Introduction
Medulloblastoma (MB), a cancer of the cerebellum, is
the most common malignant embryonal neuroepithelial tumor in
children. It is one of the leading causes of morbidity and
mortality in pediatric cancer (1).
Recently, the survival rates have shown improvement; yet, patients
undergoing current treatment regimes suffer from serious
therapy-related side-effects, such as loss of hearing and cognitive
impairment (2). Due to its high
mortality rate and the post-treatment disabilities of current
therapies, there is an urgent need for the development of non-toxic
and efficient agents for MB.
Curcumin (diferuloymethane,
C21H20O6), a yellow natural
product, is a non-toxic, major component of the spice turmeric
derived from the plant Curcumin longa (3). It has shown a wide range of
pharmacological activities including anti-inflammatory,
anti-oxidative, immunomodulating, anti-atherogenic and
anticarcinogenic effects (4,5). For
the last few decades, in vivo and in vitro studies
have demonstrated the ability of curcumin to effectively inhibit
tumor growth (6). This antitumor
capacity of curcumin is associated with its property to interact
with a wide variety of signaling pathways and the ability to
regulate their activity (5).
However, the precise molecular mechanisms of curcumin-mediated
inhibition of tumor growth need to be elucidated.
Recent studies have shown that carcinogenesis and
progression of MB are related to multiple molecular dysfunctions.
The major molecular pathways known to have important roles in
cerebellum development, include Wnt/β-catenin, Sonic Hedgehog
(SHH), Notch and Akt/nuclear factor-κB (NF-κB) (7,8). The
Wnt signaling pathway contributes to the control of processes
involved in embryonic development including cell proliferation,
differentiation and oncogenesis (9). β-catenin is a key activator of the
canonical Wnt signaling pathway. In the absence of Wnt stimulation,
β-catenin is complexed with adenomatosis polyposis coli (APC) and
scaffold protein axis inhibition protein (Axin), and is
phosphorylated by glycogen synthase kinase 3β (GSK-3β), leading to
ubiquitination and proteasome-dependent degradation. In the
presence of Wnt stimulation, Wnt ligands bind to the family of cell
surface Frizzled receptors and low density lipoprotein
receptor-related protein (LRP), resulting in an intracellular
cascade allowing β-catenin release from phosphorylation by GSK-3β
and degradation by proteosome. Then the accumulated β-catenin
translocates into the nucleus and binds to the T cell
factor/lymphoid enhancer factor (TCF/LEF) transcription factors
inducing unabated transcription of several oncogenes, including
cyclin D1 and c-Myc, resulting in enhanced cellular proliferation
(10). Aberrant expression of
Wnt/β-catenin signaling components and inappropriate activation of
Wnt signaling have been found in a variety of human cancers
(11). Mutations in Wnt signaling
complex have been identified in MB, accounting for 25% of sporadic
MB (12). These include activating
mutations in CTNNB1 (which codes for β-catenin) and inactivating
mutations in APC and Axin (13,14).
In fact, the abnormal accumulation and location of β-catenin play a
crucial role in all of these mutations. Thus, inhibiting the
expression and nuclear translocation of β-catenin can be a
potential therapy for the treatment of MB.
In the present study, we investigated the effect of
curcumin on the proliferation of DAOY cells, and demonstrate its
inhibitory effect on the Wnt/β-catenin signaling pathway.
Therefore, we hypothesized that curcumin inhibits cell
proliferation by increasing the activity of GSK-3β and by
inhibiting the Wnt/β-catenin signaling pathway through nuclear
β-catenin loss.
Materials and methods
Chemicals
Minimum Essential Medium (MEM/EBSS), fetal bovine
serum (FBS) and penicillin-streptomycin solution were purchased
from HyClone Laboratories (South Logan, UT, USA). OPTI-MEM was
purchased from Life Technologies (Carlsbad, CA, USA). Curcumin
[1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-hepatadiene-3,5-dione;
diferuloymethane] and MTT
[3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] were
purchased from Sigma-Aldrich (Bangalore, India).
Cell culture
The medulloblastoma cell line DAOY was a generous
gift from Dr Xiuwu Bian (Institute of Pathology and Southwest
Cancer Center, Southwest Hospital, Third Military Medical
University, Chongqing, China). Cells were cultured in MEM
containing 10% FBS, 2 mM glutamine, 100 μg/ml streptomycin, and 100
U/ml penicillin and aseptically grown at 37°C in a humidified
incubator containing 5% CO2.
Antibodies
Rabbit anti-human GSK-3β antibody (27C10; Cell
Signaling Technology, Danvers, MA, USA), rabbit anti-human
phospho-GSK-3β (Ser9) antibody (5B3; Cell Signaling Technology),
rabbit anti-human β-catenin monoclonal antibody (1247-1; Epitomics
Inc., Burlingame, CA, USA), rabbit anti-human phospho-β-catenin
(Ser37) antibody (11219-2; Signalway Antibody, College Park, MD,
USA), rabbit anti-human cyclin D1 monoclonal antibody (2261-1;
Epitomics) and rabbit anti-human histone H2 monoclonal antibody
(3522-1; Epitomics) were used to detect the expression of the
corresponding proteins. Rabbit anti-human β-actin polyclonal
antibody (CW0097; CWBIO, Beijing, China) was used as an internal
control. Peroxidase conjugated goat anti-rabbit IgG (GAR007;
MultiSciences Biotech Co.) and fluorescein-conjugated Affinipure
goat anti-rabbit IgG (ZF-0311; ZSGB-BIO, Beijing, China) were used
as secondary antibodies.
Cell proliferation assay
The effect of curcumin on cell proliferation was
assessed by MTT assay as previously described (15). DAOY cells were seeded in triplicate
in 96-well plates at a density of 5,000/well and incubated
overnight. Curcumin was dissolved in dimethyl sulfoxide (DMSO) and
diluted in MEM. The cells were treated with varying concentrations
(0, 20, 40, 60, 80 and 100 μM) of curcumin for 24, 48 and 72 h.
After the indicated times, 10 μl of the MTT solution was added to
each well, and the plates were incubated for 4 h at 37°C. The
absorbance in individual wells was quantified using an
enzyme-linked immunosorbent assay (ELISA) reader at 595 nm.
Cell cycle analysis as detected by flow
cytometry
In order to evaluate the effect of curcumin on the
cell cycle, DAOY cells were treated with curcumin at a dose of 30
μM for different time periods (12, 24 and 48 h). After the
treatment, the cells were harvested by trypsinization, washed twice
with ice-cold PBS, fixed with ice-cold 70% ethanol and maintained
overnight at −20°C. DNA was stained with 100 μg/ml propidium iodide
(PI) solution. The cell cycle distribution was analyzed by flow
cytometry.
Immunofluorescence
For immunofluorescence, DAOY cells were cultured on
glass coverslips in a 6-well plate and treated with curcumin (30
μM) for 48 h. Thereafter, the cells were washed with PBS
[phosphate-buffered saline (0.01 M, pH 7.2)] and fixed in 4%
paraformaldehyde for 15 min at room temperature. The coverslips
were washed and permeablized for 10 min in 0.5% Triton X-100
followed by blocking with normal serum for 30 min at room
temperature. The cells were then incubated with the anti-β-catenin
antibody (1:100) overnight at 4°C. Expression of β-catenin was
evaluated using FITC-conjugated goat anti-rabbit antibody (1:100)
and the cells were incubated for 1 h at 37°C. Subsequently, cells
were washed again with PBS and observed under a confocal laser
microscope.
Western blot analysis
For protein analysis, DAOY cells were grown and
treated with curcumin (30 μM) for 48 h. The cells were washed with
PBS and then lysed in RIPA buffer [150 mM of NaCl, 1 mM of EDTA, 1%
Nonidet P-40, 0.5% sodium deoxycolate, 0.1% SDS, 50 mM of Tris-HCl
(pH 7.5)], containing protease inhibitors. Lysates were clarified
by centrifugation (14,000 × g for 15 min at 4°C); the supernatant
was removed and stored at −80°C. Protein concentration was
determined by BCA (bicinchoninic acid) and lysates were
electrophoretically resolved on an 8–12% SDS-PAGE and transferred
onto a PVDF membrane. After blocking with 5% non-fat milk in
Tris-buffered saline with 0.1% Tween-20 (TBST), membrances were
incubated with respective primary antibodies directed against
GSK-3β (1:1,000), p-GSK-3β (Ser9) (1:1,000), β-catenin (1:1,000),
p-β-catenin (Ser37) (1:300), cyclin D1 (1:1,000) and β-actin
(1:3,000) at 4°C overnight. Membranes were washed and then
incubated with HRP-conjugated rabbit anti-IgG (1:5,000) for 1 h at
room temperature. Protein bands were assessed by enhanced
chemiluminescence system (ECL; KeyGen, Nanjing, China) and
quantitated using Quantitive One Image Analysis. The specific
western blot process was performed according to the study by Wang
et al (16).
RT-PCR
Total RNA was extracted from DAOY cells using the
TRIpurer Reagent (Bioteke Corp., Beijing, China). The RNA (200 ng)
was reverse transcribed (RT) into single-stranded cDNA using
BioTeke Super RT kit according to the manufacturer’s instructions
(Bioteke Corp.). PCR amplification of target cDNAs, and an internal
control (GAPDH) cDNA, was carried out using the specific primer
pairs. The PCR cycling conditions used included a denaturation step
at 94°C for 3 min; 30 cycles of denaturation at 94°C for 30 sec,
annealing at 58°C for 30 sec and extension at 72°C for 30 sec; and
a final extension at 72°C for 5 min. The PCR products were
separated by electrophoresis on a 1.5% agarose gel at 100 V for 30
min. The sequences of the primers were as follow: GSK-3β (261 bp)
forward, ATTTCCAGGGGATAGTGGTGTG and reverse,
GTTAGTCGGGCAGTTGGTGTAT. β-catenin (305 bp) forward,
GAACTGTCTTTGGACTCTCAGG and reverse, CCATCTCTGCTTCTTGGTGTC; cyclin
D1 (175 bp) forward, CGGAGGAGAACAAACAGATCT and reverse, AGGCGGT
AGTAGGCGGGT; GAPDH (542 bp) forward, GAGCCAA AAGGGTCATCATCTC and
reverse, AAAGGTGGAGGA GTGGGTGTC.
Silencing of β-catenin by siRNA
The expression of β-catenin in DAOY cells was
blocked using synthetic siRNA. The cells were plated in 6-well
plates at a density of 3×105 cells/well and grown for 24
h. The cells were transfected according to the manufacturer’s
directions using 200 pM synthetic siRNA duplexes (sense
5-GGACACAGCAGCAAUUUGUTT, antisense 5′-ACAAAUUGCUGCUGUGUCCTT;
GenePharma) or negative control sense siRNA (sense 5′-UUCUCCGAACG
UGUCACGUTT and antisense 5′-ACGUGACACGUUCGGA GAATT; GenePharma) for
24 h to silence β-catenin expression or using Lipofectamine 2000
reagent (Invitrogen, Carlsbad, CA, USA). Then DAOY cells were grown
for 6 h, and washed once with PBS and switched to 10% FBS media.
After 24 h, plates were replaced with 10% FBS media or curcumin (30
μM). Cell viability analysis was performed 48 h after
treatment.
Statistical analysis
All data are expressed as means ± SD; the
homogeneity test for variance was evaluated using the SPSS 17.0
software. The significance of difference between the groups was
analyzed using two-way ANOVA test or the two-tailed unpaired
Student’s t-test. A P-value <0.05 was considered to indicate a
statistically significant result.
Results
Curcumin inhibits medulloblastoma cell
proliferation
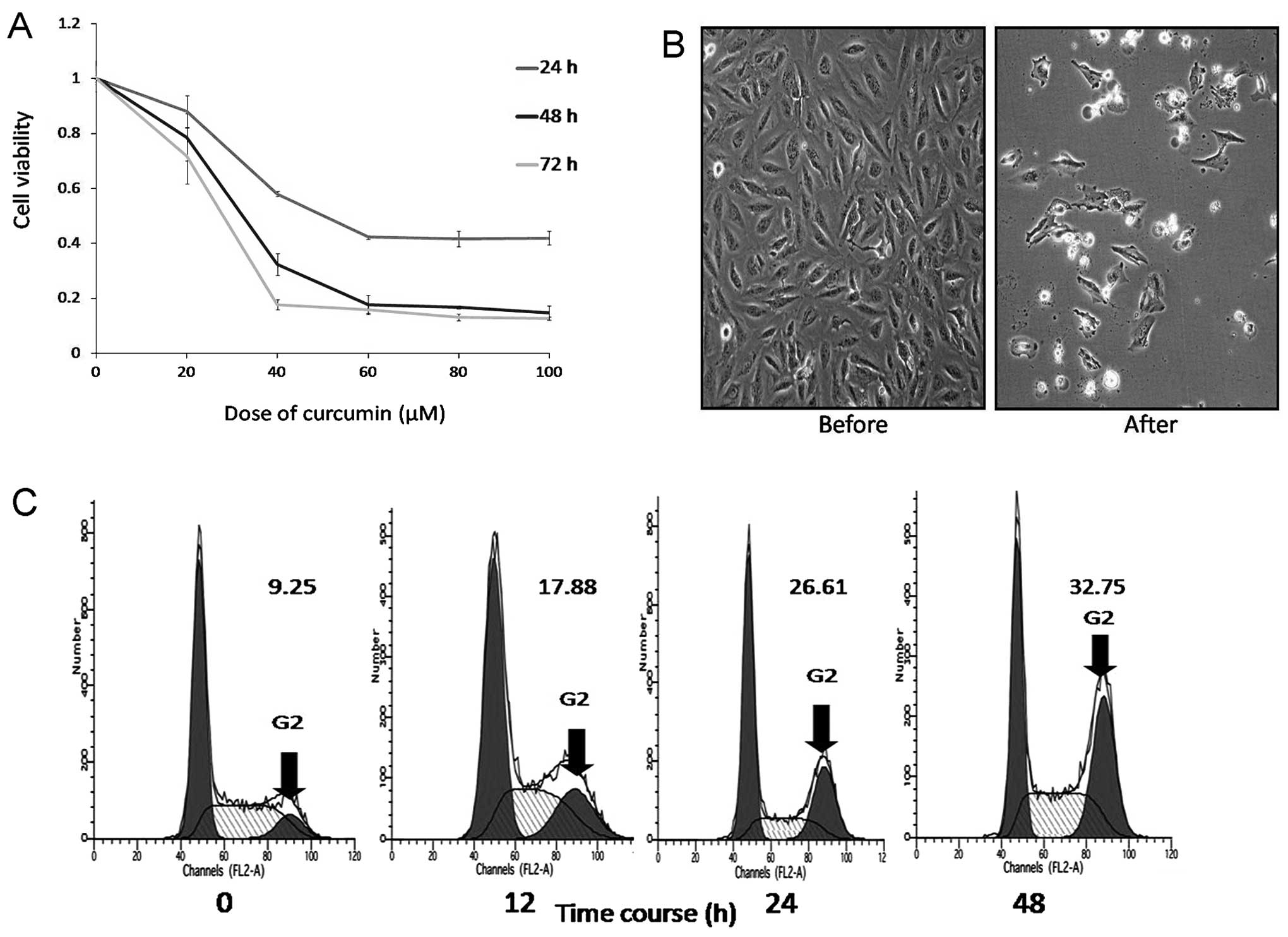
We investigated the effect of curcumin on DAOY cells
using the MTT assay. The cells were incubated in the presence of
varying concentrations of curcumin (0–100 μM) for 24, 48 and 72 h,
and then the effects of curcumin were measured. The MTT assay
showed that curcumin treatment exhibited dose- and time-dependent
inhibition of cell growth (Fig.
1A). Curcumin had an 50% antiproliferative effect on DAOY cells
at an inhibitory concentration and time of 35 μM and 48 h. In
addition, we observed that after 48 h, curcumin-treated DAOY cells
underwent morphological changes, such as cell rounding, shrinking,
vacuolation and detachment (Fig.
1B). In order to explore the mechanism of the antiproliferation
effect of curcumin on DAOY cells, the optimum cytostatic dose of 30
μM of curcumin for 48 h was used.
To further study the effect of curcumin on the cell
cycle, DAOY cells were analyzed by flow cytometry after 12, 24 and
48 h of treatment with 30 μM curcumin. This showed that 17.88,
26.61 and 32.73% of cells reached the G2/M phase
compared to 9.25% (control). There was a time-dependent increase of
cells at the G2/M phase (Fig. 1C). This indicated that curcumin
induced cell cycle arrest at the G2/M phase, which led
to inhibition of proliferation.
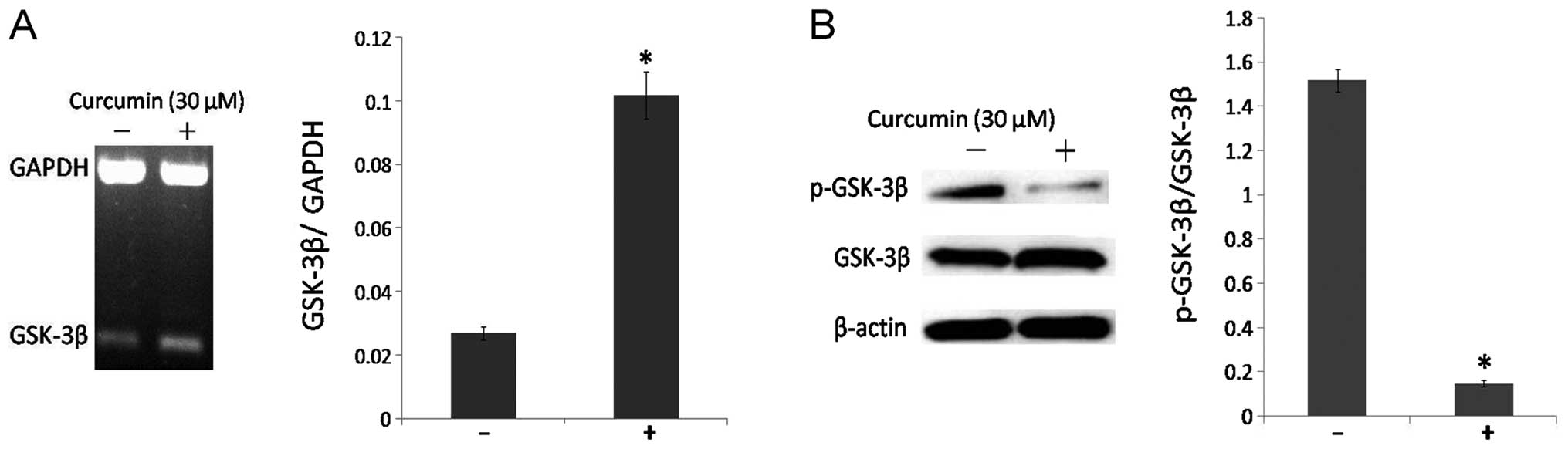
GSK-3β is activated in curcumin-treated
DAOY cells
GSK-3β is a negative regulator of the Wnt/β-catenin
signaling pathway. The main action of GSK-3β is to induce the
phosphorylation of β-catenin and to inhibit its translocation to
the cell nucleus. RT-PCR and western blot analysis assessed the
action of curcumin in DAOY cells for activating GSK-3β function. In
particular, curcumin treatment significantly increased the mRNA
level of GSK-3β (Fig. 2A). At the
protein level, the percentage between phospho-GSK-3β (inactive) and
total GSK-3β was reduced following curcumin treatment compared to
the control cells (Fig. 2B). These
data revealed that curcumin treatment can induce the activation of
GSK-3β in DAOY cells (P<0.05).
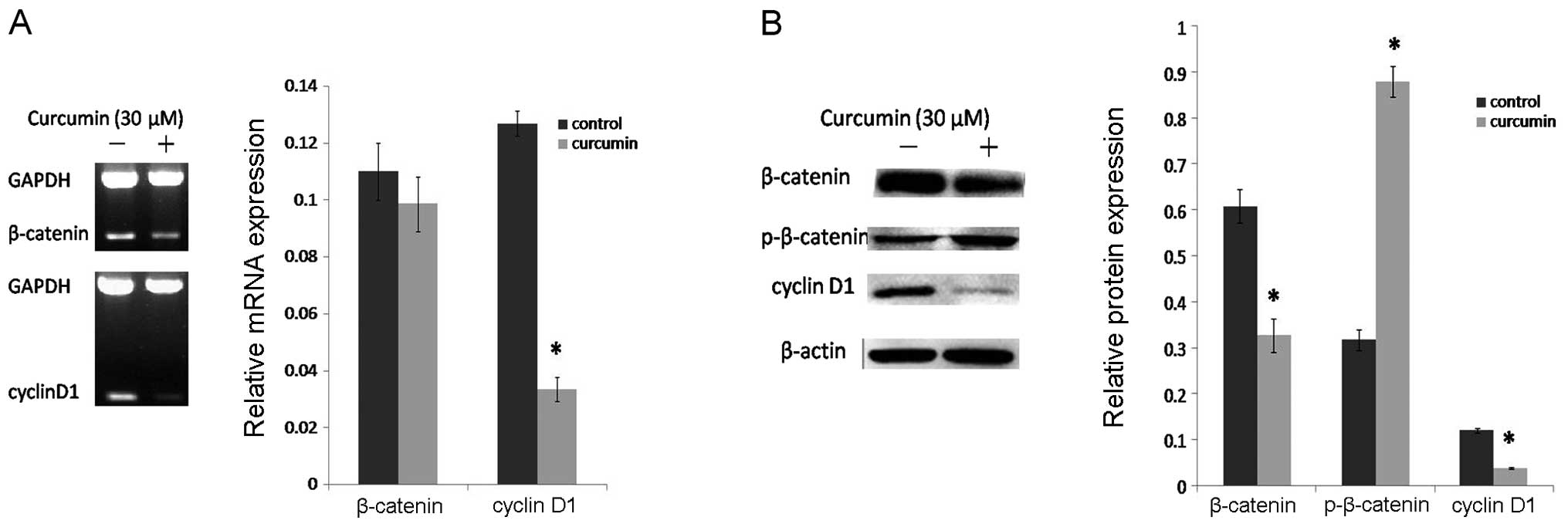
Curcumin suppresses the Wnt/β-catenin
signaling pathway in DAOY cells
β-catenin is a critical component of the Wnt
signaling pathway and GSK-3β is known to affect β-catenin
phosphorylation, thus, we investigated whether curcumin was able to
inhibit the Wnt/β-catenin signaling cascade by RT-PCR and western
blot assay. The results showed that curcumin decreased the
expression of β-catenin and increased p-β-catenin (inactive) at the
protein level (Fig. 3B), but had no
effect on β-catenin at the mRNA level (Fig. 3A). Together with the changes in
β-catenin and p-β-catenin, the mRNA (Fig. 3A) and protein levels (Fig. 3B) of cyclin D1 were markedly
decreased after curcumin treatment. All of these changes suggest
that curcumin has a prominent inhibitory effect on the
Wnt/β-catenin signaling pathway.
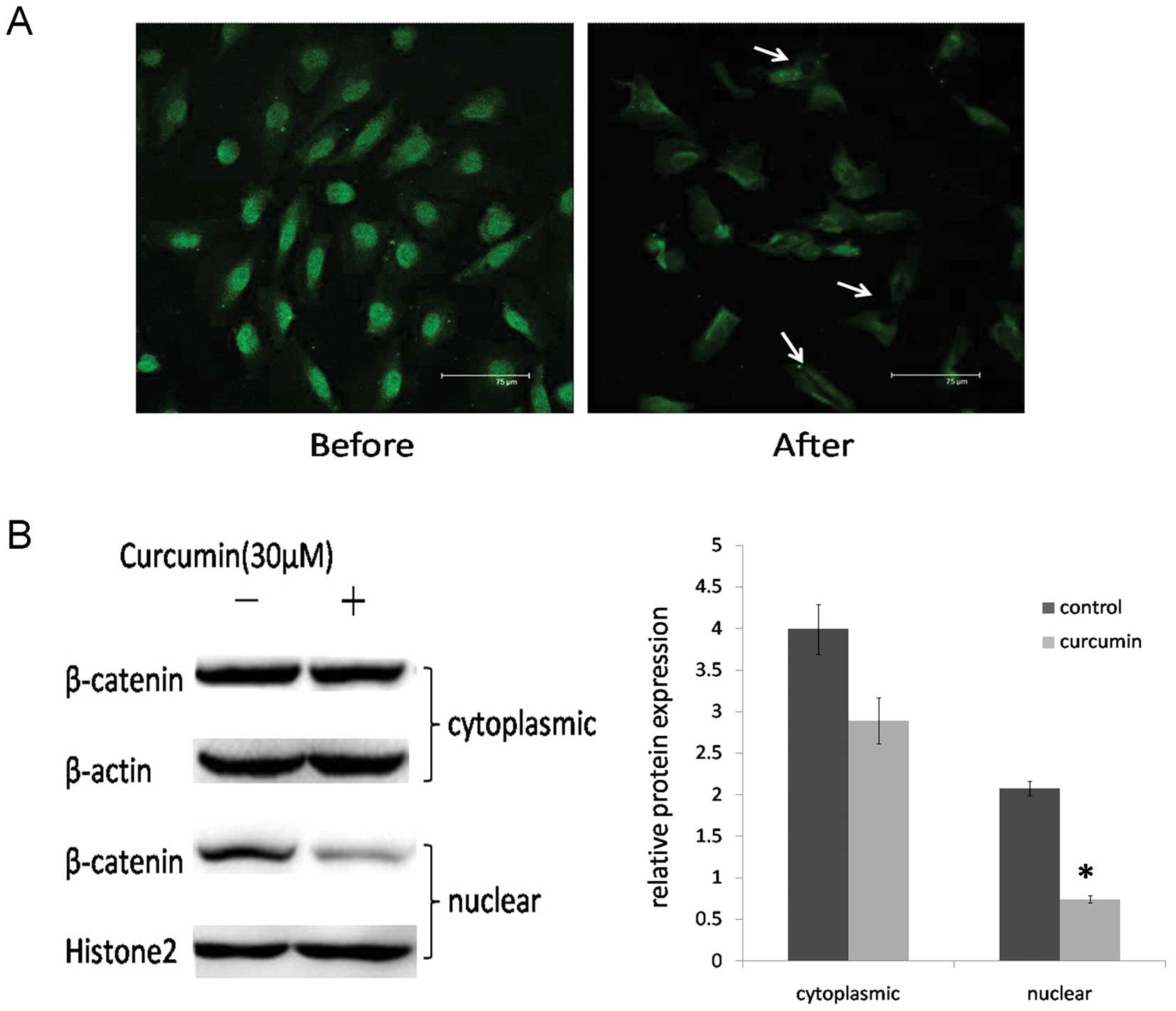
Curcumin inhibits Wnt/β-catenin signaling
through nuclear β-catenin loss
The above results showed that curcumin inhibits the
activation of the Wnt/β-catenin signaling pathway. Since the
nuclear translocation of β-catenin is a key event in activation of
the Wnt/β-catenin signaling pathway, we checked the effect of
curcumin on the expression of nuclear β-catenin. Immunofluorescence
and western blot assays in DAOY cells were carried out to determine
the location of β-catenin. After curcumin treatment, the
cytoplasmic and nuclear expression levels of β-catenin were
decreased, particularly in the nucleus (P<0.05) (Fig. 4).
Curcumin mediates the antiproliferation
of DAOY cells dependent on the downregulation of β-catenin
β-catenin is the key regulator of the Wnt signaling
pathway, which is a major event in the equilibrium between cell
proliferation and cell death, and, therefore, it is implicated in
the development of the cerebellar tumor medulloblastoma. Thereby,
we sought to determine the role of β-catenin in the
curcumin-mediated antiproliferation of DAOY cells. We transiently
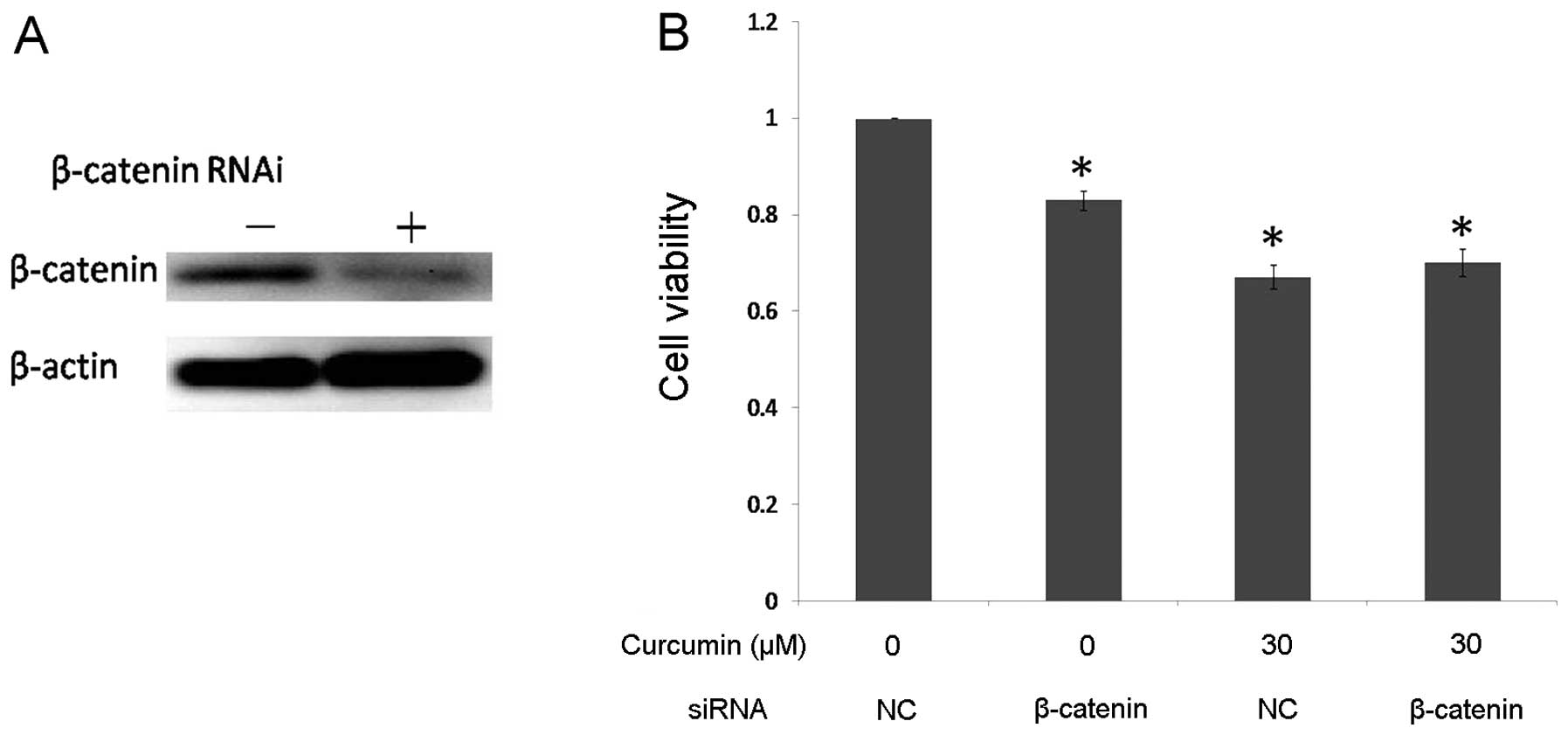
reduced β-catenin expression in DAOY cells using siRNA. Western
blot analysis confirmed a >75% decrease in β-catenin expression
in the DAOY cells compared with the negative control siRNA
(Fig. 5A). If downregulation of
β-catenin is a critical antiproliferation mechanism of curcumin,
one would predict that in the context of already-reduced β-catenin,
curcumin treatment would not cause a significant additional
increase in the antiproliferation effect. After inhibition of
β-catenin expression, DAOY cells were exposed to 30 μM curcumin,
and cell proliferation was assayed by MTT. In the negative control
siRNA-transfected group, curcumin treatment efficiently inhibited
cell growth compared to the control group. In the β-catenin
siRNA-transfected group, this decrease in β-catenin expression was
associated with a significant reduction in the percentage of cell
viability, but there was no significant difference in cell
proliferation after curcumin treatment (Fig. 5B). These data strongly indicate that
curcumin-mediated antiproliferation of DAOY cells is dependent on
the downregulation of β-catenin.
Discussion
Medulloblastoma is a multifactorial disease,
characterized by a disorder of signaling pathways at multiple steps
(8). Treatment for MB remains
highly problematic. The ineffectiveness, lack of safety, and high
cost of chemoradiotherapy have limited their use in MB management.
For this reason, major emphasis is being given to the development
of multi-targeted drugs (1). In the
present study, curcumin was shown that it could constitute a potent
anti-medulloblastoma agent. Firstly, curcumin has an outstanding
safety profile. In fact, different phase I and II clinical trials
have shown that curcumin is safe for children and adults (17). Secondly, studies of curcumin in
various central nervous system (CNS) disorders including
Alzheimer’s, Parkinson’s, and stroke showed the potent effect of
orally delivered curcumin in the brain (18). Due to the less accessible anatomic
location of MB, when drugs cannot cross the blood brain barrier
(BBB) it limits their inclusion in any chemotherapeutic protocol.
Therefore, the treatment will be more effective and there will be
less neurotoxic injury if the biological agents can cross the BBB.
A recent study showed that curcumin crossed the BBB and inhibited
tumor growth in orthotopic glioblastoma models (19). Finally, curcumin had potential
antitumor effects in a variety of cancers (5), including MB. Curcumin has effects on
multiple levels within the transcriptional network to restrict MB
growth (20). Curcumin was reported
to downregulate bcl-2 and bcl-xl, reduce histone deacetylase (HDAC)
4 expression and activity inhibiting MB cell proliferation.
Curcumin inhibited the Shh-Gli1 signaling pathway by downregulating
Shh protein. In in vivo medulloblastoma xenografts, curcumin
reduced tumor growth and significantly increased survival in
Smo/Smo transgenic MB mouse model (20,21).
Although, multiple biological functions have been attributed to
curcumin, the prime driving mechanism underlying its action remains
to be clarified.
Gene-expression profiling studies have identified
four molecular subgroups of MB associated with a different genetic
profile, different activation of oncogenic pathways and distinct
clinical outcomes (12). Thus, the
activation of the Wnt/β-catenin, Shh, Notch and Akt/NF-κB signaling
pathways play key roles in different subgroups of MB. These
pathways are differentially activated in different subsets of MB,
but engage in considerable crosstalk and cooperation (8,22).
Wnt-activated tumors are an independent molecular subgroup in MB
which are characterized by a distinct pattern of genomic
aberrations. Additional studies have identified activation of the
Wnt/β-catenin pathway in 25% MBs with the majority associated with
activating mutations in β-catenin (23). Recently, studies show that
inhibition of the Akt/NF-κB signaling pathway can affect
Wnt/β-catenin signaling, thus, resulting in cytoplasmatic retention
of β-catenin (24). The
Wnt/β-catenin signaling is also required for Hh pathway-driven
tumorigenesis (25). Inhibition of
the Wnt/β-catenin signaling pathway can impair MB growth in
vitro and in vivo (26).
Due to the wide involvement of the Wnt/β-catenin signaling pathway
in MB, inhibition of this pathway can be considered as an
attractive target for MB.
In the present study, we investigated the antitumor
activity of curcumin on DAOY cells and demonstrated that curcumin
exerted multiple modulatory effects on the Wnt/β-catenin signaling
pathway components GSK-3β, β-catenin and cyclin D1. In the present
study our data suggest that curcumin can inhibit cell proliferation
of MB cells by enabling the arrest of the cell cycle at the
G2/M phase. Moreover, our results also showed that
inhibition of Wnt/β-catenin signaling pathway through nuclear
β-catenin loss may be one of the mechanisms implicated in the
suppression of cell proliferation in MB by curcumin.
Curcumin is known to be a good inhibitor of the
Wnt/β-catenin signaling pathway in gastric, colon, intestinal and
prostatic cancer cell lines (27).
Studies also exist on the downregulation of β-catenin in MB cells
(20). However, the detailed
molecular mechanisms of curcumin-mediated reduction of β-catenin
are not fully understood.
GSK-3β is a kinase loaded with serine (Ser) and
threonine (Thr). The phosphorylation or dephosphorylation of Ser9
is an important gating switch for regulating the activity of GSK-3β
(28). It is also an important
regulator in the Wnt/β-catenin signaling pathway, and plays an
important role in the proliferation and differentiation of
progenitor cells during brain development (29). In the canonical Wnt signaling
pathway, activated GSK-3β phosphorylates and translocates nuclear
β-catenin from the nucleus to the cytoplasm resulting in the
inhibition of the subsequent activation of T cell factor 4
(TCF4)-dependent gene transcription (such as cyclin D1 and c-Myc)
(30). That is to say GSK-3β
regulates β-catenin by controlling its protein level and nuclear
localization. In particular, most of the Wnt pathway mutations
reported in sporadic MBs target residues of serine 33 and 37 of
β-catenin, which prevent phosphorylation-dependent degradation of
β-catenin by GSK-3β. As a consequence, β-catenin levels are
increased in an uncontrolled manner, leading to the development of
a transformed phenotype (31). In
the present study, our data showed for the first time that curcumin
attenuates the Wnt/β-catenin signaling in MB cells by promoting
phosphorylation-dependent degradation of β-catenin by GSK-3β. Thus,
it is no surprise that after curcumin treatment the changes in
β-catenin were only at the protein level, not the mRNA level. The
reason might be that the GSK-3β-mediated effect of curcumin on
β-catenin is more important than the direct effect of curcumin on
β-catenin in MB. But more evidence confirming this hypothesis needs
to be found.
Cyclin D1 is an oncoprotein that plays a key role in
the development of MB (32). High
expression of cyclin D1 is considered to be indicative of a poor
prognosis as it is related to an unfavorable therapeutic outcome.
Morever, overexpression of cyclin D1 protein leads to increased
cell proliferation, which gives neoplastic cells a growth advantage
and may also favor the occurrence of additional genetic lesions
with potential oncogenic effects (33). Here, we showed that suppression of
the nuclear translocation of β-catenin resulted in the decreased
expression of cyclin D1, a downstream oncogene of the Wnt/β-catenin
signaling pathway. We also showed that curcumin effectively
inhibited MB cell proliferation in vitro.
Through mechanistic studies, we found that curcumin
promoted the activity of GSK-3β, enhanced GSK-3β binding to
β-catenin, increased the phosphorylation of β-catenin, and reduced
the levels of nuclear β-catenin and cyclin D1, suggesting that
curcumin could inactivate Wnt/β-catenin signaling to suppress the
proliferation of MB cells. Our study found that the viability of MB
cells was attenuated after β-catenin was silenced. This verified
that β-catenin has an important role in the onset and maintainance
of MB. Yet, the antiproliferation following the silencing of
β-catenin was found to be weaker than the treatment of curcumin.
This phenomenon may occur due to the following reasons. On the one
hand, Wnt-activated signals undergo crosstalk with additional
signaling pathways, for example, those of Hh (34), TGF (35) and Notch (36), which play important roles in the
development of MB. Curcumin modulates various molecular targets
including transcription factors, growth factors and their
receptors, cytokines, enzymes, and genes regulating cell
proliferation and apoptosis (5).
Thus, there may be other mechanisms involved in the
antiproliferative effect of curcumin.
In conclusion, these findings provide evidence that
the inhibitory effect of curcumin on cell proliferation involves
the inhibition of the Wnt/β-catenin pathway by activating GSK-3β,
attenuating the Wnt/β-catenin pathway via reducing nuclear
β-catenin, accompanied by the downregulation of cyclin D1, which is
tightly connected to the development and prognosis of MB. Thus,
curcumin has the potential to be developed as a safe therapeutic
for medulloblastoma. Further studies are needed to verify the
antitumor ability of curcumin in vivo.
Acknowledgements
The present study was supported by funds from the
National Science Foundation of China (NSFC: 81272571).
References
|
1
|
de Bont JM, Packer RJ, Michiels EM, den
Boer ML and Pieters R: Biological background of pediatric
medulloblastoma and ependymoma: a review from a translational
research perspective. Neuro Oncol. 10:1040–1060. 2008.PubMed/NCBI
|
|
2
|
Lin J, Zheng Y, Chen K, Huang Z, Wu X and
Zhang N: Inhibition of FOXM1 by thiostrepton sensitizes
medulloblastoma to the effects of chemotherapy. Oncol Rep.
30:1739–1744. 2013.PubMed/NCBI
|
|
3
|
Maheshwari RK, Singh AK, Gaddipati J and
Srimal RC: Multiple biological activities of curcumin: a short
review. Life Sci. 78:2081–2087. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Anand P, Sundaram C, Jhurani S,
Kunnumakkara AB and Aggarwal BB: Curcumin and cancer: an ‘old-age’
disease with an ‘age-old’ solution. Cancer Lett. 267:133–164.
2008.
|
|
5
|
Kunnumakkara AB, Anand P and Aggarwal BB:
Curcumin inhibits proliferation, invasion, angiogenesis and
metastasis of different cancers through interaction with multiple
cell signaling proteins. Cancer Lett. 269:199–225. 2008. View Article : Google Scholar
|
|
6
|
Dorai T, Cao YC, Dorai B, Buttyan R and
Katz AE: Therapeutic potential of curcumin in human prostate
cancer. III Curcumin inhibits proliferation, induces apoptosis, and
inhibits angiogenesis of LNCaP prostate cancer cells in vivo.
Prostate. 47:293–303. 2001. View Article : Google Scholar
|
|
7
|
Gilbertson RJ and Ellison DW: The origins
of medulloblastoma subtypes. Annu Rev Pathol. 3:341–365. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Guessous F, Li Y and Abounader R:
Signaling pathways in medulloblastoma. J Cell Physiol. 217:577–583.
2008. View Article : Google Scholar
|
|
9
|
Angers S and Moon RT: Proximal events in
Wnt signal transduction. Nat Rev Mol Cell Biol. 10:468–477.
2009.PubMed/NCBI
|
|
10
|
Roussel MF and Hatten ME: Cerebellum
development and medulloblastoma. Curr Top Dev Biol. 94:235–282.
2011.
|
|
11
|
Reya T and Clevers H: Wnt signalling in
stem cells and cancer. Nature. 434:843–850. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Northcott PA, Korshunov A, Witt H,
Hielscher T, Eberhar CG, Mack S, Bouffet E, Clifford SC, Hawkins
CE, French P, Rutka JT, Pfister S and Taylor MD: Medulloblastoma
comprises four distinct molecular variants. J Clin Oncol.
29:1408–1414. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Thompson MC, Fuller C, Hogg TL, Dalton J,
Finkelstein D, Lau CC, Chintagumpala M, Adesina A, Ashley DM,
Kellie SJ, Taylor MD, Curran T, Gajjar A and Gilbertson RJ:
Genomics identifies medulloblastoma subgroups that are enriched for
specific genetic alterations. J Clin Oncol. 24:1924–1931. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Koch A, Hrychyk A, Hartmann W, Waha A,
Mikeska T, Schuller U, Sorensen N, Berthold F, Goodyer CG, Wiestler
OD, Birchmeier W, Behrens J and Pietsch T: Mutations of the Wnt
antagonist AXIN2 (Conductin) result in TCF-dependent transcription
in medulloblastomas. Int J Cancer. 121:284–291. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sun P, Liu Y, Ying H and Li S: Action of
db-cAMP on the bystander effect and chemosensitivity through
connexin 43 and Bcl-2-mediated pathways in medulloblastoma cells.
Oncol Rep. 28:969–976. 2012.PubMed/NCBI
|
|
16
|
Wang X, Shi Q, Xu K, Gao C, Chen C, Li XL,
Wang GR, Tian C, Han J and Dong XP: Familial CJD associated PrP
mutants within transmembrane region induced Ctm-PrP retention in ER
and triggered apoptosis by ER stress in SH-SY5Y cells. PLoS One.
6:e146022011. View Article : Google Scholar
|
|
17
|
Goel A, Kunnumakkara AB and Aggarwal BB:
Curcumin as ‘Curecumin’: from kitchen to clinic. Biochem Pharmacol.
75:787–809. 2008.
|
|
18
|
Cole GM, Teter B and Frautschy SA:
Neuroprotective effects of curcumin. Adv Exp Med Biol. 595:197–212.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Perry MC, Demeule M, Regina A, Moumdjian R
and Beliveau R: Curcumin inhibits tumor growth and angiogenesis in
glioblastoma xenografts. Mol Nutr Food Res. 54:1192–1201.
2010.PubMed/NCBI
|
|
20
|
Elamin MH, Shinwari Z, Hendrayani SF,
Al-Hindi H, Al-Shail E, Khafaga Y, Al-Kofide A and Aboussekhra A:
Curcumin inhibits the Sonic Hedgehog signaling pathway and triggers
apoptosis in medulloblastoma cells. Mol Carcinog. 49:302–314.
2010.PubMed/NCBI
|
|
21
|
Lee SJ, Krauthauser C, Maduskuie V,
Fawcett PT, Olson JM and Rajasekaran SA: Curcumin-induced HDAC
inhibition and attenuation of medulloblastoma growth in
vitro and in vivo. BMC Cancer. 11:1442011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Baryawno N, Sveinbjornsson B, Kogner P and
Johnsen JI: Medulloblastoma: a disease with disorganized
developmental signaling cascades. Cell Cycle. 9:2548–2554. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rogers HA, Miller S, Lowe J, Brundler MA,
Coyle B and Grundy RG: An investigation of WNT pathway activation
and association with survival in central nervous system primitive
neuroectodermal tumours (CNS PNET). Br J Cancer. 100:1292–1302.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Baryawno N, Sveinbjornsson B, Eksborg S,
Chen CS, Kogner P and Johnsen JI: Small-molecule inhibitors of
phosphatidylinositol 3-kinase/Akt signaling inhibit Wnt/β-catenin
pathway cross-talk and suppress medulloblastoma growth. Cancer Res.
70:266–276. 2010.PubMed/NCBI
|
|
25
|
Rogers HA, Sousa S, Salto C, Arenas E,
Coyle B and Grundy RG: WNT/β-catenin pathway activation in Myc
immortalised cerebellar progenitor cells inhibits neuronal
differentiation and generates tumours resembling medulloblastoma.
Br J Cancer. 107:1144–1152. 2012.
|
|
26
|
Cimmino F, Scoppettuolo MN, Carotenuto M,
De Antonellis P, Dato VD, De Vita G and Zollo M: Norcantharidin
impairs medulloblastoma growth by inhibition of Wnt/β-catenin
signaling. J Neurooncol. 106:59–70. 2012.PubMed/NCBI
|
|
27
|
Sundram V, Chauhan SC, Ebeling M and Jaggi
M: Curcumin attenuates β-catenin signaling in prostate cancer cells
through activation of protein kinase D1. PLoS One.
7:e353682012.
|
|
28
|
Frame S, Cohen P and Biondi RM: A common
phosphate binding site explains the unique substrate specificity of
GSK3 and its inactivation by phosphorylation. Mol Cell.
7:1321–1327. 2001. View Article : Google Scholar
|
|
29
|
Mao Y, Ge X, Frank CL, Madison JM, Koehler
AN, Doud MK, Tassa C, Berry EM, Soda T, Singh KK, Biechele T,
Petryshen TL, Moon RT, Haggarty SJ and Tsai LH: Disrupted in
schizophrenia 1 regulates neuronal progenitor proliferation via
modulation of GSK3β/β-catenin signaling. Cell. 136:1017–1031.
2009.PubMed/NCBI
|
|
30
|
Wu G, Huang H, Garcia Abreu J and He X:
Inhibition of GSK3 phosphorylation of β-catenin via phosphorylated
PPPSPXS motifs of Wnt coreceptor LRP6. PLoS One. 4:e49262009.
|
|
31
|
Raffel C: Medulloblastoma: molecular
genetics and animal models. Neoplasia. 6:310–322. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jozwiak J, Grajkowska W and Wlodarski P:
Pathogenesis of medulloblastoma and current treatment outlook. Med
Res Rev. 27:869–890. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhao X, Song T, He Z, Tang L and Zhu Y: A
novel role of cyclinD1 and p16 in clinical pathology and prognosis
of childhood medulloblastoma. Med Oncol. 27:985–991. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mimeault M and Batra SK: Frequent
deregulations in the hedgehog signaling network and cross-talks
with the epidermal growth factor receptor pathway involved in
cancer progression and targeted therapies. Pharmacol Rev.
62:497–524. 2010. View Article : Google Scholar
|
|
35
|
Guoand X and Wang XF: Signaling cross-talk
between TGF-β/BMP and other pathways. Cell Res. 19:71–88. 2009.
|
|
36
|
Li C, Zhang Y, Lu Y, Cui Z, Yu M, Zhang S
and Xue X: Evidence of the cross talk between Wnt and Notch
signaling pathways in non-small-cell lung cancer (NSCLC):
Notch3-siRNA weakens the effect of LiCl on the cell cycle of NSCLC
cell lines. J Cancer Res Clin Oncol. 137:771–778. 2011. View Article : Google Scholar : PubMed/NCBI
|