Introduction
Esophageal squamous cell carcinoma (ESCC) is one of
the most aggressive gastrointestinal cancers. Despite significant
progress in adjuvant therapy, ESCC has the poorest prognosis, and
surgical resection still appears to be the only prospect for
long-term survival. The main influences on prognosis
post-esophagectomy are the depth of tumor invasion and the presence
of lymph node metastases, which often accompany even superficial
carcinomas. Unfortunately, at the time of diagnosis, more than 50%
of patients have either non-resectable tumors or visible lymph node
metastases (1). The invasion and
spread of malignant cells is a complex multi-step process that
involves degradation and reorganization of the extracellular matrix
(ECM) via the activation of different proteolytic systems (2). Proteolysis changes the structure and
mechanics of the extracellular scaffold to allow cell migration. It
also leads to the release of membrane-anchored cytokines (in
particular chemokines) and adhesion molecules, and growth factors
stored within the extracellular matrix components (2–4).
Dipeptidyl peptidase IV or CD26 (DPPIV) and
fibroblast activation protein-α (FAP-α or F19 cell surface antigen,
also known as seprase) are two of a six-member family of serine
proteases with post-proline dipeptidyl aminopeptidase activity
(5). In their active forms, both
enzymes are transmembrane homodimeric glycoprotein complexes with a
molecular weight of ~200 kDa. The FAP-α and DPPIV
genes are located close together on chromosome 2 (q23 and q24.3).
They share 52% amino acid sequence identity, but they differ in
their cellular and substrate specificity (5–7). DPPIV
is a ubiquitously distributed peptidase that releases a number of
biologically active peptides involved in cell growth, migration,
invasion, neovascularization or immune system activation (8). These features potentially make DPPIV
one of the key players in cancer pathogenesis. FAP-α is a peptidase
that cleaves larger proteins and similarly to DPPIV, shows a
collagen type I-specific gelatinase activity (9). FAP-α is selectively expressed by
myofibroblast-like cells of the tumor stroma, by fibrotic and
granulation tissues, and by several types of cancer cells (6,7).
As previously shown, matrix metalloproteinases
(MMPs), which are zinc- and calcium-dependent peptidases, are
crucial for the initiation and maintenance of ECM degradation. They
determine the aggressiveness of cancer cells. Membrane-type
metalloproteinase 1 (MT1-MMP/MMP-14) is a member of a
membrane-anchored MMP subfamily. It is characterized as an enzyme
marker of invadopodia (10). This
endopeptidase is synthesized as a 64-kDa pro-enzyme that undergoes
furin-catalyzed proteolytic cleavage to form an active 54-kDa
protein. It not only has the capacity to degrade ECM fibrillar
components, proteoglycans or cell surface receptors and cell
adhesion molecules, but also acts as a specific initiator of
zymogen activation of other MMPs, including metalloproteinase-2
(MMP-2/gelatinase A) and indirectly metalloproteinase-9
(MMP-9/gelatinase B) (11,12). MMP-2 and MMP-9, two members of the
gelatinase subfamily of MMPs, are considered to play a particular
role in the early steps of cancer cell invasion and tumor
vascularization since they cleave type IV collagen, laminin and
elastin, which are the major components of the basement membrane
(BM) (3). MMP-2 and MMP-9 are
highly homologous proteins, but they differ in their regulation of
expression and activation, post-translational modification such as
glycosylation, and substrate preferences. Similar to all soluble
MMPs, both gelatinases are secreted as inactive pro-enzymes. Their
latent forms can be activated in an autocatalytic reaction or
through cleavage by serine proteases or other MMPs (3). In addition to their ability to
directly degrade extracellular components, MMP-2 and MMP-9 are
known to affect cell signaling by releasing the active ectodomain
of fibroblast growth factor receptor 1 (FGFR-1) or by activating
transforming growth factor β1 (TGF-β1) (13,14).
MMP-2 and MMP-9 are secreted to the extracellular milieu but both
of them localize at the surface of tumor cells. They are able to
associate with the MT1-MMP/TIMP-2 complex, αvβ3 and α3β1 integrins,
and/or CD44, and thus are involved in local matrix degradation,
which enhances the invasive capacity of cancer cells (3,14,15).
As all of the above-mentioned proteases are involved
in metastasis and in particular in invadopodia organization, it is
crucial to better characterize the role of different
membrane-associated proteolytic systems in esophageal cancer
growth. To this end, we studied the expression of two serine
proteinases, FAP-α and DPPIV, and three metalloproteinases, MMP-2,
MMP-9 and MT1-MMP, in 24 primary ESCC tissues paired with
non-cancer tissues.
Materials and methods
Tissue samples and patients
Tissue samples were obtained from 24 patients (19
men and 5 women) with a mean age of 62 years (range, 51–74) who
underwent surgery or endoscopic examination of the esophagus in
order to diagnose esophageal carcinoma at the Department of
Gastrointestinal and General Surgery of Wroclaw Medical University
between 2009 and 2012. All of the patients had tumors classified as
squamous cell carcinomas. All of the tumors were diagnosed as T3 or
T4, N1–N3 and M0–M1 according to the TNM classification. All of the
patients had lost >10% of their body weight before the
diagnosis. They did not have any other serious diseases. All of the
cancer samples were paired with non-cancer tissue samples taken
from the distal area (5–10 cm from macroscopic changes) and
confirmed by histological examinations to be R0. In all cases, 3-
to 5-mg fragments of each tissue sample were placed in a lysis
reagent for total RNA extraction using a Qiagen RNeasy Mini kit.
The remainder of each tissue sample was frozen and stored at −22°C
until use for western blotting or zymography.
Table I lists the
methods used for the study of individual membrane-associated
proteases.
 | Table IMethods used for the study of the
expression level and the status of activation of the selected
membrane-associated proteases. |
Table I
Methods used for the study of the
expression level and the status of activation of the selected
membrane-associated proteases.
| Membrane-associated
proteases | RT-PCR | Western blotting | Gelatin
zymography |
|---|
| FAP-α | Yes | Yes | Yes |
| DPPIV | Yes | Yes | Yes |
| MT1-MMP | | Yes | |
| MMP-2 | | | Yes |
| MMP-9 | | | Yes |
The data are expressed as means ± standard error
(SE). The statistical analysis of the data was performed using
Student’s t-test. The correlation coefficient (r) was estimated in
order to analyze the correlations between the expression levels of
all of the evaluated proteins. Differences were considered
significant at p<0.05.
Reverse transcription-polymerase chain
reaction (PCR)
All the RT-PCR reactions were performed using a
one-step RT-PCR kit (Clontech Laboratories, Inc.), according to the
manufacturer’s instructions. Total RNA (1 μl) extracted from each
sample was added to a 20 μl final reaction volume of RT-PCR mix
containing 0.25 pmol of each specific primer. Reverse transcription
(RT) was performed for 1 h at 50°C and 5 min at 94°C. To amplify
the FAP-α transcripts, we used the primer pair
5′-gctggagctaagaatcccgttgttcg-3′ (sense) and
5′-tgcttggaggatagcttccaatgct-3′ (antisense). The FAP-α
amplification product length was 544 bp. To amplify the DPPIV
transcripts, we used the primers 5′-ggaagatggaactgcttagt ggcacg-3′
(sense) and 5′-tctcagccctttatcattcacgctgc-3′ (antisense). The
length of the DPPIV amplification product was 473 bp. The PCR
conditions were 30 sec at 94°C, 45 sec at 60°C and 1 min at 68°C
(30 cycles). To detect genomic DNA contamination, RT-free PCR
controls were included.
The PCR products were separated and visualized in 1%
agarose gel containing ethidium bromide, digitized and assessed by
densitometry using ImageJ software. For the semi-quantitative
analysis, the transcripts were related to co-amplified actin DNA, a
housekeeping control gene. The actin primers
5′-tacaatgagctgcgtgtggctccccccg-3′ (sense) and
5′-aatggtgatgacctggccgtcaggc-3′ (antisense), yielding a 479-bp
amplification product, were used under the same amplification
conditions. The pixel density of each individual PCR product was
calculated on the basis of the pixel density for the actin
transcripts, the values of which were equal to 1. Results are shown
as the ratio of cancer to paired normal tissue.
SDS/PAGE and western blotting
The total protein was extracted from frozen tissues
by homogenization 1:15 w/v in sample buffer (0.125 M Tris-HCl, pH
6.8, 10% glycerol, 4% SDS and 10% 2-mercaptoethanol). Homogenates
were centrifuged for 20 min at 14,000 × g. All the samples were
heated at 95°C for 10 min and electrophoresed in amounts of 10
μl/well through a 10% SDS polyacrylamide gel. After
electrophoresis, the samples were transferred at 350 mA for 1.5 h
to nitrocellulose membranes (Trans-Blot Transfer Medium; Bio-Rad
Laboratories). The membranes were blocked for 1 h with 5% non-fat
milk in PBS (pH 7.4) at room temperature and then incubated
overnight at 4°C with each primary antibody. The antibodies used
were: DPP4 antibody-C-terminal region rabbit polyclonal antibody
(ARP63319_P050; Aviva Systems Biology); MT-MMP-1 (H-72) rabbit
polyclonal antibody (sc-30074); FAP-α (C-19) goat polyclonal
antibody (sc-54539) (both from Santa Cruz Biotechnology, Inc.,
Santa Cruz, CA, USA) and seprase rabbit polyclonal antibody (N1N3)
(GTX102732; GeneTex, Inc.). Actin (I-19) goat polyclonal antibody
(sc-1616; Santa Cruz Biotechnology, Inc.) was used to ensure equal
loading of the proteins.
Immunodetection was performed using HRP-conjugated
donkey anti-goat IgGs (sc-2020) or donkey anti-rabbit (sc-2313)
(both from Santa Cruz Biotechnology, Inc.) antibodies and a
chemiluminescence kit (Pierce Biotechnology, Rockford, IL,
USA).
Semi-quantitative digital image analysis was
performed with ImageJ software to assess the integral density of
particular bands. The integral density of each protein band was
normalized to β-actin (housekeeping protein), the value of which
was equal to 1. Results are shown as the ratio of cancer to paired
normal tissue.
Zymography
Tissue extracts were prepared by homogenizing tissue
fragments 1:15 w/v in a sample buffer consisting of 62.5 mM
Tris-HCl (pH 6.8) with 10% glycerol, 2% SDS and 0.05% bromophenol
blue. After a 15-min incubation at room temperature, the
homogenates were centrifuged for 15 min at 14,500 × g. The
gelanolytic activity of the MMPs in the supernatants was determined
with substrate gel SDS-PAGE zymography. A total of 3 or 7 μl of
each of the non-reduced samples was loaded in 9% SDS-polyacrylamide
gels copolymerized with gelatin (2 mg/ml). After semi-native
electrophoresis, the enzymes were renaturated by washing SDS out
twice in 50 mM Tris-HCl (pH 7.5) with 2.5% Triton X-100 for 30 min
at room temperature. Then the gels were incubated for 20 h at 37°C
in 50 mM Tris-HCl (pH 7.5) containing 150 mM NaCl, 10 mM
CaCl2, 1 μM ZnCl2 and 0.05% Brij-35. An
incubation buffer containing 5 mM EDTA was used to identify
FAP-α/DPPIV activity. To visualize the proteolytic bands, the gels
were stained with 0.12% Coomassie blue and destained with a
solution containing 5% acetic acid and 10% ethanol in water. The
pixel intensity (in inversion) of the bands, both in their latent
and active forms, was determined densitometrically using an ImageJ
gel analyzer showing MMP-2 and MMP-9 activity. The result for each
patient was expressed as the ratio of cancer to normal tissue.
Results
FAP-α and DDPIV are overexpressed in the
ESCC tumor tissues
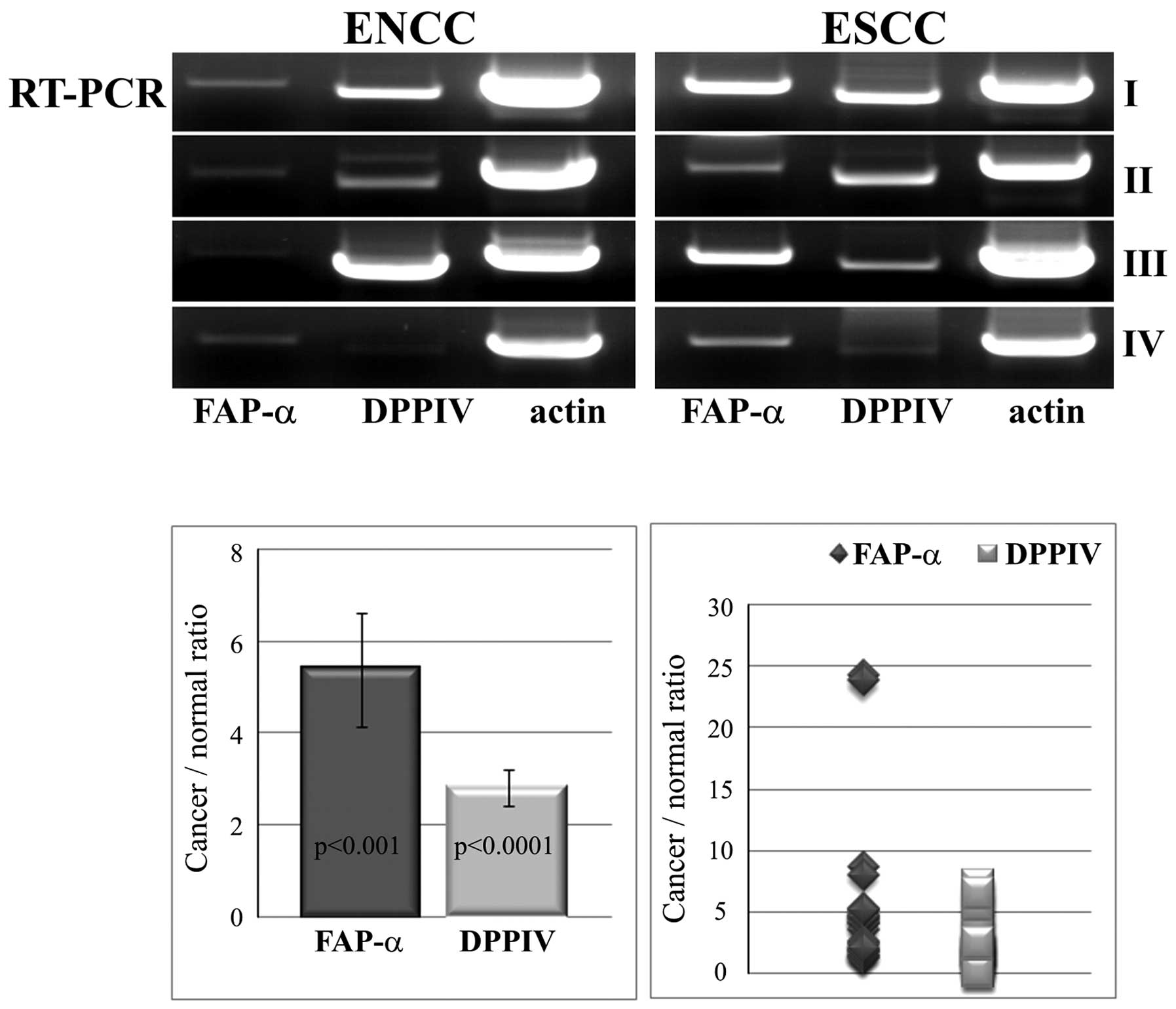
As shown in Fig. 1,
FAP-α amplification products corresponding to the predicted ones
were generated from all the tumor samples and notably, also from
all the non-cancer esophageal tissue samples. For the
semi-quantitative analysis, we co-amplified actin transcripts in
all of the samples under the same RT-PCR conditions. We found a
much lower amplification of FAP-α transcripts in the control in
comparison with the cancer tissue samples. The differences between
the groups were statistically significant (p<0.001). The mean
ratio of cancer to normal tissue was calculated to be 5.387±1.23
(range, 1.229–24.258).
DPPIV transcripts were found in all the analyzed
cancer and non-cancer tissue samples, with significantly higher
expression of DPPIV in the pathological samples than that in the
control samples (p<0.0001). However, the ratio of cancer to
normal tissue for DPPIV, calculated as 2.811±0.41 (range,
0.162–7.139), was not as high as for FAP-α (Fig. 1). Upregulation of DPPIV was found to
positively correlate with the FAP-α expression ratio (r=0.58,
p=0.002). No genomic DNA transcripts for FAP-α or DPPIV were found
in the RT-free PCR controls. The FAP-α and DPPIV transcripts were
verified by sequencing.
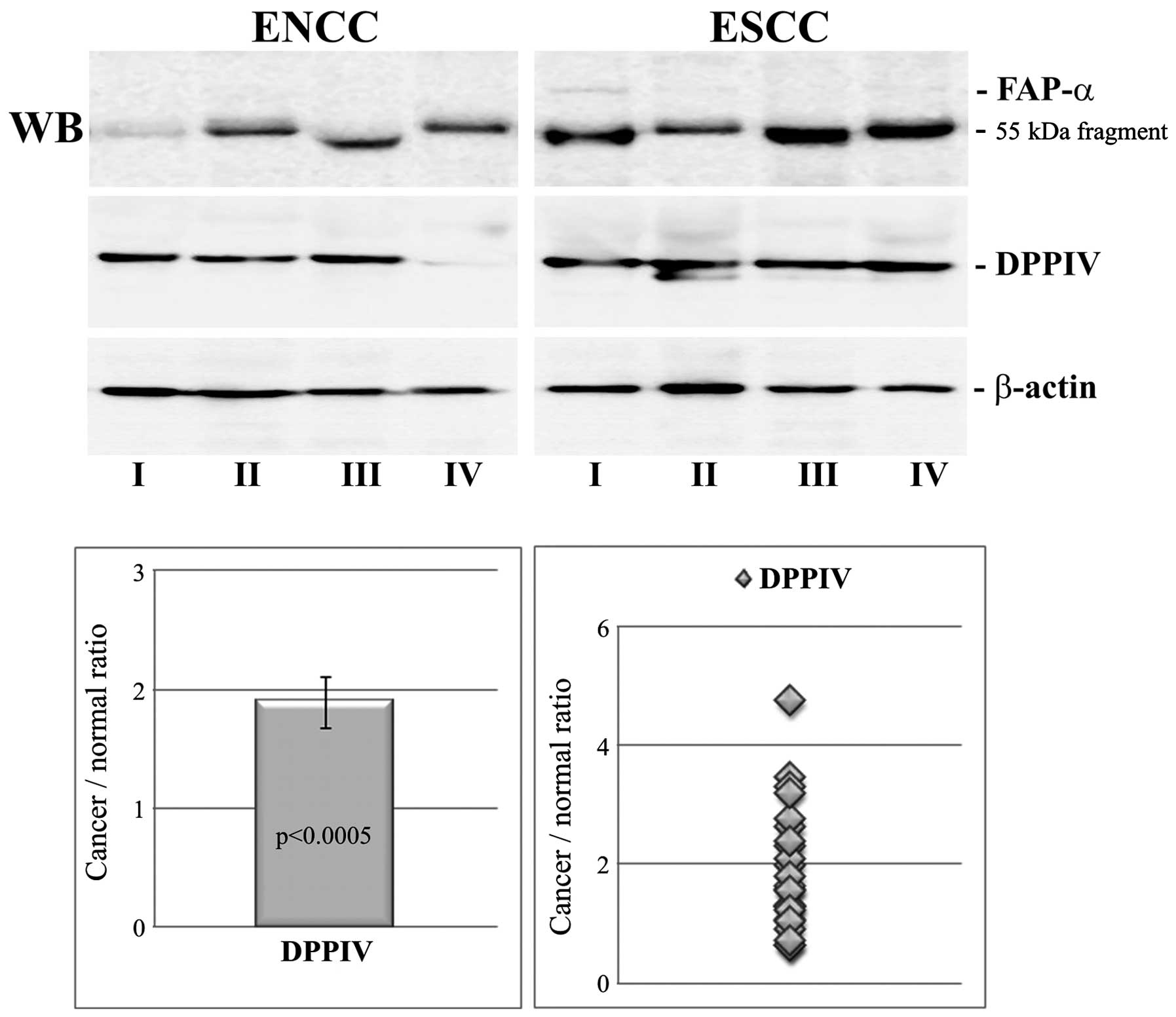
We used the western blotting technique to determine
the protease expression at the protein level. The results were
normalized to β-actin and are shown in Fig. 2. The ratio of cancer to normal
tissue for DPPIV was in the range of 0.63–4.75 with a mean value of
1.89±0.21. The increased level of DPPIV in ESCC was statistically
significant (p<0.0005) and was correlated positively with DPPIV
expression at the mRNA level (r=0.725, p=0.023).
The weak signal in immunoblots in the case of FAP-α
did not allow densitometric analysis to be performed. Of the 24
cancer tissue samples, only three were unequivocally immunoreactive
for full-length protein. All three FAP-α-positive cases are shown
in Fig. 2. In the remaining cases,
only proteolytic fragments of ~55 kDa were observed. Gelatin
zymography was also not sensitive enough to detect serine protease
activity in most of the tissue extracts.
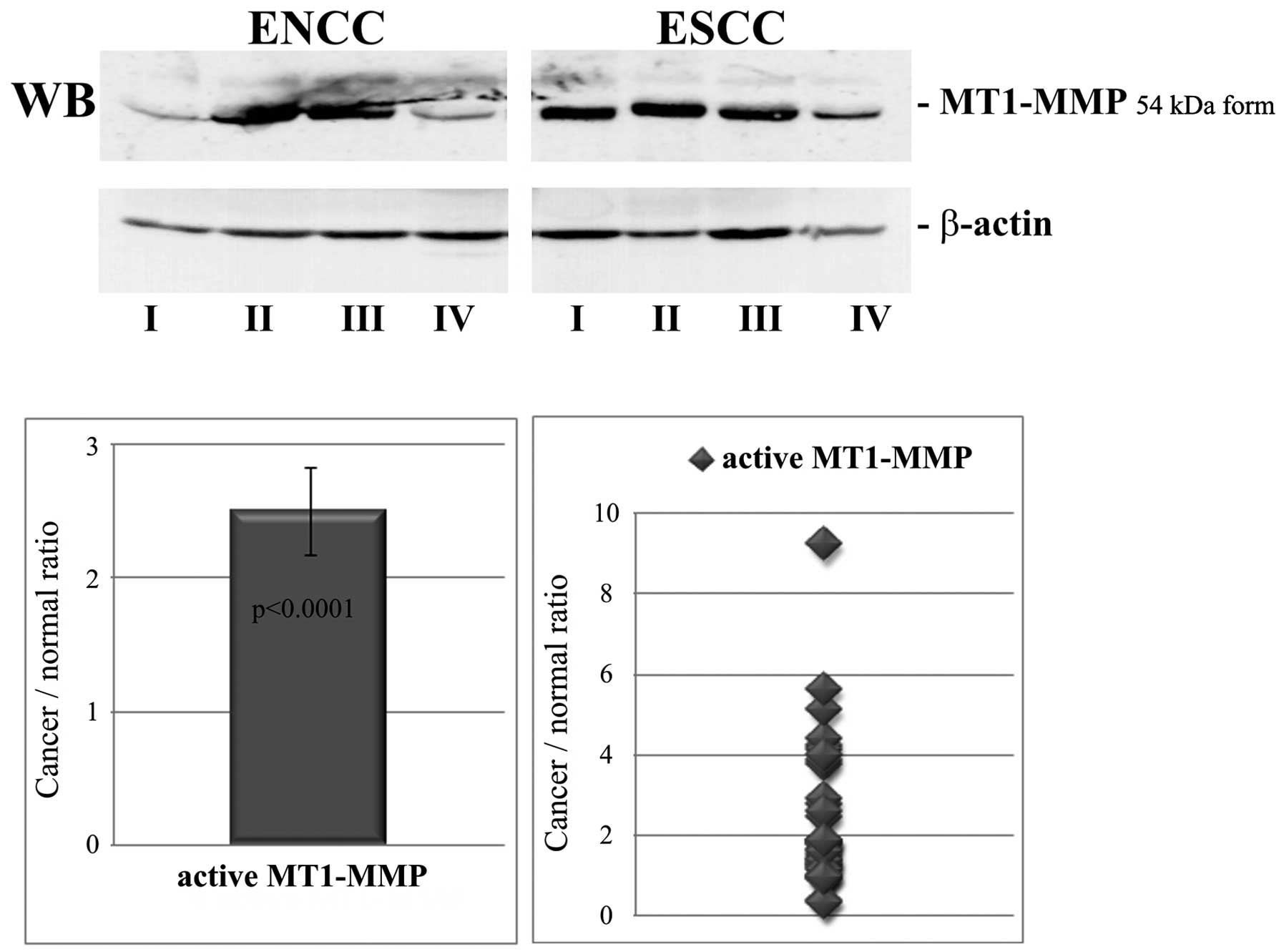
MT1-MMP, MMP-2 and MMP-9 are upregulated
in ESCC tumor tissues
The levels of the active 54-kDa form of MT1-MMP were
evaluated by western blot analysis after normalization to β-actin.
The results are shown in Fig. 3.
The ratio of cancer to normal tissue for activated MT1-MMP was in
the range of 0.33–9.25 with a mean value of 2.5±0.33. The increased
level of the MT1-MMP active form in ESCC was statistically
significant (p<0.0001).
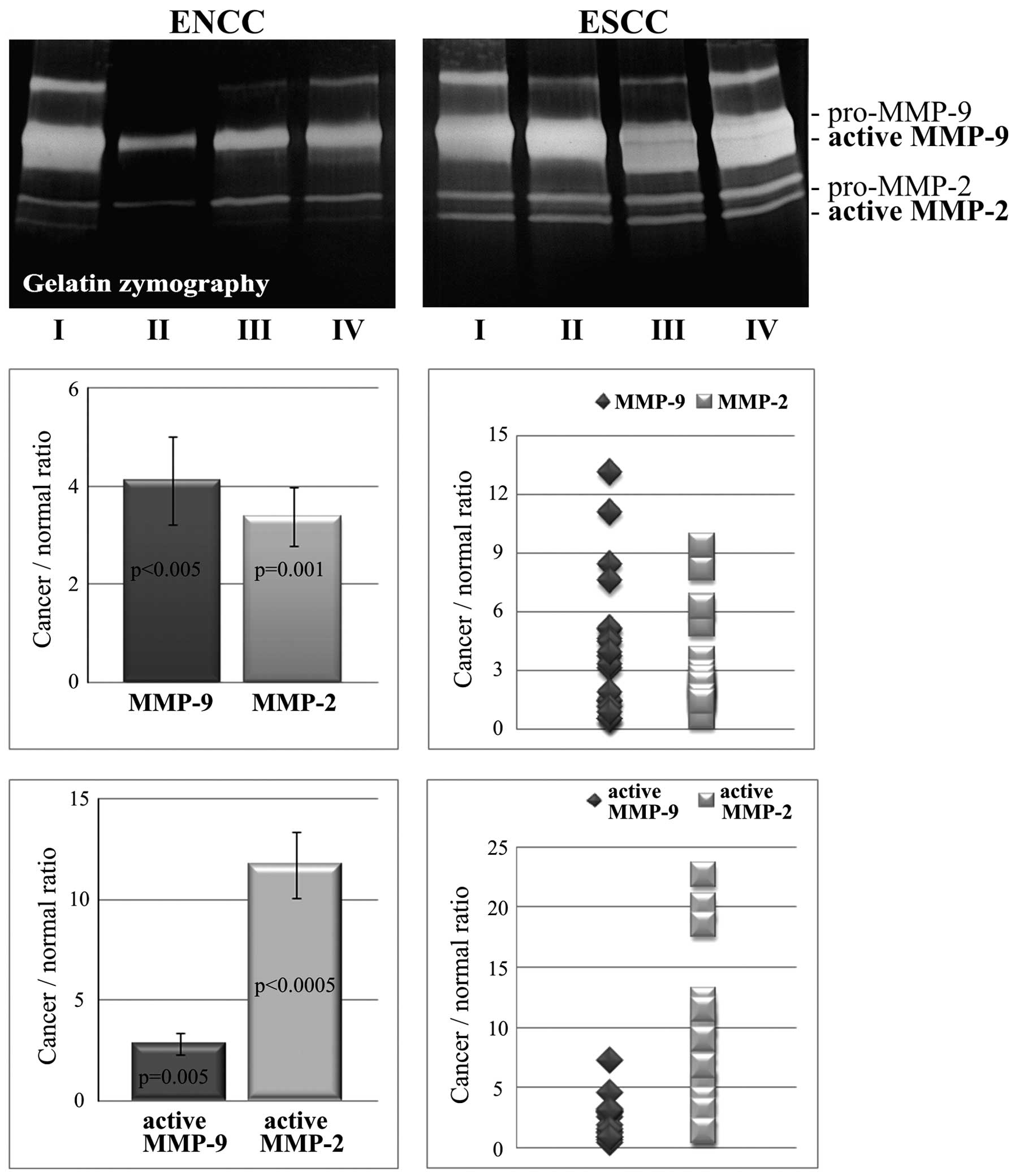
As shown in Fig. 4,
the values for the ratio of cancer to normal tissue calculated on
the basis of zymogram gels for total (active and latent form) MMP-2
(3.38±0.6; range, 0.68–9.4) and total (active and latent form)
MMP-9 (4.11±0.89; range, 0.49–13.12) were comparable. The levels of
the two gelatinases in the cancer tissues were significantly higher
than these levels in the non-cancer tissues (p=0.001 and
p<0.005, respectively). The relative increase in activity of
MMP-2 was not significantly different than the relative increase in
activity of MMP-9. However, the upregulation of the activities of
the two gelatinases was found to be positively correlated (r=0.819,
p=0.001). The ratio of cancer to normal tissue for the active MMP-2
form was 11.75±1.65 (range, 5.57–22.68) but only 2.85±0.52 (range,
0.46–7.28) for the active form of MMP-9. In ESCC, the increase in
gelatinase activity was statistically significant for MMP-2
(p<0.00005) and MMP-9 (p=0.005). A positive correlation was
found between the ratios calculated for the active form of
gelatinase A and MT1-MMP (correlation, r=0.93; p=0.007). Changes in
MMP-2 and MMP-9 activity were found to positively correlate with
the FAP-α and DPPIV expression levels (correlation, r=0.9;
p<0.05).
Discussion
Cancer invasion and the formation of metastases are
based on the ability of cells to degrade and remodel the
extracellular scaffold. Deregulation of protease expression and
activity has been reported in a large number of epithelial cancers,
including tumors of the esophagus (16,17).
The participation of proteases in tumor development and progression
not only involves their direct participation in extracellular
matrix degradation but also in maintaining communication between
tumor cells and the microenvironment.
FAP-α and DPPIV are cell surface glycoproteins that
act as post-proline-specific peptidases. They are highly expressed
in areas of active tissue remodeling. Since many biologically
active peptides contain a conserved proline residue as a
proteolytic-processed regulatory site, FAP-α and DPPIV may be
recognized as important regulators of extracellular signaling. In
addition to their typical dipeptidyl peptidase activity, FAP-α and
DPPIV possess collagenolytic activity. Due to their cellular
location, they are known to be proteases associated with sites of
cell adherence to the ECM (6,9).
The variety of functions included in the range of
DPPIV activity indicates that the mechanisms by which DPPIV affects
the behavior of tumor cells can vary in different types of cancer,
even resulting in the opposite roles. It was shown that DPPIV
overexpression in ovarian cancer cells induces downregulation of
MT1-MMP and MMP-2 and upregulates tissue inhibitors of
metalloproteases (TIMPs), leading to the suppression of the
invasive potential of the cells (18,19).
Several studies also indicated that the loss of expression of DPPIV
was linked with malignant transformation and tumor progression. On
the other hand, increased expression of DPPIV was reported in
prostate and thyroid carcinomas (20,21).
We found that the average 3-fold increase in DPPIV
gene expression and an ~2-fold protein level increase in ESCC over
the controls was positively correlated with the intensity of MMP-2
and MMP-9 activity. DPPIV was present in all of the normal and
cancer tissues. Goscinski et al investigated the level of
DPPIV expression in ESCC via immunohistochemistry (22). They observed positive staining for
DPPIV in the majority of cancer tissues. They did not find DPPIV
immunoreactivity in the normal esophageal epithelium. Mentlein
et al showed that various types of cells in healthy tissue,
such as certain fibroblasts, endothelial cells or activated T or NK
cells, express high levels of DPPIV (23). Therefore, the presence of DPPIV in
non-cancer tissues was not surprising.
In the present study, using RT-PCR, we found an
average 5-fold lower level of FAP-α expression in the normal
non-cancer tissues than that in the cancer cases, but all of the
studied controls were FAP-α mRNA-positive. It is accepted that
FAP-α is not expressed by cells of mature somatic tissues, except
by the myofibroblasts of granulation or fibrotic tissues or
myofibroblast-like cells of tumor stroma (7). In esophageal cancers, intense FAP-α
immunostaining has already been observed (24). The positive immune reaction was also
found in stroma adjacent to cancer foci and even in areas adjacent
to dysplastic changes. However, FAP-α was not detected in the
microscopic normal epithelium of the esophagus. In fact,
immunohistochemistry is much less sensitive than molecular
techniques. Therefore, the open question is whether or not the
presence of FAP-α in the surgical margin may be evidence supporting
the view that the stromal changes extend much farther into the
surrounding tumor tissues than is usually considered. Alterations
in the stromal environment are known to precede cancer invasion and
are closely associated with myofibroblast-like cells. One of the
major markers of myofibroblasts is α-smooth muscle actin (α-SMA)
(25). We estimated the expression
of α-SMA in ESCC and paired non-cancer tissues (data not shown) but
these data did not correlate with the FAP-α expression level.
MT1-MMP is a key mediator in ECM proteolysis
(4,10). MT1-MMP was the first membrane MMP
discovered on the surface of invasive cells. Various authors
demonstrated that MT1-MMP is overexpressed in different cancer cell
lines and tumor tissue types (26,27).
It was also shown that specific MT1-MMP downregulation suppresses
the migration and invasion of cancer cells (28). In the present study, we found a much
higher level of the active MT1-MMP form in cancers relative to
paired non-cancer esophageal tissues. The correlation between the
level of the MT1-MMP active form and the MMP-2 activity found in
the present study shows a tight connection between the two enzymes
and concurs with the data in previous reports describing the role
of MT1-MMP as an MMP-2 activator (29). A lack of correlation between
activated MT1-MMP and the remaining proteases may suggest that
MT1-MMP is not directly involved in the mechanisms of their
expression and/or activation in ESCC.
Upregulation of MMP-2 and MMP-9 expression was
observed in almost all human cancers and was found to significantly
correlate with the histological grade of the cancer, with
metastases and with patient survival (17,30).
Therefore, the active contribution of both gelatinases to cancer
progression is evident. In the present study, we also found the
enhanced synthesis of both MMP-2 and MMP-9 in cancer tissues. The
most significant result of our analysis was the extremely high
increase in MMP-2 activity in ESCC tissues. It was relatively
higher than that for MMP-9. Despite the differences in activation,
the ratio of cancer to normal tissues of the two active gelatinases
correlated positively with the expression levels of both serine
proteases. This suggests that MMP-2, MMP-9, FAP-α and DPPIV may
function coordinately to regulate the behavior of cancer cells.
In summary, this study demonstrated that different
membrane-associated proteolytic systems, including transmembrane
proteases, such as FAP-α, DPPIV and MT1-MMP, and the
membrane-attached matrix metalloproteinases, such as MMP-2 and
MMP-9, are highly altered in ESCC. The positive correlation between
the expression of serine proteases and the activity of both
gelatinases indicates that all these proteolytic systems may be
tightly linked to each other and may collectively improve focal ECM
degradation, which facilitates cancer cell invasion and
metastasis.
References
|
1
|
Ilson DH: Oesophageal cancer: new
developments in systemic therapy. Cancer Treat Rev. 29:525–532.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ellis V and Murphy G: Cellular strategies
for proteolytic targeting during migration and invasion. FEBS Lett.
506:1–5. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Björklund M and Koivunen E:
Gelatinase-mediated migration and invasion of cancer cells. Biochim
Biophys Acta. 1755:37–69. 2005.PubMed/NCBI
|
|
4
|
Poincloux R, Lizárraga F and Chavrier P:
Matrix invasion by tumour cells: a focus on MT1-MMP trafficking to
invadopodia. J Cell Sci. 122:3015–3024. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen WT and Kelly T: Seprase complexes in
cellular invasiveness. Cancer Metastasis Rev. 22:259–269. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kelly T: Fibroblast activation protein-α
and dipeptidyl peptidase IV (CD26): cell-surface proteases that
activate cell signaling and are potential targets for cancer
therapy. Drug Resist Updat. 8:51–58. 2005.
|
|
7
|
O’Brien P and O’Connor BF: Seprase: an
overview of an important matrix serine protease. Biochim Biophys
Acta. 1784:1130–1145. 2008.PubMed/NCBI
|
|
8
|
Boonacker E and Van Noorden CJ: The
multifunctional or moonlighting protein CD26/DPPIV. Eur J Cell
Biol. 82:53–73. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Baum O, Reutter W and Bermpohl F:
Structure-function relationship of DPP IV: insights into its
dimerisation and gelatinase activity. Adv Exp Med Biol. 524:19–27.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Itoh Y: MT1-MMP: a key regulator of cell
migration in tissue. IUBMB Life. 58:589–596. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Murphy G, Stanton H, Cowell S, et al:
Mechanisms for pro matrix metalloproteinase activation. APMIS.
107:38–44. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Toth M, Chvyrkova I, Bernardo MM, et al:
Pro-MMP-9 activation by the MT1-MMP/MMP-2 axis and MMP-3: role of
TIMP-2 and plasma membranes. Biochem Biophys Res Commun.
308:386–395. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Levi E, Fridman R, Miao HQ, et al: Matrix
metalloproteinase 2 releases active soluble ectodomain of
fibroblast growth factor receptor 1. Proc Natl Acad Sci USA.
93:7069–7074. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Egeblad M and Werb Z: New functions for
the matrix metalloproteinases in cancer progression. Nat Rev
Cancer. 2:161–174. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Monsky WL, Kelly T, Lin CY, et al: Binding
and localization of Mr 72,000 matrix metalloproteinase
at cell surface invadopodia. Cancer Res. 53:3159–3164.
1993.PubMed/NCBI
|
|
16
|
Etoh T, Inoue H, Yoshikawa Y, et al:
Increased expression of collagenase-3 (MMP-13) and MT1-MMP in
oesophageal cancer is related to cancer aggressiveness. Gut.
47:50–56. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Roh MR, Zheng Z, Kim HS, et al:
Differential expression patterns of MMPs and their role in the
invasion of epithelial premalignant tumors and invasive cutaneous
squamous cell carcinoma. Exp Mol Pathol. 92:236–242. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kajiyama H, Kikkawa F, Khin E, et al:
Dipeptidyl peptidase IV overexpression induces up-regulation of
E-cadherin and tissue inhibitors of matrix metalloproteinases,
resulting in decreased invasive potential in ovarian carcinoma
cells. Cancer Res. 63:2278–2283. 2003.
|
|
19
|
Kikkawa F, Kajiyama H, Shibata K, et al:
Dipeptidyl peptidase IV in tumor progression. Biochim Biophys Acta.
1751:45–51. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kotani T, Aratake Y, Ogata Y, et al:
Expression of dipeptidyl aminopeptidase IV activity in thyroid
carcinoma. Cancer Lett. 57:203–208. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wilson MJ, Ruhland AR, Quast BJ, et al:
Dipeptidylpeptidase IV activities are elevated in prostate cancers
and adjacent benign hyperplastic glands. J Androl. 21:220–226.
2000.PubMed/NCBI
|
|
22
|
Goscinski MA, Suo ZH, Nesland JM, et al:
Dipeptidyl peptidase IV expression in cancer and stromal cells of
human esophageal squamous cell carcinomas, adenocarcinomas and
squamous cell carcinoma cell lines. APMIS. 116:823–831. 2008.
View Article : Google Scholar
|
|
23
|
Mentlein R, Hattermann K, Hemion C, et al:
Expression and role of the cell surface protease seprase/fibroblast
activation protein-α (FAP-α) in astroglial tumors. Biol Chem.
392:199–207. 2011.PubMed/NCBI
|
|
24
|
Goscinski MA, Suo ZH, Nesland JM, et al:
Seprase, dipeptidyl peptidase IV and urokinase-type plasminogen
activator expression in dysplasia and invasive squamous cell
carcinoma of the esophagus. A study of 229 cases from Anyang Tumor
Hospital, Henan Province, China. Oncology. 75:49–59. 2008.
View Article : Google Scholar
|
|
25
|
Yamashita M, Ogawa T, Zhang X, et al: Role
of stromal myofibroblasts in invasive breast cancer: stromal
expression of alpha-smooth muscle actin correlates with worse
clinical outcome. Breast Cancer. 19:170–176. 2012. View Article : Google Scholar
|
|
26
|
Jiang WG, Davies G, Martin TA, et al:
Expression of membrane type-1 matrix metalloproteinase, MT1-MMP in
human breast cancer and its impact on invasiveness of breast cancer
cells. Int J Mol Med. 17:583–590. 2006.PubMed/NCBI
|
|
27
|
Adley BP, Gleason KJ, Yang XJ and Stack
MS: Expression of membrane type 1 matrix metalloproteinase (MMP-14)
in epithelial ovarian cancer: high level expression in clear cell
carcinoma. Gynecol Oncol. 112:319–324. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ueda J, Kajita M, Suenaga N, et al:
Sequence-specific silencing of MT1-MMP expression suppresses tumor
cell migration and invasion: importance of MT1-MMP as a therapeutic
target for invasive tumors. Oncogene. 22:8716–8722. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nakamura H, Ueno H, Yamashita K, et al:
Enhanced production and activation of progelatinase A mediated by
membrane-type 1 matrix metalloproteinase in human papillary thyroid
carcinomas. Cancer Res. 59:467–4573. 1999.PubMed/NCBI
|
|
30
|
Rosenthal EL and Matrisian LM: Matrix
metalloproteases in head and neck cancer. Head Neck. 28:639–648.
2006. View Article : Google Scholar : PubMed/NCBI
|