Introduction
Lung cancer is considered a major cause of
cancer-related mortality worldwide. The incidence of lung cancer
has increased by 51% since 1985. Lung cancer is clinically
classified into two types: small cell lung carcinoma (SCLC) and
non-small cell lung carcinoma (NSCLC). SCLC is known as a type of
highly malignant cancer and corresponds to 20% of all lung cancer
cases; NSCLC represents approximately 80% of all cases. It is
clinically considered that NSCLC is relatively insensitive to
chemotherapy compared with small cell carcinoma (1,2).
Although several treatments including chemoradiation and
combination treatment have been developed to treat patients with
lung cancer, the survival rates of lung cancer patients remain very
low (1,3,4).
It has recently been considered that autophagy is a
physiological process for the temporary survival of cells, and
cellular stresses such as oxidative stress, nutrient starvation,
misfolded protein accumulation and chemotherapy can induce
autophagy (5,6). At the beginning of the processes
related with the autophagy induction, autophagosomes are formed by
elongation of double-membrane, and they sequester cellular
components such as damaged proteins and/or organelles.
Autophagosomes are then merged with lysosome to autolysosome
formation, which produces the degradation of organelles in the
cells (7,8).
Chemotherapy is a very important option for the
treatment of various types of cancer, including lung cancer.
Pemetrexed (PTX) is one of the most popular chemotherapies
currently used for cancer patients. PTX is a multi-targeted
antifolate cytotoxic agent and has been widely used as a single or
combination chemotherapy agent with platinum compounds such as
cisplatin for the treatment of patients with NSCLC (4,9).
Several studies have reported the cellular and molecular roles of
PTX in the regulation of apoptosis, cell cycle arrest and DNA
synthesis (10–12). It is known that PTX induces
apoptosis through inhibiting cellular enzymes responsible for the
synthesis of pyrimidine and purine, and they mainly indicate the
thymidylate synthase, glycinamide ribonucleotide formyl transferase
and dihydrofolate reductase (13,14).
In addition to the apoptotic property of PTX, recent
studies have focused on the molecular property of PTX to induce
autophagy, since apoptotic processes may not fully explain the
chemotherapeutic values of PTX, and PTX revealed several
limitations to the application in clinical study (15). Therefore, to date, the cellular
mechanisms involved in the PTX-induced death of NSCLC are not fully
understood.
Based on previous knowledge and information, we
hypothesized that PTX may accelerate the autophagy induction in the
lung adenocarcinoma cells of humans, and these cellular events
possibly regulate the chemotherapeutic effects of PTX through
induction of apoptotic and/or necrotic cell death. To examine this
hypothesis, the present study investigated whether PTX concurrently
induces autophagy and apoptosis in the cultured A549 lung
adenocarcinoma cells.
Materials and methods
Cell line
A549 cells were purchased from the American Type
Culture Collection (ATCC). A549 cells were cultured with RPMI-1640
medium supplemented with 10% fetal bovine serum (FBS), 1%
antibiotics and 10 mM HEPES. The cells were incubated in 95%
O2/5% CO2 at 37°C. The medium was replaced
every 24 h. All experiments were conducted under conditions of
cells in the log phase.
Reagents
RPMI-1640, penicillin/streptomycin, trypsin, FBS and
HEPES were obtained from Gibco-BRL (Grand Island, NY, USA). The
culture plates were purchased from Nunc (Thermo Fisher Scientific,
Roskilde, Denmark). PTX was obtained from Eli Lilly Ltd. Propidium
iodide (PI), 3-methyladenine (3-MA),
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
and acridine orange (AO) were purchased from Sigma-Aldrich
Chemicals (St. Louis, MO, USA). Poly(ADP-ribose) polymerase-1/2
(PARP1/2) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH)
antibodies were obtained from Santa Cruz Biotechnology, Inc. (Santa
Cruz, CA, USA). The LC3 antibody was obtained from Cell Signaling
(Boston, MA, USA). The secondary antibody was obtained from
Amersham (Buckinghamshire, UK), and the polyvinylidine fluoride
(PVDF) membranes and the enhanced chemiluminescent (ECL) kits were
purchased from Millipore Co. (Billerica, MA, USA).
Measurement of cell viability
The cell viability was determined by MTT assay. A549
cells were seeded in 24-well plates at a density of
1×104 cells and incubated for 24 h. After treatment with
PTX (0.5, 1.0 and 1.5 μM) in the presence or absence of 3-MA (10
mM), assay reagent (5 mg/ml) was added to each well and plates were
incubated in 5% CO2 at 37°C for 4 h. Crystals were
dissolved in 200 μl of 99.0% dimethyl sulfoxide (DMSO). To measure
cell death, the absorbance was spectrophotometrically measured at
570 nm with a microplate ELISA reader (Thermo Scientific). The
absorbance of PTX-treated cells was expressed as percentage of the
absorbance produced by control cells.
Immunoblotting assay
The cells were then pretreated with 10 mM of 3-MA
for 1 h, and PTX (0.5 μM) were then subsequently added to the 60-mm
culture dishes, followed by incubation for 48 h. Subsequently, the
cells were harvested and washed twice with ice-cold PBS and lysed
in lysis buffers (50 mM HEPES, 150 mM NaCl, 1% deoxycholate, 1 mM
EDTA, 1 mM PMSF and 1 μg/ml aprotinin, pH 7.4). Following
incubation of samples for 1 h on ice, the cells were centrifuged at
10,000 rpm for 30 min at 4°C, and the supernatants were carefully
collected. The protein concentration of supernatants was determined
using the Bradford method. For western blot analysis, equal amounts
of total proteins were loaded onto each well of 10 or 15% sodium
dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and
then transferred onto PVDF membranes. The PVDF membranes were
blocked with 5% skimmed milk in PBS for 90 min and briefly washed
in PBS. The immunoblots were analyzed using specific antibodies
recognizing LC3 and PARP proteins. The membrane was then washed
with PBS and treated with secondary antibodies for 1 h. Proteins
were then visualized using an ECL kit.
Analysis of apoptosis
For the quantitative analysis of cellular apoptosis,
the cells were inoculated in 6-well plates and incubated for 24 h.
The cells were pretreated with 10 mM of 3-MA for 1 h, and PTX at
0.5 μM was then added, followed by incubation for 48 h. After
completion of treatment, the cells were harvested using 0.25%
trypsin and then washed twice in PBS. They were subsequently
treated with Annexin V/fluorescein isothiocyanate (FITC; 0.5 μg/ml
at final concentration), PI (2 μg/ml at final concentration) and
apoptosis detection kit for 10 min at room temperature. The cells
were then immediately examined using a flow cytometer (FACSVantage
Flow Cytometer, Becton-Dickinson Immunocytometry System; San Jose,
CA, USA) after addition of 250 μl of binding buffer. Analysis was
performed using CellQuest software (Becton-Dickinson, Franklin
Lakes, NJ, USA).
Analysis of autophagy
Quantitative analysis of autophagy was performed by
staining the cells with AO and using flow cytometry as previously
described (16). The cells were
grown in 6-well plates and incubated for 24 h. After reaching the
log phase, the cells were pretreated with 10 mM of 3-MA for 1 h,
and PTX at 0.5 μM was subsequently added, followed by incubation
for 48 h. After completion of treatment, the cells were stained
with AO (1 μg/ml) for 15 min at 37°C and harvested with trypsin
(0.25%), followed by washing in PBS. The samples were then combined
with 500 μl of FACS buffer (1% FBS in PBS). The cells were
immediately counted using FACS system, and then analysis was
performed using CellQuest software.
Statistical analysis
All experiments were conducted in triplicate, and
results are expressed as the means ± SEM. One-way analysis of
variance (ANOVA) was used to compare data between groups. When a
statistically significant difference was encountered, post hoc test
confirmed Tukey’s multiple comparison test. Significance was set at
p<0.05. The GraphPad Prism 4.0 (GraphPad Software, San Diego,
CA, USA) was used for statistical analysis.
Results
PTX-induced autophagy in A549 cells
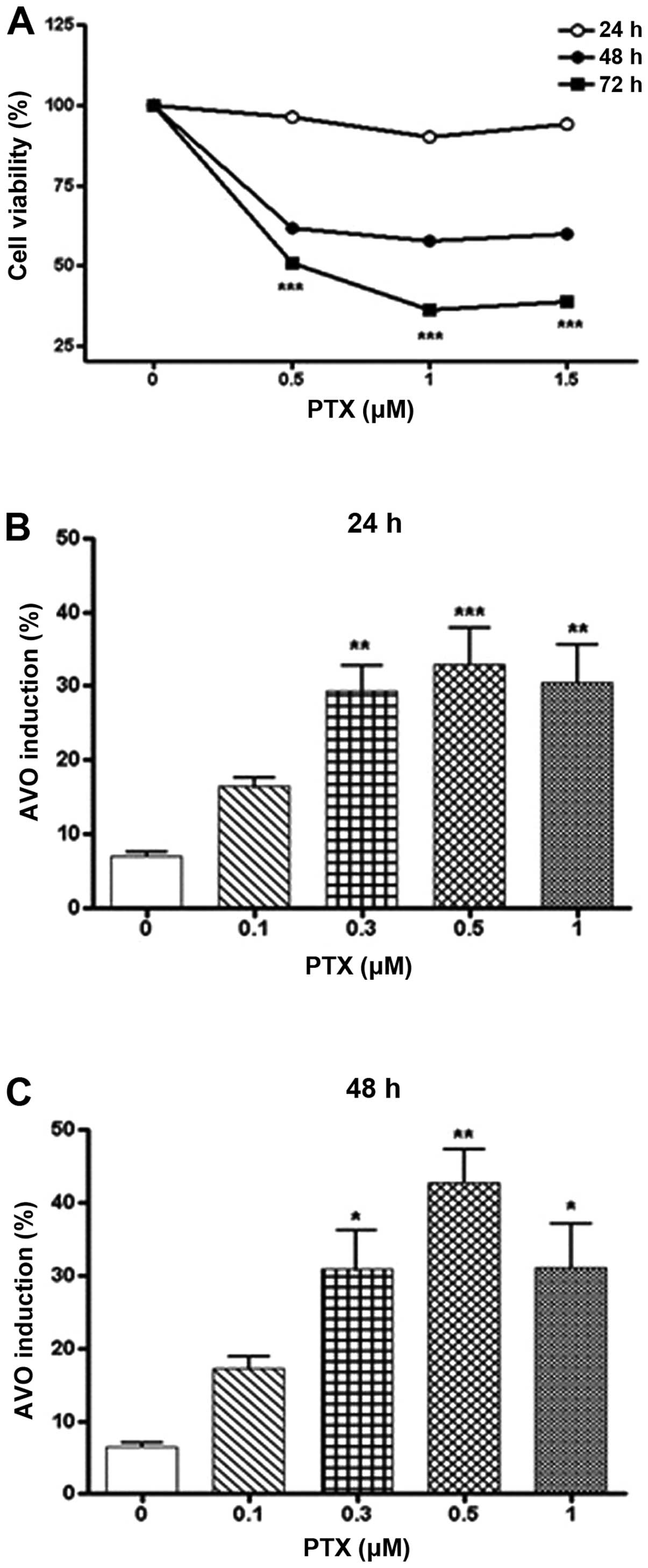
Firstly, MTT assay was performed to measure the
changes of cell viability by treatment of PTX. As shown in Fig. 1A, when A549 cells were incubated
with PTX (0.5–1.5 μM) for 24–72 h, the cell viability decreased in
a dose- and time-dependent manner. Incubation of A549 cells with
PTX for 24 and 48 h did not significantly affect the cell
viability, whereas all concentrations of PTX examined in this study
significantly decreased the cell viability by incubation for 72 h.
Next, the present study investigated whether PTX induces autophagy
in A549 cells. To examine this hypothesis, the cells were treated
with PTX (0.1–1.0 μM) for 24 and 48 h and stained with AO to detect
the formation of acidic vesicular organelles (AVOs). As shown in
Fig. 1B and C, PTX produced the
formation of AVOs in a dose-dependent manner, and a maximal effect
of PTX was observed at a concentration of 0.5 μM. When A549 cells
were incubated with 0.5 μM of PTX for 24 and 48 h, the formation of
AVOs was found to be 32.9 and 52.7%, respectively. Based on these
results, the following studies employed the concentration of 0.5 μM
of PTX and the 48-h incubation period.
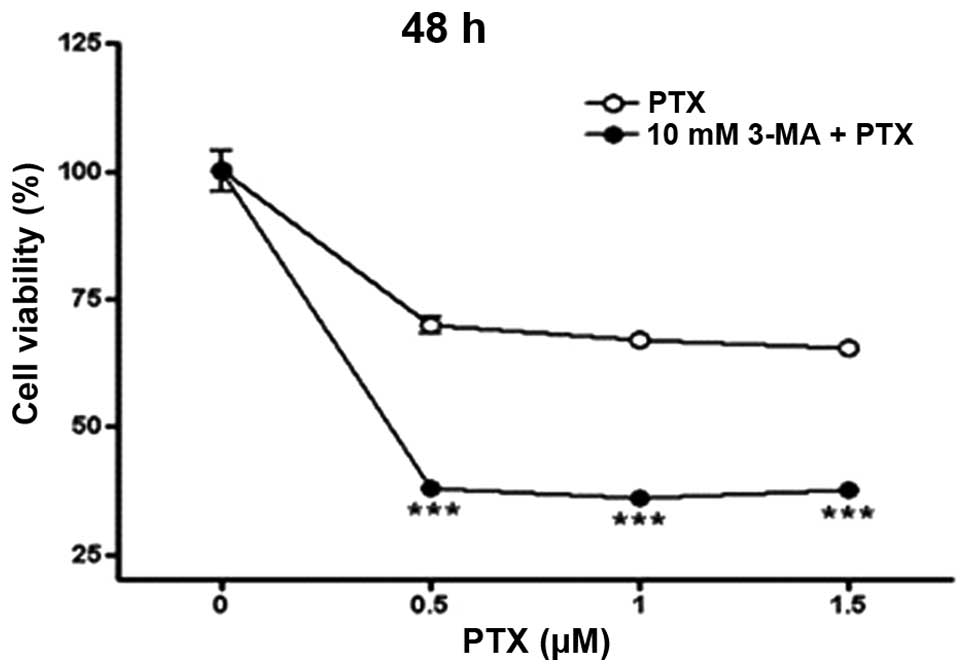
Effects of 3-MA on the PTX-induced cell
death
To investigate whether autophagy regulates the cell
death caused by PTX, A549 cells were incubated with PTX in the
presence or absence of 10 mM 3-MA, an autophagy inhibitor, for 48
h, and the obtained results are shown in Fig. 2. Consistent with the results shown
in Fig. 1A, the cell viability was
significantly reduced by treatment of PTX (0.5–1.5 μM) for 48 h. Of
note, treatment of A549 cells with 10 mM of 3-MA significantly
accelerated the reduction of cell viability induced by PTX. These
results clearly indicate that autophagy is implicated in the
PTX-induced death of lung adenocarcinoma cells.
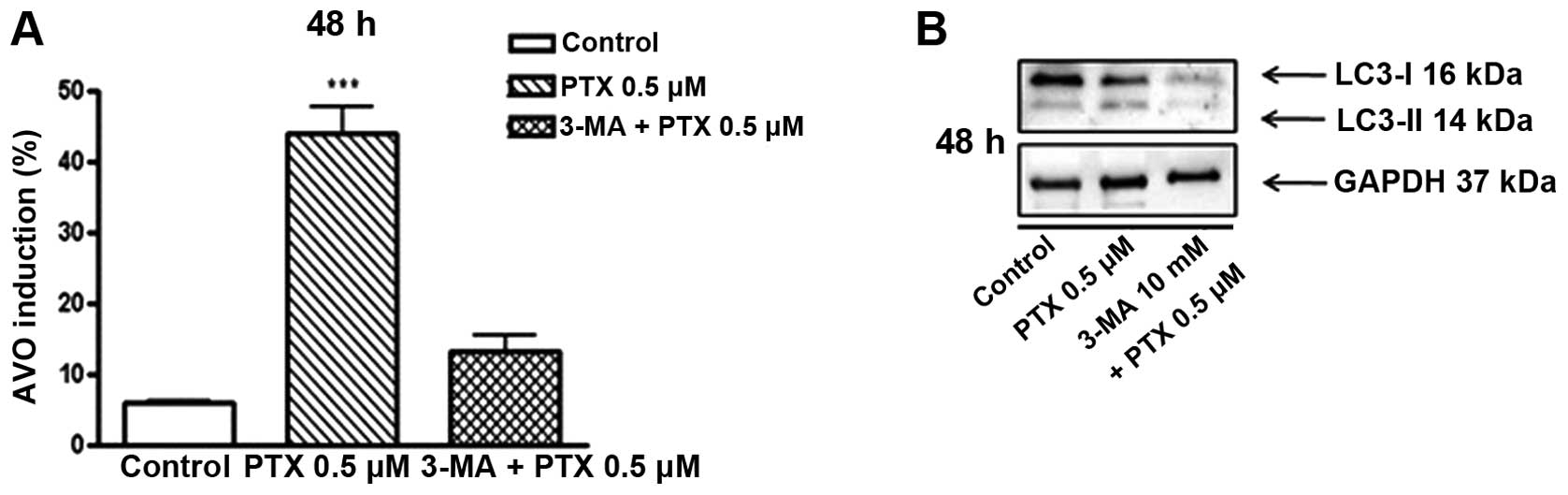
Effects of 3-MA on the PTX-induced
autophagy induction
To confirm the results shown in Fig. 2, we then performed the AO stain to
estimate the 3-MA-induced variation of autophagy, and the obtained
results are shown in Fig. 3A. The
rates of AO-positive cells in none-treated cells were found to be
6.1% of total cells, and these rates were significantly increased
by treatment of PTX (0.5 μM) to 43.9%. Notably, the PTX-induced
increase of AO-positive cells was markedly attenuated by
co-treatment of 3-MA (10 mM), and its rate was revealed as 13.2% of
total cells.
Additionally, the present study further examined the
inhibitory effects of 3-MA on the PTX-induced autophagy induction
by measuring the expression of LC3-II which is widely accepted as
an autophagy marker, and the obtained results are shown in Fig. 3B. PTX at 0.5 μM clearly elevated the
expression of LC3-II in cultured A549 cells, and this increment was
markedly reduced by co-treatment of 3-MA (10 mM). These results
clearly suggest that autophagy is involved in the death of lung
adenocarcinoma cells caused by PTX.
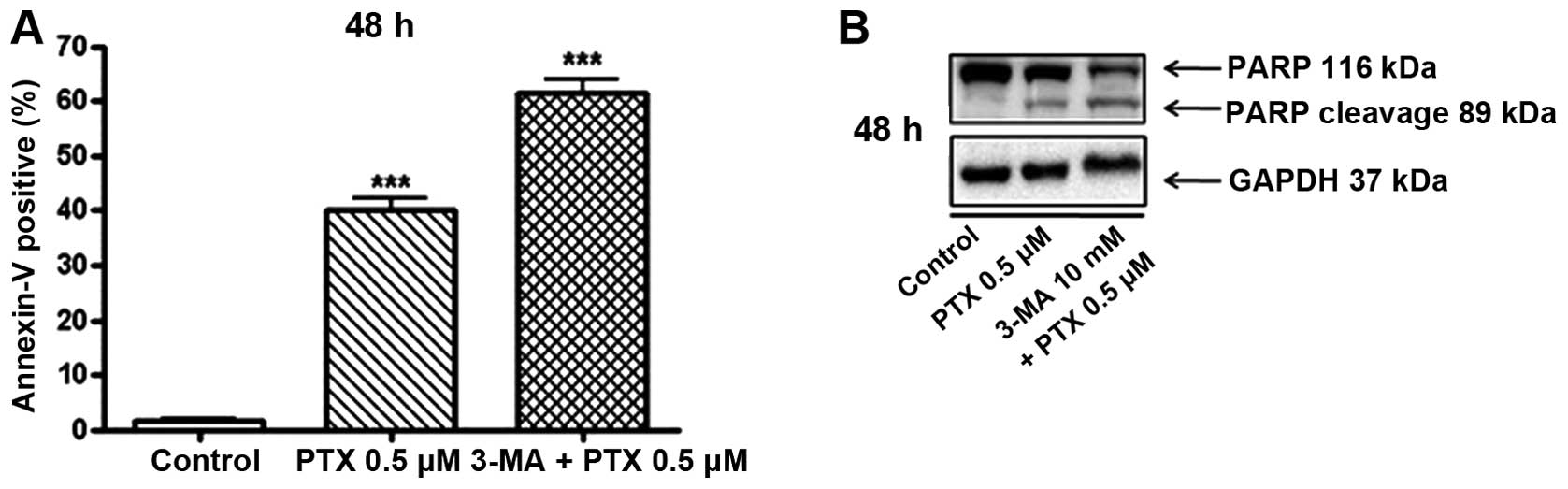
Effects of 3-MA on the PTX-induced
apoptosis
Furthermore, to confirm the results shown in
Fig. 2, that PTX-induced death of
cultured lung adenocarcinoma cells is increased by inhibiting the
autophagy induction, and to clarify the type of cell death, the
present study performed Annexin V/PI analysis, and the obtained
results are shown in Fig. 4A. When
A549 cells were cultured with 0.5 μM of PTX for 48 h, Annexin
V-positive apoptotic cells were increased to 38.4% compared to
those of control cells. Importantly, 3-MA at 10 mM further elevated
the PTX-induced increase of Annexin V-positive cells to 59.7% in
comparison with control cells. In addition to the results showing
Annexin V-positive cells, the present study examined the PARP
expression using western blot analysis, and the obtained results
are shown in Fig. 4B. When A549
cells were incubated with PTX (0.5 μM) for 48 h, in comparison with
control, expression of the cleaved PARP was markedly increased.
These cleavages of PARP by PTX treatment were accelerated by
co-treatment of 3-MA (10 mM). These results may indicate that
inhibition of autophagy formation promotes the apoptotic pathways
in cultured human lung adenocarcinoma cells.
Discussion
Although pemetrexed (PTX) is one of the most popular
chemotherapies for treating patients with lung cancer, its
pharmacotherapeutical mechanisms responsible for the anticancer
activity are not completely understood. Therefore, using A549
cells, the present study investigated the hypothesis that PTX may
accelerate the autophagy formation in A549 cells, and these
cellular events possibly mediate the chemotherapeutic effects of
PTX through induction of apoptotic and/or necrotic cell death. PTX
caused a significant reduction in the viability of cultured A549
lung adenocarcinoma cells, whereas it significantly increased the
autophagy induction. The maximal concentration of PTX to induce
autophagy formation was revealed to be 0.5 μM. An autophagy
inhibitor, 3-MA, accelerated the decrease in the cell viability
induced by PTX, and inhibition of autophagy induction promoted the
apoptotic cell death which were demonstrated by expressional
changes of LC3 and PARP in cultured A549 cells. These results
likely indicate the cellular and molecular mechanisms involved in
the chemotherapeutic activity of PTX to be used for treating human
lung adenocarcinoma.
Lung cancer in humans has shown little improvement
in survival rates over the past 30 years and the therapeutic
effects of radiotherapy and chemotherapy remain unsatisfactory
(1). It is known that human lung
cancer is clinically classified into small cell lung carcinoma
(SCLC) and non-small cell lung carcinoma (NSCLC), and NSCLC
constitutes ~80% of lung cancer cases. PTX has generally been used
as a single agent or first-line chemotherapy in combination with
platinum agents in the treatment of the advanced NSCLC (17–19).
However, it is clinically considered that PTX often induces
specific side-effects such as myelotoxicity and macular skin rash,
and this drug also has a limited therapeutic window and drug
resistance with regard to pharmacokinetical and pharmacodynamical
aspects (15).
Accumulating evidence suggests that PTX induces
autophagy (20), and autophagy is a
cellular self-catabolic process to enhance the cell survival under
certain conditions such as oxidative stress, chemotherapy agent and
starvation (5,6). Autophagy is also known to play an
important role in the development and progression of tumors. For
example, autophagy is predominantly observed in the incipient stage
of tumor development and also maintains cell survival from the
several different types of stresses including tumor progression and
chemotherapy to treat the already formed tumor (21,22).
However, the cellular pathophysiological roles of autophagy in the
development and/or progression of human cancer remain
controversial. Most evidence indicates the roles of autophagy in
supporting the cell survival property. In contrast to these
suggestions, it has also been reported that autophagy contributes
to cell death resulting from progression self-consumption (23,24).
However, the cellular properties of autophagy have not yet been
fully confirmed, and several questions regarding
pharmacotherapeutical pathways of PTX remain to be elucidated.
The present study investigated whether PTX induces
autophagy in the cultured A549 human lung adenocarcinoma cells, and
examined the pathophysiological and cellular roles of autophagy in
the lung adenocarcinoma cells treated with PTX. Our results
confirmed that PTX induces autophagy in a dose- and time-dependent
manner, and the autophagy induced by PTX is likely involved in the
regulation of survival of lung adenocarcinoma cells. Here, the
pharmacological strategy was employed to evaluate the
pathophysiological relationship between autophagy and apoptosis and
the 3-MA autophagy inhibitor was used. This drug disturbs the
autophagy progression by inhibiting class III phosphoinositide
3-kinase (PI3K), which is essential for the autophagosome formation
in the isolated membrane or phagophore (25,26).
Several studies have suggested that autophagy inhibition increases
the antitumor effects of chemotherapy through targeting apoptotic
pathways (27,28). Similar findings to the previous
suggestions were also observed in this study, indicating that
compared with PTX alone, combination of PTX with 3-MA attenuates
the survival of the cultured A549 cells. Therefore, it is
conceivable that autophagy inhibition by 3-MA augments the
autophagy formation caused by PTX through regulation of PI3K
signaling pathways, and these phenomena possibly indicate the
pharmacotherapeutical values of combination therapy of PTX with
3-MA to treat human lung adenocarcinoma.
In the present study, we also observed that
autophagy is critically related to the apoptotic cell death with
regard to cell signaling. Therefore, this study investigated the
expressional changes of signaling proteins, which are known as a
mediator for the modulation of the autophagy and apoptotic
pathways. It is known that LC3 forms the double membranes of
autophagosome, and this protein is then generally considered as a
useful marker to demonstrate the induction and/or formation of
autophagy. Also, AO can be used as a marker to detect autophagy. As
expected in the working hypothesis, 3-MA significantly reduced the
LC3-II expression and AVO formation in cultured A549 cells. These
results clearly indicate that the experimental method used in this
study may be sufficient to demonstrate the autophagy induction and
apoptotic cell death, and autophagy may be affected by apoptotic
cell death progress.
Additionally, it is known that Annexin V and PARP
probably act as main factors for regulating the cellular apoptosis,
and PARP repairs the cancer cells from DNA damage. In comparison
with PTX alone, the Annexin V expression was significantly
increased by co-treatment of PTX with 3-MA, and the expression of
cleaved PARP was also obviously increased. These results clearly
indicate that apoptotic cell death is possibly augmented by
inhibition of autophagy in A549 cells.
Taken together, the results of the present study
suggest the cellular and molecular mechanisms responsible for the
chemotherapeutic effects of PTX in the treatment of lung
adenocarcinoma in humans. PTX decreases viability of cultured A549
cells through regulation of autophagy induction and apoptotic cell
death, and these effects of PTX are augmented by combination with
3-MA. Based on the results of the present study, although it is
difficult to tell conclusively the pharmacotherapeutical roles of
PTX in the treatment of patients with lung adenocarcinoma, the
inhibition of PTX-induced autophagy seems to be a more important
tumor suppressive mechanism. Therefore, this study considers that
autophagy inhibition is likely a useful method to treat NSCLC,
including lung adenocarcinoma of humans, and the combination
chemotherapy of PTX with autophagy inhibitors may be a significant
strategy for obtaining the chemotherapeutic effects in patients
with advanced NSCLC.
Acknowledgements
The present study was supported by Wonkwang
University in 2013.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar
|
|
2
|
D’Addario G and Felip E; ESMO Guidelines
Working Group. Non-small-cell lung cancer: ESMO clinical
recommendations for diagnosis, treatment and follow-up. Ann Oncol.
20(Suppl 4): 68–70. 2009.
|
|
3
|
Gkiozos I, Charpidou A and Syrigos K:
Developments in the treatment of non-small cell lung cancer.
Anticancer Res. 27:2823–2827. 2007.PubMed/NCBI
|
|
4
|
Scagliotti GV, Parikh P, von Pawel J, et
al: Phase III study comparing cisplatin plus gemcitabine with
cisplatin plus pemetrexed in chemotherapy-naive patients with
advanced-stage non-small-cell lung cancer. J Clin Oncol.
26:3543–3551. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Katayama M, Kawaguchi T, Berger MS and
Pieper RO: DNA damaging agent-induced autophagy produces a
cytoprotective adenosine triphosphate surge in malignant glioma
cells. Cell Death Differ. 14:548–558. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Levine B and Yuan J: Autophagy in cell
death: an innocent convict? J Clin Invest. 115:2679–2688. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ravikumar B, Sarkar S, Davies JE, et al:
Regulation of mammalian autophagy in physiology and
pathophysiology. Physiol Rev. 90:1383–1435. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gozuacik D and Kimchi A: Autophagy as a
cell death and tumor suppressor mechanism. Oncogene. 23:2891–2906.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rollins KD and Lindley C: Pemetrexed: a
multitargeted antifolate. Clin Ther. 27:1343–1382. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ramirez JM, Ocio EM, San Miguel JF and
Pandiella A: Pemetrexed acts as an antimyeloma agent by provoking
cell cycle blockade and apoptosis. Leukemia. 21:797–804.
2007.PubMed/NCBI
|
|
11
|
Yang TY, Chang GC, Chen KC, et al:
Pemetrexed induces both intrinsic and extrinsic apoptosis through
ataxia telangiectasia mutated/p53-dependent and -independent
signaling pathways. Mol Carcinog. 52:183–194. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tonkinson JL, Worzalla JF, Teng CH and
Mendelsohn LG: Cell cycle modulation by a multitargeted antifolate,
LY231514, increases the cytotoxicity and antitumor activity of
gemcitabine in HT29 colon carcinoma. Cancer Res. 59:3671–3676.
1999.PubMed/NCBI
|
|
13
|
McLeod HL, Cassidy J, Powrie RH, et al:
Pharmacokinetic and pharmacodynamic evaluation of the glycinamide
ribonucleotide formyltransferase inhibitor AG2034. Clin Cancer Res.
6:2677–2684. 2000.PubMed/NCBI
|
|
14
|
Shih C, Chen VJ, Gossett LS, et al:
LY231514, a pyrrolo[2,3-d]pyrimidine-based antifolate that inhibits
multiple folate-requiring enzymes. Cancer Res. 57:1116–1123.
1997.
|
|
15
|
D’Angelo SP, Kris MG, Pietanza MC, Rizvi
NA and Azzoli CG: A case series of dose-limiting peripheral edema
observed in patients treated with pemetrexed. J Thorac Oncol.
6:624–626. 2011.PubMed/NCBI
|
|
16
|
Mujumdar N, Mackenzie TN, Dudeja V, et al:
Triptolide induces cell death in pancreatic cancer cells by
apoptotic and autophagic pathways. Gastroenterology. 139:598–608.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hanna N, Shepherd FA, Fossella FV, et al:
Randomized phase III trial of pemetrexed versus docetaxel in
patients with non-small-cell lung cancer previously treated with
chemotherapy. J Clin Oncol. 22:1589–1597. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ciuleanu T, Brodowicz T, Zielinski C, et
al: Maintenance pemetrexed plus best supportive care versus placebo
plus best supportive care for non-small-cell lung cancer: a
randomised, double-blind, phase 3 study. Lancet. 374:1432–1440.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vogelzang NJ, Rusthoven JJ, Symanowski J,
et al: Phase III study of pemetrexed in combination with cisplatin
versus cisplatin alone in patients with malignant pleural
mesothelioma. J Clin Oncol. 21:2636–2644. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bareford MD, Hamed HA, Tang Y, et al:
Sorafenib enhances pemetrexed cytotoxicity through an
autophagy-dependent mechanism in cancer cells. Autophagy.
7:1261–1262. 2011. View Article : Google Scholar
|
|
21
|
Nakai A, Yamaguchi O, Takeda T, et al: The
role of autophagy in cardiomyocytes in the basal state and in
response to hemodynamic stress. Nat Med. 13:619–624. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kondo Y, Kanzawa T, Sawaya R and Kondo S:
The role of autophagy in cancer development and response to
therapy. Nat Rev Cancer. 5:726–734. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Baehrecke EH: Autophagy: dual roles in
life and death? Nat Rev Mol Cell Biol. 6:505–510. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Debnath J, Baehrecke EH and Kroemer G:
Does autophagy contribute to cell death? Autophagy. 1:66–74. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zeng X, Overmeyer JH and Maltese WA:
Functional specificity of the mammalian Beclin-Vps34 PI 3-kinase
complex in macroautophagy versus endocytosis and lysosomal enzyme
trafficking. J Cell Sci. 119:259–270. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pattingre S, Espert L, Biard-Piechaczyk M
and Codogno P: Regulation of macroautophagy by mTOR and Beclin 1
complexes. Biochimie. 90:313–323. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kanematsu S, Uehara N, Miki H, et al:
Autophagy inhibition enhances sulforaphane-induced apoptosis in
human breast cancer cells. Anticancer Res. 30:3381–3390.
2010.PubMed/NCBI
|
|
28
|
Liu D, Yang Y, Liu Q and Wang J:
Inhibition of autophagy by 3-MA potentiates cisplatin-induced
apoptosis in esophageal squamous cell carcinoma cells. Med Oncol.
28:105–111. 2011. View Article : Google Scholar : PubMed/NCBI
|