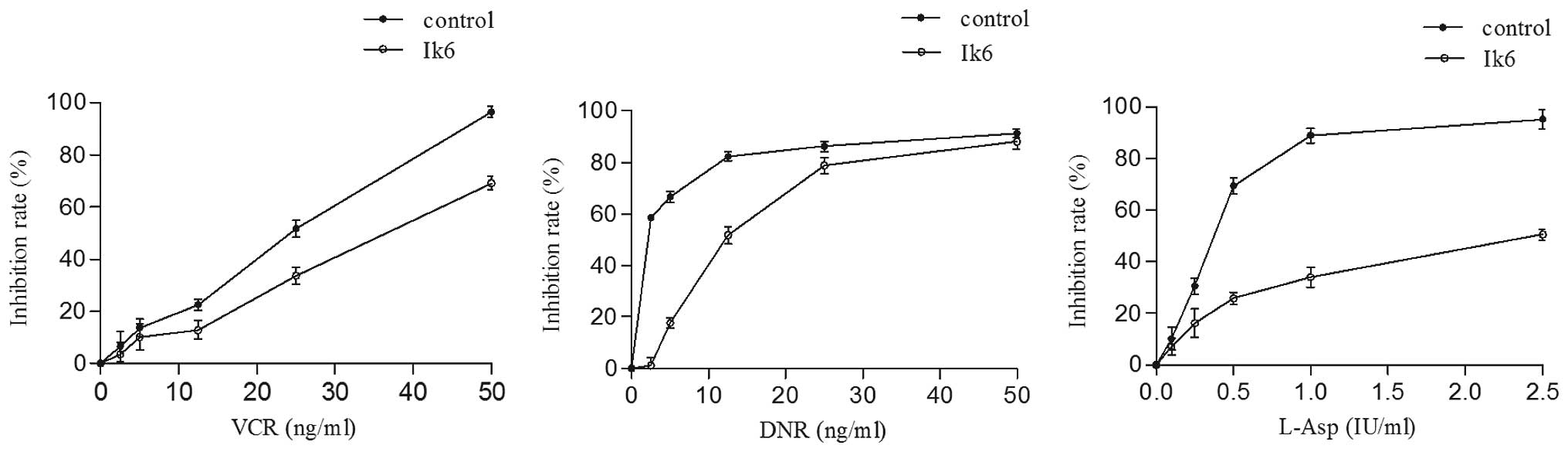
The effects of VCR, DNR and L-Asp on the growth of
leukemia cells were evaluated, and the results indicated that the
resistance to VCR, DNR and L-Asp was increased in the Ik6
transfectants. The IC50 values of VCR (34.94 vs. 20.51
ng/ml), DNR (12.25 vs. 1.89 ng/ml) and L-Asp (2.37 vs. 0.36I U/ml)
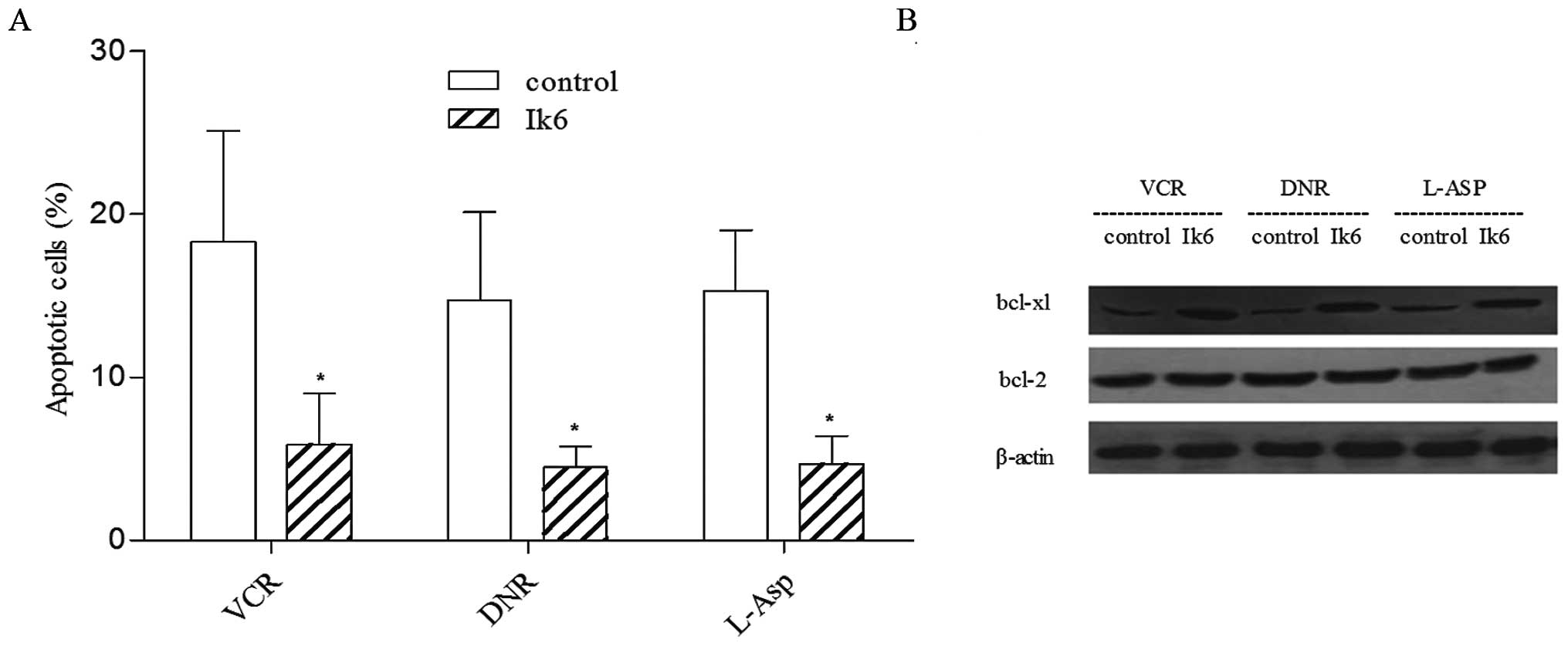
were higher than that of the control (P<0.05) (Fig. 5). The results from flow cytometry
and western blotting revealed that Ik6 decreased the drug-induced
apoptosis together with the upregulation of the bcl-xl protein
(Fig. 6A and B).
Ik6 does not affect the invasiveness of
Nalm-6 cells
Real-time PCR was performed to measure the
expression of genes regulating invasion, and the results revealed
no significant differences in the expression levels for the mRNA
coding of VEGF, Flt-1, PlGF, Ang1, Ang2, MMP-2 and MMP-9 between
Nalm-6/Ik6 cells and the control (P>0.05) (Fig. 7A). In the migration and invasion
assays, cells from both groups transmigrated from the upper to the
lower chamber, but the quantity of cells in the lower chamber was
not statistically different (P>0.05) (Fig. 7B).
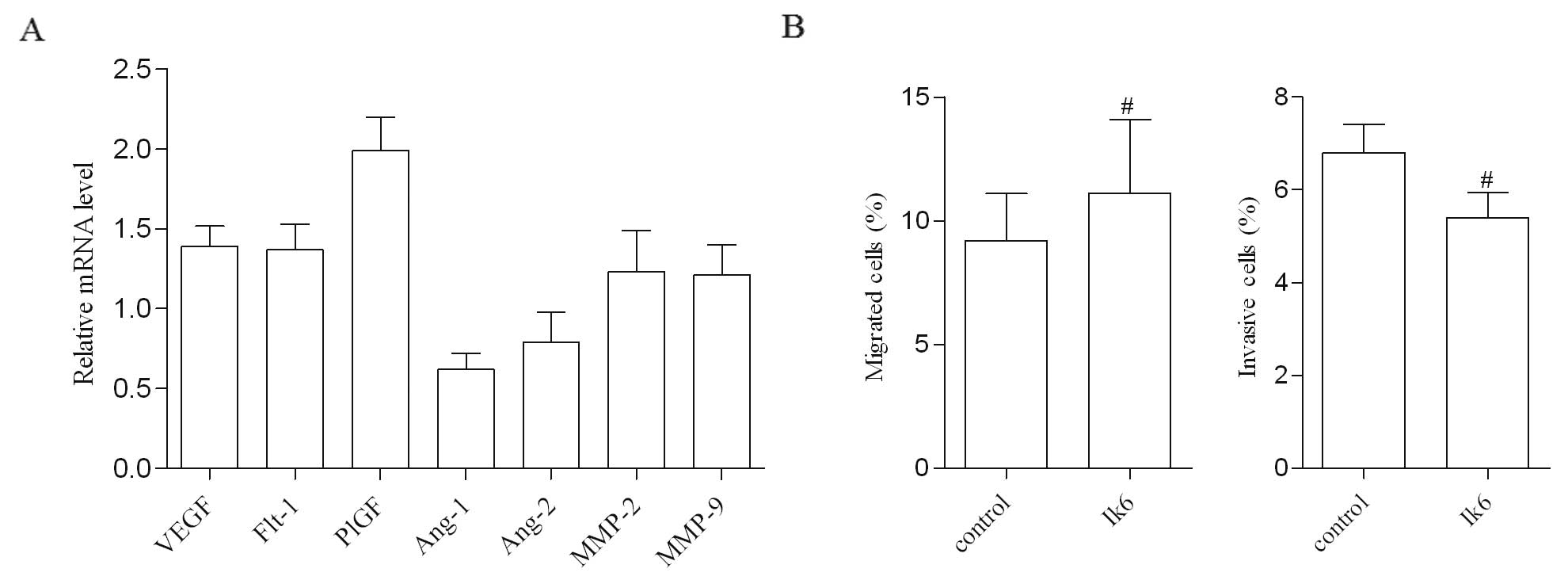 | Figure 7Ik6 overexpression has no effect on
the invasive ability of leukemia cells. (A) The mRNA level of genes
regulating invasion, VEGF, Flt-1, PlGF, Ang-1, Ang-2, MMP-2 and
MMP-9, in Nalm-6/Ik6 cells was detected by real-time RT-PCR. Using
Nalm-6/mock cells as the control, the result showed that there was
no differences in the expression of these genes (n=5). (B) The
migration and invasion assays showed that there was no difference
in migratory and invasive abilities between the Nalm-6/Ik6 cells
and control cells (#P>0.05, n=3). Ik6, Ikaros isoform
6; VEGF, vascular endothelial growth factor; Flt-1, vascular
endothelial growth factor receptor; PlGF, placenta growth factor
fragment; Ang-1, angiogenin-1; Ang-2, angiogenin-2; MMP-2, matrix
metalloproteinase-2; MMP-9, matrix metalloproteinase-9. |
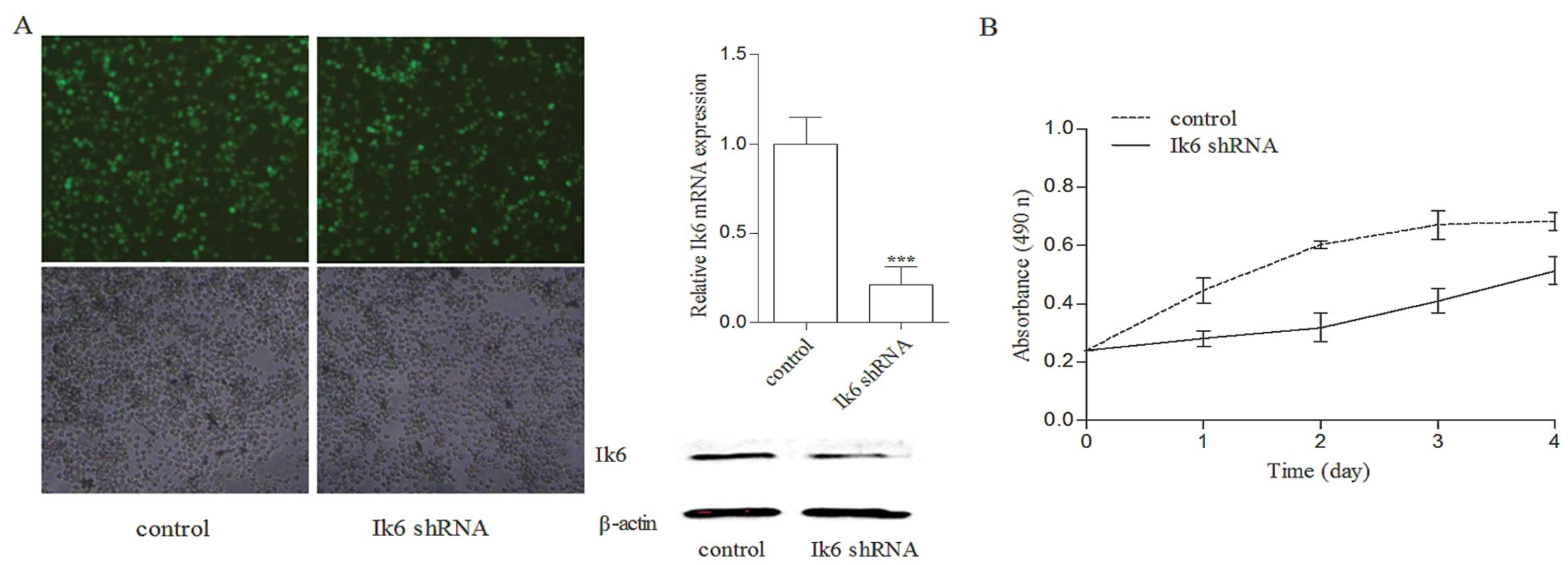
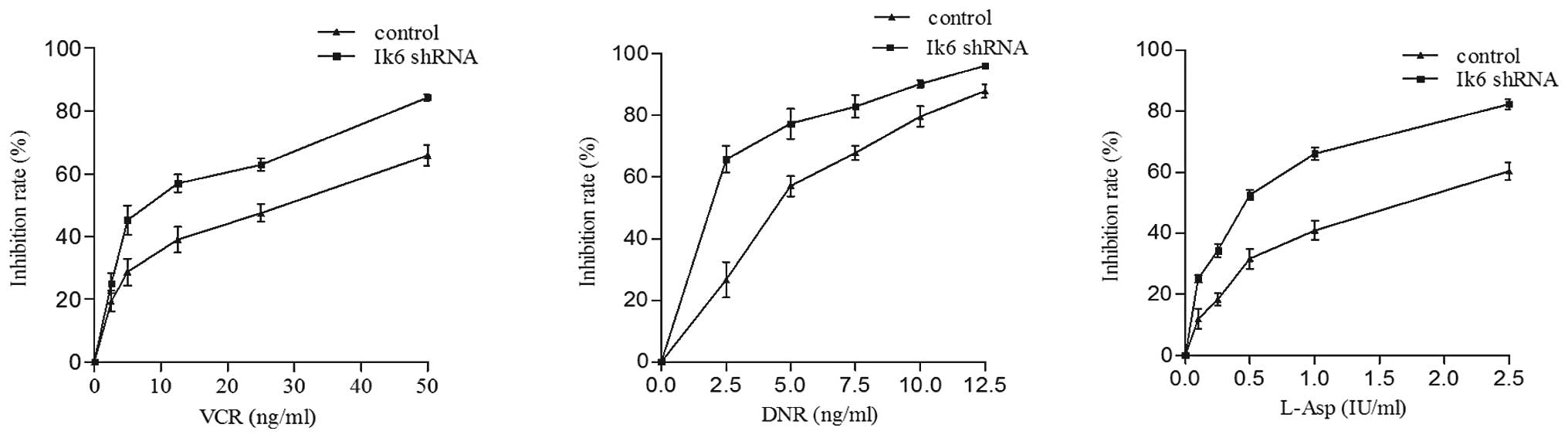
Silencing of Ik6 in Sup-B15 cells
inhibits proliferation and increases chemosensitivity
To further confirm the effect of Ik6 on
proliferation and chemosensitivity of leukemia cells, Sup-B15 cells
were modified to block Ik6 expression via a lentiviral-mediated
shRNA vector. As shown in Fig. 8A,
Ik6 mRNA and protein expression was downregulated in the
Sup-B15/Ik6 shRNA cells when compared with the control. Ik6 shRNA
significantly inhibited the proliferative activity of Sup-B15 cells
(Fig. 8B, P<0.05) and enhanced
the chemosensitivity to VCR, DNR and L-Asp. The IC50
values of control cells to VCR, DNR and L-Asp were 2.6-, 2.9- and
3.4-fold higher than these values in the Sup-B15/Ik6 shRNA cells
(P<0.05, Fig. 9).
Discussion
In an effort to understand the phenomenon of
leukemia relapse, several predictors of the ultimate outcome have
been identified in the hopes of providing clues that may lead to
more effective treatment (17,18).
The Ik6 variant of the IKZF1 gene, an unfavorable prognostic marker
in the outcome analysis of ALL, was independently associated with
both overall survival and relapse-free survival (16). Ph/BCR-ABL was also known as a
high-risk prognostic factor, but the emergence of tyrosine kinase
inhibitors has significantly improved complete remission rates and
the outcome of Ph-positive ALL patients (19,20).
Therefore, just as Ph not only indicates risk but is also a
therapeutic target, Ik6 may not only provide insight into
leukemogenesis but may also lead to the establishment of new
treatment strategies targeting ALL.
We previously reported that Ik6 expression in the
bone marrow cells of newly diagnosed ALL patients is associated
with a higher level of 33-day minimal residual disease, which
indicated excessive proliferation and primary chemoresistance of
leukemia cells (16). However,
there have been few published data concerning the dynamic
expression of Ik6 during chemotherapy. The present study
demonstrated that Ik6 expression is downregulated by
chemotherapeutic agents in vivo and in vitro. A high
level of Ik6 mRNA expression was detected in relapsed patients.
Thus, Ik6 is not only a predictor of poor prognosis at initial
diagnosis but is also a marker for monitoring chemotherapeutic
efficacy and relapse during treatment. Certainly, more data from
Ik6-positive patients are needed to provide the relationship
between the exact level of Ik6 mRNA expression and response to
treatment and relapse.
To explore the potential role of Ik6 in the
treatment of ALL, we evaluated the effect of Ik6 on leukemia cell
growth and found that overexpression of Ik6 increased cell
proliferation. The results were in accordance with these of studies
on in vitro systems, which demonstrated that Ik6
transfection stimulated the proliferation of pituitary cells
(21) and human CD34+
cord blood cells (22).
Furthermore, Ik6-expressing cells progressed more rapidly through
the cell cycle than the control cells, in as much as they peaked in
the S phase earlier.
Additionally, we analyzed the role of Ik6 in
leukemia cell chemosensitivity. Our study demonstrated that the
overexpression of Ik6 increased the chemoresistance of leukemia
cells in vitro. The Ik6-expressing Nalm-6 cells were 1.7
times more resistant to VCR, 6.5 times more resistant to DNR and
6.6 times more resistant to L-Asp. The following clinical studies
support the above-mentioned results. Tonnelle et al
(14) reported that the response of
patients with positive expression of Ik6 to induction treatment was
not favorable; 7 of 8 patients did not reach complete remission and
1 achieved remission at the end of the induction therapy. A large
sample of clinical data showed that 16.07% of the patients with Ik6
did not achieve remission and 48.44% suffered from relapse
(16). VCR, DNR and L-Asp are
currently the first-line chemotherapeutic drugs for the treatment
of pediatric ALL. All of these drugs can induce apoptosis of
leukemic cells. In the present study, we found that when Ik6
expression was increased in Nalm-6 cells, following treatment with
the three drugs, significantly enhanced proliferative activity of
Nalm-6 cells and a decreased level of apoptosis were noted with
upregulation of bcl-xl. To further confirm the role of Ik6 in
therapy, we found that silencing of Ik6 significantly inhibited
proliferation and sensitizes Sup-B15 cells to the chemotherapeutic
agents.
As well as acquired drug resistance, extramedullary
tissue infiltration of leukemic cells is a major obstacle to
leukemia treatment (23). Excessive
egress of leukemia cell blasts results in invasion into various
organs or tissues, such as the central nervous system (CNS) and
testis (24,25). The results of our studies on
leukemia cell invasion indicated that there was no effect of Ik6 on
the invasive ability of leukemia cells in vitro.
In the present study, we found that Ik6 may be
utilized as a gene marker to predict the clinical efficacy of
chemotherapy. The patients with Ik6 overexpression should receive
more intensive therapy, and detection for multidrug resistance
should be carried out. More importantly, Ik6 regulates the
proliferation and chemosensitivity of leukemia cells, and
anti-apoptosis may be the mechanism of action. Regulatory apoptosis
pathways that are associated with Ik6 are a potential target for a
novel strategy for the chemotherapy of ALL.
Acknowledgements
The authors would like to thank Dengli Hong and Zhen
Li of the Department of Medical Stem Cell Biology of Shanghai
Jiaotong University for supporting the present study. The study was
supported by a grant from the National Nature Science Foundation of
China (81300414).
References
|
1
|
Pui CH, Robison LL and Look AT: Acute
lymphoblastic leukaemia. Lancet. 371:1030–1043. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rivera GK, Zhou Y, Hancock ML, et al: Bone
marrow recurrence after initial intensive treatment for childhood
acute lymphoblastic leukemia. Cancer. 103:368–376. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fielding AK, Richards SM, Chopra R, et al:
Outcome of 609 adults after relapse of acute lymphoblastic leukemia
(ALL); an MRC UKALL12/ECOG 2993 study. Blood. 109:944–950. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Thomas X, Boiron JM, Huguet F, et al:
Outcome of treatment in adults with acute lymphoblastic leukemia:
analysis of the LALA-94 trial. J Clin Oncol. 22:4075–4086. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Marjerrison S, Antillon F, Fu L, et al:
Outcome of children treated for relapsed acute lymphoblastic
leukemia in Central America. Cancer. 119:1277–1283. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Oriol A, Vives S, Hernández-Rivas JM, et
al: Outcome after relapse of acute lymphoblastic leukemia in adult
patients included in four consecutive risk-adapted trials by the
PETHEMA Study Group. Haematologica. 95:589–596. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang JH, Nichogiannopoulou A, Wu L, et al:
Selective defects in the development of the fetal and adult
lymphoid system in mice with an Ikaros null mutation. Immunity.
5:537–549. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Winandy S, Wu P and Georgopoulos K: A
dominant mutation in the Ikaros gene leads to rapid
development of leukemia and lymphoma. Cell. 83:289–299. 1995.
|
|
9
|
Rebollo A and Schmitt C: Ikaros, Aiolos
and Helios: transcription regulators and lymphoid malignancies.
Immunol Cell Biol. 81:171–175. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mullighan CG, Miller CB, Radtke I, et al:
BCR-ABL1 lymphoblastic leukaemia is characterized by the
deletion of Ikaros. Nature. 453:110–114. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Iacobucci I, Lonetti A, Cilloni D, et al:
Identification of different Ikaros cDNA transcripts in
Philadelphia-positive adult acute lymphoblastic leukemia by a
high-throughput capillary electrophoresis sizing method.
Haematologica. 93:1814–1821. 2008. View Article : Google Scholar
|
|
12
|
Koipally J and Georgopoulos K: A molecular
dissection of the repression circuitry of Ikaros. J Biol Chem.
277:27697–27705. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mullighan CG, Su X, Zhang J, et al:
Deletion of IKZF1 and prognosis in acute lymphoblastic
leukemia. N Engl J Med. 360:470–480. 2009.PubMed/NCBI
|
|
14
|
Tonnelle C, Imbert MC, Sainty D, Granjeaud
S, N’Guyen C and Chabannon C: Overexpression of dominant-negative
Ikaros 6 protein is restricted to a subset of B common adult acute
lymphoblastic leukemias that express high levels of the CD34
antigen. Hematol J. 4:104–109. 2003. View Article : Google Scholar
|
|
15
|
Zhou F, Mei H, Jin R, Li X and Chen X:
Expression of Ikaros isoform 6 in Chinese children with acute
lymphoblastic leukemia. J Pediatr Hematol Oncol. 33:429–432. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mi JQ, Wang X, Yao Y, et al: Newly
diagnosed acute lymphoblastic leukemia in China (II): prognosis
related to genetic abnormalities in a series of 1091 cases.
Leukemia. 26:1507–1516. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Harrison CJ: Cytogenetics of paediatric
and adolescent acute lymphoblastic leukaemia. Br J Haematol.
144:147–156. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Szczepański T, Harrison CJ and van Dongen
JJ: Genetic aberrations in paediatric acute leukaemias and
implications for management of patients. Lancet Oncol. 11:880–889.
2010.PubMed/NCBI
|
|
19
|
Mizuta S, Matsuo K, Yagasaki F, et al:
Pre-transplant imatinib-based therapy improves the outcome of
allogeneic hematopoietic stem cell transplantation for
BCR-ABL-positive acute lymphoblastic leukemia. Leukemia.
25:41–47. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wassmann B, Pfeifer H, Goekbuget N, et al:
Alternating versus concurrent schedules of imatinib and
chemotherapy as front-line therapy for Philadelphia-positive acute
lymphoblastic leukemia (Ph+ALL). Blood. 108:1469–1477.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ezzat S, Zhu X, Loeper S, Fischer S and
Asa SL: Tumor-derived Ikaros 6 acetylates the Bcl-XL promoter to
up-regulate a survival signal in pituitary cells. Mol Endocrinol.
20:2976–2986. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Suzuki K, Ono R, Ohishi K, et al: IKAROS
isoform 6 enhances BCR-ABL1-mediated proliferation of human
CD34+ hematopoietic cells on stromal cells. Int J Oncol.
40:53–62. 2012.PubMed/NCBI
|
|
23
|
Song JH, Kim SH, Cho D, Lee IK, Kim HJ and
Kim TS: Enhanced invasiveness of drug-resistant acute myeloid
leukemia cells through increased expression of matrix
metalloproteinase-2. Int J Cancer. 125:1074–1081. 2009. View Article : Google Scholar
|
|
24
|
Suminoe A, Matsuzaki A, Hattori H, Koga Y,
Ishii E and Harav T: Expression of matrix metalloproteinase (MMP)
and tissue inhibitor of MMP (TIMP) genes in blasts of infant acute
lymphoblastic leukemia with organ involvement. Leuk Res.
31:1437–1440. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kaddu S, Zenahlik P, Beham-Schmid C, Kerl
H and Cerroni L: Specific cutaneous infiltrates in patients with
myelogenous leukemia: a clinicopathologic study of 26 patients with
assessment of diagnostic criteria. J Am Acad Dermatol. 40:966–978.
1999. View Article : Google Scholar : PubMed/NCBI
|