Introduction
Nasopharyngeal cancer (NPC) is currently the most
lethal head and neck neoplasm in Southeast Asia, especially in the
Cantonese region, and radiotherapy is a cornerstone in the
treatment of this malignancy. However, the efficacy of
radiotherapy, particularly for advanced patients, is limited due to
the development of radioresistance. Evidence has suggested that
cancer stem cells (CSCs) are involved in resistance to various
forms of therapies, including radiotherapy, and represent
potentially useful pharmacologic targets (1–3).
However, the interaction between radioresistance and CSCs and its
underlying mechanisms have not been previously explored.
B-cell-specific Moloney murine leukemia virus integration site 1
(Bmi-1), a member of the Polycomb family of transcriptional
repressors, is essential for maintaining the self-renewal and
differentiation of human stem cells (4–7). Bmi-1
has been demonstrated to be overexpressed in various of tumors
(8), such as lung (9), breast (10) and prostate cancer (11), esophageal carcinoma (12) and NPC (13). Bmi-1 inhibition has been shown to
sensitize those tumor cells to radiation (10,14,15).
However, whether Bmi-1 inhibition can potentiate the cytotoxic
effects of radiation on nasopharyngeal CSCs remains to be
elucidated.
Our previous study identified that the
CD44+ NPC cells, derived from the human NPC cell line
SUNE-1 5–8F, with high capacity of self-renewal, differentiation
abilities, tumorigenesis and radiochemoresistance, may be assumed
to be one of the markers of nasopharyngeal carcinoma cancer stem
cell-like cells (CSC-LCs) and Bmi-1 is overexpressed in
CD44+ NPC (16). To
further explore the functional role of Bmi-1 in
NPC-CD44+ cells, we used a lentiviral vector expressing
shRNA to knock down Bmi-1 expression (sh-Bmi-1) in
NPC-CD44+ cells and evaluated the effects of Bmi-1
inhibition by shRNA on the sensitivity of NPC-CD44+
cells to radiation, DNA damage and repair, cell cycle distribution
and apoptosis, and Bmi-1 downstream related protein for the purpose
of improving the radiosensitivity of nasopharyngeal cancer
stem-like cells, which may provide a broad potential therapeutic
paradigm against NPC.
Materials and methods
Cell culture and reagents
The human NPC cell line SUNE-1 5–8F was purchased
from Xiang-Ya Central Experiment Laboratory, and was maintained in
RPMI-1640 medium supplemented with 10% fetal bovine serum in a
humidified incubator (37°C, 5% carbon dioxide). Purified
CD44+ nasopharyngeal cancer stem-like cells were
cultured in DMEM/F12 medium supplemented with 20 ng/ml EGF, 10
ng/ml bFGF, 5 μg/ml insulin and 0.4% bovine serum albumin
(BSA).
Flow cytometric analysis and cell sorting
(FACS)
The cell sorting procedures were the same as
previously described (16). After
sorting, an aliquot of sorted cells was always reanalyzed to check
for purity, which was generally >95%.
Generation of stable Bmi-1 knockdown (KD)
cell lines
Sequence-specific oligonucleotide stretch shRNA
designed to target the Bmi-1 (gene sequence no. NM_005180) were
synthesized as follows: Bmi1-RNAi (19426-1), 5′-TAATACTT
TCCAGATTGAT-3′; Bmi1-RNAi (19427-1), 5′-GAAAGTAA ACAAAGACAAA-3′;
Bmi1-RNAi (19428-1), 5′-AGAACAG ATTGGATCGGAA-3′; loop sequence
CTCGAG. Retroviral vector Bmi-1 short hairpin RNA (shRNA) was
constructed by GeneChem (Shanghai, China). Bmi-1 gene was
introduced into CD44+ NPC cells by infecting cells with
a retroviral vector LV-BMI1-RNAi (19428-1) KD. Negative control
cells (NC) were infected with the empty retroviral vector
GV118-U6-MCS-Ubi-EGFP (GeneChem). The lentivirus stock
[LV-BMI1-RNAi (19428-1)] was added to CD44+ cells at a
multiplicity of infection (MOI) of 30. The cells without
transfection were used as a blank control (CON). Transfection rates
were monitored with fluorescence microscopy. The silencing of Bmi-1
was confirmed by western blotting and real-time PCR analysis.
Real-time PCR
Total RNA was isolated using TRIzol reagent
(Invitrogen). Reverse transcription (RT) was performed using 1 μg
of total RNA and the MML-V reverse transcriptase (Invitrogen).
Real-time PCR was performed using the Platinum SYBR-Green Super Mix
(Invitrogen) and a real-time PCR apparatus (ABI Prism 7000). Primer
sets used were: Bmi-1 (F), 5′-CCACCTGATGTGTGTGCTTTG-3′ and (R),
5′-TTCAGTAGTGGTCTGGTCTTGT-3′; p16 (F), 5′-GGAGT
TTTCAGAAGGGGTTTGT-3′ and (R), 5′-CCTCATTCCTCT TCCTTGGTTT-3′; p14
(F), 5′-TTCTGCCTTTTCACTGTGT TGGA-3′ and (R),
5′-CTCAAGAGAAGCCAGTAACCC-3′; GAPDH (F), 5′-TGACTTCAACAGCGACACCCA-3′
and (R), 5′-CACCCTGTTGCTGTAGCCAAA-3′; β-actin (F), 5′-GTCC
ACCGCAAATGCTTCTA-3′ and (R), 5′-TGCTGTCACCTTC ACCGTTC-3′. GAPDH and
β-actin were used as internal standard for data calibration, which
was for Bmi-1, and p16 and p14, respectively. The 2ΔΔCt
formula was used for the calculation of differential gene
expression.
Western blot analysis
Cells were lysed in lysis buffer [25 mM Tris-HCl pH
7.5, 2.5 mM EDTA, 137 mM NaCl, 2.7 mM KCl, 1% sodium deoxycholic
acid, 0.1% SDS, 1% Triton X-100, 2 mM PMSF and protease inhibitor
cocktail (Nacalai Tesque)] for 30 min at 4°C. Lysates were
clarified by centrifugation at 12,000 rpm for 15 min at 4°C, and
the supernatants were collected. Protein concentrations were
measured using a DC Protein Assay kit (Bio-Rad Laboratories,
Hercules, CA, USA). An equal amount of the protein from each sample
was separated by sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) and transferred to a polyvinylidene
difluoride (PVDF) membrane (Immobilon; Millipore). The membranes
were developed using Immobilon Western Detection Reagents
(Millipore) according to the manufacturer’s instructions. The
chemiluminescence of the membrane was detected using VersaDoc
(Bio-Rad). Densitometric analyses of the band intensities were
performed using ImageJ software (version 1.38x; National Institutes
of Health). The following antibodies were used: Bmi-1 (1:1,000;
rabbit anti-human); p16 (1:200; rabbit anti-human) (both from
Epitomics); p14 (1:200; rabbit anti-human) and p53 (1:1,000; rabbit
anti-human) (both from Cell Signaling Technology). β-actin protein
levels were used as a control to verify equal protein loading.
Immunofluorescence staining
Cells were seeded on coverslips in 24-well plates
and allowed to grow overnight. Cells were irradiated with a single
dose of 2 Gy. Then, cells were washed and fixed with 4%
paraformaldehyde at specific measuring times (0.5, 6, 12 and 24 h)
and cells were rinsed in phosphate-buffered saline (PBS). After
blocking in 5% BSA at room temperature for 30 min, slides were
incubated with γH2AX antibody, mouse anti-human (Abcam) at 4°C
overnight and then incubated with goat anti-mouse IgG-conjugated
with PE (Abbkine). Images were captured by Olympus laser scanning
confocal microscopy (Olympus Optical Co., Tokyo, Honshu, Japan).
Cells were judged as ‘positive’ for γH2AX foci when they displayed
10 or more discrete dots of brightness. For quantitation of foci, a
minimum of 100 cells were analyzed for each time point. All data
points represent means ± SD of three experiments.
Colony formation assay
The exponential growth cells were plated on six-well
plates with 200–6,000 cells/well, irradiated the next day at the
distinct doses (0, 2, 4, 6 and 8 Gy). The cells were maintained in
culture for an additional 14 days to allow colony formation, then
fixed with methanol, stained with 0.5% crystal violet (Sigma) and
colonies containing at least 50 cells in size were counted. Each
treatment was carried out in triplicate. Cell survival fraction
(SF), radiation dose (D), the bottom of the natural logarithm (e),
the mean death dose (D0)and extrapolate number (N) were
used for cell survival curves. Finally, the sensitization enhancing
ratio (SER) was calculated (a ratio of SF2).
Cell cycle analysis
The cells were harvested and fixed in 70% ethanol at
4°C overnight. Next day, cells were suspended in 200 μl PBS and 2
μl RNase A (5 mg/ml). After 30 min incubation, 10 μl PI (1 mg/ml)
was added and the cells were incubated for 30 min in the dark for
analysis. The flow cytometry data was acquired using FACSCanto II
flow cytometer (BD Biosciences, San Jose, CA, USA) and the results
were analyzed by ModFit LT2.0 software (Coulter Electronics).
Cell apoptosis analysis
The cells were exposed to 6 Gy X-rays, 24 h after IR
and cells were treated according to the Annexin V-PE/7-AAD
apoptosis detection kit (KeyGen, Nanjing, Jiangsu, China). Cells
were harvested and resuspended in 50 μl of binding buffer and 7-AAD
(5 μl) in the dark for 10 min, at room temperature. Then, binding
buffer (450 μl) and 1 μl Annexin V-PE were added in the dark for 10
min, at room temperature, and cells were analyzed by flow
cytometry. PE-positive cells were regarded as apoptotic cells. This
experiment was repeated three times.
Statistical analysis
Results are expressed as the means ± SD. Analysis
was performed using a two-way Student’s t-test. P<0.05 was
considered to indicate a statistically significant difference.
Results
FACS detects CD44 expression in the
SUNE-1 5–8F cells, and the sorting purity
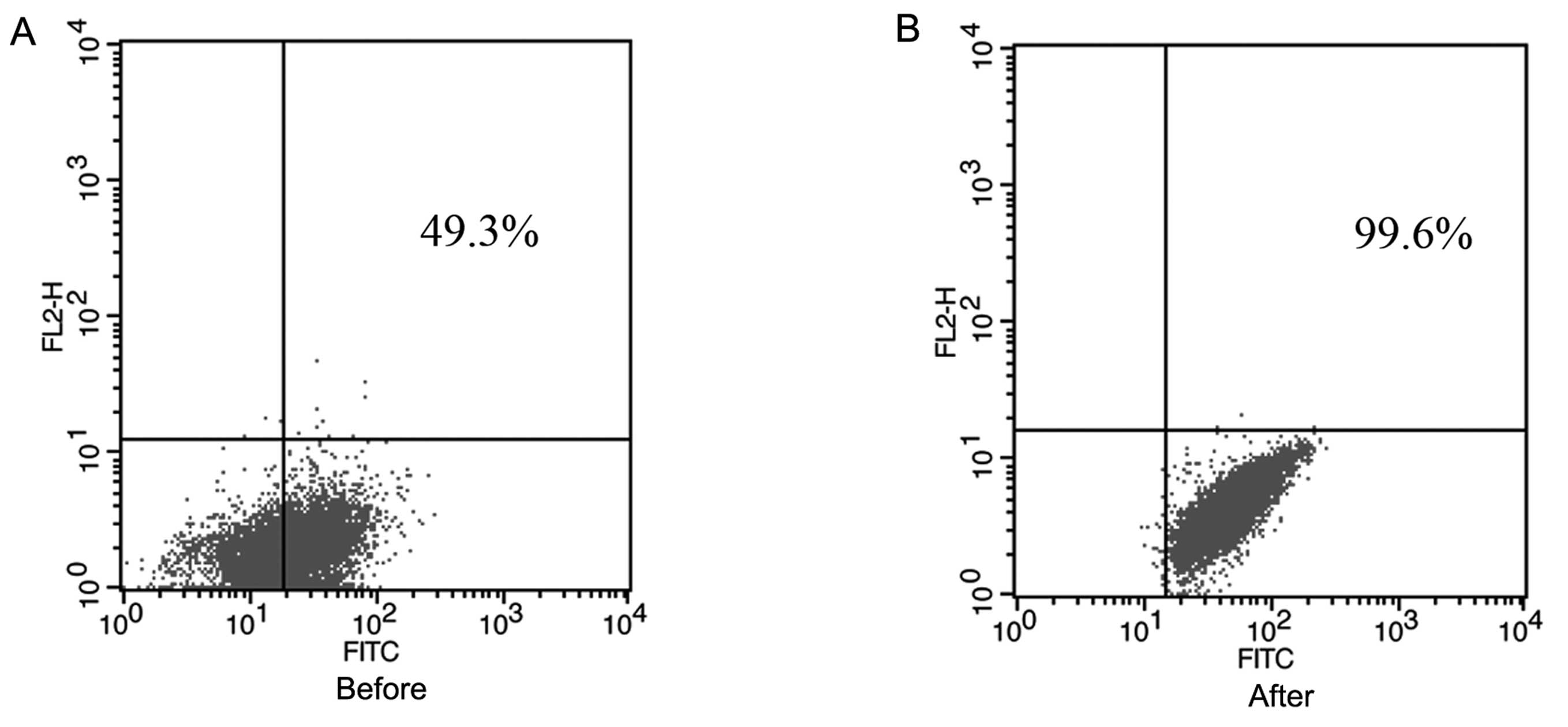
We examined CD44 expression in the human NPC SUNE-1
5–8F cell line by flow cytometry. As shown in Fig. 1, in the NPC cell line SUNE-1 5–8F,
CD44+ cells occupied ~49.3% of the total cells, which
was in agreement with our previous study (43–52%) (16). CD44+ cells were collected
for subsequent experiments. The purity of these cells was
99.6%.
Knockdown of Bmi-1 by lentivirus-mediated
RNA interference in CD44+ NPC cells
Previous studies in other systems suggested that
Bmi-1 expression was associated with significantly reduced patient
survival (17–19) after RT with or without chemotherapy,
indicating that Bmi-1 overexpression may be mediating
radioresistance. To address this possibility, CD44+
NPC-CLCs cells were transfected with lentivirus-mediated RNA
targeting Bmi-1 [LV-BMI1-RNAi (19428-1), KD]. Control cells were
infected with the empty retroviral vector GV118-U6-MCS-Ubi-EGFP,
NC. To ensure the transfection efficiency of lentivirus into
CD44+ NPC CLCs cells, GFP expression was monitored by
fluorescence microscopy and FACS analysis. It showed a
high-efficiency infection that the majority of cells displayed
green fluorescence 72 h after lentivirus transfection, with the
efficiency reaching >90% in both KD and NC-treated cells
(Fig. 2A). LV-Bmi-1-RNAi showed 90%
reduction in Bmi-1 transcript level, observed at 72 h
post-transfection by real-time PCR (Fig. 2B), which resulted in nearly
undetectable Bmi-1 protein expression (Fig. 2C).
Real-time PCR and western blot detection
of the Bmi-1 downstream related genes p16, p14 and p53 mRNA and
protein changes
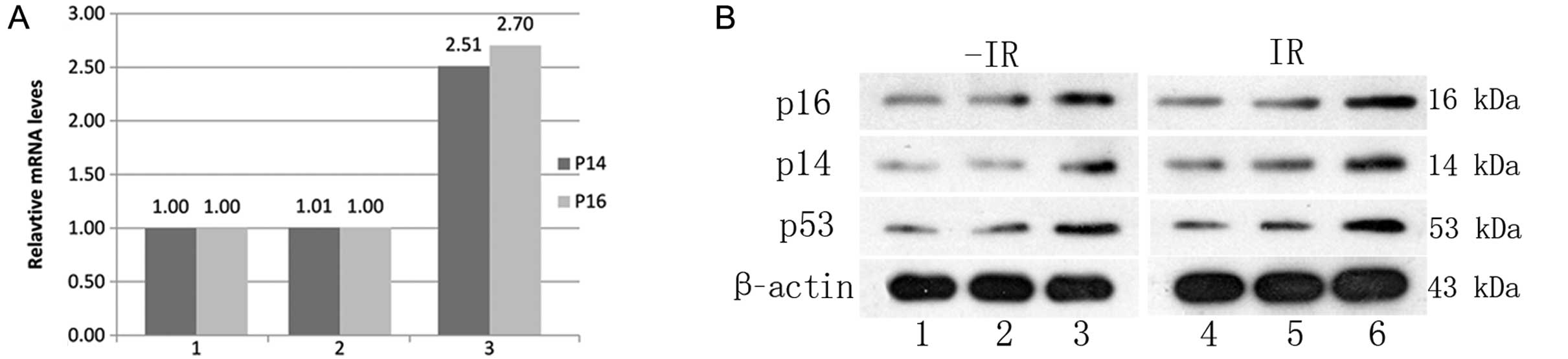
Emerging evidence indicates that Bmi-1 is
upregulated in various malignancies and promotes tumor progression
by inhibiting the transcription of tumor suppressors, such as p53,
p16INK4a and p14Arf (15,20–23).
As shown in Fig. 3, knockdown of
Bmi-1 increased the downstream related genes p16, p14 and p53 mRNA
and protein levels.
Bmi-1 knockdown sensitizes
CD44+ NPC CLCs to radiation
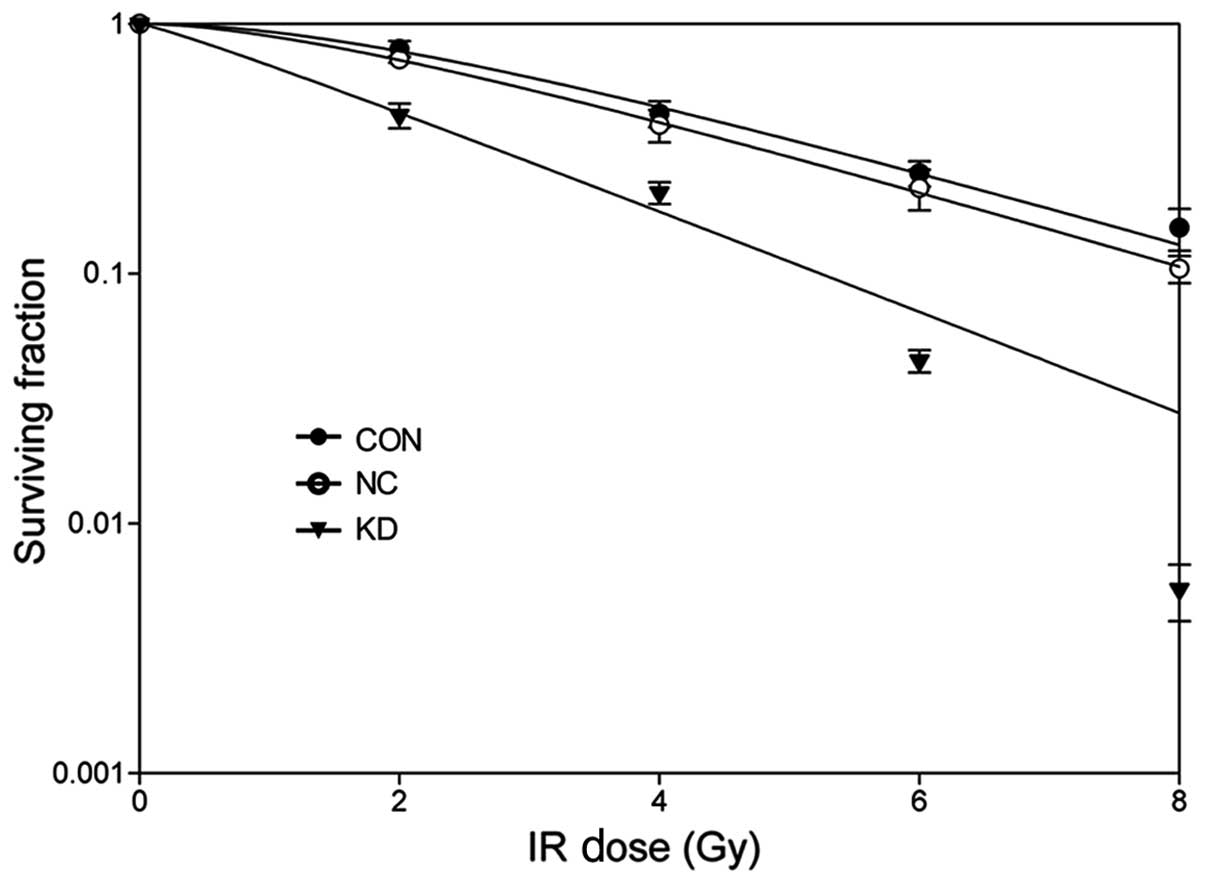
To determine the radiosensitizing effect of Bmi-1
depletion on the CD44+ NPC CLCs, a clonogenic formation
assay was performed. As depicted in Fig. 4, the shoulder area of the survival
curves was significantly narrowed and the surviving fractions (SFs)
at each dose (2, 4, 6 and 8 Gy) decreased in Bmi-1-depleted cells.
The values of SF2, D0, Dq and N
were all lower in Bmi-1 KD cells (Table
I). SF2 was reduced to 44% in KD cells from 78% in
CON cells and the enhancement ratio was 1.77. Thus, we concluded
that downregulation of Bmi-1 could radiosensitize CD44+
NPC CLCs cells.
 | Table IThe main parameters of cell survival
curves after ionizing radiation.a |
Table I
The main parameters of cell survival
curves after ionizing radiation.a
| Parameters | CON | NC | KD |
|---|
| D0 | 2.89 | 2.83 | 2.14 |
| Dq | 3.36 | 2.42 | 0.34 |
| N | 2.16 | 1.85 | 1.16 |
| SF2 | 0.78 | 0.71 | 0.44 |
| SER | 1.10 | 1.77 | |
Knockdown of Bmi-1 increases DNA double
strand break (DSB) and decreases DSB repair
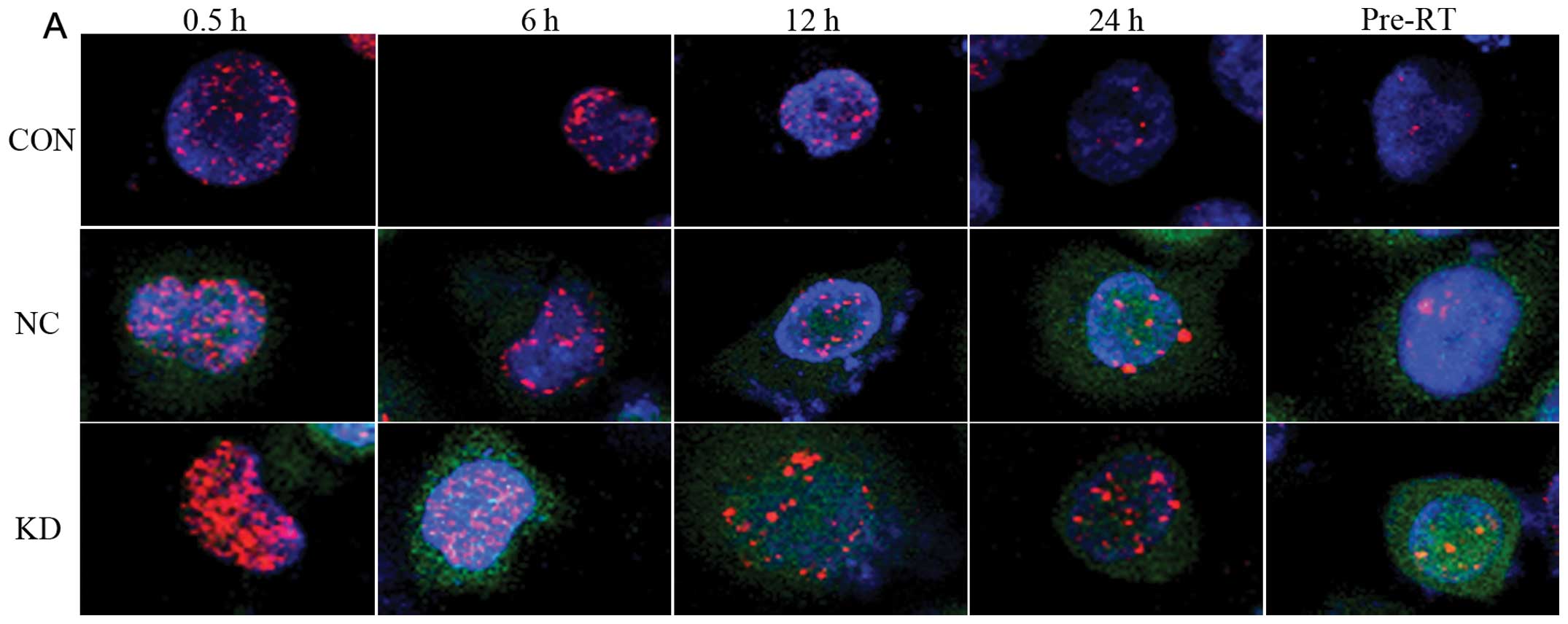
γH2AX is commonly utilized to assess DSBs inflicted
by RT (24). When CD44+
NPC were depleted of Bmi-1, we monitored kinetics of DSB repair by
immunofluorescent γH2AX foci at different time points after
exposure to 2 Gy of X-rays. As shown in Fig. 5, γH2AX foci appeared immediately
following IR treatment in the three groups, indicating that Bmi-1
is dispensable for γH2AX focus formation. The average number of
γH2AX foci/cell in KD cells was significantly higher than in cells
treated with NC or untreated/control cells at 0.5, 6, 12 and 24 h
after radiation (P<0.05). This delayed clearance of γH2AX foci
in Bmi-1-depleted cells demonstrated that DSB repair is severely
impaired in the absence of Bmi-1 (25).
Effects of Bmi-1 downregulation on
IR-induced cell cycle distribution and apoptosis
To further evaluate potent reasons conferring
radiation sensitivity induced by Bmi-1 knockdown, we tested cell
cycle distribution and cell apoptosis following treatment of
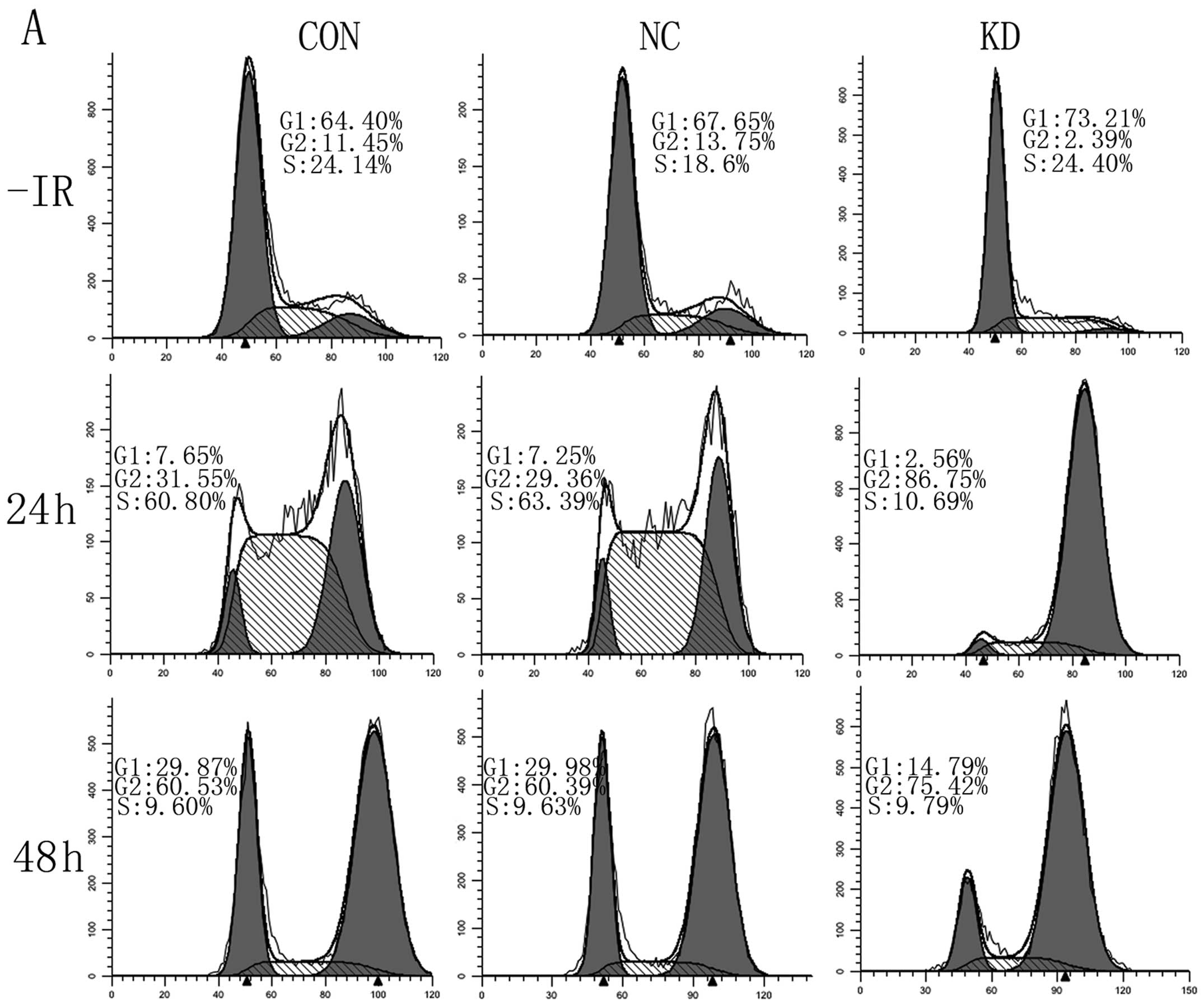
lentivirus and/or IR by flow cytometry assays. As shown in Fig. 6A, compared with the blank CON group,
cells treated with LV-BMI1-RNAi showed prolonged G1 from 64.40 to
73.21%. G2/M phase accumulation came to max within 24 h in the KD
group after IR; however, CON and NC G2/M phase increased by 48 h
after irradiation, the number of cells in G2/M in KD had to some
extent decreased at 48 h after IR. The levels were still
significantly greater than that for NC-treated or control cells.
Depletion of Bmi-1 exhibited a markedly prolonged accumulation of
cells in G2/M. In addition, there was an increase in IR-induced
apoptosis in the absence of Bmi-1 (Fig.
6B). Based on these results, we concluded that knockdown of
Bmi-1 leads to an extended G2/M accumulation and an increase of
IR-induced apoptosis.
Discussion
Cancer stem cell theory is based on the hypothesis
that cancers arise from a rare population of cells, which are
responsible for the chemo- and radioresistance of the tumors.
Therefore, the cancer stem cell population is thought to be an
important determinant of treatment failure. Polycomb group proteins
(PcG) are a family of proteins that is essential for proper
embryonic development and for the specification of stem cell gene
expression profiles, controlling self-renewal, pluripotency and
differentiation (25), and PcG may
confer radioresistance to stem cells (25). Bmi-1, a member of the Polycomb
family of transcriptional repressors, is essential for maintaining
the self-renewal abilities of adult stem cells. Facchino et
al observed that Bmi-1 was responsible for the radioresistance
in both glioma stem cells and normal stem cells (26). Our results demonstrate that the
isolated CD44+ subpopulation from SUNE-1 5–8F human NPC
cell line presents the key biological properties of CSCs and Bmi-1
was overexpressed in our previous study (16).
In the present study, we provided evidence that
Bmi-1 was correlated with radiation response in CD44+
NPC CSC-LCs. We showed that Bmi-1 overexpression developed
radiation resistance, whereas Bmi-1 inhibition significantly
increased radiation sensitivity with a DER of 1.77 by radiation
clonogenic survival assay. It suggested that Bmi-1 may play an
important role in the regulation of cellular response to radiation
in CD44+ NPC CSC-LCs. Thus, the results underscore the
importance of Bmi-1 targeting in combination with irradiation in
nasopharyngeal neoplasm therapy. This finding is consistent with
the reports of Wang et al (13) and Alajez et al (15).
Bmi-1 is a transcriptional repressor of the
Ink4a/Arf locus. This locus encodes 2 separate tumor suppressor
genes, namely, p16Ink4a and p19Arf
(p14Arf in humans) (27). The p16INK4a
(pRb/p16INK4a/cyclin D1) and p53 (p14ARF/mdm2/p53) pathways are the
two main cell cycle control pathways. p16INK4a is a
potent inhibitor of the kinase activities of CDK4/6, resulting in
Rb hypophosphorylation, binding to E2F1, the initiation of cell
cycle arrest in the G1 phase (28).
In addition, cell cycle arrest and apoptosis are promoted by p14
through its regulation of p53 stability stages, and p14 effectively
prevents degradation of the tumor suppressor protein p53, which is
required for cell cycle arrest. Hence, Bmi-1 knockdown inhibited
cell cycle progression through derepression of the p16INK4a/p14ARF
locus.
It is well known that DSBs are suggestive of
critical lesions in DNA caused by ionizing radiation (29). DSB is the main mechanism of tumor
cell death after irradiation (30),
DNA DSB repair shows strong cell cycle dependency and radiation
therapy may mediate cell cycle redistribution of tumor cells. Our
findings showed that knockdown of Bmi-1 prolonged G1, after 6 Gy
irritation, and the proportion of the cells in G2/M phase was
increased, which was the most radiosensitive phase of the cell
cycle. In addition, Gilbert et al showed that low-expression
of p16 has been linked to decreased chemoradiotherapy
responsiveness, p16Ink4a negative patients had
significantly poorer overall survival (31). The present study showed that p16
increased after Bmi-1 inhibition and this may be one of the reasons
resulting in increased DSBs and decreased DSB repair in Bmi-1
knockdown cells. Bmi-1 inhibition markedly increased DSB and
significantly decreased the rate of DNA DSB repair induced by
irradiation, suggesting a participation of Bmi-1 in DNA strand
damage and repair (26,32). If complete DNA damage repair fails,
apoptosis is triggered for the elimination of damaged cells. We
noted that the number of apoptotic cells in Bmi-1 knockdown cells
was apparently much higher compared with the controls using FACS
analysis (Fig. 6B). The mechanism
of Bmi-1 inhibition combined radiation induced apoptosis is
complex. A possible reason is that the P53 pathway for apoptosis
was activated in response to combined Bmi-1 inhibition with
ionizing radiation (15).
In conclusion, we reported the enhanced
radiosensitivity of CD44+ NPC CSCs after Bmi-1
inhibition using shRNA. The increased sensitivity was associated
with increased DSBs and decreased DSB repair. We also showed that
combination Bmi-1 inhibition with irradiation could induce
apoptosis, elevated protein p53, p16 and p14 expression. These
results suggest that Bmi-1 knockdown combined radiotherapy could
induce a synergistic effect on increasing the radiosensitivity of
CD44+ CSC-LCs. Bmi-1 inhibition in NPC stem cells may be
an effective therapeutic strategy against NPC. However, further
study is required to confirm these results using in vivo
xenograft models.
Acknowledgements
This study was supported by The Foundation of Health
Department of Hubei Province, China (no. JX4B52). We thank Jing-Hua
Ren, Wei-Hong Chen, Hong-Xia Zhou and other personnel in the
Laboratory of Cancer Center, the Union Hospital, the Tongji Medical
College, the Huazhong University of Science and Technology (Wuhan,
China) for their technical assistance.
References
|
1
|
Chargari C, Moncharmont C, Lévy A, et al:
Cancer stem cells, cornerstone of radioresistance and perspectives
for radiosensitization: glioblastoma as an example. Bull Cancer.
99:1153–1160. 2012.(In French).
|
|
2
|
Malik B and Nie D: Cancer stem cells and
resistance to chemo and radio therapy. Front Biosci. 4:2142–2149.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Krause M, Yaromina A, Eicheler W, Koch U
and Baumann M: Cancer stem cells: targets and potential biomarkers
for radiotherapy. Clin Cancer Res. 17:7224–7229. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Park IK, Qian D, Kiel M, et al: Bmi-1 is
required for maintenance of adult self-renewing haematopoietic stem
cells. Nature. 423:302–305. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Grinstein E and Mahotka C: Stem cell
divisions controlled by the proto-oncogene BMI-1. J Stem Cells.
4:141–146. 2009.PubMed/NCBI
|
|
6
|
Lukacs RU, Memarzadeh S, Wu H and Witte
ON: Bmi-1 is a crucial regulator of prostate stem cell self-renewal
and malignant transformation. Cell Stem Cell. 7:682–693. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yamazaki H, Mori T, Yazawa M, et al: Stem
cell self-renewal factors Bmi1 and HMGA2 in head and neck squamous
cell carcinoma: clues for diagnosis. Lab Invest. 93:1331–1338.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sinha N, Mukhopadhyay S, Das DN, Panda PK
and Bhutia SK: Relevance of cancer initiating/stem cells in
carcinogenesis and therapy resistance in oral cancer. Oral Oncol.
49:854–862. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shien K, Toyooka S, Ichimura K, et al:
Prognostic impact of cancer stem cell-related markers in non-small
cell lung cancer patients treated with induction chemoradiotherapy.
Lung Cancer. 77:162–167. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu ZG, Liu L, Xu LH, et al: Bmi-1 induces
radioresistance in MCF-7 mammary carcinoma cells. Oncol Rep.
27:1116–1122. 2012.PubMed/NCBI
|
|
11
|
Moscatelli D and Lynette Wilson E: Bmi-1,
stem cells and prostate carcinogenesis. Asian J Androl. 13:353–354.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yu X, Li H, Jiang X, Guo L, Jiang W and Lu
SH: miR-203 inhibits the proliferation and self-renewal of
esophageal cancer stem-like cells by suppressing stem renewal
factor Bmi-1. Stem Cells Dev. 23:576–585. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang HB, Liu GH, Zhang H, et al: Sp1 and
c-Myc regulate transcription of BMI1 in nasopharyngeal
carcinoma. FEBS J. 280:2929–2944. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang G, Liu L, Sharma S, et al: Bmi-1
confers adaptive radioresistance to KYSE-150R esophageal carcinoma
cells. Biochem Biophys Res Commun. 425:309–314. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Alajez NM, Shi W, Hui AB, et al: Targeted
depletion of BMI1 sensitizes tumor cells to P53-mediated apoptosis
in response to radiation therapy. Cell Death Differ. 16:1469–1479.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Su J, Xu XH, Huang Q, et al:
Identification of cancer stem-like CD44+ cells in human
nasopharyngeal carcinoma cell line. Arch Med Res. 42:15–21. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen YC, Chang CJ, Hsu HS, et al:
Inhibition of tumorigenicity and enhancement of
radiochemosensitivity in head and neck squamous cell cancer-derived
ALDH1-positive cells by knockdown of Bmi-1. Oral Oncol. 46:158–165.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Häyry V, Mäkinen LK, Atula T, et al: Bmi-1
expression predicts prognosis in squamous cell carcinoma of the
tongue. Br J Cancer. 102:892–897. 2010.PubMed/NCBI
|
|
19
|
Häyry V, Tynninen O, Haapasalo HK, et al:
Stem cell protein BMI-1 is an independent marker for poor prognosis
in oligodendroglial tumours. Neuropathol Appl Neurobiol.
34:555–563. 2008.PubMed/NCBI
|
|
20
|
Smith KS, Chanda SK, Lingbeek M, et al:
Bmi-1 regulation of INK4A-ARF is a downstream requirement for
transformation of hematopoietic progenitors by E2a-Pbx1. Mol Cell.
12:393–400. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Calao M, Sekyere EO, Cui HJ, et al: Direct
effects of Bmi1 on p53 protein stability inactivates oncoprotein
stress responses in embryonal cancer precursor cells at tumor
initiation. Oncogene. 32:3616–3626. 2013. View Article : Google Scholar
|
|
22
|
Fujii H, Honoki K, Tsujiuchi T, et al:
Reduced expression of INK4a/ARF genes in stem-like sphere cells
from rat sarcomas. Biochem Biophys Res Commun. 362:773–778. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim K, Kim DH, Chae SW, et al: Expression
of cell cycle-related proteins, p16, p53 and p63 as important
prognostic markers in gallbladder adenocarcinoma. Pathol Oncol Res.
20:409–415. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Scully R and Xie A: Double strand break
repair functions of histone H2AX. Mutat Res. 750:5–14. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gieni RS, Ismail IH, Campbell S and
Hendzel MJ: Polycomb group proteins in the DNA damage response: a
link between radiation resistance and ‘stemness’. Cell Cycle.
10:883–894. 2011.PubMed/NCBI
|
|
26
|
Facchino S, Abdouh M, Chatoo W and Bernier
G: BMI1 confers radioresistance to normal and cancerous neural stem
cells through recruitment of the DNA damage response machinery. J
Neurosci. 30:10096–10111. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Iwama A, Oguro H, Negishi M, et al:
Enhanced self-renewal of hematopoietic stem cells mediated by the
polycomb gene product Bmi-1. Immunity. 21:843–851. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huber GF, Albinger-Hegyi A, Soltermann A,
et al: Expression patterns of Bmi-1 and p16 significantly correlate
with overall, disease-specific, and recurrence-free survival in
oropharyngeal squamous cell carcinoma. Cancer. 117:4659–4670. 2011.
View Article : Google Scholar
|
|
29
|
Santivasi WL and Xia F: Ionizing
radiation-induced DNA damage, response, and repair. Antioxid Redox
Signal. Feb 3–2014.(Epub ahead of print).
|
|
30
|
Mladenov E, Magin S, Soni A and Iliakis G:
DNA double-strand break repair as determinant of cellular
radiosensitivity to killing and target in radiation therapy. Front
Oncol. 3:1132013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gilbert DC, Williams A, Allan K, et al:
p16INK4A, p53, EGFR expression and KRAS mutation status
in squamous cell cancers of the anus: correlation with outcomes
following chemo-radiotherapy. Radiother Oncol. 109:146–151.
2013.
|
|
32
|
Dong Q, Oh JE, Chen W, et al:
Radioprotective effects of Bmi-1 involve epigenetic silencing of
oxidase genes and enhanced DNA repair in normal human
keratinocytes. J Invest Dermatol. 131:1216–1225. 2011. View Article : Google Scholar : PubMed/NCBI
|