Introduction
Lung cancer is the leading cause of cancer-related
mortality in both men and women, resulting in ~221,130 new cases
and 156,940 deaths in the United States in 2011 (1). Non-small cell lung cancer (NSCLC)
represents 85% of all lung cancer cases, and most NSCLC patients
present with advanced disease with a very poor rate of cure
(2,3). Since the 5-year survival of patients
with metastatic NSCLC is <15%, prognostic assessment of the
patient is essential for the choice of better therapeutic
strategies. The current challenge demands the discovery of accurate
and non-invasive biomarkers for diagnosis, prognosis and prediction
of recurrence to improve the clinical management of NSCLC
patients.
During the past decades, several screening tests,
including CT scan and bronchoscopy, have been used for early
detection of lung cancer (4).
However, compliance with these screening tests has been far from
adequate. An ideal screening method should have a high sensitivity
and specificity for lung cancer. Currently, one of the most
important prognostic factors in NSCLC is the anatomical extent of
disease, as described by the tumor node metastasis (TNM)
classification (5). However, it
fails to consider the variety of NSCLC patients, tumor and
environmental factors that influence prognosis, and a large
variability in disease outcomes that has been observed in subsets
of patients with the same clinical features (6). Therefore, there is a clear need to
identify biomarkers for the early detection of NSCLC, and to
categorize different prognostic groups and improve the clinical
management of NSCLC patients.
MicroRNAs (miRNAs) are non-coding RNA molecules of
~21–23 nucleotides in length that play an important role in
regulation of mRNA expression (7).
miRNAs are known to be involved in various cellular processes and
are associated with various diseases including cancer (8,9).
Several studies have demonstrated that miRNAs play important roles
in the initiation and progression of cancer. In addition, miRNA
expression profiles and specific miRNAs have been shown to be
potential diagnostic or prognostic tools for cancer (10–12).
Recent studies (13,14)
have also shown that circulating miRNAs may constitute accurate
methods for diagnosis and prognosis of NSCLC. A study by Markou
et al (15) demonstrated
that high expression of serum miR-21 and miR-30e-5p was associated
with shorter overall survival (OS). Hu et al (14) also reported a 4-miRNA signature
(miR-486, miR-30d, miR-1 and miR-499) that predicted survival of
stage I–IIIa NSCLC. However, whether the expression profile of
circulating miR-499 reflects the miRNA profile of tumor tissue
remains unclear. In addition, the relationship between dysregulated
miRNAs and tumor stage is also uncertain. In the present study, we
evaluated the possible association of miR-499 with early detection
of NSCLC and survival of NSCLC patients in an attempt to further
clarify the impact of miRNAs on the diagnosis and prognosis of lung
cancer.
Materials and methods
Study design
The present study was approved by the institutional
review board of Shanghai Pulmonary Disease Hospital. All
participants provided written consent and indicated willingness to
donate their blood and tissue samples for research. A total of 568
subjects were enrolled in this study, including 514 NSCLC patients
and 54 age- and gender-matched healthy volunteers as controls.
NSCLC patients were recruited at Shanghai Pulmonary Disease
Hospital affiliated to Tongji University in Shanghai, China,
between January 2007 and December 2011. Patients were excluded if
they had any of the following: self-reported previous cancer
history, metastasis from other organs, or if they underwent
chemotherapy or radiotherapy before blood collection. They were
followed-up at 3-month intervals by telephone after the first visit
to the hospital.
The present study was designed as an initial
screening phase and a subsequent validation phase. In the screening
phase, serum levels of miR-499 (14,16)
were analyzed in a subset of 40 patients with stage I (n=20) and
stage IV (n=20) NSCLC. To further assess the specificity of miRNA
expression, serum samples were collected from 12 patients with
NSCLC and 12 gender- and age-matched normal controls. In the
validation phase, miR-499 expression levels in serum (n=514) and
tissues (n=136) from NSCLC patients were evaluated in a large and
independent cohort of 514 patients.
RNA isolation and qRT-PCR from serum and
tissues
miRNAs were extracted from blood samples using the
Qiagen miRNeasy kit (Qiagen, Valencia, CA, USA). Briefly, 250 μl
serum was centrifuged at 10,000 rpm for 10 min at 4°C. miRNAs were
enriched and purified according to the manufacturer’s protocol. To
allow for normalization of sample-to-sample variation in the RNA
isolation step, synthetic C. elegans miRNA (cel-miR-39) was
added to each sample (12,17).
MiRNAs were extracted from fresh frozen tissue
samples using PureLink™ miRNA Isolation kit (Life Technologies
Corporation, Carlsbad, CA, USA). Briefly, fresh frozen tissue
samples were microdissected to enrich for neoplastic cells.
Homogenized samples were centrifuged, followed by deparaffinization
and RNA extraction using the manufacturer’s protocol.
PCR reactions for quantifying miR-499, miR-39 and
miR-16 were performed in triplicate using the TaqMan microRNA
Reverse Transcription kit (Applied Biosystems, USA). qRT-PCR was
performed in an ABI Prism 7000 sequence detection system (Applied
Biosystems) according to the manufacturer’s instructions, with the
following cycling conditions: 95°C for 10 min, followed by 40
cycles at 95°C for 15 sec and 60°C for 1 min.
Calculation of miRNA expression
Relative quantification of miR-499 expression was
calculated with the 2−ΔΔCt method (18) [Applied Biosystems; User Bulletin no.
2 (P/N 4303859)] and normalized using cel-miR-39 (for serum
samples) and miR-16 (19) (for
tissue samples) using the 2−ΔΔCt method, knowing that it
facilitates detecting and quantifies exclusively mature miRNAs but
not their precursors.
Statistical analysis
Mann-Whitney U and Kruskal-Wallis analyses of
variance were used to evaluate statistical differences in serum or
tissue miRNA expression between unpaired groups and multiple
comparison groups. χ2 test was used for categorical
data. A multivariable logistic regression model was used to
calculate odds ratios (ORs) for patients associated with NSCLC
according to serum miRNA levels.
Disease-free survival (DFS) and OS were measured for
each patient. Survival curves were estimated using the Kaplan-Meier
method, and differences between them were evaluated by the log-rank
test. Cox proportional hazard regression test was used to estimate
univariate and multivariate hazard ratios for recurrence and
prognosis with a step-down method.
Receiver operating characteristic (ROC) curves were
established for discriminating NSCLC and controls. The optimal
miRNA expression cut-off threshold values were determined at the
point on the ROC curve at which Youden’s index (20) was maximal.
All P-values are two-sided and P<0.05 was
considered to indicate a statistically significant difference. All
statistical analyses were carried out using MedCalc version 11.2
(Mariakerke, Belgium).
Results
Serum and tissue miR-499 expression
during the screening phase
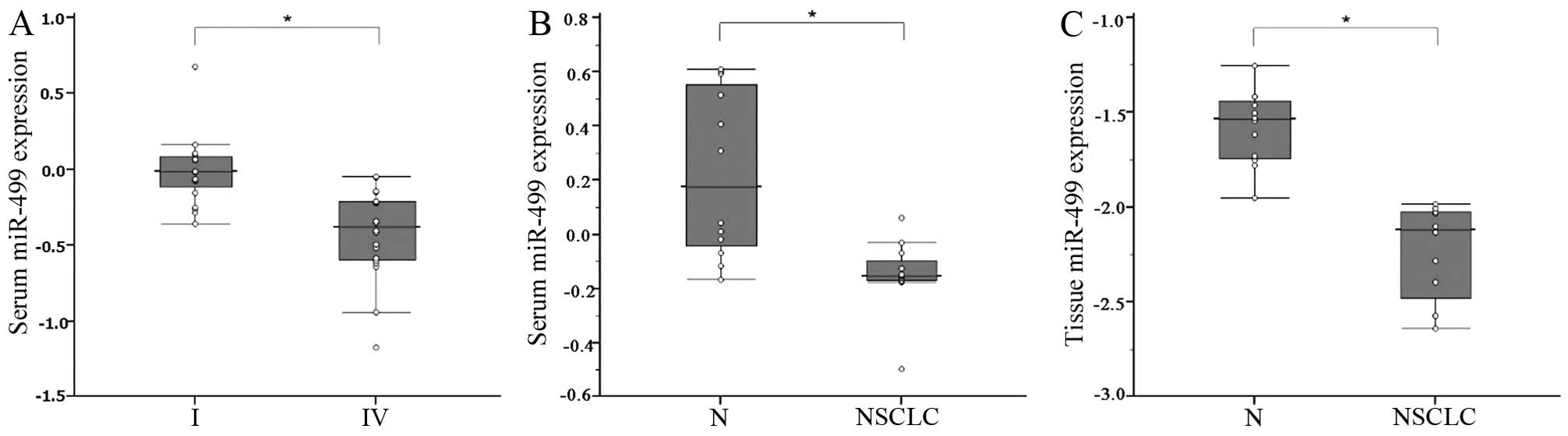
In the screening phase, we investigated the relative
expression levels of the miR-499 in a subset of serum specimens
from 20 NSCLC patients with stage IV compared with 20 patients with
stage I. It was found that miR-499 was significantly elevated in
the serum of NSCLC patients with stage I compared with stage IV
(P<0.001; Fig. 1A). We next
examined the feasibility of detecting the expression of serum
miR-499 in 12 NSCLC patients and 12 healthy control subjects. It
was found that serum miR-499 levels were significantly decreased in
the sera of NSCLC patients (P<0.001; Fig. 1B). Expression of miR-499 was
determined in a small set of 12 NSCLCs compared with the adjacent
normal tissue. miR-499 level was also found to be reduced in NSCLC
tissues compared with normal tissues (P<0.001; Fig. 1C). These results indicated that
serum miR-499 was downregulated in NSCLC patients compared with
adjacent tissues.
Based on these observations, we focused the rest of
our study on miR-499 for further assessment of its efficacy as a
diagnostic, prognostic and recurrence predictive biomarker in NSCLC
patients.
Serum miR-499c expression level as a
diagnostic biomarker in NSCLC patients
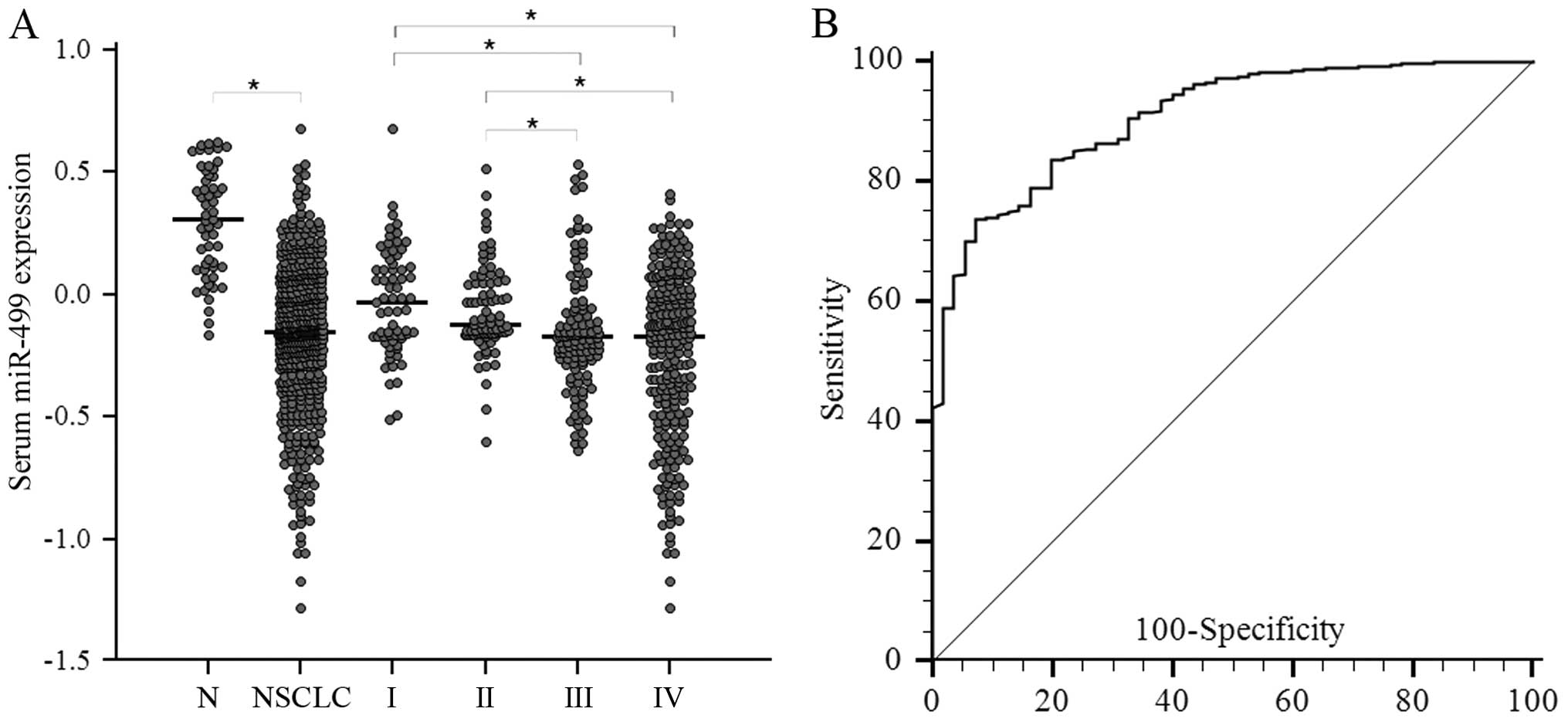
To examine whether miR-499 had diagnostic potential,
568 serum samples, including 514 from NSCLC patients and 54 from
normal controls, were analyzed. It was found that miR-499
expression levels were downregulated markedly in sera of NSCLC
patients as compared with those in normal controls (P<0.001;
Fig. 2A). In addition, when all
NSCLC patients were grouped based on TNM stage, miR-499 expression
levels were significantly lower in stage III and IV patients than
those in stage I or II patients (both P<001, Fig. 2A). The potential clinical
significance of serum miR-499 expression is presented in Table I.
 | Table IAssociation between miR-499 expression
in serum and tissue specimens from NSCLC patients and various
clinicopathological characteristics. |
Table I
Association between miR-499 expression
in serum and tissue specimens from NSCLC patients and various
clinicopathological characteristics.
| Serum miR-499
(n=514) | Tissue miR-499
(n=136) |
|---|
|
|
|
|---|
| Factors | High (n=257) | Low (n=257) | P-value | High (n=68) | Low (n=68) | P-value |
|---|
| Age (years) | | | 0.28 | | | 0.36 |
| ≤65 | 111 | 98 | | 20 | 26 | |
| >65 | 146 | 159 | | 48 | 42 | |
| Gender | | | 0.79 | | | 0.17 |
| Male | 133 | 137 | | 39 | 30 | |
| Female | 124 | 120 | | 29 | 38 | |
| Smoking status | | | 0.36 | | | 0.39 |
| Non-smoker | 105 | 94 | | 34 | 40 | |
| Ever-smoker | 152 | 163 | | 34 | 28 | |
| Stage | | | 0.28 | | | 0.22 |
| I | 34 | 26 | | 28 | 22 | |
| II | 57 | 45 | | 35 | 44 | |
| III | 46 | 56 | | 5 | 2 | |
| IV | 120 | 130 | | 0 | 0 | |
| Histologic
type | | | 0.81 | | | 0.016 |
|
Adenocarcinoma | 140 | 146 | | 52 | 40 | |
| Squamous cell | 99 | 92 | | 14 | 28 | |
| Others | 18 | 19 | | 2 | 0 | |
| Surgical
operation | | | 0.02 | | | 0.93 |
| No | 172 | 196 | | 0 | 0 | |
| Yes | 85 | 61 | | 68 | 68 | |
| Chemotherapy or
radiotherapy | | | 0.08 | | | 0.12 |
| No | 55 | 39 | | 5 | 12 | |
| Yes | 202 | 218 | | 63 | 56 | |
Next, we determined whether miR-499 possessed any
significance in sensitivity and specificity in lung cancer
patients. ROC curves were analyzed, indicating that serum miR-499
levels were robust in differentiating patients with NSCLC from
control subjects with an area under the ROC curve (AUC) value of
0.906 (95% CI=0.879 to 0.929) (Fig.
2B). At the cut-off value of 1.330 for miR-499, the optimal
sensitivity and specificity were 73.7 and 92.7%, respectively.
Multivariate logistic regression analyses on variables including
age, gender and serum miRNAs showed that miR-499 was a potential
biomarker for NSCLC diagnosis (P<0.0001). The OR for patients
with miR-499 <1.0247 associated with NSCLC was 64.1 (95% CI:
20.83–197.49). These results indicated that serum miR-499 had
potential significance with respect to the sensitivity and
specificity in the diagnosis of NSCLC.
Correlation between serum/tissue miR-499
expression and survival of NSCLC patients
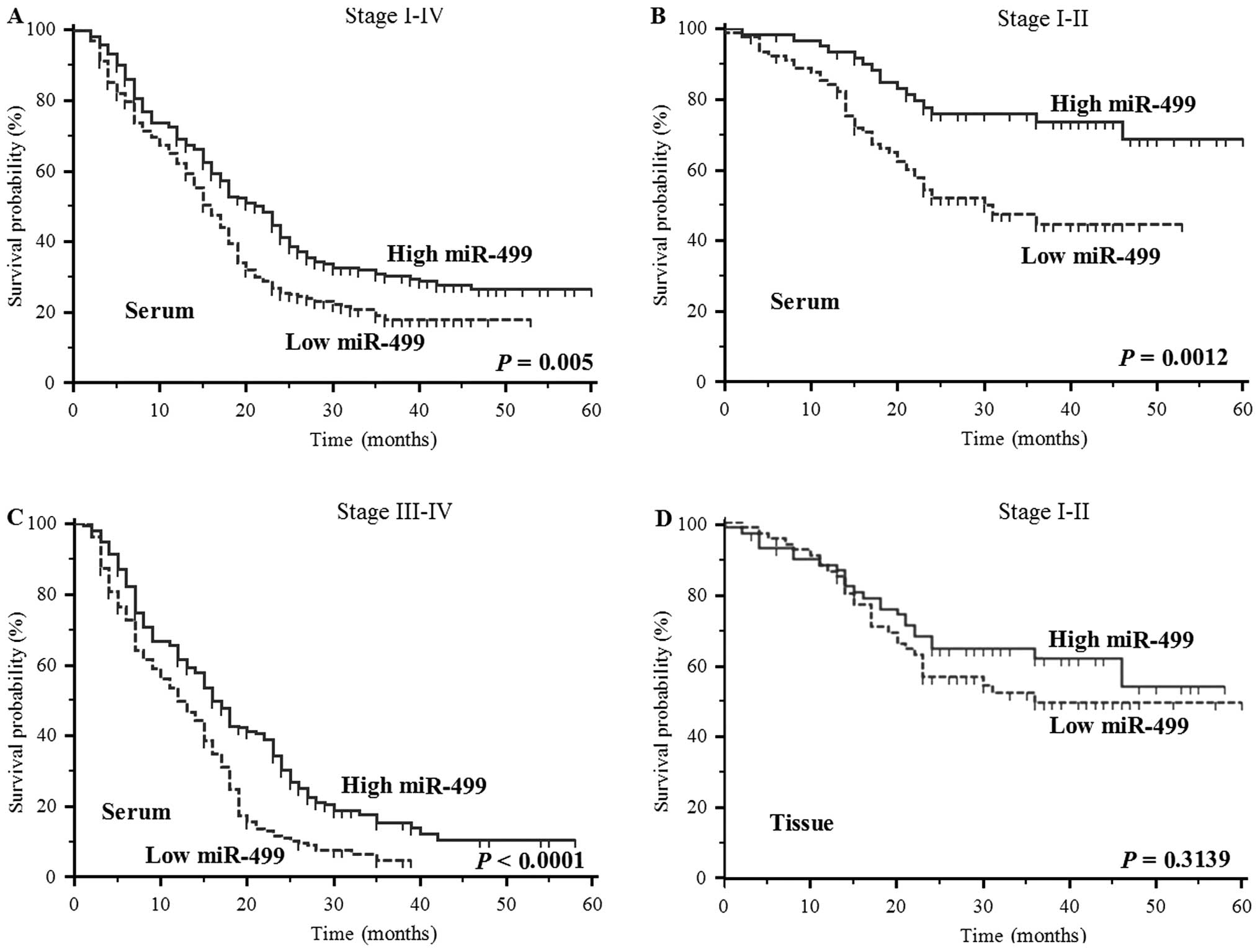
Based on the above findings, we further evaluated
whether serum miR-499 levels may predict the prognosis of NSCLC
patients. As anticipated, OS was poorer in patients with lower
serum levels of miR-499 (P=0.005, log-rank test; Fig. 3A). In addition, lower serum levels
of miR-499 also predicted poorer survival in patients with stage
I–II and III–IV NSCLC (P=0.0012 and <0.0001, log-rank test;
Fig. 3B and C). However, tissue
miR-499 levels were not significantly associated with survival in
NSCLC patients (P=0.3139, log-rank test; Fig. 3D) (Table II). Furthermore, the Cox
proportional hazard regression model was used to clarify whether
serum miR-499 expression was an independent risk factor for
prognosis. The result of univariate analysis showed that poor
prognosis in NSCLC patients was significantly associated with low
serum miR-499 levels but not with tissue miR-499 levels (P=0.0005
and P=0.3139, respectively), high T stage (T3/4, P<0.0001),
lymph node metastasis (N1/2/3, P<0.0001), distant metastasis
(M1, P<0.0001), and high TNM stage (III/IV, P<0.0001)
(Table II). The result of
multivariate analysis showed that downregulation of serum miR-499
expression was an independent prognostic marker for predicting
poorer OS in NSCLC patients (HR=1.63, 95% CI=1.33–2.0, P<0.0001;
Table II). These results indicated
that downregulation of serum miR-499 expression was an independent
prognostic marker for predicting poorer OS in NSCLC patients.
 | Table IIUnivariate and multivariate analyses
for prognostic factors in patients with NSCLC. |
Table II
Univariate and multivariate analyses
for prognostic factors in patients with NSCLC.
| Univariate | Multivariate |
|---|
|
|
|
|---|
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age, years (>65
vs. ≤65) | 0.84 | 0.61–1.16 | 0.2855 | | | |
| Gender (female vs.
male) | 0.98 | 0.80–1.20 | 0.8212 | - | - | - |
| Histology (squamous
vs. non-squamous) | 0.91 | 0.74–1.12 | 0.37 | - | - | - |
| Pathological T
(T3/4 vs. T1/2) | 2.39 | 1.94–2.94 | <0.0001 | 1.45 | 1.10–1.89 | 0.0077 |
| Lymph node
metastasis (N1–3 vs. N0) | 1.80 | 1.46–2.20 | <0.0001 | 1.21 | 0.95–1.54 | 0.1333 |
| Distant metastasis
(M1 vs. M0) | 2.67 | 2.14–3.32 | <0.0001 | 1.68 | 1.32–2.14 | <0.0001 |
| TNM stage (III/IV
vs. I/II) | 3.57 | 2.91–4.40 | <0.0001 | 21.63 | 2.98–156.85 | 0.0025 |
| Chemotherapy or
radiotherapy (no vs. yes) | 1.49 | 0.94–2.38 | 0.0928 | - | - | - |
| miR-499 in serum
(low vs. high) | 1.42 | 1.16–1.75 | 0.0005 | 1.63 | 1.33–2.00 | <0.0001 |
| miR-499 in tumor
(low vs. high) | 1.31 | 0.77–2.21 | 0.3139 | - | - | - |
Serum miR-499 as a predictive biomarker
of tumor recurrence in NSCLC
Next, we analyzed DFS. Patients with low serum
miR-499 in stage I–II had shorter DFS (P=0.045, log-rank test). To
further evaluate whether serum miR-499 levels may be used as a
predictor of tumor recurrence after surgery (stage I–II), the Cox
proportional hazard regression model was performed (Table III). The result of univariate
analysis showed that poor DFS was significantly associated with low
serum levels of miR-499 (P=0.0304), high TNM stage (II,
P<0.0001), and no chemotherapy or radiotherapy (P<0.0001).
The result of multivariate analysis showed that low serum miR-499
expression was an independent predictor for tumor recurrence in
patients with stage I–II NSCLC (HR=1.96, 95% CI=1.03–3.73, P=0.04).
These results indicated that serum miR-499 levels may serve not
only as a diagnostic and prognostic marker, but also as a predictor
of early recurrence of NSCLC as well.
 | Table IIIUnivariate and multivariate analyses
for predictive factors of recurrence in patients with stage I–II
NSCLC. |
Table III
Univariate and multivariate analyses
for predictive factors of recurrence in patients with stage I–II
NSCLC.
| Univariate | Multivariate |
|---|
|
|
|
|---|
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age, years (>65
vs. ≤65) | 1.11 | 0.62–1.97 | 0.7346 | | | |
| Gender (female vs.
male) | 0.66 | 0.31–1.42 | 0.29 | - | - | - |
| Pathological T
(T2/3 vs. T1) | 2.22 | 0.62–8.01 | 0.22 | - | - | - |
| Lymph node
metastasis (N1 vs. N0) | 1.24 | 0.23–6.69 | 0.8024 | - | - | - |
| TNM stage (II vs.
I) | 2.54 | 1.13–5.71 | 0.024 | 2.76 | 1.32–5.77 | 0.007 |
| Chemotherapy or
radiotherapy (no vs. yes) | 1.39 | 1.06–1.81 | 0.0158 | 1.61 | 1.15–2.26 | 0.0057 |
| miR-499 in serum
(low vs. high) | 3.03 | 1.11–8.27 | 0.0304 | 1.96 | 1.03–3.73 | 0.04 |
Discussion
In the present study, we investigated the potential
clinical utility of serum miR-499 and found that serum miR-499
could serve as a non-invasive biomarker for the diagnosis and
prediction of prognosis and tumor recurrence in NSCLC patients. The
miR-499 levels in serum samples from NSCLC patients were
significantly lower than those in healthy controls. miR-499 had a
significant diagnostic value for NSCLC and yielded AUC of 0.906
with 73.7% sensitivity and 92.7% specificity in discriminating
NSCLC from normal controls. In addition, the OR for case subjects
with low levels of miR-499 expression associated with NSCLC was
64.1 (95% CI; 20.83–197.49), indicating that miR-499 expression may
be exploited as a promising non-invasive biomarker for early
detection of NSCLC.
Our study also strongly suggested that serum miR-499
expression may serve as a prognostic biomarker for NSCLC. We found
that serum miR-499 levels were significantly lower in patients with
stage III or IV NSCLC than those in stage I or II NSCLC. In
addition, low serum miR-499 levels (rather than tissue) were
associated with shorter OS and may prove to be an independent
prognostic biomarker in NSCLC patients. In addition, low serum
levels of miR-499 expression indicate a poor DFS in stage I–II
NSCLC. The multivariable Cox proportional hazards model showed that
low serum miR-499 expression was an independent predictor of tumor
recurrence in patients with stage I–II NSCLC.
To the best of our knowledge, this is the first
report to demonstrate the potential role of serum miR-499 in the
early detection of NSCLC. Although the observation of the
prognostic value of miR-499 is similar to a previous report
(14), our results are the first to
demonstrate that low serum miR-499 expression is associated with
advanced TNM stage and poor DFS.
Several studies (21,22)
have reported that miRNAs are potential diagnostic biomarkers and
prognostic factors in lung cancer. In 2004, lung tumor-derived
miRNAs were first described in tissue by Takamizawa et al
(23), who reported that reduced
let-7 expression was significantly associated with shortened
postoperative survival. A study by Yanaihara et al (21) showed that the tissue miRNA
expression profile was a diagnostic and prognostic marker of lung
cancer. Serum miRNAs are resistant to RNase digestion, suggesting
that miRNAs in serum are sufficiently stable to serve as a clinical
biomarker (12). miR-21 was the
first serum miRNA biomarker discovered by Lawrie et al
(24), who reported that high serum
levels of miR-21 in patients with diffuse large B cell lymphoma
were associated with increased relapse-free survival. Chen et
al (9) also demonstrated that
serum let-7 level was increased in lung cancer patients as compared
with healthy controls. In addition, serum miRNAs are prognostic
factors for lung cancer. Hu et al (14) found that serum levels of 4 miRNAs
(miR-486, miR-30d, miR-1 and miR-499) were significantly associated
with OS of NSCLC patients. Boeri et al (25) described plasma miRNA signatures for
high risk of lung cancer, diagnosis and prognosis. These findings
indicate that circulating miRNAs may be non-invasive diagnostic or
prognostic markers for lung cancer.
Previous studies usually focused on the association
between miR-499 and the muscle and heart. Wang et al
(26) detected the presence of
plasma miR-499 in patients with acute myocardial infarction with
very low abundance, suggesting it may be a biomarker for early
detection of myocardial injury. Donaldson et al (27) found that miR-499 was elevated in the
plasma of COPD patients as compared with controls. Recently, Vinci
et al (16) found that the
expression of miR-499 in NSCLC tissues tended to be lower than
controls but the reduction was not statistically significant
(P=0.123). However, they found that increased expression of miR-499
was associated high tumor grade. Hu et al (14) showed that low serum levels of
miR-499 were significantly associated with short OS. Therefore, the
clinical significance of circulating miR-499 levels in NSCLC
remains unclear.
The present study demonstrated that the relative
expression of serum miR-499 was significantly different between
NSCLC patients and normal controls, suggesting that serum miR-499
level may be a useful biomarker for the clinical diagnosis of
NSCLC. Screening for lung cancer has long been of interest, with
the hope of reducing the number of patients diagnosed with advanced
disease. Although screening with low-dose computed tomography
(LDCT) has substantially reduced the risk of mortality due to lung
cancer, there is a significant chance of a false-positive result
(28,29). Therefore, serum miR-499 level with
high specificity for early detection of NSCLC may be a powerful
adjunct to LDCT. Our study showed that preoperative serum miR-499
expression was an independent factor for detecting early tumor
recurrence in patients with stage I–II NSCLC. In clinical practice,
these patients can significantly benefit from timely clinical
intervention, thus improving cancer survival. Our data also support
the use of miR-499 as a non-invasive biomarker that can facilitate
disease risk assessment, severity and survival time in NSCLC
patients.
Although our study suggests that miR-499 is a
promising screening and assessment tool for NSCLC, there are
potential limitations in using miR-499 as a diagnostic, prognostic
and recurrence predictive biomarker. Firstly, as no consensus
internal controls for circulating miRNA have been established, we
used the ideal approach to normalize experimental miRNA data using
spiked-in synthetic, non-human mature miRNA from Caenorhabditis
elegans. Although cel-miR-39 had given consistent expression
across all patients and controls, and the method of quantifying
relative expression of serum miRNAs was widely recognized, absolute
quantization of serum miR-499 expression may further improve the
translation of these data into clinical application. Secondly,
although selection criteria for lung cancer screening were
uncertain, it is unclear whether high-risk people benefit from
serum miRNAs tests. Plasma miRNA tests may be more functional for
non-invasive diagnosis of lung cancer in individuals with solitary
pulmonary nodules (30,31). Thirdly, as serum expression of
miR-499 has been described in other diseases including COPD and
AMI, it may be difficult to differentiate whether serum miR-499
expression is specifically associated with NSCLC. Finally, it is
difficult to ensure that the control subjects are healthy, and our
clinical materials are solely from Chinese people. A large sample
size including diverse ethnic populations may be helpful to
eliminate potential sampling error.
In conclusion, serum miR-499 appears to be a novel
diagnostic, prognostic and predictive biomarker in patients with
NSCLC. Nevertheless, large prospective studies are required to
further evaluate our theory before serum miR-499 can be
incorporated into routine clinical practice.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (81172229, 81372175) and the China
Postdoctoral Science Foundation (First Class, 2013M530212).
References
|
1
|
Siegel R, Ward E, Brawley O and Jemal A:
Cancer statistics, 2011: the impact of eliminating socioeconomic
and racial disparities on premature cancer deaths. CA Cancer J
Clin. 61:212–236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Parkin DM: Global cancer statistics in the
year 2000. Lancet Oncol. 2:533–543. 2001.PubMed/NCBI
|
|
3
|
Li M, Zhang Q, Fu P, et al: Pemetrexed
plus platinum as the first-line treatment option for advanced
non-small cell lung cancer: a meta-analysis of randomized
controlled trials. PLoS One. 7:e372292012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Smith RA, Brooks D, Cokkinides V, et al:
Cancer screening in the United States, 2013: a review of current
American Cancer Society guidelines, current issues in cancer
screening, and new guidance on cervical cancer screening and lung
cancer screening. CA Cancer J Clin. 63:88–105. 2013. View Article : Google Scholar
|
|
5
|
Goldstraw P, Ball D, Jett JR, et al:
Non-small-cell lung cancer. Lancet. 378:1727–1740. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rami-Porta R and Goldstraw P: Strength and
weakness of the new TNM classification for lung cancer. Eur Respir
J. 36:237–239. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Filipowicz W, Bhattacharyya SN and
Sonenberg N: Mechanisms of post-transcriptional regulation by
microRNAs: are the answers in sight? Nat Rev Genet. 9:102–114.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
van Kouwenhove M, Kedde M and Agami R:
MicroRNA regulation by RNA-binding proteins and its implications
for cancer. Nat Rev Cancer. 11:644–656. 2011.PubMed/NCBI
|
|
9
|
Chen X, Ba Y, Ma L, et al:
Characterization of microRNAs in serum: a novel class of biomarkers
for diagnosis of cancer and other diseases. Cell Res. 18:997–1006.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kong YW, Ferland-McCollough D, Jackson TJ
and Bushell M: microRNAs in cancer management. Lancet Oncol.
13:e249–e258. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lu J, Getz G, Miska EA, et al: MicroRNA
expression profiles classify human cancers. Nature. 435:834–838.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mitchell PS, Parkin RK, Kroh EM, et al:
Circulating microRNAs as stable blood-based markers for cancer
detection. Proc Natl Acad Sci USA. 105:10513–10518. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Voortman J, Goto A, Mendiboure J, et al:
MicroRNA expression and clinical outcomes in patients treated with
adjuvant chemotherapy after complete resection of non-small cell
lung carcinoma. Cancer Res. 70:8288–8298. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hu Z, Chen X, Zhao Y, et al: Serum
microRNA signatures identified in a genome-wide serum microRNA
expression profiling predict survival of non-small-cell lung
cancer. J Clin Oncol. 28:1721–1726. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Markou A, Sourvinou I, Vorkas PA, et al:
Clinical evaluation of microRNA expression profiling in non small
cell lung cancer. Lung Cancer. 81:388–396. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vinci S, Gelmini S, Pratesi N, et al:
Genetic variants in miR-146a, miR-149,
miR-196a2, miR-499 and their influence on relative
expression in lung cancers. Clin Chem Lab Med. 49:2073–2080.
2011.
|
|
17
|
Kroh EM, Parkin RK, Mitchell PS and Tewari
M: Analysis of circulating microRNA biomarkers in plasma and serum
using quantitative reverse transcription-PCR (qRT-PCR). Methods.
50:298–301. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods.
25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Peltier HJ and Latham GJ: Normalization of
microRNA expression levels in quantitative RT-PCR assays:
identification of suitable reference RNA targets in normal and
cancerous human solid tissues. RNA. 14:844–852. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ruopp MD, Perkins NJ, Whitcomb BW and
Schisterman EF: Youden Index and optimal cut-point estimated from
observations affected by a lower limit of detection. Biom J.
50:419–430. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yanaihara N, Caplen N, Bowman E, et al:
Unique microRNA molecular profiles in lung cancer diagnosis and
prognosis. Cancer Cell. 9:189–198. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yu SL, Chen HY, Chang GC, et al: MicroRNA
signature predicts survival and relapse in lung cancer. Cancer
Cell. 13:48–57. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Takamizawa J, Konishi H, Yanagisawa K, et
al: Reduced expression of the let-7 microRNAs in human lung
cancers in association with shortened postoperative survival.
Cancer Res. 64:3753–3756. 2004.PubMed/NCBI
|
|
24
|
Lawrie CH, Gal S, Dunlop HM, et al:
Detection of elevated levels of tumour-associated microRNAs in
serum of patients with diffuse large B-cell lymphoma. Br J
Haematol. 141:672–675. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Boeri M, Verri C, Conte D, et al: MicroRNA
signatures in tissues and plasma predict development and prognosis
of computed tomography detected lung cancer. Proc Natl Acad Sci
USA. 108:3713–3718. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang GK, Zhu JQ, Zhang JT, et al:
Circulating microRNA: a novel potential biomarker for early
diagnosis of acute myocardial infarction in humans. Eur Heart J.
31:659–666. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Donaldson A, Natanek SA, Lewis A, et al:
Increased skeletal muscle-specific microRNA in the blood of
patients with COPD. Thorax. 68:1140–1149. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
National Lung Screening Trial Research
Team. Aberle DR, Adams AM, Berg CD, et al: Reduced lung-cancer
mortality with low-dose computed tomographic screening. N Engl J
Med. 365:395–409. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Infante M, Cavuto S, Lutman FR, et al: A
randomized study of lung cancer screening with spiral computed
tomography: three-year results from the DANTE trial. Am J Respir
Crit Care Med. 180:445–453. 2009.PubMed/NCBI
|
|
30
|
Shen J, Liu Z, Todd NW, et al: Diagnosis
of lung cancer in individuals with solitary pulmonary nodules by
plasma microRNA biomarkers. BMC Cancer. 11:3742011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cazzoli R, Buttitta F, Di Nicola M, et al:
microRNAs derived from circulating exosomes as noninvasive
biomarkers for screening and diagnosing lung cancer. J Thorac
Oncol. 8:1156–1162. 2013. View Article : Google Scholar : PubMed/NCBI
|