Introduction
ASAP1, also known as AMAP1, DDEF1, DEF1 or centaurin
β4, is an ADP-ribosylation factor GTP-ase activating protein that
induces the hydrolysis of GTP molecules (1–3). ASAP1
contains multiple domains, including BAR, Ankyrin repeat,
proline-rich, SH3 and pleckstrin homology (PH) domains. The
N-terminal BAR domain induces membrane tubulation and can function
as a protein binding site (4,5), while
the Ankyrin and SH3 domains facilitate signaling and interactions
by ASAP1 with focal adhesion kinase (FAK), CD2AP, Src and paxillin
(6–10). Using its pleckstrin homology domain,
ASAP1 also regulates ADP-ribosylation factors (ARFs) which cycle
between GDP and GTP states (11).
Organization of the actin cytoskeleton represents a
key factor in the formation of membrane ruffles, filopodia and
actin-rich protrusions which occur in tumor cells (1,7,12–14).
ASAP1 has been shown to have a role in the remodeling of the actin
cytoskeleton and in the regulation of intracellular membrane
traffic (12). Correspondingly,
overexpression of ASAP1 has proved to be a malignant indicator for
a variety of tumors such as breast, colorectal, uveal melanomas and
prostate (15–18). In addition, ASAP1 has been shown to
play an important role in regulating the motility, invasion and
metastasis of tumor cells (19,20).
Rac and Cdc42 are small GTP-binding proteins of the
Rho family, a subset of the Ras superfamily (21). Rac1 and Cdc42 have been
well-characterized, and similar to the other GTPases, they cycle
between a GTP-bound and a GDP-bound form. It is hypothesized that
their activity is regulated by a combination of GTPase-activating
proteins (GAPs), guanine nucleotide exchange factors and guanine
nucleotide dissociation inhibitors (GDIs). As such, these small
GTP-binding proteins serve as molecular switches within numerous
signal transduction pathways and regulate a diverse set of cellular
functions, including control of cell morphology, cell cycle
progression, cell migration and invasion, cell growth and
proliferation, actin dynamics and the transcriptional activation of
apoptotic signaling (22–29). However, it is currently unknown
whether ASAP1 affects the activation of Rac and Cdc42 in LSCC.
Carcinoma of the larynx is the most common malignant
neoplasm of the head and neck and is associated with a high rate of
mortality. Moreover, many of these patients are diagnosed with
squamous cell carcinomas. Currently, the treatment options for
laryngeal carcinoma include radical laryngectomy and conservative
chemotherapy or radiotherapy. However, despite advances in these
treatment methods, the mortality rate of advanced stage laryngeal
cancer remains largely unchanged. Metastasis of primary laryngeal
cancers also contributes to the high mortality rates associated
with these tumors, and the mechanisms associated with this process
remain largely uncharacterized. Thus, mechanistic studies of LSCC
metastasis continue to be an important area of research, as well as
the identification of more effective biological markers for the
diagnosis and prognosis of LSCC.
Currently, it remains unknown whether ASAP1 plays a
role in laryngeal carcinoma. Therefore, in the present study,
expression of ASAP1 mRNA was detected in laryngeal carcinoma
tissues and matched non-tumor tissues using real-time PCR and
immunohistochemistry assays. The potential for ASAP1 to regulate
the invasive phenotype of Hep-2 cells in vitro was also
evaluated using a lentivirus vector system. Finally, the prognostic
value associated with ASAP1 mRNA levels was analyzed.
Materials and methods
Patients and specimens
A retrospective review of 80 adult patients with
pathologically confirmed primary LSCC was carried out. Between 2006
and 2007, these patients underwent a partial or total laryngectomy
at the Department of Otorhinolaryngology, at the Second Affiliated
Hospital of Harbin Medical University. Cancer tissues and
corresponding adjacent normal tissues were collected during surgery
and were subsequently fixed in formalin and embedded in paraffin
(FFPE) according to standard pathology protocols. Patient
characteristics for this cohort are listed in Table I. Following treatment, patients
underwent follow-up through December 2007, with endpoints
classified as: surviving without LSCC, death due to primary
recurrent LSCC, death due to other causes and follow-up status
unknown. Surviving patients were confirmed by phone or by checking
census records. For 64/80 patients, complete clinicopathological
data and tumor specimens in a good state of preservation were
available. Therefore, these patients were examined in the present
study. The study protocol used was in accordance with the
institutional guidelines for human research and was approved by the
ethics committee.
 | Table ILevels of ASAP1 mRNA and the
clinicopathological characteristics of the LSCC patients
examined. |
Table I
Levels of ASAP1 mRNA and the
clinicopathological characteristics of the LSCC patients
examined.
| Patient
characteristics (No. of patients) | Levels of
ASAP1 mRNA (T/N ratioa) | P-value |
|---|
| Gender | | 0.31 |
| Male (n=42) | 4.42±0.24 | |
| Female (n=22) | 4.49±0.29 | |
| T
classification | | 0.29 |
| T1–2 (n=43) | 4.40±0.26 | |
| T3–4 (n=21) | 4.48±0.31 | |
| Lymph node
metastasis | | <0.01 |
| Negative
(n=39) | 4.32±0.26 | |
| Positive
(n=25) | 4.58±0.22 | |
|
Differentiation | | 0.32 |
| G1 (n=47) | 4.40±0.25 | |
| G2–G3 (n=17) | 4.48±0.34 | |
| Clinical stage | | <0.01 |
| I–II (n=36) | 4.44±0.26 | |
| III–IV (n=28) | 4.59±0.22 | |
| Patient age
(years) | | 0.88 |
| <60 (n=24) | 4.44±0.23 | |
| ≥60 (n=40) | 4.45±0.26 | |
| ASAP1 mRNA
levels | | <0.01 |
| Low (n=32) | 4.22±0.21 | |
| High (n=32) | 4.63±0.16 | |
Lentiviral vector system
A recombinant lentivirus was generated that
contained siRNA designed to target the human mRNA sequence of ASAP1
(siRNA-ASAP1) (5′-GACCAG AUCUCUGUCUCGGAGUUCA-3′ and 5′-UGAACUCCGA
GACAGAGAUCUGGUC-3′), and these were synthesized by Shanghai
GeneChem Co., Ltd. (Shanghai, China). A control lentivirus was also
generated. To monitor transfection efficiency, both lentiviruses
included a green fluorescent protein (GFP) cassette. The
lentiviruses containing siRNAs targeting ASAP1 and GFP control
lentiviruses were titered to 109 U/ml according to the
manufacturer’s guidelines (GeneChem).
Cell culture and transfection
The human LSCC cell line, Hep-2, was purchased from
the Cell Bank of the Chinese Academy of Science (Shanghai, China).
Cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM;
Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10%
fetal bovine serum (FBS; Shanghai Shenggong Co., Ltd., Shanghai,
China) and were maintained at 37°C under a humidified atmosphere
containing 5% CO2. Hep-2 cells in the logarithmic phase
of growth were seeded in 6-well plates at a concentration of
1×105 cells/well for transfection. After 12 h, when
cells reached ~40–50% confluency, 1 ml of complete medium
containing lentivirus (108 TU/ml) preparations and
polybrene (8 mg/ml) were added to each well. The cells were
incubated at 37°C for 12 h. The supernatant from each cell was then
removed, and DMEM containing 10% FBS and 1% penicillin-streptomycin
was added. After 24 h, the culture medium was replaced with fresh
DMEM. Seventy-two hours post transfection, the mean percentage of
GFP-positive cells observed in each well was calculated from three
random fields of view (FOV) at ×200 magnification using a
fluorescence microscope (IX70; Olympus, Tokyo, Japan).
RNA isolation and quantitative real-time
PCR
RNA was isolated from well-preserved FFPE tissues
using a High Pure RNA Paraffin kit (Roche Applied Science,
Mannheim, Germany), according to the manufacturer’s instructions.
RNA was also isolated from Hep-2 cells using TRIzol reagent
(Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s
instructions. RNA concentrations were determined by absorbance
readings at 260 nm, while RNA purity was evaluated according to the
OD260/OD280 absorption ratios obtained. cDNA
was reverse transcribed using an All-in-One™ miRNA qPCR Detection
kit (GeneCopoeia, Rockville, MD, USA). The reverse transcription
reactions were incubated at 37°C for 60 min, then at 70°C for 5
min. Real-time PCR was performed using a SYBR-Green Master Mix
(Applied Biosystems, Carlsbad, CA, USA) and a 7500 Fast Real-Time
PCR system (Applied Biosystems). Reactions were incubated at 95°C
for 10 min, followed by 45 cycles at 95°C for 10 sec, 60°C for 20
sec, and 72°C for 15 sec. Expression data were normalized to human
U6 gene expression data obtained in parallel as an external
reference using the 2−ΔΔCt method. The forward and
reverse primers used for detection of ASAP1 included: 5′-TG
TAGTCTTACTTGAAGAGGATGGACC-3′ and 5′-CCCTC CCAGCCCACTACCT-3′,
respectively, and were synthesized by GeneCore BioTechnologies Co.,
Ltd. (Shanghai, China). Each reaction was performed in
triplicate.
Invasion assay
Seventy-two hours post-transduction, cells
(2×104) were resuspended in 200 μl serum-free medium and
were plated in the upper chambers of Boyden chambers (24-well, 8-mm
pores) coated with Matrigel (Becton-Dickinson Labware, NJ, USA).
The lower chambers contained 1 ml medium containing 10% FBS as a
chemoattractant. Untreated Hep-2 cells were also plated as
controls. After 24 h, cells were mechanically removed from the
upper side of the filters, while cells that had migrated to the
lower side of the filters were fixed in 4% paraformaldehyde and
stained with hematoxylin and eosin (H&E). The number of cells
present in three ×200 microscopic fields for each well were
averaged and reported. Five independent experiments were
performed.
Western blotting
Cells from the siRNA lentivirus group, the GFP
control lentivirus group, and untreated Hep-2 cells were harvested
72 h post-transfection and incubated in cell lysis buffer for 30
min on ice. Cell lysates were separated using 10% sodium dodecyl
sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and then
were transferred to polyvinylidene fluoride (PVDF) membranes. After
incubating the membranes in 5% skim milk in Tris-buffered saline
containing 0.05% Tween-20 (TBST), the membranes were incubated with
primary antibodies overnight at 4°C. The primary antibodies used
included rabbit anti-human ASAP1 (1:500; Abcam, Cambridge, MA,
USA), rabbit anti-human rac1 (1:100; Abnova, Taipei, Taiwan), and
rabbit anti-human Cdc42 (1:200; Bioss, Beijing, China). The
membranes were then washed with TBTS and incubated with
species-appropriate horseradish peroxidase (HRP)-conjugated
secondary antibodies for 1 h at 37°C. β-actin served as a loading
control, and bands were quantified using ImageJ software (National
Institutes of Health, Bethesda, MD, USA). Three independent
experiments were performed.
Immunohistochemistry
FFPE specimens were sequentially sectioned (4 μm
each). After deparaffinization and rehydration, sections were
treated with 0.3% H2O2, and then were
incubated with 10% normal goat serum. Antigen retrieval was
performed using EDTA (pH 8.0) at 100°C for 20 min. Sections were
then washed, and incubated with the primary antibodies at 4°C
overnight. Sections were subsequently incubated at 37°C for 45 min
before being washed and incubated with secondary antibodies at room
temperature. After 1 h, sections were washed and incubated with
diaminobenzidine tetrachloride for 10 min. Sections were also
counterstained with haematoxylin. Negative controls were prepared
in parallel and were incubated with PBS instead of the primary
antibodies.
ASAP1 scoring
ASAP1 immunoreactivity was evaluated in blinded
analyses performed by two pathologists. Staining intensity was
graded from 1 to 4, and these scores indicated an absence of
staining (receiving a score of 1) up to strong staining of ASAP1
(receiving a score of 4). Mean score values for each sample were
reported.
Statistical analysis
The SPSS statistical software package was used for
all calculations. An independent t-test was used to analyze
differences in ASAP1 mRNA levels, ASAP1 protein scoring, and the
invasive phenotype between groups. Overall survival (OS) was
defined as the time period from the date of surgery to the date of
death due to LSCC or other causes, or to the end of the study
(2011-12-31). The log-rank test was used to evaluate the
association of risk factors with time-to-event endpoints. OS curves
were also calculated using the Kaplan-Meier method. A P-value
<0.05 was considered to indicate a statistically significant
result.
Results
ASAP1 is overexpressed in human LSCC both
in vivo and in vitro
Real-time PCR was used to determine the expression
status of ASAP1 mRNA for both FFPE LSCC tissue samples and matched
normal tissue samples obtained from 64 patients diagnosed with
LSCC. For the LSCC samples, the mean mRNA level for ASAP1 was
4.45-fold higher than that of the corresponding matched samples
(range, 3.76–4.97; median, 4.41) (P<0.05). Based on these data,
the LSCC patients were divided into a low expression group (ASAP1
mRNA level ≤4.41) and a high expression group (ASAP1 mRNA
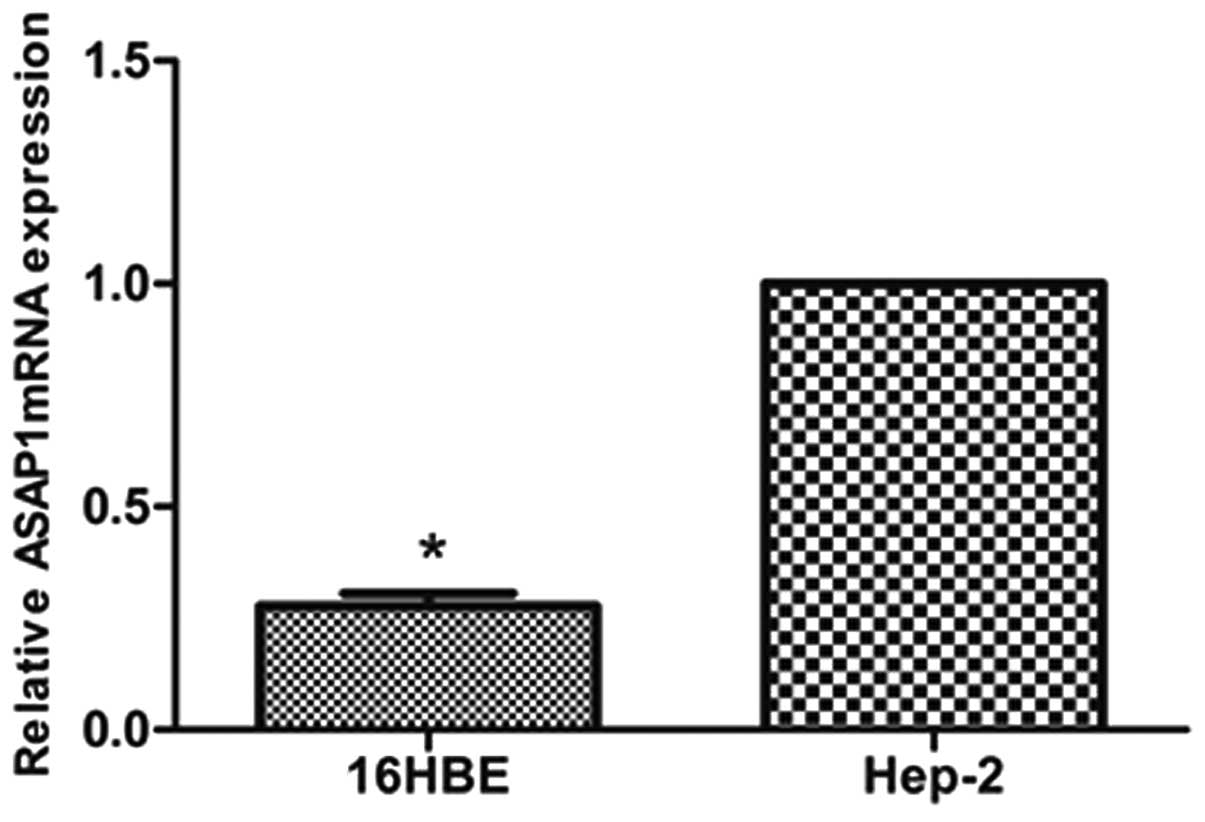
>4.47). A similar observation was made in vitro, where
the Hep-2 cells exhibited higher levels of ASAP1 mRNA than the
16HBE cells (Fig. 1).
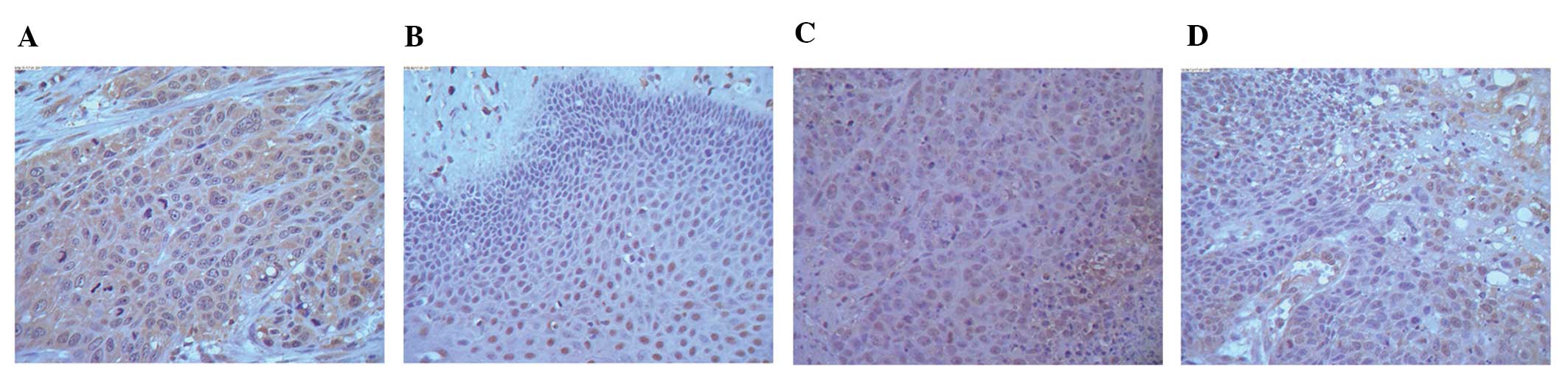
Consistent with the mRNA levels detected for ASAP1,
immunohistochemistry assays detected stronger expression of ASAP1
in tissue sections from the high expression group compared to
tissue sections from the low expression group. Moreover, the mean
ASAP1 score for the high expression group (2.63±0.87) was
significantly higher than that of the low expression group
(2.19±0.82) (P<0.05). ASAP1 expression was also detected in a
subset of the normal tissues stained, although the staining
intensity was much weaker compared to that observed for the cancer
tissues, particularly in the cytoplasm (Fig. 2).
Levels of ASAP1 mRNA and
clinicopathological factors
We analyzed the relationships between levels of
ASAP1 mRNA in the LSCC tumors and the clinical data from these
patients (Table I). There were no
obvious differences in the mRNA levels associated with gender,
tumor grade and patient age. However, significantly higher levels
of ASAP1 mRNA present in LSCC tissues were associated with lymph
node metastasis and clinical tumor stage. For example, patients
with higher ASAP1 mRNA levels tended to have an advanced clinical
stage of LSCC or lymph node metastasis. These results suggest that
upregulated expression of ASAP1 mRNA correlates with the
progression of LSCC and an invasive phenotype.
Targeting of ASAP1 mRNA in the Hep-2
cells
In order to investigate the biological function of
ASAP1 in LSCC, recombinant lentiviruses containing specific siRNA
designed to target the human mRNA sequence of ASAP1, as well as a
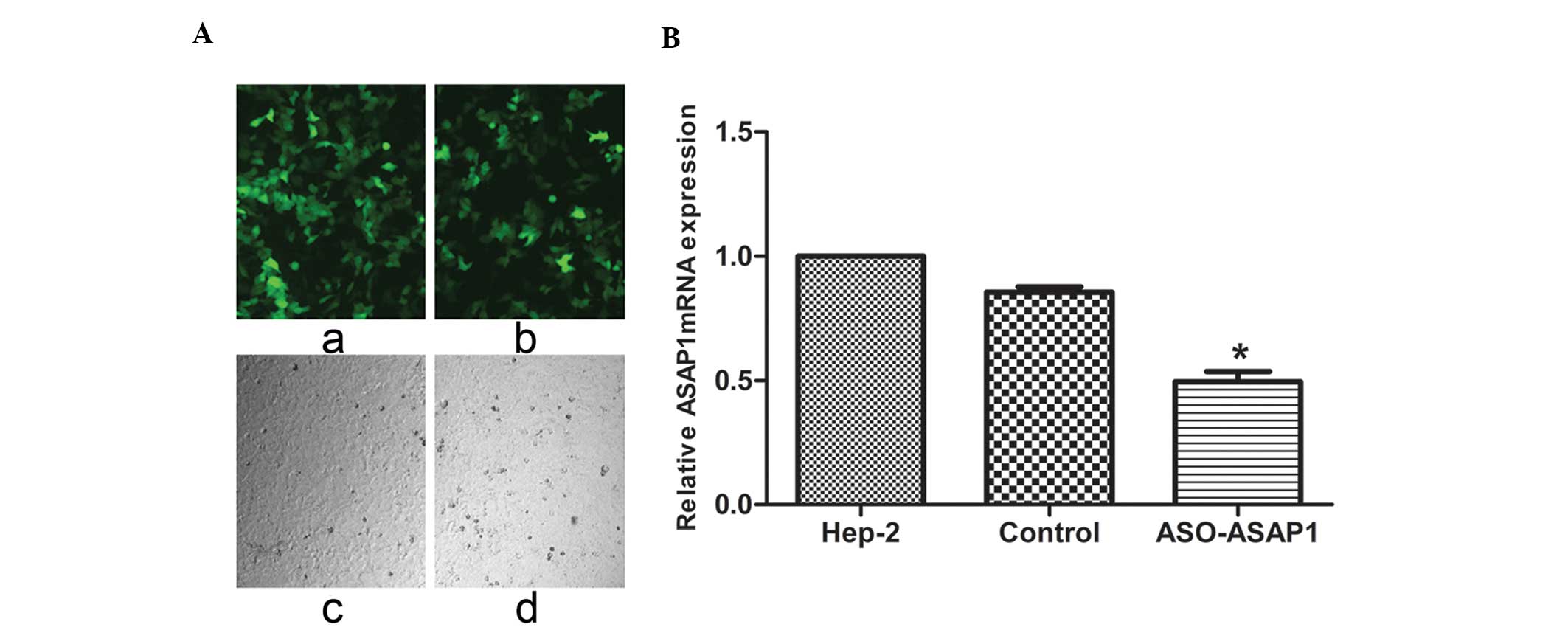
GFP cassette, were created. Seventy-two hours after Hep-2 cells
were transfected with siRNA-ASAP1 and GFP control lentiviruses,
>80% of the Hep-2 cells were observed to express GFP, indicating
the efficiency and stability of the transductions performed
(Fig. 3A). These cells were then
subjected to quantitative real-time PCR. Levels of ASAP1 mRNA were
found to be considerably lower in the cells transfected with the
siRNA-ASAP1 lentivirus compared to the GFP control and untreated
cells, thereby validating the use of this lentiviral vector system
for studies of ASAP1 (P<0.05) (Fig.
3B).
Downregulation of ASAP1 suppresses the
invasion of Hep-2 cells in vitro
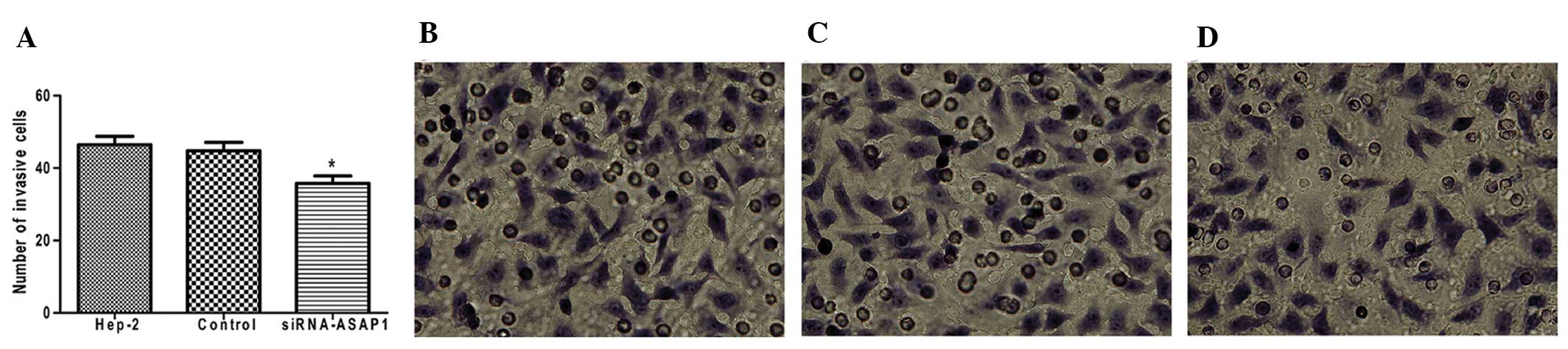
To investigate whether ASAP1 contributes to the
invasive phenotype of Hep-2 cells, invasion assays were performed
using 24-well Boyden chambers coated with Matrigel. As shown in
Fig. 4, the number of
siRNA-ASAP1-treated Hep-2 cells exhibiting an invasive phenotype 72
h after transfection (35.8±4.49) was less than that observed for
GFP control-treated Hep-2 cells (44.8±5.07) and untreated Hep-2
cells (46.4±5.32) (P<0.05). These data strongly suggest that
downregulation of ASAP1 may mediate a reduction in the invasiveness
of laryngeal carcinoma cells.
ASAP1 expression positively correlates
with Rac1 and Cdc42 expression
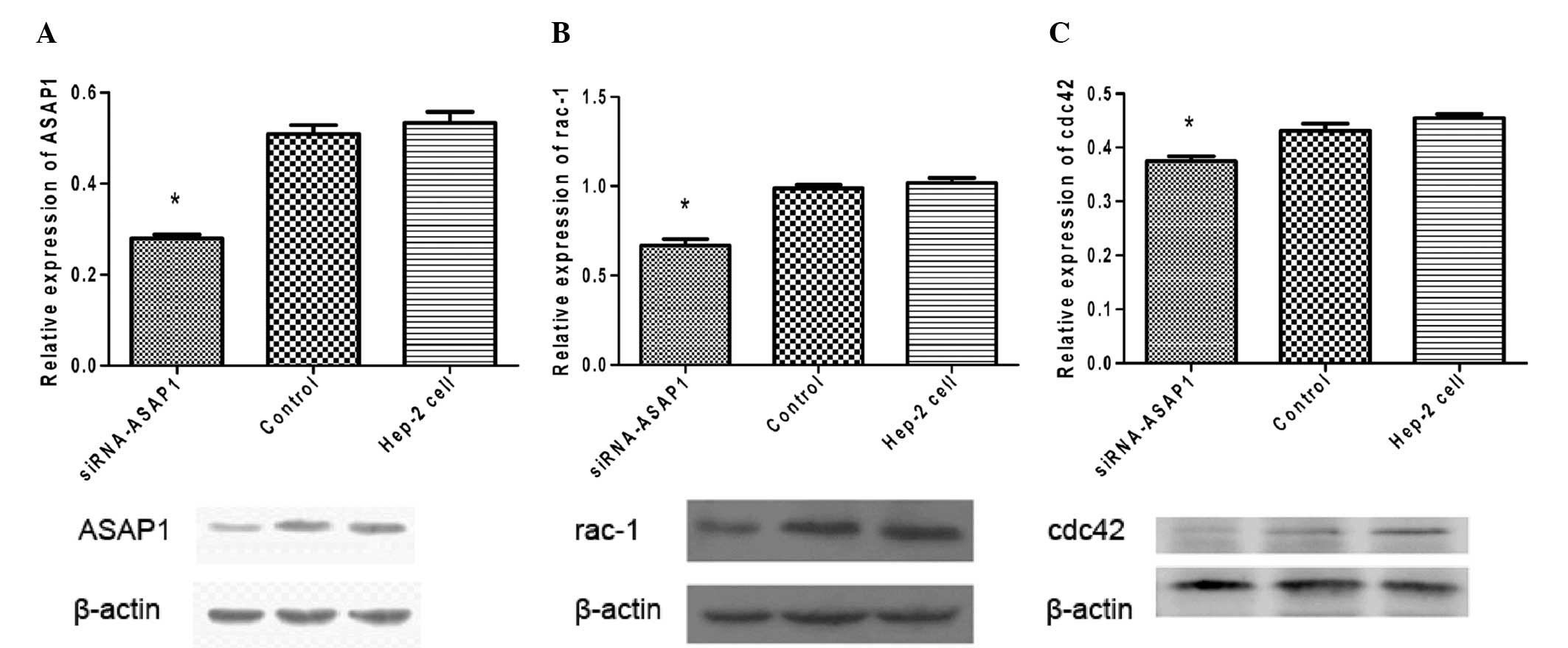
Western blotting was used to measure expression
levels of ASAP1, Rac1 and Cdc42 in the Hep-2 cells transfected with
siRNA-ASAP1 and GFP control lentiviruses. When Hep-2 cells were
transfected with the siRNA-ASAP1 lentivirus, lower levels of ASAP1
were detected compared to cells transfected with the GFP control
lentivirus or untransfected cells (P<0.05) (Fig. 5). In these cells, lower levels of
Rac1 and Cdc42 expression were also detected. In contrast, cells
transfected with the GFP control lentivirus did not show any
significant changes in the expression of these three proteins
compared to the untransfected Hep-2 cells (P>0.05).
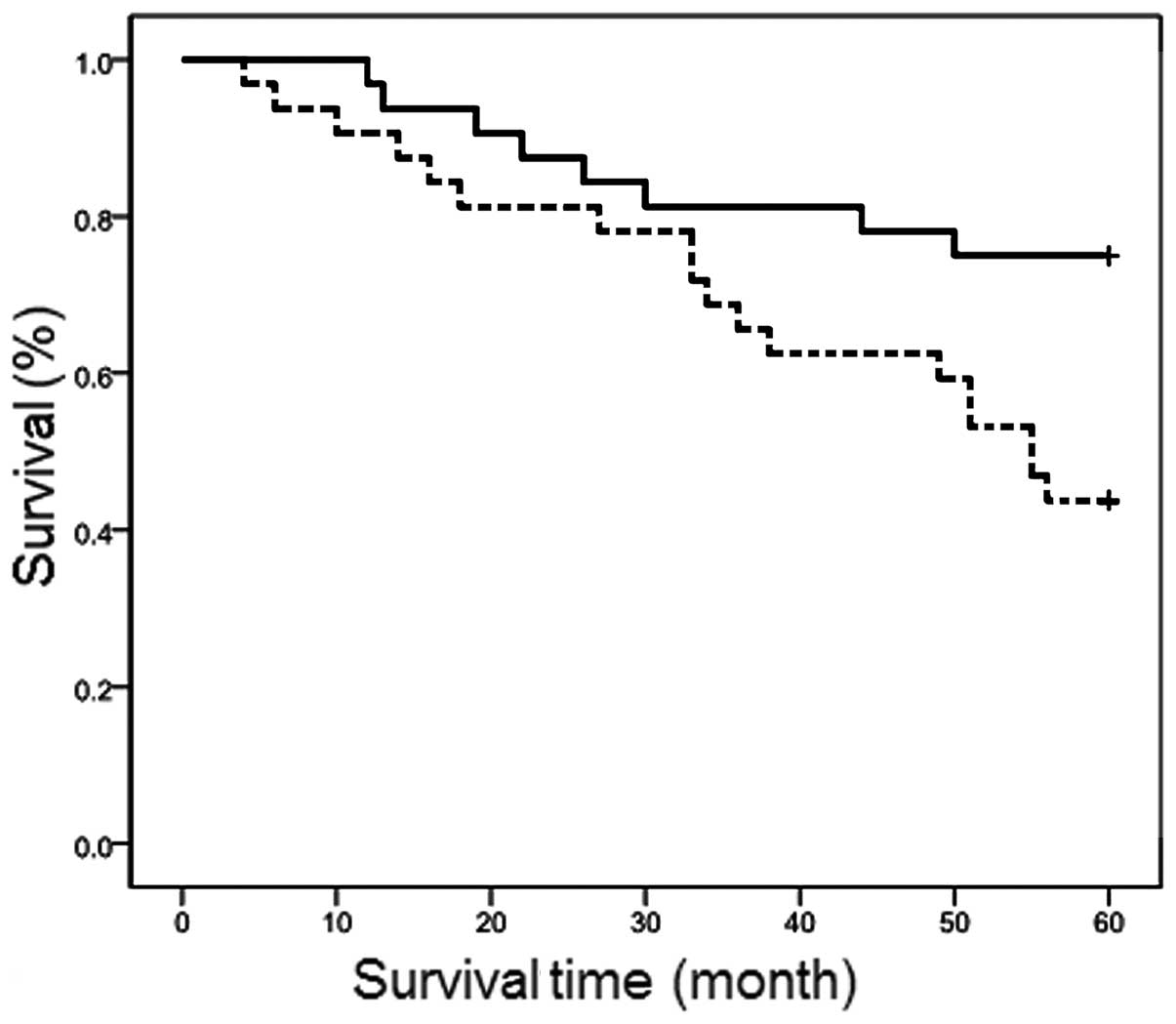
Prognostic significance of ASAP1 mRNA
levels
Of the 64 patients examined, 26 died during the
follow-up period. Depending on the levels of ASAP1 that were
detected, the 3-year survival probability was 65.6 and 81.3% for
cases with high vs. low levels of ASAP1 mRNA, respectively. The
5-year survival probability for the same groups was 43.8 and 75.0%,
respectively (P<0.05) (Fig. 6).
Taken together, these results suggest that patients with LSCC
tumors that express high levels of ASAP1 have a poor prognosis and
will experience a shorter survival period compared with patients
presenting with LSCC tumors that express low levels of ASAP1.
Discussion
Despite advances achieved in the diagnosis and
treatment of LSCC in recent years, the long-term survival rate for
LSCC, and especially the survival rate for advanced stages of LSCC,
have not significantly improved. In addition, metastasis of LSCC
still represents a significant challenge, and contributes to the
mortality rates reported for LSCC patients each year. While the
expression of various genes has been correlated with the
development of metastatic LSCC, and some of these have the
potential to serve as markers of tumor progression in the clinic
(30,31), the mechanistic details for LSCC
remain to be determined.
Previously, Miyata et al (32) identified a region in the 5′-UTR of
AMAP1 that exhibits a significant internal ribosome entry site
(IRES) activity in differentiated U937 cells. Moreover, this
activity appears to be necessary for enhanced AMAP1 expression in
this cell line. However, this IRES-dependent mechanism for AMAP1
expression may not be conserved in all tumor cells. Currently, the
role of ASAP1 in LSCC remains largely uncharacterized. In
colorectal tumors, ASAP1 expression is strongly upregulated
(16), and Lin et al
(18) reported that levels of ASAP1
were elevated in 80% of primary prostate cancer samples. The
results of the present study are consistent with these results, and
further demonstrated that both mRNA and protein levels of ASAP1
were upregulated in LSCC tissues compared to paired normal tissues.
A similar observation was made in vitro for the Hep-2 and
16HBE cell lines. Based on these findings, it is hypothesized that
high levels of ASAP1 expression contribute to LSCC.
Cell dissemination is a complex cell motility
phenomenon that requires the coordination of protrusion,
chemotaxis, invasion and contractile activities of cancer cells in
order to achieve directed cell migration. Roles for ASAP1 in actin
cytoskeleton remodeling and local adhesion have previously been
demonstrated (6,12). More recently, ASAP1 has been
implicated in mediating the invasive phenotypes of tumor cells
(33,34). In the present study, Boyden chamber
assays were used to characterize the role of ASAP1 in relation to
the invasive phenotype of LSCC in vitro, and overexpression
of ASAP1 was found to promote the invasive activity of Hep-2 cells.
Similarly, ASAP1 has been shown to mediate the invasion and
metastasis of breast cancer cells via the EGFR-GEP100-Arf6-AMAP1
signaling pathway, or in cooperation with CIN85 or Rab5c (35–37).
Overexpression of ASAP1 has also been associated with increased
invasion and metastatic potential of high-grade uveal melanomas
(17). Therefore, the findings of
the present study are consistent with the results obtained using
other tumor models, and further support a role for ASAP1 in tumor
invasion.
Directed cell migration involves modulation of the
actin cytoskeleton by Rho GTPases. In fibroblasts, Rac proteins
regulate the formation of lamellipodia and membrane ruffles, as
well as the subsequent formation of stress fibers (38,39).
In contrast, Cdc42 plays a key role in the formation of filopodia
at the cell periphery, and this is followed by the formation of
lamellipodia and membrane ruffles (40). When ASAP1 was downregulated in Hep-2
cells using an siRNA targeting ASAP1 lentivirus, cell invasion was
inhibited. Furthermore, reduced ASAP1 expression also positively
correlated with the protein levels detected for Rac1 and Cdc42.
Taken together, these results suggest that decreased expression of
Rac1 and Cdc42 may also contribute to the invasive phenotype of
Hep-2 cells.
To determine whether ASAP1 expression levels affect
the prognosis of laryngeal cancer patients, the median level of
ASAP1 mRNA that was identified in 64 LSCC patients was used to
establish a high ASAP1 expression level group and a low ASAP1
expression level group. The former was associated with a higher
mortality rate, as well as a higher rate of relapse. To the best of
our knowledge, this is the first study to investigate the effect of
ASAP1 expression on the recurrence and mortality of LSCC. Given the
small sample size of this study, additional studies are needed to
validate the present results. However, the results of the present
study do highlight the potential for ASAP1 to be considered as a
risk factor for human laryngeal carcinoma.
Based on the results obtained, ASAP1 appears to have
an oncogenic role in the metastasis of laryngeal tumors. Therefore,
it is hypothesized that ASAP1 mRNA and ASAP1 represent therapeutic
targets and prognostic biomarkers that should be considered and
evaluated for LSCC.
Acknowledgements
We thank the Department of Otorhinolaryngology at
the Second Affiliated Hospital of Harbin Medical University for
providing human laryngeal tissue samples. The present study was
supported by grants from the National Science Foundation of China
(81241085); the Key Project of Natural Science Foundation of
Heilongjiang Province (ZD201215/H1302); the Research Fund for the
Doctoral Program of Higher Education of China (20102307110007) and
the Postdoctoral Science Foundation of Heilongjiang Province
(LBH-Z11087).
References
|
1
|
Inoue H and Randazzo PA: Arf GAPs and
their interacting proteins. Traffic. 8:1465–1475. 2007. View Article : Google Scholar
|
|
2
|
Buffart TE, Coffa J, Hermsen MA, et al:
DNA copy number changes at 8q11–24 in metastasized colorectal
cancer. Cell Oncol. 27:57–65. 2005.
|
|
3
|
Martin RK and Jackson TR: Centaurin β4 in
cancer. Biochem Soc Trans. 33:1282–1284. 2005.
|
|
4
|
Inoue H, Ha VL, Prekeris R and Randazzo
PA: Arf GTPase-activating protein ASAP1 interacts with Rab11
effector FIP3 and regulates pericentrosomal localization of
transferrin receptor-positive recycling endosome. Mol Biol Cell.
19:4224–4237. 2008. View Article : Google Scholar
|
|
5
|
Jian X, Brown P, Schuck P, et al:
Autoinhibition of Arf GTPase-activating protein activity by the BAR
domain in ASAP1. J Biol Chem. 284:1652–1663. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu Y, Loijens JC, Martin KH, Karginov AV
and Parsons JT: The association of ASAP1, an ADP ribosylation
factor-GTPase activating protein, with focal adhesion kinase
contributes to the process of focal adhesion assembly. Mol Biol
Cell. 13:2147–2156. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu Y, Yerushalmi GM, Grigera PR and
Parsons JT: Mislocalization or reduced expression of Arf
GTPase-activating protein ASAP1 inhibits cell spreading and
migration by influencing Arf1 GTPase cycling. J Biol Chem.
280:8884–8892. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Brown MT, Andrade J, Radhakrishna H, et
al: ASAP1, a phospholipid-dependent arf GTPase-activating protein
that associates with and is phosphorylated by Src. Mol Cell Biol.
18:7038–7051. 1998.PubMed/NCBI
|
|
9
|
Bharti S, Inoue H, Bharti K, et al:
Src-dependent phosphorylation of ASAP1 regulates podosomes. Mol
Cell Biol. 27:8271–8283. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
King FJ, Hu E, Harris DF, Sarraf P,
Spiegelman BM and Roberts TM: DEF-1, a novel Src SH3 binding
protein that promotes adipogenesis in fibroblastic cell lines. Mol
Cell Biol. 19:2330–2337. 1999.PubMed/NCBI
|
|
11
|
Kam JL, Miura K, Jackson TR, et al:
Phosphoinositide-dependent activation of the ADP-ribosylation
factor GTase-activating protein ASAP1. Evidence for the pleckstrin
homology domain functioning as an allosteric site. J Biol Chem.
275:9653–9663. 2000. View Article : Google Scholar
|
|
12
|
Randazzo PA, Andrade J, Miura K, et al:
The Arf GTPase-activating protein ASAP1 regulates the actin
cytoskeleton. Proc Natl Acad Sci USA. 97:4011–4016. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nie Z and Randazzo PA: Arf GAPs and
membrane traffic. J Cell Sci. 119:1203–1211. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Donaldson JG and Jackson CL: ARF family G
protein and their regulators: roles in membrane transport,
development and disease. Nat Rev Mol Cell Biol. 12:362–375. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Onodera Y, Hashimoto S, Hashimoto A, et
al: Expression of AMAP1, an ArfGAP, provides novel targets to
inhibit breast cancer invasive activities. EMBO J. 24:963–973.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Muller T, Stein U and Poletti A: ASAP1
promotes tumor cell motility and invasiveness, stimulates
metastasis formation in vivo, and correlates with poor survival in
colorectal cancer patients. Oncogene. 29:2393–2403. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ehlers JP, Worley L, Onken MD and Harbour
JW: DDEF1 is located in an amplified region of chromosome 8q
and is overexpressed in uveal melanoma. Clin Cancer Res.
11:3609–3613. 2005. View Article : Google Scholar
|
|
18
|
Lin D, Watahiki A, Bayani J, et al:
ASAP1, a gene at 8q24, is associated with prostate cancer
metastasis. Cancer Res. 68:4352–4359. 2008. View Article : Google Scholar
|
|
19
|
Randazzo PA, Inoue H and Bharti S: Arf
GAPs as regulators of the actin cytoskeleton. Biol Cell.
99:583–600. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Furman C, Short SM, Subramanian RR, Zetter
BR and Roberts TM: DEF-1/ASAP1 is a GTPase-activating protein (GAP)
for ARF1 that enhances cell motility through a GAP-dependent
mechanism. J Biol Chem. 277:7962–7969. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bar-Sagi D and Hall A: Ras and Rho
GTPases: a family reunion. Cell. 103:227–238. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wagner AC and Williams JA: Low molecular
weight GTP-binding proteins: molecular switches regulating diverse
cellular functions. Am J Physiol. 266:G1–G14. 1994.PubMed/NCBI
|
|
23
|
Waschke J, Burger S, Curry FR, Drenckhahn
D and Adamson RH: Activation of Rac-1 and Cdc42 stabilizes the
microvascular endothelial barrier. Histochem Cell Biol.
125:397–406. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lamarche N, Tapon N, Stowers L, et al: Rac
and Cdc42 induce actin polymerization and G1 cell cycle progression
independently of p65PAK and the JNK/SAPK MAP kinase cascade. Cell.
87:519–529. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang FC, Atkinson SJ, Gu Y, et al: Rac and
Cdc42 GTPases control hematopoietic stem cell shape, adhesion,
migration, and mobilization. Proc Natl Acad Sci USA. 98:5614–5618.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang M, Satchell L, Duhadaway JB,
Prendergast GC and Laury-Kleintop LD: RhoB links PDGF signaling to
cell migration by coordinating activation and localization of Cdc42
and Rac. J Cell Biochem. 112:1572–1584. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kaibuchi K, Kuroda S and Amano M:
Regulation of the cytoskeleton and cell adhesion by the Rho family
GTPases in mammalian cells. Annu Rev Biochem. 68:459–486. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Engers R, Springer E, Michiels F, Collard
JG and Gabbert HE: Rac affects invasion of human renal cell
carcinomas by up-regulating tissue inhibitor of metalloproteinases
(TIMP)-1 and TIMP-2 expression. J Biol Chem. 276:41889–41897. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Filipenko NR, Attwell S, Roskelley C and
Dedhar S: Integrin-linked kinase activity regulates Rac- and
Cdc42-mediated actin cytoskeleton reorganization via α-PIX.
Oncogene. 24:5837–5849. 2005.PubMed/NCBI
|
|
30
|
Fidler IJ: The pathogenesis of cancer
metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev
Cancer. 3:453–458. 2003.
|
|
31
|
Foley R, Hollywood D and Lawler M:
Molecular pathology of prostate cancer: the key to identifying new
biomarkers of disease. Endocr Relat Cancer. 11:477–488. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Miyata M, Raven JF, Baltzis D, Koromilas
AE and Sabe H: IRES-mediated translational control of AMAP1
expression during differentiation of monocyte U937 cells. Cell
Cycle. 7:3273–3281. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jackson TR, Brown FD, Nie Z, et al: ACAPs
are arf6 GTPase-activating proteins that function in the cell
periphery. J Cell Biol. 151:627–638. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kondo A, Hashimoto S, Yano H, Nagayama K,
Mazaki Y and Sabe H: A new paxillin-binding protein,
PAG3/Papα/KIAA0400, bearing an ADP-ribosylation factor
GTPase-activating protein activity, is involved in paxillin
recruitment to focal adhesions and cell migration. Mol Biol Cell.
11:1315–1327. 2000.PubMed/NCBI
|
|
35
|
Sabe H, Hashimoto S, Morishige M, et al:
The EGFR-GEP100-Arf6-AMAP1 signaling pathway specific to breast
cancer invasion and metastasis. Traffic. 10:982–993. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nam JM, Onodera Y, Mazaki Y, Miyoshi H,
Hashimoto S and Sabe H: CIN85, a Cbl-interacting protein, is a
component of AMAP1-mediated breast cancer invasion machinery. EMBO
J. 26:647–656. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Onodera Y, Nam JM, Hashimoto A, et al:
Rab5c promotes AMAP1-PRKD2 complex formation to enhance beta1
integrin recycling in EGF-induced cancer invasion. J Cell Biol.
197:983–996. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ridley AJ, Paterson HF, Johnston CL,
Diekmann D and Hall A: The small GTP-binding protein rac regulates
growth factor-induced membrane ruffling. Cell. 70:401–410. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chianale F, Cutrupi S, Rainero E, et al:
Diacylglycerol kinase-alpha mediates hepatocyte growth
factor-induced epithelial cell scatter by regulating Rac activation
and membrane ruffling. Mol Biol Cell. 18:4859–4871. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Nobes CD and Hall A: Rho, rac, and cdc42
GTPases regulate the assembly of multimolecular focal complexes
associated with actin stress fibers, lamellipodia, and filopodia.
Cell. 81:53–62. 1995. View Article : Google Scholar
|