Introduction
Gastric cancer is one of the most common causes of
cancer-related mortality worldwide (1). In China, the incidence rate of gastric
cancer ranks the third highest amongst the most common cancers
(2). Wnt/β-catenin signaling is one
of the major pathways in gastric carcinogenesis. When Wnt binds to
the cell surface receptor Frizzled and activates disheveled, GSK3β
is dissociated from their complex. As a result, free β-catenin
accumulates and translocates into the nucleus and subsequently
binds to T-cell factor, initiating transcription of its target
genes which may be relevant for tumor development and progression
(3). These target genes include
myelocytomatosis viral oncogene homolog (c-myc) and cyclin D1,
matrix metalloproteinase 7 (MMP7), CD44. Epithelial-to-mesenchymal
transition is another phenotype caused by β-catenin activation,
which gives cancer cells migration ability accompanied by EMT
marker alterations, such as downregulation of epithelial marker
E-cadherin and upregulation of mesenchymal markers such as
N-cadherin, MMP2 and MMP9 (4). This
activation of β-catenin as a transcriptional factor is the major
requirement for cancer initiation in cancer triggered by aberrant
Wnt signaling. Hence, more attention has been paid to the
regulation of β-catenin in cancer studies.
Wnt/β-catenin can be regulated in multiple steps.
One of the key steps in deciding the activation of β-catenin is the
destruction complex APC/Axin/GSK3β. The role of this complex is to
degrade β-catenin and prevent its nuclear translocation. In
addition to intracellular regulation, outside signals are other
regulators of Wnt/β-catenin signaling. Secreted inhibitors are
other regulators of Wnt signaling including Wnt inhibitory factor
(WIF), secreted forms of frizzled proteins (sFRPs) and Dickkopf
family proteins (DKKs). FRZB, also known as sFRP3, is one of the
Wnt signaling pathway regulators. FRZB contains a 24-amino acid
putative transmembrane segment (5),
a cysteine-rich domain (CRD) which is similar to the putative
Wnt-binding region of the frizzled family of transmembrane
receptors, a netrin-like domain (NTR) which is homologous with
tissue inhibitors of metalloproteases (TIMPs) (6). Polymorphisms in the FRZB gene have
been associated with osteoarthritis (7) and are considered one of the osteoblast
regulatory genes. FRZB affects the cartilage integrity as well as
cortical bone thickness and density. The mechanism of this
protection can be partly attributed to FRZB suppression of the
expression of WNT/β-catenin target genes, including genes for MMP3
and cyclooxygenase 2 (COX2) (8).
Further study demonstrated that FRZB may bind and inhibit MMP3
proteinase activity through its NTR domain (8).
FRZB is also involved in malignant tumor generation
and progression. Deregulation of FRZB is found in bone-originated
malignant diseases. Expression of FRZB was also found to be related
to bone involvement at diagnosis in myeloma plasma cells (9). Loss of FRZB expression was commonly
found in osteogenic sarcoma tissues (10). FRZB was reportedly highly expressed
in gastric cancer tissues, especially in intestinal-type and
well-differentiated gastric cancer tissues (11). Expression of FRZB suppresses
epithelial original prostate cancer cell in vivo growth and
progression (12). FRZB can
function as a melanoma migration and invasion suppressor by
interfering with Wnt5a signaling (13). FRZB decreases growth and
invasiveness of fibrosarcoma cells and this inhibition is
associated with downregulation of c-Met expression and inhibited
Met-mediated signaling (14). These
data strongly suggest a tumor suppressor role of FRZB and
involvement of FRZB in regulating the Wnt signaling pathway. Here,
we examined the expression of FRZB and β-catenin, a key downstream
factor of Wnt signaling, to observe the expression pattern and
association of these two proteins. We further investigated the
function of FRZB by knockdown of FRZB expression in gastric cancer
cells.
Materials and methods
Gene expression analysis
For gene expression analysis, 111 gastric cancer and
20 normal gastric tissues were collected from Ruijin Hospital.
Total RNA was extracted using TRIzol reagent (Invitrogen, Carlsbad,
CA, USA), according to the manufacturer’s instructions. Gene
expression profiling was conducted using U133 plus 2.1 array
(Affymetrix, Santa Clara, CA, USA).
Immunohistochemistry
Gastric cancer tissues, confirmed by pathological
diagnosis, were obtained from 89 patients who underwent radical
resection for gastric cancer between 2006 and 2008 at the
Department of Surgery, Ruijin Hospital, Shanghai, China. The
corresponding non-tumor gastric tissues were obtained at least 6 cm
from the tumors. All tissue samples were formalin-fixed and
paraffin-embedded. TNM staging was classified based on the criteria
of the American Joint Committee on Cancer (AJCC, 7th edition) for
gastric cancer. The present study was approved by the Shanghai Jiao
Tong University Medical School Institutional Review Board.
Immunohistochemistry staining was performed by using
a highly sensitive streptavidin-biotin-peroxidase detection system
with gastric cancer tissue microarrays. Rabbit monoclonal anti-FRZB
(working dilution 1:100) was purchased from LifeSpan Biosciences
(Seattle, WA, USA) and Rabbit anti-β-catenin (working dilution
1:100) was purchased from Cell Signaling (Danvers, MA, USA).
Immunolabeling was conducted using Envision+ Rabbit Polymer (Dako
Carpinteria, CA, USA). The slides were counterstained with
hematoxylin and coverslipped.
Immunohistochemistry scoring
The histology of the samples was examined by two
histopathologists independently without knowing the
clinicopathological information. We scored the slides according to
a previous publication (15). The
percentage of positive tumor cells was assigned to 5 categories:
≤5% (0), 5–25% (1), 25–50%
(2), 50–75% (3), and ≥75% (4). ≤5% positive cells were used as the
cutoff to define negative tumors. The intensity of immunostaining
was scored as: weak (1), moderate
(2), and strong (3). The percentage of positivity of tumor
cells and staining intensity were multiplied to produce a weighted
score for each tumor specimen. The intensity scores were grouped as
low (which included scores 0 to +4) and high (which included scores
+6 and +12).
Cell culture
Human gastric cancer cell line MKN45 was obtained
from Shanghai Institute of Cell Biology, Chinese Academy of
Sciences. The cells were grown in RPMI-1640 medium containing 10%
fetal bovine serum (FBS), penicillin and streptomycin (Gibco-BRL,
Gaithersburg, MD, USA).
Cell proliferation and soft agar
assay
Cell proliferation was assessed using the Cell
Counting Kit-8 (CCK-8; Dojindo Laboratories, Kumamoto, Japan)
assay. Soft agar colony formation assay was performed by using 0.3%
agar in complete medium (RPMI-1640 medium containing 10% FBS) with
cells as the feeder layer and 0.6% agar in complete medium as the
bottom layer.
FRZB-specific shRNA and transfection
The sequence used for construct of FRZB-shRNA was
3′-GGAGATTCTAAAGTCCTCTTTCAAGAGAAGAGGACTTTAGAATCTCC-5′. The shRNAs
were cloned into pGPU6/Neo (Shanghai GenePharma, Shanghai, China).
Plasmids were transfected into gastric cancer cells using
Lipofectamine 2000 (Invitrogen). G418 was used for stable clone
selection.
RT-PCR
RT-PCR was carried out and the set of primers for
FRZB was: F, 5′-GAGGAGCTGCCAGTGTACGAC-3′ and R,
5′-GAAAATCAGCTCCGTCCGC-3′; GAPDH: F, 5′-GGACCTGACCTGCCGTCTAG-3′ and
R, 5′-GTAGCCCAGGATGCCCTTGA-3′ respectively (11). Primers for Ecad were: F,
5′-TTCCCTCGACACCCGATTCA-3′ and R, 5′-CCAGAAACGGAGGCCTGATG-3′; Ncad:
F, 5′-CCCGGTTCATTTGAGGGCA-3′ and R, 5′-GGCATTGGGATCGTCGCAT-3′;
MMP7: F, 5′-GTCTCTGGACGGCAGCTATG-3′ and R,
5′-TAGTCCTGAGCCTGTTCCCA-3′; cyclin D1: F,
5′-GATGCCAACCTCCTCAACGA-3′ and R, 5′-GGAAGCGGTCCAGGTAGTTC-3′.
Relative density of target gene levels were measured by the
following equation: Relative density = density of band of target
genes/density of GAPDH.
Western blotting
Whole cell lysates were harvested using RIPA cell
lysis buffer supplemented with a protease inhibitor cocktail
(Sigma). A total of 50 μg protein were separated by
SDS-polyacrylamide gel electrophoresis and blotted onto 0.22 μm
polyvinylidene difluoride membranes (Millipore, Billerica, MA,
USA). For extracting nuclear protein, nuclear extract kit (Active
Motif, Carlsbad, CA, USA) was used following the manufacturer’s
instructions. Antibodies against FRZB (LifeSpan) were used at
1:1,000 dilutions. Antibodies against GAPDH (Sigma) were used at a
1:5,000 dilution. Antibodies against Lamin B1 and β-catenin (Cell
Signaling, Boston, MA, USA) were used at 1:1,000 dilutions. The
signals were visualized using Odyssey-Sa model 9260 (Li-COR,
Lincoln, NE, USA) and images were captured and managed using
Odyssey-Sa Infrared Image System (Li-COR).
Cell aggregation assay
Cells were trypsinized in the presence of EDTA,
washed twice in PBS and suspended at a concentration of
2.5×105 cells/ml in DMEM with 10% FBS. Cell culture
dishes (diameter, 10 cm) were pre-coated with
poly-2-hydroxyethyl-methacrylate (poly-HEMA; Sigma, St. Louis, MO,
USA) to block cell attachment. Same amount of MKN45 (parental),
vector-only (MKN45/vector) and FRZB-shRNA (MKN45/FRZB-KD) cells
were seeded and cultured for 24 h (16). Cells were observed using IX71
microscope (Olympus, Tokyo, Japan). Images were captured using
Digital Sight DS-U1 (Nikon, Tokyo, Japan) and NIS elements F3.0
software was used (Nikon).
Cell migration and invasion assay
Cell migration was analyzed by a Transwell chamber
assay. Cell invasion assays were performed using BioCoat Matrigel
Invasion Chambers (BD Biosciences, Franklin Lakes, NJ, USA) and 10%
FBS was used as the chemoattractant. Cells on the lower surface of
the insert were fixed and stained followed by counting under a
light microscope. Cells were visualized using BX50 microscope
(Olympus). Images were captured using Digital Sight DS-U2 (Nikon)
and NIS elements F3.0 software was used (Nikon).
Statistical analysis
For IHC staining, the differences in
clinicopathological features between the different groups were
determined using Pearson’s χ2 test. P<0.05 was
considered to indicate a statistically significant difference. The
significance of differences between experimental groups was
analyzed using the Student’s t-test and two-tailed distribution.
Statistical Package for the Social Sciences version 13.0 (SPSS,
Inc., Chicago, IL, USA) was used for all statistical analyses.
Results
FRZB levels are negatively correlated
with β-catenin levels in gastric cancer tissues
As demonstrated by previous studies (3,5), FRZB
is associated with the activity of β-catenin. To investigate the
correlation between FRZB and β-catenin, we first analyzed mRNA
levels of FRZB and β-catenin in non-tumor and tumor gastric
tissues. cDNA microarray dataset using a cohort of 111 gastric
cancer tissues and 20 normal gastric mucosal tissues was analyzed.
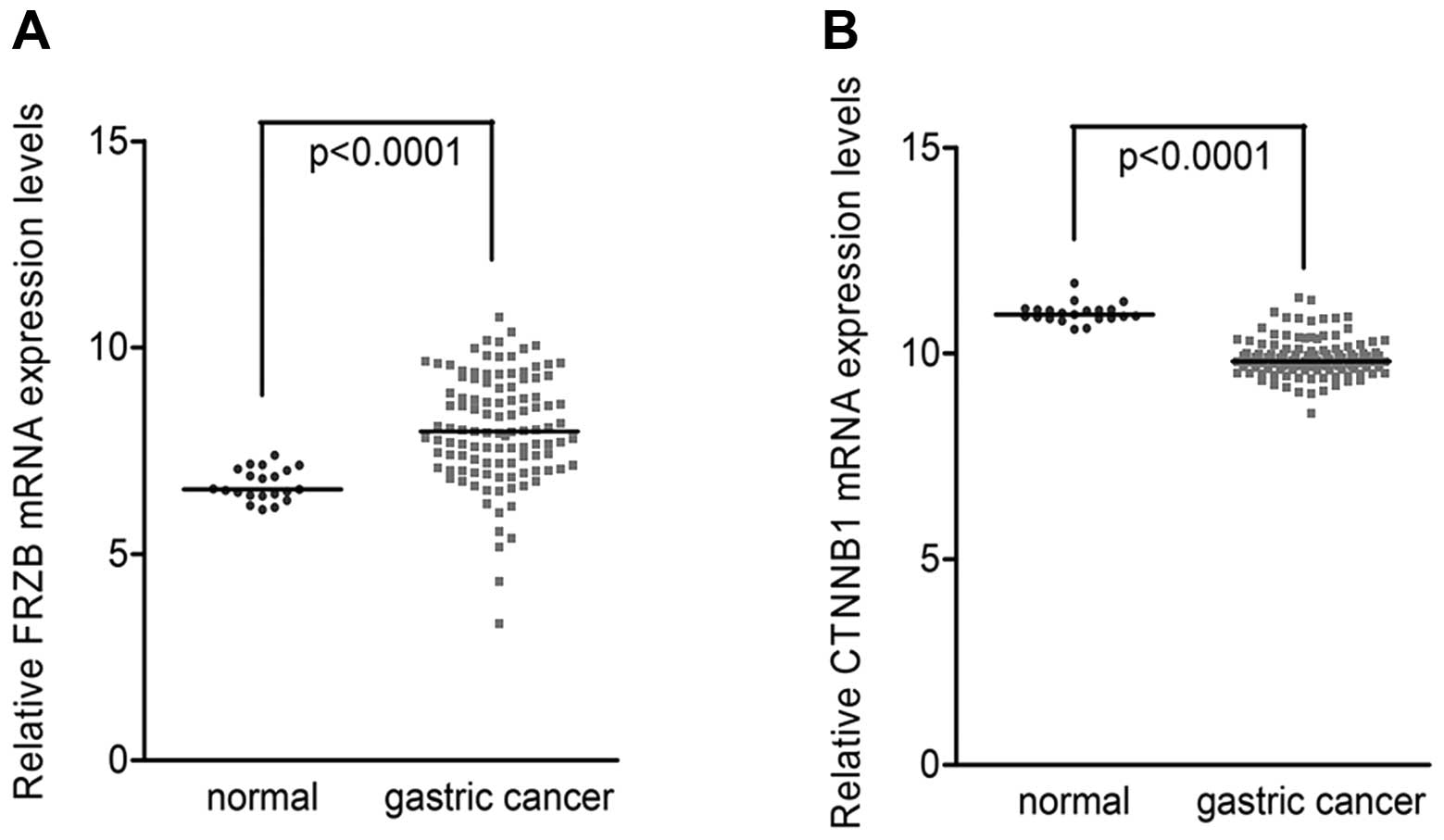
This analysis demonstrated that FRZB mRNA levels were higher in
gastric cancer tissues than in non-tumor tissues (Fig. 1A). On the contrary, β-catenin mRNA
levels were lower in gastric cancer tissues than in non-tumor
tissues (Fig. 1B). Pearson
correlation analysis revealed that the negative correlation between
FRZB and β-catenin levels was statistically significant in gastric
cancer tissues (Corr=−0.263, P=0.005) but not in normal gastric
tissues (Corr=−0.063, P=0.402) (Table
I). These data showed a negative correlation between FRZB and
β-catenin in gastric cancer tissues.
 | Table ICorrelation between FRZB and β-catenin
RNA analyzed using cDNA microarray dataset. |
Table I
Correlation between FRZB and β-catenin
RNA analyzed using cDNA microarray dataset.
| Tissues | Corr
FRZB-β-catenin | P-value |
|---|
| Gastric cancer | −0.263 | 0.005 |
| Non-tumor | −0.063 | 0.402 |
FRZB levels are correlated with β-catenin
localization in gastric cancer tissues
To investigate the possible role of FRZB in
regulating the function of β-catenin, we performed IHC staining
using the same cohort of specimens as we used for FRZB staining. We
discovered that FRZB was correlated with the sub-cellular
localization of β-catenin, which might suggest the activation of
β-catenin related signaling pathway. Among these 90 cases, 14 cases
displayed membrane staining of β-catenin, while 18 cases showed
nuclear β-catenin. As shown in Table
II, membrane β-catenin tended to exist in the high FRZB
expression group (11/14 cases), while nuclear β-catenin tended to
exist in the low FRZB expression group (13/18 cases). The FRZB
protein levels were associated with β-catenin membrane location
with statistical significance (P=0.005). Although not statistically
significant (P=0.112), gastric cancers in the FRZB-low group had
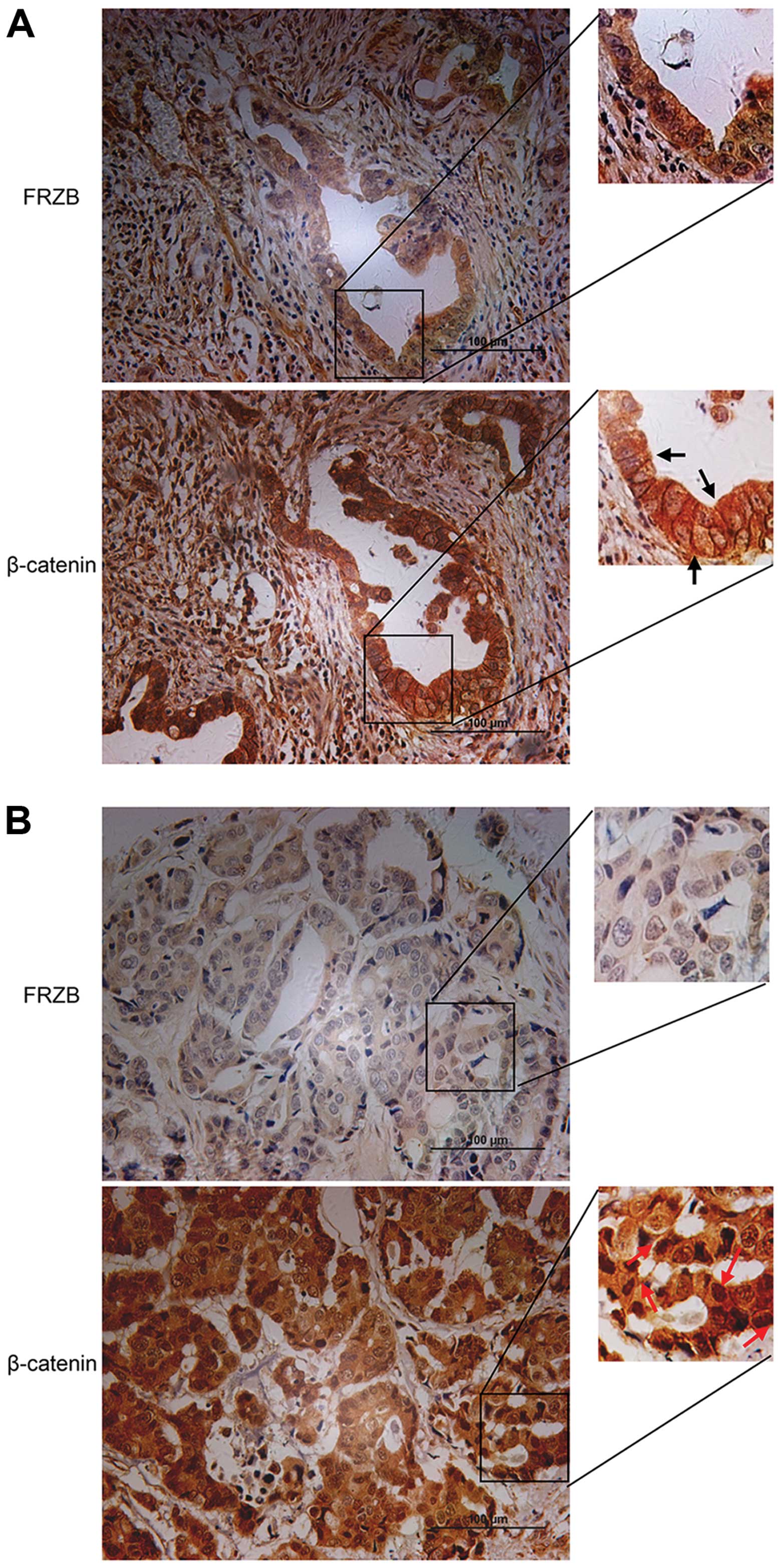
more nuclear β-catenin. As shown in Fig. 2A, strong FRZB expression was shown
in an intestinal-type gastric cancer specimen (upper left) and
membrane β-catenin was also observed in the same case (upper right,
black arrows). Weak FRZB expression was shown in a diffuse-type
gastric cancer specimen (lower left) and nuclear β-catenin
translocation was observed in the same case (lower right, red
arrows) (Fig. 2B). These data
suggested FRZB expression levels were negatively correlated with
β-catenin levels and strongly associated with sub-cellular location
of β-catenin.
 | Table IIAssociation between FRZB and
subcellular localization of β-catenin in gastric cancer
tissues. |
Table II
Association between FRZB and
subcellular localization of β-catenin in gastric cancer
tissues.
| FRZB | |
|---|
|
| |
|---|
| β-catenin | Low (%) | High (%) | P-value |
|---|
| Membrane |
| Negative | 47 (94.0) | 29 (72.5) | 0.005 |
| Positive | 3 (6.0) | 11 (27.5) | |
| Nucleus |
| Negative | 37 (74.0) | 35 (87.5) | 0.112 |
| Positive | 13 (26.0) | 5 (12.5) | |
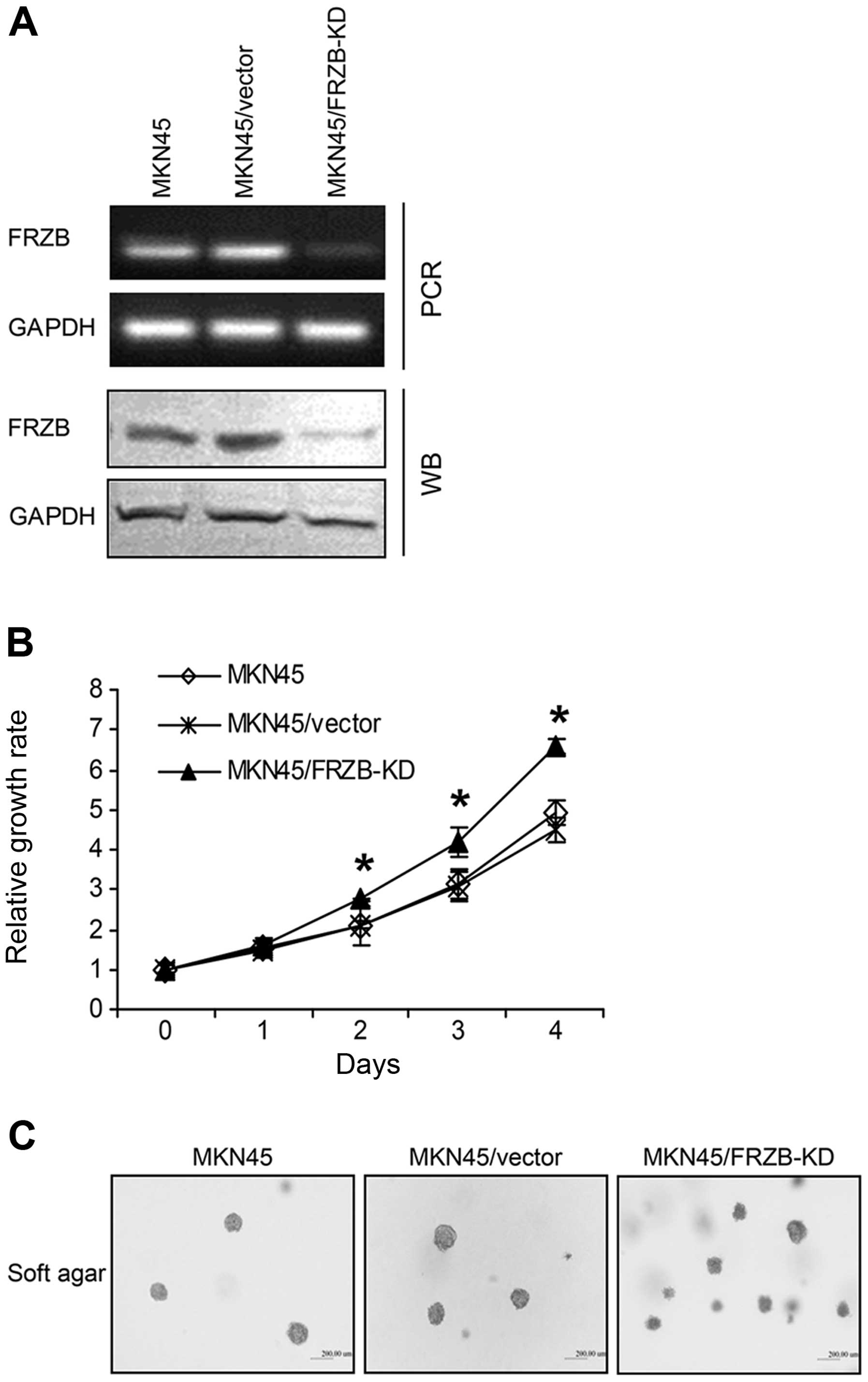
FRZB knockdown increases gastric cancer
cell growth
Since we observed strong associations between FRZB
and β-catenin in gastric cancer tissue, we next investigated
whether the alteration of FRZB levels changed the activity of
β-catenin. Thus, we established an FRZB-knockdown (FRZB-KD) model
by stable transfection of FRZB-specific shRNA into gastric cancer
cells. FRZB mRNA and protein levels were significantly inhibited by
shRNA (Fig. 3A). We first examined
the effect of FRZB knockdown on cell growth using CCK-8 analysis.
FRZB knockdown significantly increased cell growth rate from day 2
after plating in monolayer culture (Fig. 3B). We then examined whether FRZB-KD
had an effect on clonogenicity, using an in vitro assay to
investigate tumorigenicity. We performed soft agar culture using
parental cells and cells stably transfected with either
control-vector or FRZB-shRNA-vector. After 14 days of culture, we
observed more clones growing in soft agar in FRZB-KD cells than
vector control and parental cells (Fig.
3C, upper). These data suggested that FRZB knockdown increased
gastric cancer cell growth.
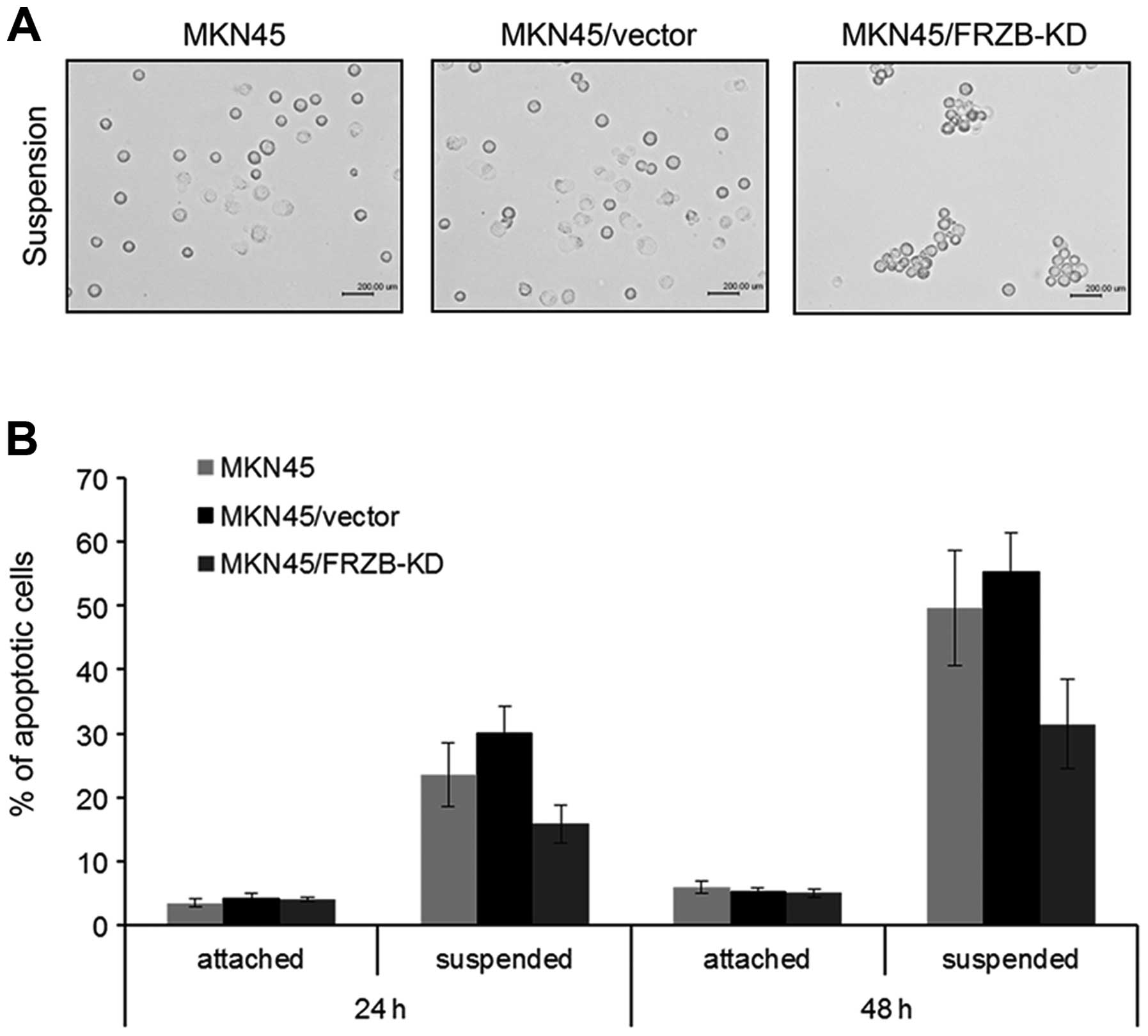
FRZB knockdown increases anoikis
resistance in gastric cancer cells
Metastasis is a multi-step process which includes
the capacity of detaching from original site, escaping from anoikis
and forming secondary tumors (17).
We next employed suspension culture assay to examine the
anti-anoikis activity, which reflected anchorage-independent growth
ability and correlated with metastatic ability of tumor cells.
After 24 h of suspension culture, FRZB-KD cells showed bigger cell
aggregation clusters than the other two control groups (Fig. 4A). Apoptosis analysis also showed a
lower apoptotic rate in FRZB-KD cells which was consistent with the
results from suspension culture (Fig.
4B). These data suggested that anoikis resistance was enhanced
by FRZB knockdown.
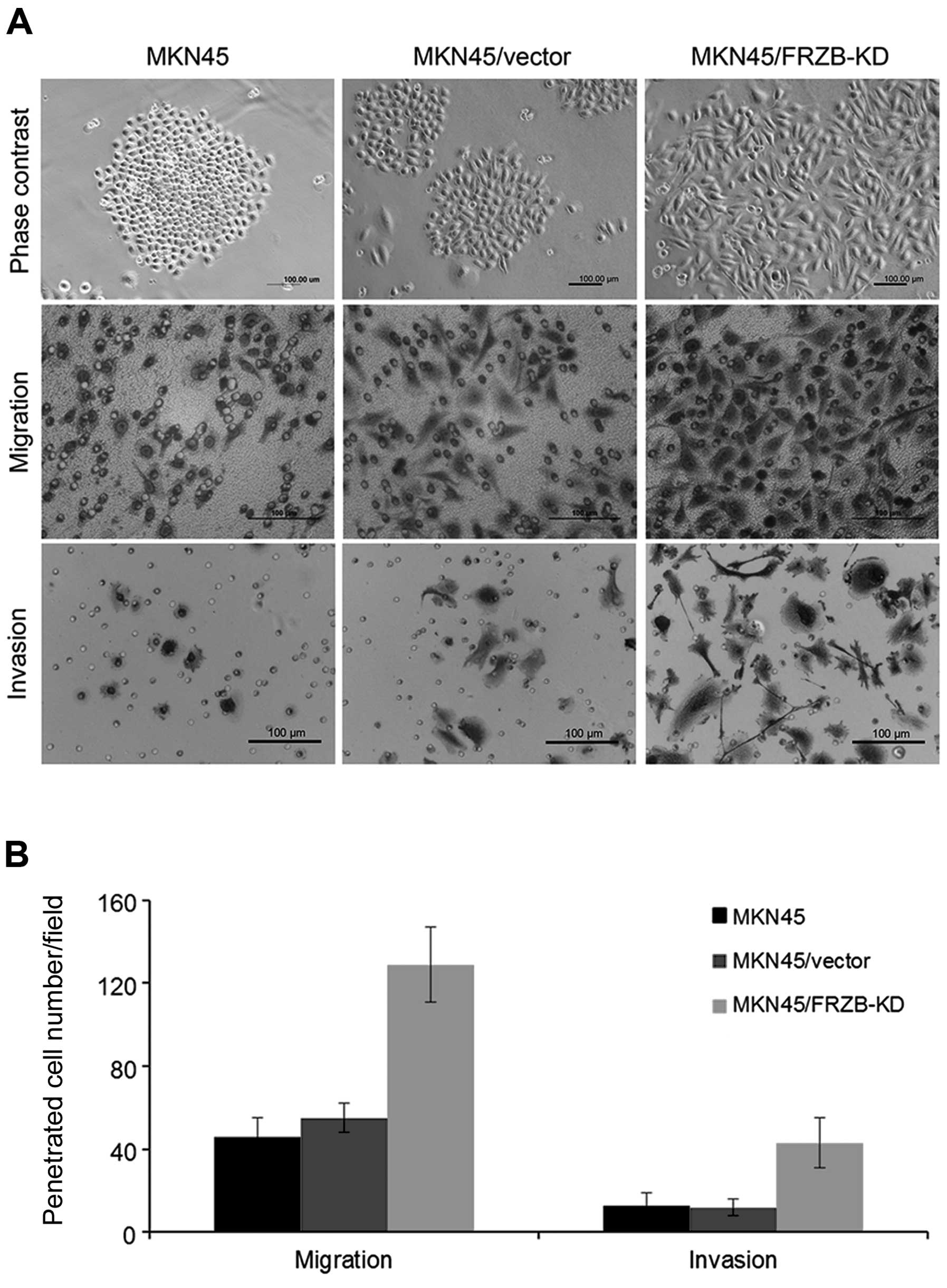
FRZB knockdown increases migration and
invasion in gastric cancer cells
In addition to anoikis, we observed
epithelial-to-mesenchymal (EMT) morphological changes in FRZB-KD
cells. FRZB-KD cells exhibited scattered clone, while the
vector-only and parental cells grew intense clone which showed
strong cell interactions (Fig. 5A).
We further performed in vitro migration and Matrigel
invasion assay to investigate the effect of FRZB-KD on cell
motility. As expected, FRZB-KD cells migrated and invaded faster
than the other two control cells (Fig.
5B). These data suggested that FRZB knockdown promoted
metastatic ability in gastric cancer cells.
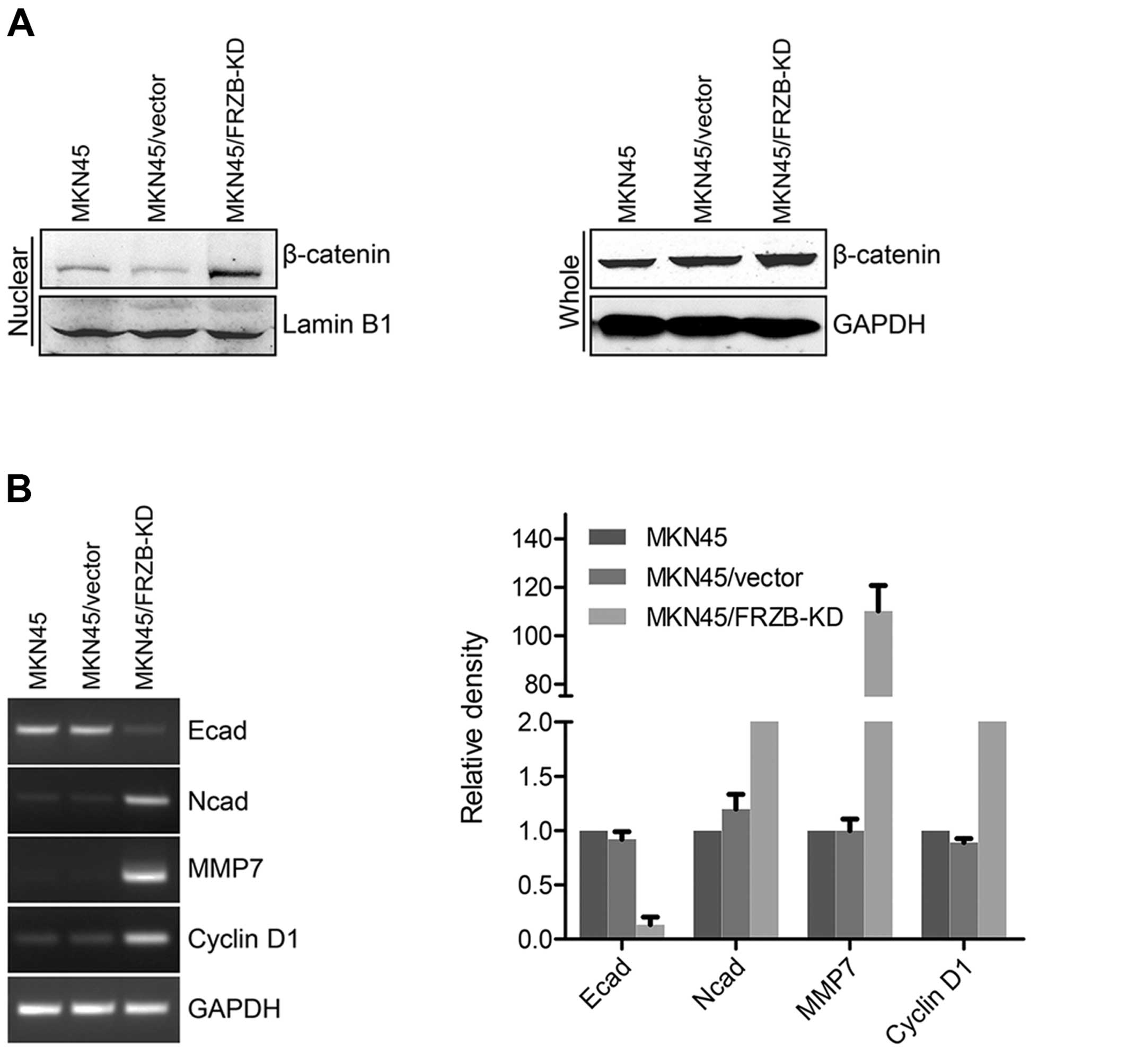
FRZB knockdown upregulates β-catenin
activity
As we discovered earlier that FRZB and β-catenin
showed negative correlation in gastric cancer tissues, we used an
FRZB-KD gastric cancer cell model to further validate this
correlation. We first found nuclear β-catenin level was increased
in FRZB-KD cells and total β-catenin level was also slightly
increased (Fig. 6A). We further
examined the expression levels of EMT markers and downstream
targets which are known to be altered by β-catenin signaling, such
as E-cadherin, N-cadherin, MMP7 and cyclin D1. As expected, levels
of the epithelial marker E-cadherin were downregulated while the
levels of the mesenchymal marker N-cadherin were upregulated by
FRZB knockdown (Fig. 6B). MMP7 and
cyclin D1, two direct downstream targets of β-catenin, were
upregulated by FRZB knockdown. Our data indicated activation of
β-catenin signaling in FRZB knockdown gastric cancer cells.
Discussion
In the present study, we observed a negative
concurrence of FRZB levels with β-catenin subcellular localization
in gastric cancer tissues. We further showed a functional
correlation of FRZB levels with anoikis and mobility in gastric
cancer cells using FRZB knockdown. Furthermore, an activation of
Wnt/β-catenin downstream targets in FRZB knockdown cells was
observed, which is consistent with our findings in gastric cancer
tissues.
The various secreted Wnt antagonists interact
directly and indirectly to affect Wnt signaling and influence a
wide variety of biological processes, including developmental cell
fate, differentiation and tumorigenesis. The interactions of Wnt
ligands and Fz receptors are modulated by the secreted Wnt
antagonists (sWAs), which can be divided into two functional
classes: the soluble frizzled related protein (sFRP) class and the
DKK class. Members of the sFRP family include Wnt inhibitory
factor-1 (Wif1), sFRP1, 2, 4 and 5 and FRZB (sFRP3). They bind
directly to Wnts, thus altering their ability to bind the Fz
receptors. Four members of the Dkk family, Dkk1-4, bind to low
density lipoprotein receptor related proteins (LRP5 and 6)
contained within the Wnt receptor complex and influence Wnt
signaling by preventing normal LRP-Fz-Wnt interactions (18). FRZB is one of the classic Wnt
signaling inhibitors which have been studied in bone diseases
(19,20). In cancer studies, FRZB was found to
play a tumor suppressor role in many malignancies including
melanoma (13), medulloblastoma
(21), gastric cancer (11) and renal cell carcinoma (22). However, there are also studies
showing the opposite results. FRZB was found highly expressed in
metastatic renal cancer tissues and promoted cell growth, invasion
and inhibition of apoptosis (23).
Although FRZB is a member of the secreted Wnt
signaling inhibitors and its downregulation has been reported in
many malignant tumors, whether FRZB inhibits β-catenin in gastric
cancer remains unclear.
β-catenin is a multifunctional protein which travels
from the cell membrane to the nucleus, thus functioning as an
intercellular connecting protein or transcriptional factor.
Membrane β-catenin is part of the cytoskeleton components.
Hyperactivation of β-catenin activity has been reported to play
critical role in regulating anoikis resistance in tumor cells
(24). As anchorage-independent
growth and epithelial-mesenchymal transition are two features
associated with anoikis resistance, they are considered to be vital
steps during cancer progression and metastatic colonization.
Anoikis provides a prerequisite for the dissemination of carcinoma.
The ability of cancer cells to resist anoikis is a hallmark of
cancer providing tumor cells with metastatic ability. Nuclear
accumulation caused activation of β-catenin was found to increase
anoikis in hepatocellular carcinoma cells (25). Further studies revealed direct
activation of Wnt/β-catenin signaling was the mediator of this
phenotype. We also observed larger cell aggregates and less
apoptosis in FRZB-knockdown cells under suspension which highly
suggested increased anoikis resistance in gastric cancer cells.
Downstream of β-catenin was also activated by FRZB knockdown.
Consistent with previous findings, our data suggest that FRZB plays
a tumor suppressor role by inhibiting β-catenin signaling.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Yang L: Incidence and mortality of gastric
cancer in China. World J Gastroenterol. 12:17–20. 2006.
|
|
3
|
Cho KH, Baek S and Sung MH: Wnt pathway
mutations selected by optimal beta-catenin signaling for
tumorigenesis. FEBS Lett. 580:3665–3670. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hoang B, Moos M Jr, Vukicevic S and Luyten
FP: Primary structure and tissue distribution of FRZB, a novel
protein related to Drosophila frizzled, suggest a role in skeletal
morphogenesis. J Biol Chem. 271:26131–26137. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Banyai L and Patthy L: The NTR module:
domains of netrins, secreted frizzled related proteins and type I
procollagen C-proteinase enhancer protein are homologous with
tissue inhibitors of metalloproteases. Protein Sci. 8:1636–1642.
1999. View Article : Google Scholar
|
|
7
|
Killock D: Osteoarthritis: Frzb knockout
reveals the complexity of Wnt signaling in joint homeostasis. Nat
Rev Rheumatol. 8:1232012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lories RJ, Peeters J, Bakker A, et al:
Articular cartilage and biomechanical properties of the long bones
in Frzb-knockout mice. Arthritis Rheum. 56:4095–4103. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kristensen IB, Haaber J, Lyng MB, et al:
Myeloma plasma cell expression of osteoblast regulatory genes:
overexpression of SFRP3 correlates with clinical bone involvement
at diagnosis. Leuk Lymphoma. 54:425–427. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mandal D, Srivastava A, Mahlum E, et al:
Severe suppression of Frzb/sFRP3 transcription in osteogenic
sarcoma. Gene. 386:131–138. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qu Y, Li JF, Cai Q, et al: Over-expression
of FRZB in gastric cancer cell suppresses proliferation and induces
differentiation. J Cancer Res Clin Oncol. 134:353–364. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zi X, Guo Y, Simoneau AR, et al:
Expression of Frzb/secreted Frizzled-related protein 3, a secreted
Wnt antagonist, in human androgen-independent prostate cancer PC-3
cells suppresses tumor growth and cellular invasiveness. Cancer
Res. 65:9762–9770. 2005. View Article : Google Scholar
|
|
13
|
Ekström EJ, Sherwood V and Andersson T:
Methylation and loss of Secreted Frizzled-Related Protein 3
enhances melanoma cell migration and invasion. PLoS One.
6:e186742011.PubMed/NCBI
|
|
14
|
Guo Y, Xie J, Rubin E, et al: Frzb, a
secreted Wnt antagonist, decreases growth and invasiveness of
fibrosarcoma cells associated with inhibition of Met signaling.
Cancer Res. 68:3350–3360. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sinicrope FA, Ruan SB, Cleary KR, Stephens
LC, Lee JJ and Levin B: bcl-2 and p53 oncoprotein expression during
colorectal tumorigenesis. Cancer Res. 55:237–241. 1995.PubMed/NCBI
|
|
16
|
Geiger TR, Song JY, Rosado A and Peeper
DS: Functional characterization of human cancer-derived TRKB
mutations. PLoS One. 6:e168712011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Klein CA: Cancer. The metastasis cascade.
Science. 321:1785–1787. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kawano Y and Kypta R: Secreted antagonists
of the Wnt signalling pathway. J Cell Sci. 116:2627–2634. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhao X, Huang H, Chen Y, et al: Dynamic
expression of secreted Frizzled-related protein 3 (sFRP3) in the
developing mouse spinal cord and dorsal root ganglia. Neuroscience.
248C:594–601. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kristensen IB, Christensen JH, Lyng MB, et
al: Expression of osteoblast and osteoclast regulatory genes in the
bone marrow microenvironment in multiple myeloma: only upregulation
of Wnt inhibitors SFRP3 and DKK1 is associated with lytic bone
disease. Leuk Lymphoma. Aug 5–2013.(Epub ahead of print).
|
|
21
|
Kongkham PN, Northcott PA, Croul SE, Smith
CA, Taylor MD and Rutka JT: The SFRP family of WNT inhibitors
function as novel tumor suppressor genes epigenetically silenced in
medulloblastoma. Oncogene. 29:3017–3024. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nikuseva-Martic T, Serman L, Zeljko M, et
al: Expression of secreted frizzled-related protein 1 and 3, T-cell
factor 1 and lymphoid enhancer factor 1 in clear cell renal cell
carcinoma. Pathol Oncol Res. 19:545–551. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hirata H, Hinoda Y, Ueno K, Majid S, Saini
S and Dahiya R: Role of secreted frizzled-related protein 3 in
human renal cell carcinoma. Cancer Res. 70:1896–1905. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lin DC, Zhang Y, Pan QJ, et al: PLK1 is
transcriptionally activated by NF-κB during cell detachment and
enhances anoikis resistance through inhibiting beta-catenin
degradation in esophageal squamous cell carcinoma. Clin Cancer Res.
17:4285–4295. 2011.PubMed/NCBI
|
|
25
|
Fischer AN, Fuchs E, Mikula M, Huber H,
Beug H and Mikulits W: PDGF essentially links TGF-beta signaling to
nuclear beta-catenin accumulation in hepatocellular carcinoma
progression. Oncogene. 26:3395–3405. 2007. View Article : Google Scholar : PubMed/NCBI
|