Introduction
DJ-1 protein was discovered in 1997 by Nagakubo
et al; it is widely expressed in various human tissues, with
high expression levels in testis, kidney, pancreas, heart and
skeletal muscle (1). The total
length of the human DJ-1 gene is 24 ku, including 8 exons of which
the first two do not encode proteins. The mRNA of DJ-1 gene is 570
bp, and encoding a DJ-1 protein containing 189 amino acids, with a
molecular weight of ~20 kDa. The DJ-1 gene is highly conserved in
evolution, the homology of human and monkey DJ-1 gene is up to 98%
and the homology to mouse is 91% (2,3).
Formation, positive regulation of androgen receptor and the
fertilization process, participation in the regulation of RNA
binding complex (4), and
anti-oxidation, inhibition of apoptosis (5). Previous studies on the DJ-1 gene were
mostly on Parkinson’s disease (PD)-related research, and they found
that its mutations are related to human early-onset PD. However,
some studies have found that the DJ-1 gene is closely related to
tumor as well. Several studies found that DJ-1 protein
overexpression is present in a variety of tumors such as breast
cancer, non-small cell lung cancer, esophageal squamous cell
carcinoma and prostate cancer, and this overexpression is related
to proliferation, metastasis and poor prognosis of malignant tumors
(6–10). Currently, carcinogenic DJ-1 gene
downstream molecular signaling network and transduction mechanism
remain unclear, and the role of the DJ-1 gene in the tumor
formation and growth process is also unclear. Kim et al
found that DJ-1 expression in breast cancer specimens was
negatively correlated with PTEN, while positively correlated with
PKB/Akt (6). Studies have also
indicated that the DJ-1 gene can promote the PI3K/AKT/mTOR pathway
by regulating PTEN genes, thereby enabling increased expression of
a variety of detoxifying enzymes to improve the cell viability and
promote cell proliferation (11).
Davidson et al detected PTEN protein expression levels of
cancer tissues of nearly 300 cases of ovarian cancer patients with
immunohistochemistry, and analyzed DJ-1 mRNA levels with RT-PCR.
The results suggested that the levels of DJ-1 and PTEN were not
correlated (12). Therefore,
whether DJ-1 is involved in regulation of PTEN remains to be
confirmed with further research. In the present study, retrovirus
was used for stable transfection of DJ-1 gene to laryngeal squamous
carcinoma cell line SNU-46, and the cell line was screened out to
compare the changes in tumor biological characteristics
(proliferation, apoptosis, invasion and migration) with untreated
laryngeal cancer cell lines. In the present study, we preliminarily
studied the effect of DJ-1 gene overexpression on laryngeal cancer
cell line SNU-46 biological activities, PI3K/AKT/mTOR pathway and
PTEN protein expression.
Materials and methods
Materials
Cell line and culture
Human laryngeal squamous cell carcinoma SNU-46 cells
were cultured in RPMI-1640 medium containing 10% fetal bovine serum
(FBS; HyClone) and incubated in a 37°C, 5% CO2
humidified incubator. GP2-293 packaging cells were cultured in high
glucose Dulbecco’s modified Eagle’s medium (DMEM) containing 10%
FBS.
Biomaterials
pCDNA3.1(+)-DJ-1 plasmid carrying DJ-1 gene coding
sequence was stored in our laboratory. pLNCX2 plasmid, pVSV-G
plasmid and GP2-293 packaging cells were kindly donated by Dr Lei
Jinju from the Sun Yat-sen University Cancer Center (SYSUCC).
Restriction endonucleases NotI and XhoI were
purchased from Fermentas (USA), DJ-1 primers were purchased from
Guangzhou RiboBio Co., DJ-1 monoclonal antibody was purchased from
Abcam Co., GAPDH monoclonal antibody was purchased from BioWorld
Co., HRP-labeled goat anti-mouse monoclonal antibody was purchased
from Santa Cruz Co., AKT, phospho-Akt (Ser473), mTOR, phospho-mTOR
(Ser2448) and PTEN antibody were purchased from Cell Signaling
Technology, Inc.
Methods
Vector construction
Using PCR method, according to the DJ-1 gene
sequence information and planned inserting restriction endonuclease
gene site sequence, we designed the coding region amplification
primers. pCDNA3.1(+)-DJ-1 plasmid was used as template to amplify
its coding sequence (CDS) by PCR. The gene sequence and vector
sequence were analyzed. Two restriction endonucleases NotI
and XhoI may be used to clone destination gene fragment into
the vector. Designed primers were: F, 5′CCGCTCGAGACCATGGCTTC
CAAAAGAGCTCTGGTCAT3′ and R, 5′ ATTTGCGGCCG
CCTAGTCTTTAAGAACAAGTGGAGCCTTCA3′ (primer synthesis, Shanghai Sangon
Co., Ltd.). The PCR products were purified by gel electrophoresis
and recovered for plasmid splicing. Gel extraction kit was the
product of Qiagen Co. (USA). Restriction endonucleases NotI
and XhoI were used for double digestion of the above PCR
product and pLNCX2 plasmids, followed by agarose gel
electrophoresis, purification and recovery of the digested
products. DNA Ligation kit (Takara) was used for connection. The
connected plasmids were used to transform DH-5α competent E.
coli, followed by expanding culture, extraction and sequencing
detection.
Transfection
The transfection was performed with the
Lipofectamine 2000 Reagent (Invitrogen, Carlsbad, CA, USA),
according to the manufacturer’s protocol.
Retroviral packaging
The day before transfection, GP2-293 cells were
inoculated in a 10 cm petri dish. High glucose DMEM medium
containing 10% fetal calf serum was used for culture and
transfection began at 50% cell fusion. Six micrograms of each
pVSV-G and pLNCX2-DJ-1 plasmid was added to 1.5 ml Opti-MEM
serum-free medium, and then 20 μl Lipofectamine 2000 liposome
transfection reagent was added to 1.5 ml Opti-MEM serum-free
medium. The two liquids were placed at room temperature for 5 min,
and then gently mixed together. The mixture was stored for 20 min
and was added to GP2-293 cells. Six hours later, the medium was
changed and 10 ml fresh high glucose DMEM medium containing 10% FBS
was added. After 48 h, GP2-293 cell culture supernatant in the 10
cm petri dish was drawn and filtered through 0.45 μm filter to
remove cell debris and other impurities, and it was stored at
−80°C. The same method was used to transfect pVSV-G and pLNCX2
empty vector plasmid to obtain empty vector virus of the control
group, which was stored at −80°C.
Retroviral infection of SNU-46
cells
Twenty four hours before infection, the SNU-46 cells
were inoculated in 10 cm petri dishes, with inoculation density of
~50%. Polybrene (8 μg/ml) was added to 5 ml virus-containing
supernatant which was added to SNU-46 cells. After 6 h, the culture
medium and virus-containing supernatant in SNU-46 cells were
discarded. RPMI-1640 medium (5 ml) and 5 ml viral-containing
supernatant (8 μg/ml of polybrene added) were added again. After 6
h, RPMI-1640 medium containing 10% FBS was replaced. The above
method was applied to the infection of SNU-46 cells with retrovirus
carrying DJ-1 gene and empty vector virus of the control group.
Stable cell line selection
Cells were cultured continuously at 37°C for 48 h.
Antibiotic G418 (200 μg/ml) was initially used to screen the
positive clones. After 10–12 days, cells were maintained
continuously in the medium with G418 (100 μg/ml), until the cell
colony was macroscopic. The surviving cells from G418 screening
were prepared to cell suspensions. The cells were counted and
diluted with cell culture medium to 1/10 μl. G418 screening medium
prepared according to the best screening concentration was added to
a 96-well plate, with 150 μl/well, then cell suspension was added,
with 10 μl/hole. Twenty-four hours later, the plate was observed
under a microscope. The well which had only one living cell was
selected and labelled. Standard RPMI-1640 medium containing 15% FBS
medium was replaced once every two days. When single cell was
proliferated to ~100 cells, the cells in the wells were moved to a
48-well plate for continued culture after digestion. After
proliferation, cells were moved to a 24-well plate, and so on, so
cells were cultured progressively to obtain G418-resistant cell
lines after several successful and stable transfections.
CCK-8 assay
Cell Counting Kit-8 from Biyuntian Company was used
in the experiment. Cell proliferation in each group was detected at
24, 48 and 72 h, respectively, after cells were inoculated. The
inoculated cells were suspended in 96-well plates, with 100
μl/well, and each well contained 2,000 cells. The plates were
pre-cultured in an incubator (37°C, 5% CO2 overnight
stable condition). During the experiment to determine the number of
cells, 10 μl CCK-8 solution was added to each well, and the plate
was incubated for 1.5 h. The absorbance at 450 nm (OD) was measured
by a microplate reader, and a cell growth curve was drawn with time
as the abscissa and absorbance values as the vertical.
In vitro migration and invasion
assays
For the Transwell migration or invasion assays,
cells in 0.2 ml RPMI-1640 without FBS were placed on the top
chamber of each insert (BD Biosciences, Franklin Lakes, NJ, USA)
with or without 40 μl of 1 mg/ml Matrigel. The lower chamber was
filled with 600 μl of RPMI-1640 medium with 10% FBS to act as the
nutritional attractant. Twenty-four hours later, the migrant cells
that had attached to the lower surface were fixed with 20% methanol
and stained for 20 min with crystal violet. The membranes were then
carved and embedded under cover slips with the cells on the top.
Cells in three different fields of view at ×100 magnification were
counted and expressed as the average number of cells per field of
view. All assays were performed in triplicate.
Real-time RT-PCR
Real-time RT-PCR was performed to detect the
relative level of DJ-1 transcription and mature miRNA. Briefly,
complement DNA (cDNA) was generated through reverse transcription
using M-MLV reverse transcriptase (Promega) with 1 μg of total RNA.
This cDNA product was used to amplify the DJ-1 gene. The PCR
conditions were: 95°C for 5 min, followed by 40 cycles of 95°C for
15 sec, 60°C for 15 sec and 72°C for 32 sec.
Western blot analysis
The cells were lysed with RIPA lysis buffer and the
proteins were harvested. All proteins were resolved on a 10% SDS
denatured polyacrylamide gel and then transferred onto a
nitrocellulose membrane. The membranes were incubated overnight at
4°C with mouse monoclonal to PARK7/DJ1 (Abcam, USA) and rabbit
monoclonal anti-human GAPDH, PTEN, AKT/phospho-Akt (Ser473),
mTOR/phospho-mTOR (Ser2448) (Cell Signaling Technology, Inc.). Goat
anti-mouse IgG-HRP (1:1,000; Jackson Co.) and goat anti-rabbit
IgG-HRP (1:500; Santa Cruz Inc.) were used for secondary antibody
and DAB coloring.
Flow cytometry
Apoptosis detection kit from Nanjing Kaiji Co. was
used in the experiment. Cells were collected with EDTA-free trypsin
and washed twice with PBS, followed by centrifugation (2,000 rpm)
for 5 min. Binding buffer (500 μl) was added for cell suspension.
Annexin V-EGFP (5 μl) was added and mixed well, followed by
addition of 5 μl propidium iodide and mixed well. The mixture was
reacted at room temperature for 5–15 min in a dark environment,
followed by detection with flow cytometry, excitation wavelength
Ex, 488 nm; emission wavelength Em, 530 nm. Green fluorescence of
Annexin V-EGFP was detected with FITC channel (FL1); PI red
fluorescence was detected by PI channel (FL2 or FL3). Fluorescence
compensation adjustment; normal untreated cells were used as
control for fluorescence compensation adjustment to remove spectra
overlap and set the cross gate position.
Statistical analysis
All measurement data are expressed as the means ±
SD. Differences among groups were analyzed by one-way ANOVA and
Student-Newman-Keuls (SNK) q-test using SPSS 12.0 software for
Windows. Statistical significance was defined as having a p-value
<0.05.
Results
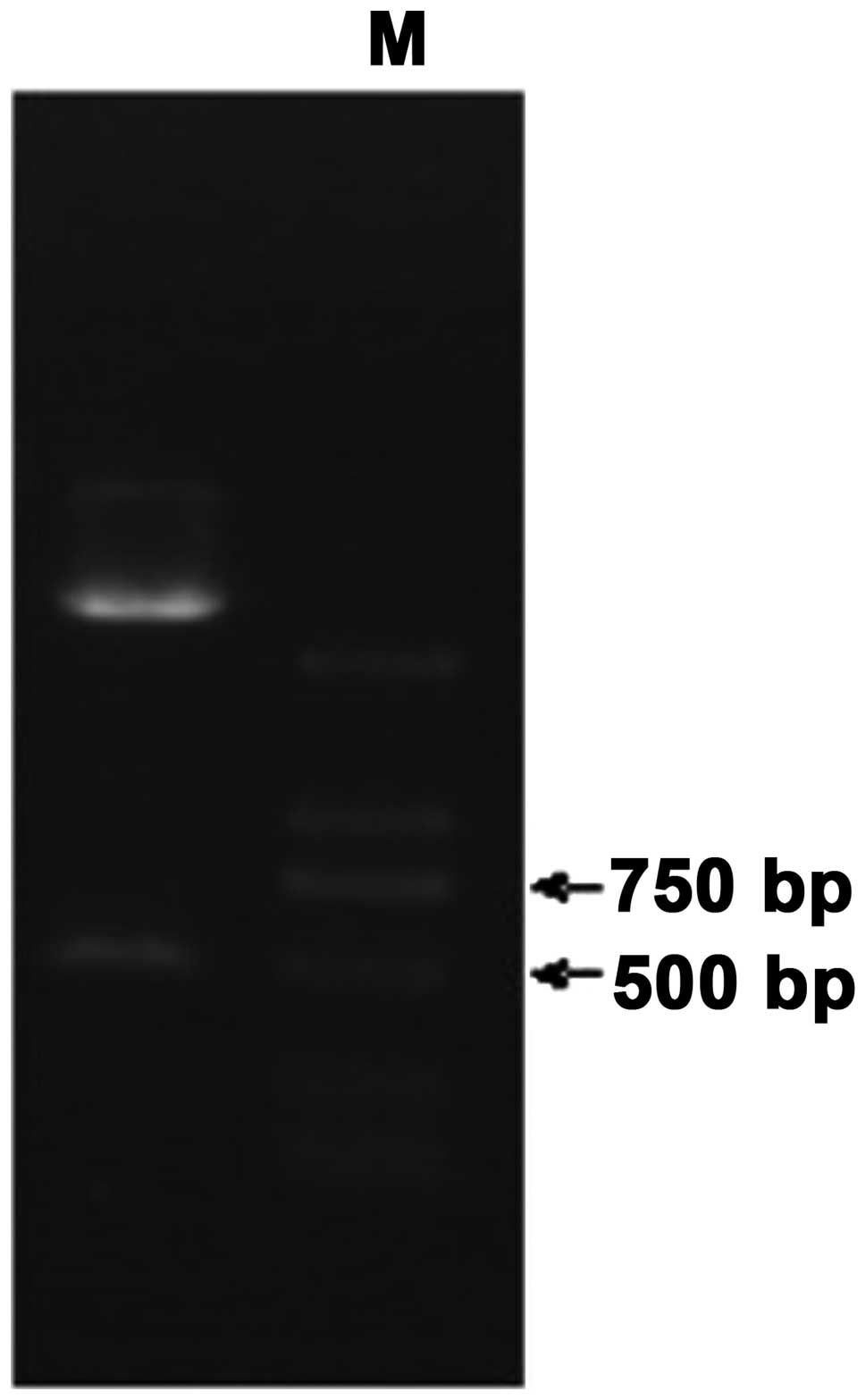
Double enzyme digestion of the
recombinant plasmid pLNCX2-DJ-1
The recombinant plasmid pLNCX2-DJ-1 was transformed
into competent E. coli, and then the plasmid was extracted
after shaking bacteria. NotI, XhoI double digestion
was performed on the exacted plasmid. The digestion result was
detected with 1% agarose gel electrophoresis. There was clearly
visible strip for the double digestion products near 600 bp
(Fig. 1). Forward sequencing was
carried out for the recombinant plasmid pLNCX2-DJ-1 according to
pLNCX2 carrier up and downstream primer sequences. BLAST alignment
was performed for the sequencing results (Beijing Liuhehua
Biotechnology) with DJ-1 sequence in NCBI and matching degree was
100%.
Determination of G418 screening
concentration
After 10 concentration gradient tests of G418 in
100–1,000 μg/ml, the minimum G418 concentration in which cells died
within 10 days was 100 μg/ml. Therefore, the optimum concentration
of G418 screening was 100 μg/ml.
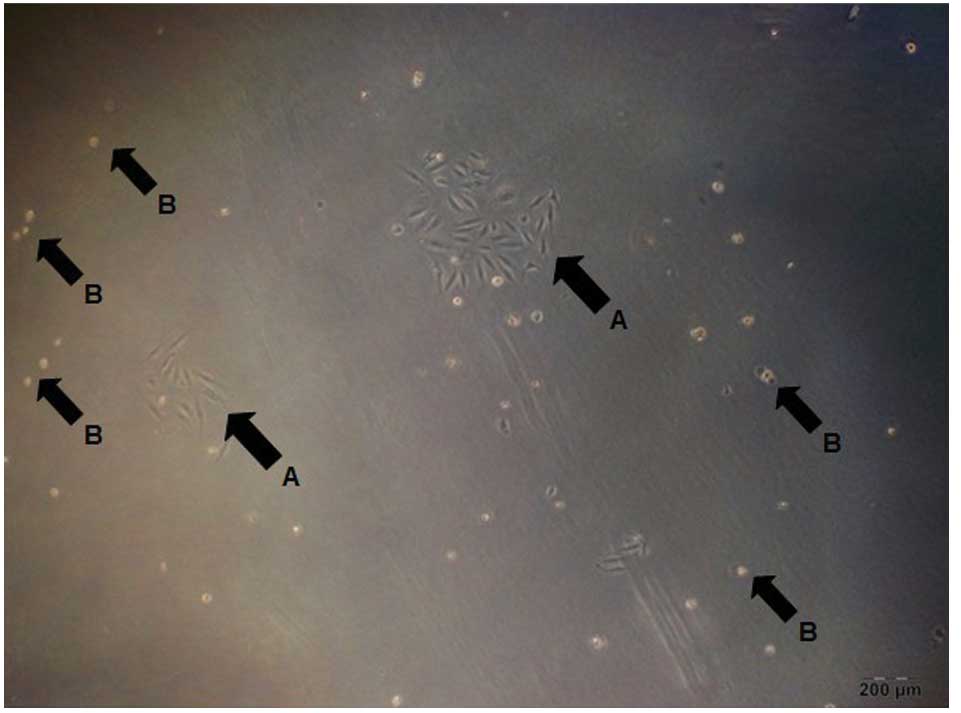
Screening of laryngeal carcinoma cell
lines of stably transfected DJ-1 protein overexpression
SNU-46 cells infected with the virus were screened
in a normal medium without G418. As shown in Fig. 2, cells infected with retroviral that
did not obtain stable transfection gradually died without G418
resistance, and cells that stably transfected had G418 resistance
and continued proliferation. Cells which obtained G418 resistance
continued to culture in the screening of antibiotics environment to
make a large amount. Then, several monoclonal cell lines were
selected with the limiting dilution method. Three monoclonal cell
lines that were infected with retrovirus carrying DJ-1 gene and
screened through G418 were selected and termed SNU-46-D1 (Fig. 3), SNU-46-D2 (Fig. 4) and SNU-46-D3 (Fig. 5). One monoclonal cell line which was
infected with empty vector retrovirus not carrying gene DJ-1 and
screened after G418 was selected and termed SNU-46-CON (Fig. 6). Fig.
7 shows untreated normal SNU-46 cells.
qRT-PCR detection of DJ-1 mRNA expression
levels of monoclonal cell lines
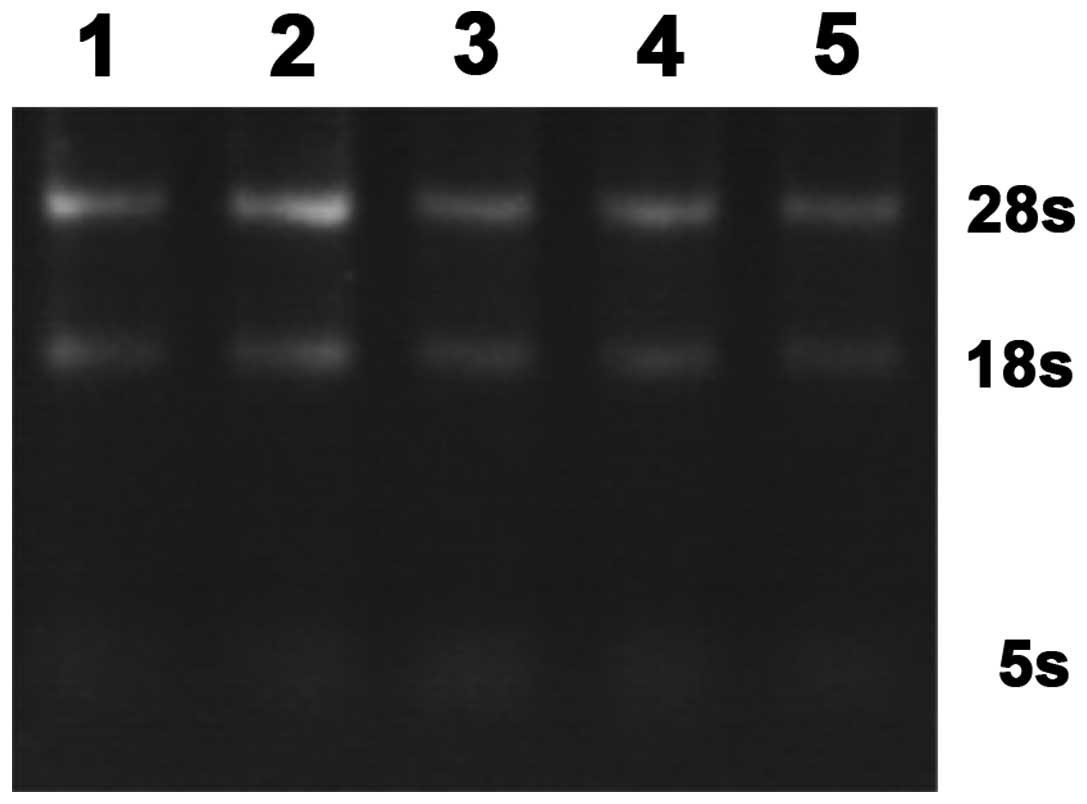
RNA sample (1 μl) was obtained for 1% agarose gel
electrophoresis 80 V × 20 min. Total RNA 5s, 18s and 28s rRNA bands
were observed by gel imaging system. Three complete bands proved a
complete extraction of total RNA (Fig.
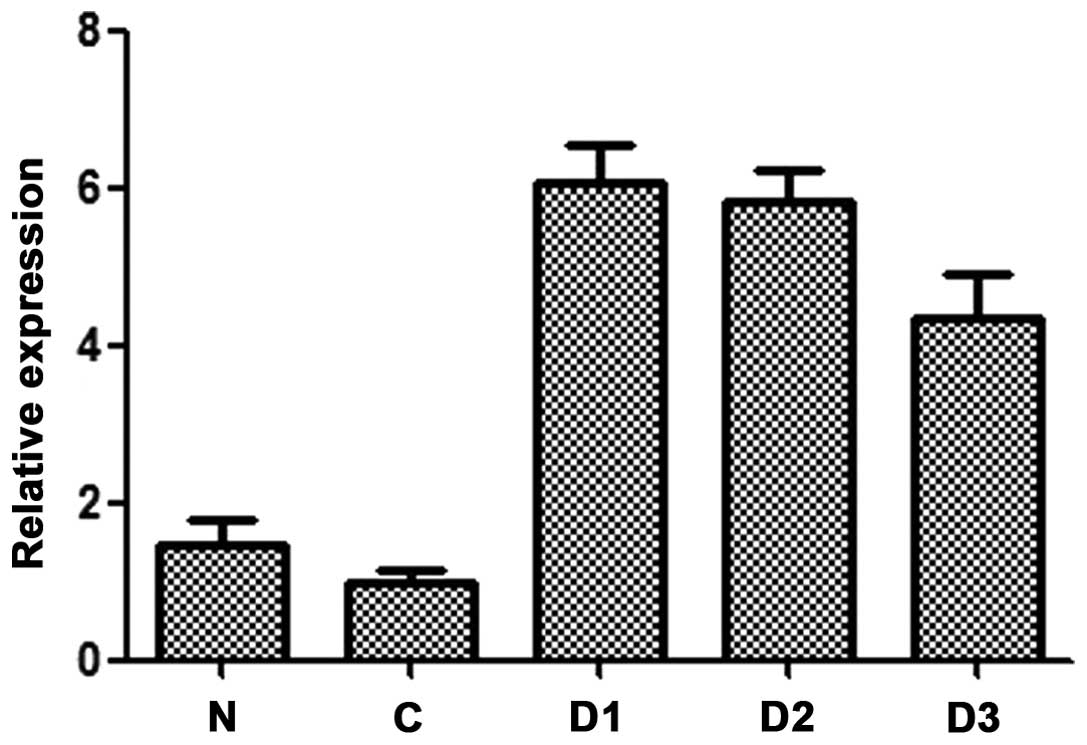
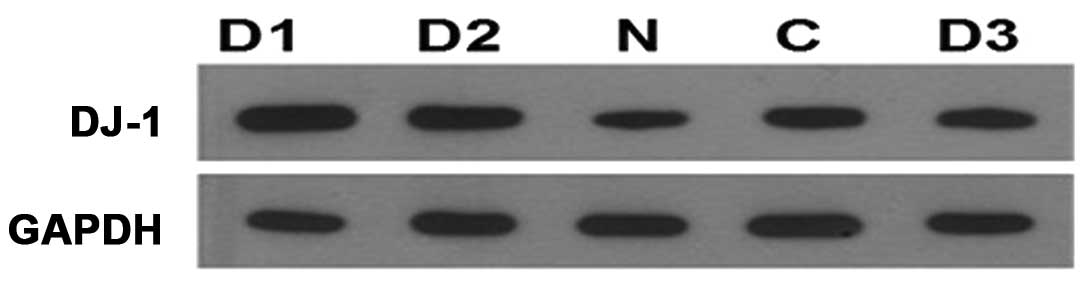
8). DJ-1 gene expression in five types of samples is shown in
Fig. 9, and DJ-1 gene expression
levels of SNU-46-D1, SNU-46-D2 and SNU-46-D3 are significantly
higher than SNU-46-CON and SNU-46 cells.
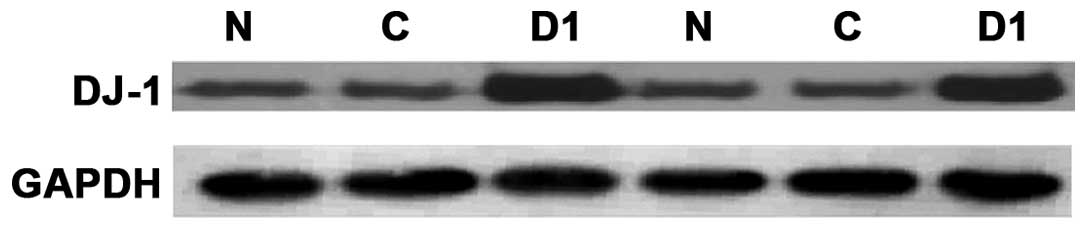
Western blot detection of DJ-1 protein
expression levels in each monoclonal cell line
The total protein was extracted after SNU-46-D1,
SNU-46-D2, SNU-46-D3, SNU-46-CON and SNU-46 cell proliferation, and
DJ-1 protein expression levels of monoclonal cell lines were
detected by western blotting. The results showed that DJ-1 protein
expression of SNU-46-D1 cells was significantly higher than other
cell groups (Fig. 10). SNU-46-D1
cell protein was extracted again and SNU-46-CON and SNU-46 cells
were used as control. Western blotting was repeated twice to verify
if DJ-1 protein expression of SNU-46-D1 was higher than normal
SNU-46 and SNU-46-CON empty vector control cells (Fig. 11). The results of the three repeats
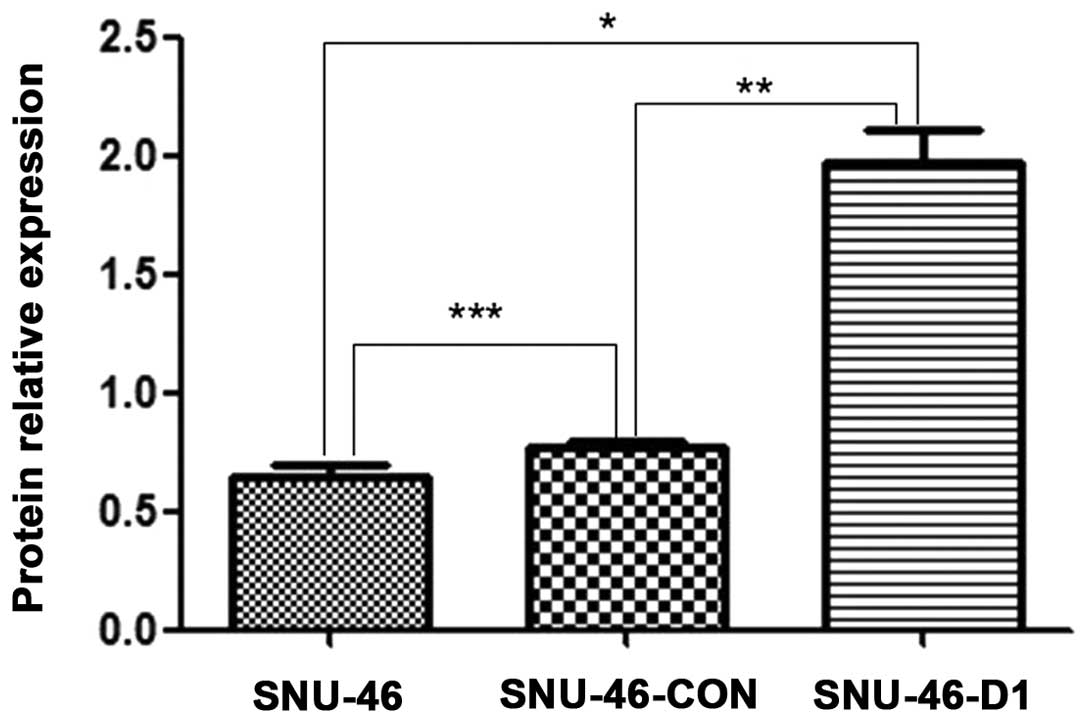
were subjected to grey and statistical analysis, and it showed the
relative DJ-1 protein expression of SNU-46-D1 cells was
1.969±0.137. There was a significant difference
(*p<0.001; **p<0.001; Fig. 12) compared to SNU-46 (0.624±0.079)
and SNU-46-CON empty vector control cells (0.783±0.279), while the
relative DJ-1 protein expression levels of SNU-46 and SNU-46-CON
empty vector control cells showed no significant difference
(***p=0.081). Compared with the blank control SNU-46
cells, DJ-1 protein expression levels increased 2.16-fold,
therefore monoclonal cell line SNU-46-D1 of DJ-1 protein
overexpression was successfully constructed.
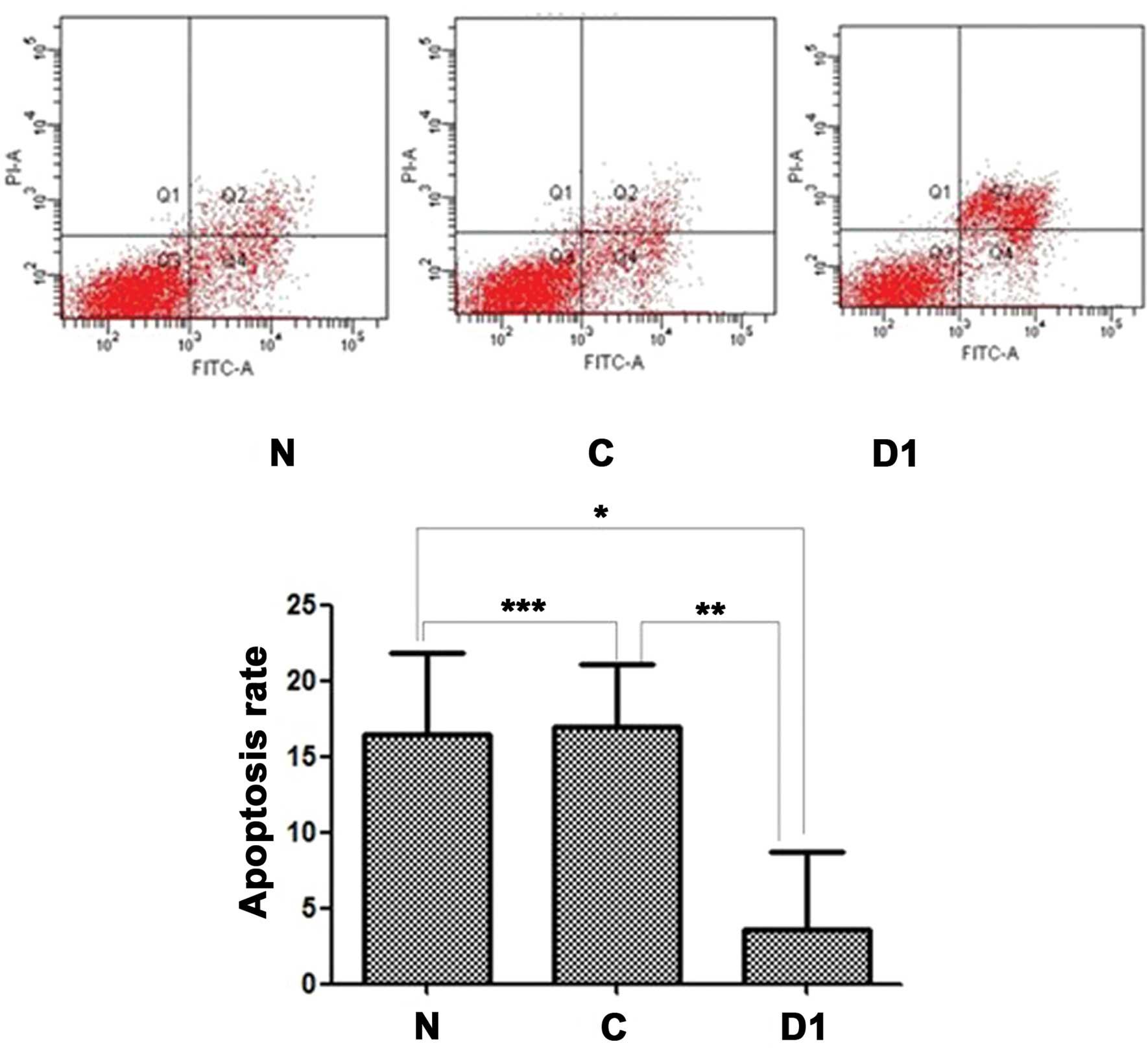
Overexpression of DJ-1 protein reduces
SNU-46 cell apoptosis rate
Fig. 13 shows
results of the apoptosis rate of three cell lines using flow
cytometry with the Annexin V/PI apoptosis kit. There was a
significant difference (p<0.05) of the apoptotic cell rate among
SNU-46-D1, SNU-46-CON and SNU-46. Combining multiple comparison
results showed the apoptotic ratio of SNU-46-D1 cell line
(3.533±5.167%) was significantly lower than that of SNU-46
(16.397±5.447%) and SNU-46-CON cell lines (16.980±4.124%)
(*p=0.019; **p=0.016). There was no
statistical difference between cell line SNU-46 apoptosis rate and
cell line SNU-46-CON (***p=0.890).
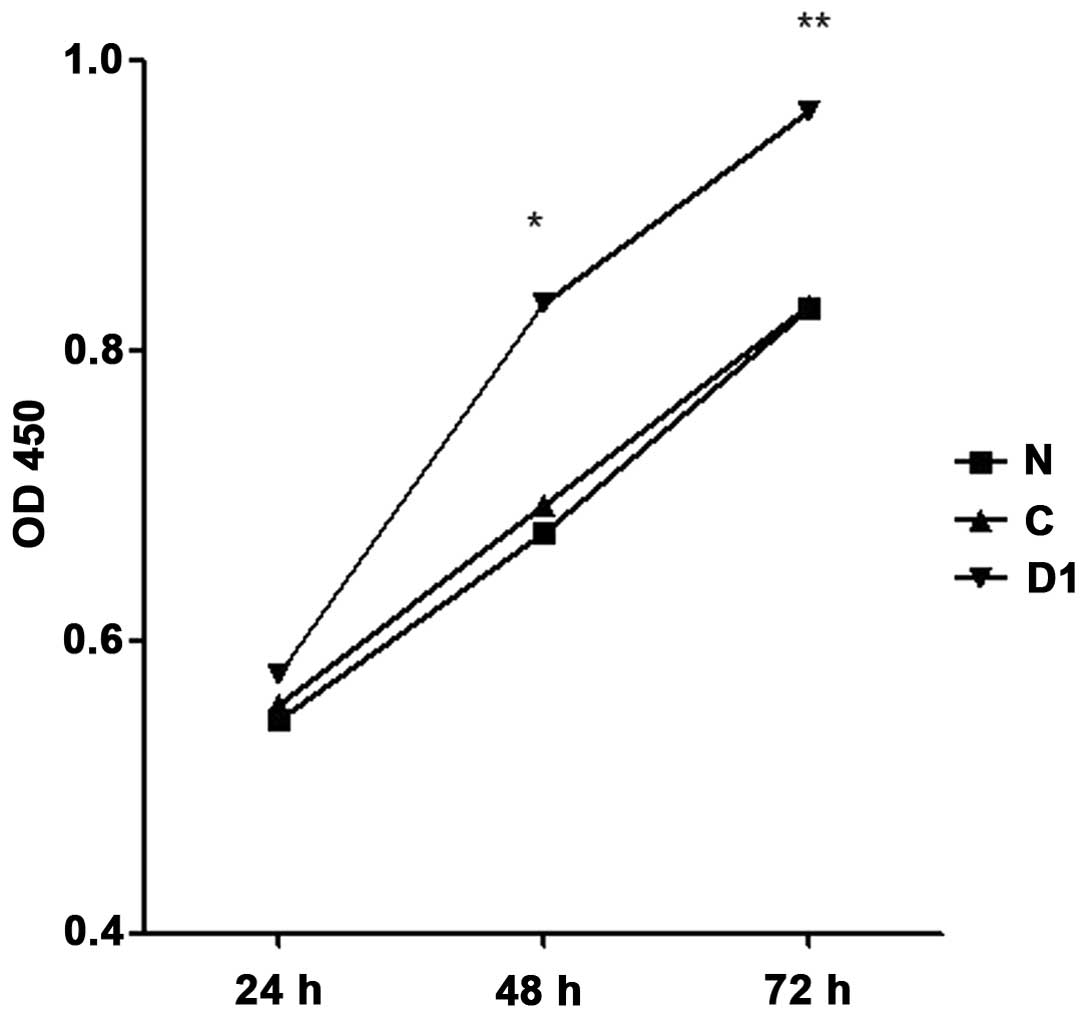
Overexpression of DJ-1 protein promotes
SNU-46 cell proliferation
Results of CCK-8 detection of cell proliferation are
shown in Fig. 14. Forty-eight and
72 h after inoculation, SNU-46-D1 cell proliferation rate was
higher than SNU-46 and SNU-46-CON cells (48 h, 0.834±0.336 vs.
0.676±0.112, *p<0.001; 0.834±0.336 vs. 0.694±0.210,
*p<0.001; 72 h, 0.965±0.177 vs. 0.830±0.172,
**p<0.001; 0.965±0.177 vs. 0.831±0.180,
**p<0.001).
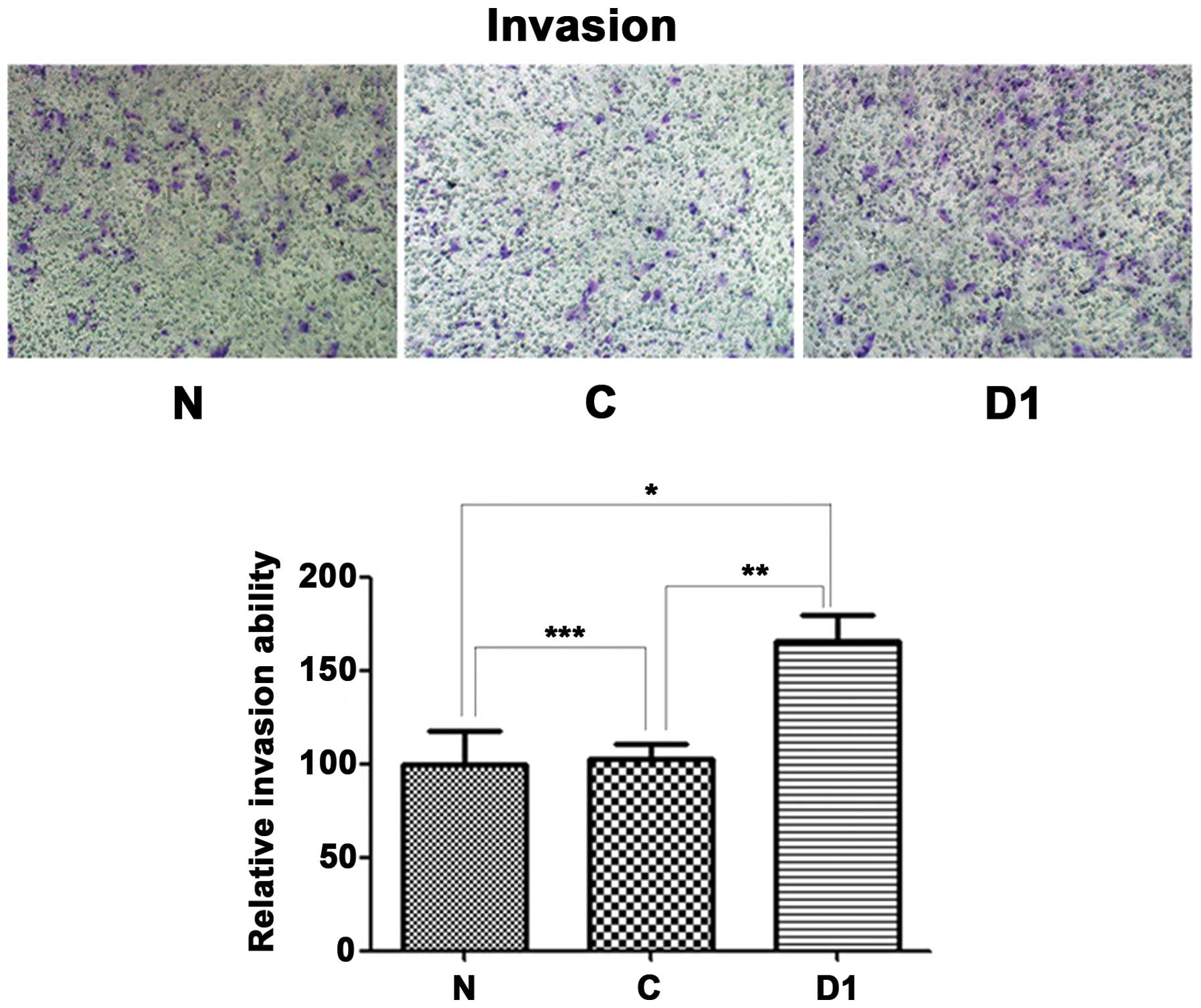
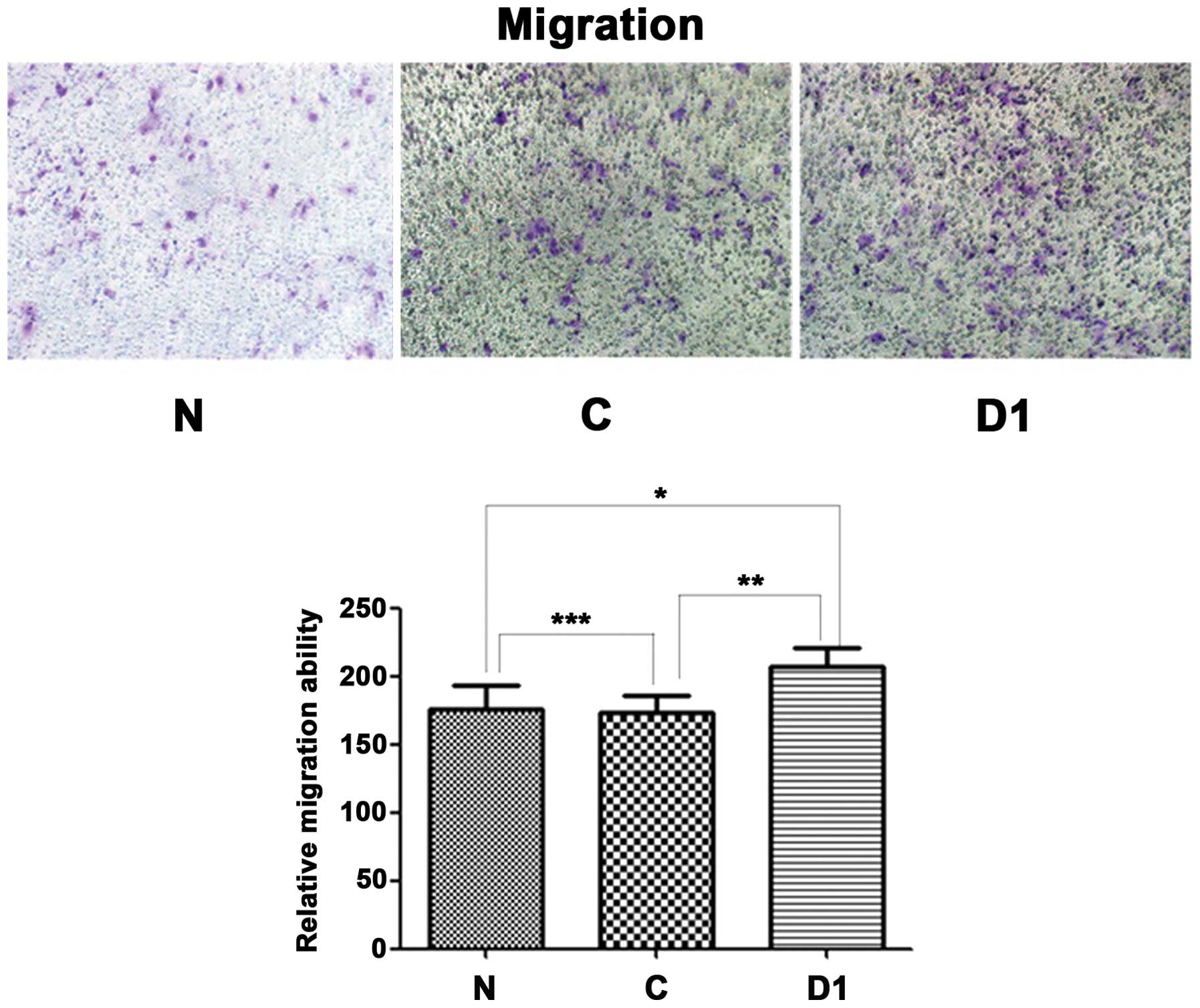
Overexpression of DJ-1 protein enhances
SNU-46 cell invasion and migration
As shown in Fig.
15, SNU-46-D1 cell invasion force was higher than SNU-46 and
SNU-46-CON cells (165.7±13.6 vs. 100.0±17.4, *p=0.001;
165.7±13.6 vs. 103.0±7.6, *p=0.001); as shown in
Fig. 16, SNU-46-D1 cell migration
was higher than SNU-46 and SNU-46-CON cells (207.3±13.1 vs.
175.3±13.3, *p=0.036; 207.3±13.1 vs. 173.0±12.5,
*p=0.027).
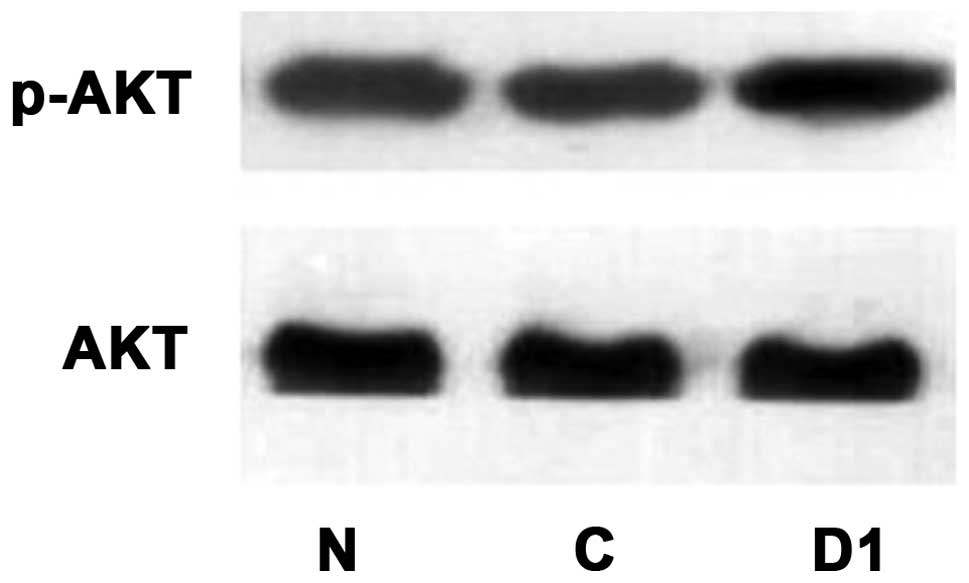
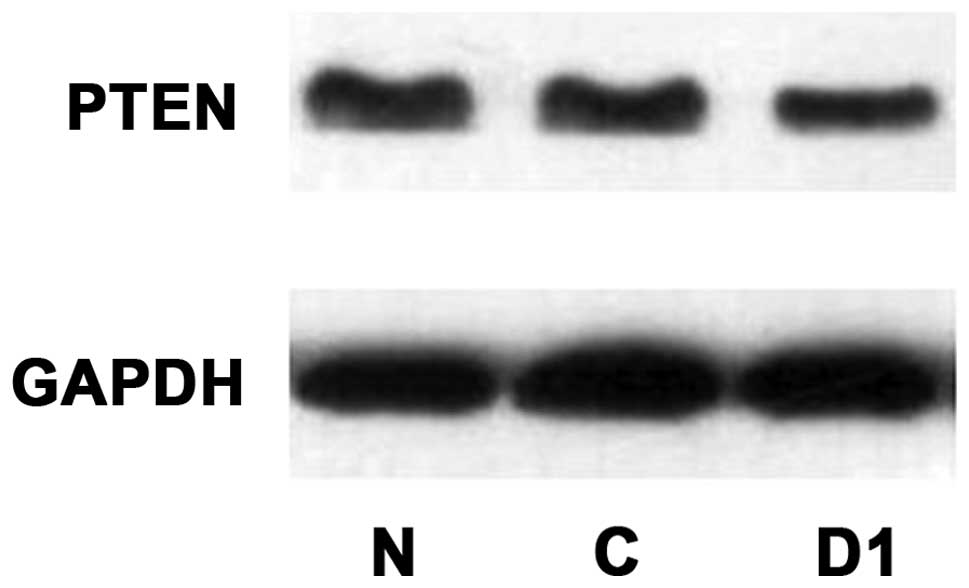
Western blot detection of changes in
p-AKT, AKT, p-mTOR, mTOR, PTEN protein expression levels after DJ-1
overexpression
As shown in Figs.
17–19, p-AKT protein
expression levels improved after overexpression of DJ-1, while AKT
protein expression did not change. p-mTOR protein expression
increased and mTOR protein expression did not change. PTEN protein
expression decreased.
Discussion
Laryngeal cancer is ranked second in the incidence
of head and neck tumors, and the most common histological type is
squamous cell carcinoma (13–15).
Currently, the overall 5-year survival rate is still hovering at
55–65% (16–21). The molecular biological mechanisms
of the pathogenesis of laryngeal cancer remain to be explored.
Previous studies found that there was overexpression of DJ-1 of
laryngeal tissue and Hep-2 cells, and poorly differentiated
laryngeal cancer had higher DJ-1 expression level than well
differentiated laryngeal cancer. Silencing DJ-1 gene can
effectively inhibit the proliferation of Hep-2 cells, inducing
apoptosis (22). However, the
effect of DJ-1 gene overexpression in laryngeal cancer cells on the
biological behavior of laryngeal tumor cells has not been reported.
Also, the effect of DJ-1 gene overexpression on laryngeal cancer
proliferation, migration, invasion, apoptosis and other biological
characteristics of the tumor cells remains unclear. The signaling
pathway through which the DJ-1 gene mediates to affect laryngeal
cancer is also unknown. Therefore, on the basis of previous
studies, we stably transfected DJ-1 gene through retrovirus to
laryngeal squamous cell carcinoma SNU-46 cell line and successfully
screened out laryngeal squamous cell lines with stable
overexpression of DJ-1 protein. The differences of biological
characteristics between laryngeal carcinoma cell line SNU-46-D1
with DJ-1 gene overexpression and normal laryngeal cancer cells was
detected, thereby we preliminarily studied the role of DJ-1
overexpression in cell proliferation, invasion, migration,
apoptosis, and established a research basis for future studies in
nude mice.
The early pre-experiment showed that the stable
transfection efficiency of SNU-46 laryngeal squamous cell line with
electroporation and lipofection is very low. In the present study,
retrovirus pLNCX2-DJ-1 vector carrying DJ-1 gene was constructed;
pVSV-G packaging plasmids and GP2-293 packaging cells were used to
successfully package viral particles carrying DJ-1 gene, which in
turn infected SNU-46 cells to achieve stable DJ-1 gene expression
in SNU-46 laryngeal cancer cells. Since the pLNCX2-DJ-1 vector also
contains the neo gene in addition to DJ-1 gene sequence,
successfully transfected cells obtained resistance to antibiotic
G418. Therefore, cells that were not successfully transfected could
be eliminated through G418 screening. In the present study, a
laryngeal carcinoma cell line with stable overexpression of DJ-1
protein was successfully screened out, SNU-46-D1. After western
blotting, DJ-1 protein expression increased 2.16-fold compared to
normal SNU-46 cells. We also screened an SNU-46 cell line as the
empty vector control (SNU-46-CON), of which DJ-1 expression level
was no different to untreated cells.
Apoptosis is a suicide mechanism of cell active
participation (22) and evasion
apoptosis is one of the basic characteristics of tumor cells. The
defect of apoptotic signaling pathway is widespread in cancer. In
the present study, after flow cytometry detection with Annexin V/PI
apoptosis kit, the results showed that the apoptosis rate of
SNU-46-D1 was significantly lower than the rates of cell lines
SNU-46 and SNU-46-CON, suggesting that overexpression of the DJ-1
gene inhibited apoptosis of laryngeal cancer cells. Previous
reports showed that DJ-1 gene silencing can induce apoptosis of
laryngeal cancer Hep-2 cells (23).
Therefore, the DJ-1 gene may play a role in inhibiting apoptosis of
laryngeal cancer cells to prolong laryngeal cell survival time and
provide the conditions for the growth of laryngeal cancer cells.
CCK-8 detection of cell proliferation showed that SNU-46-D1 cell
proliferation rate was faster than normal SNU-46 cells. The
difference was statistically significant. This suggests that the
DJ-1 gene plays a facilitating role in the proliferation of SNU-46
cells. After detecting the invasion and migration ability of SNU-46
laryngeal cancer cells and SNU-46-D1 cells with stably transfected
DJ-1 protein overexpression by Transwell chamber experiments, we
found that after stable transfection by up to 2× the DJ-1 protein
expression, invasion of SNU-46-D1 cells increased by ~65% compared
to normal SNU-46, and migration increased by ~20%. This suggests
that overexpression of DJ-1 protein can enhance the ability of
laryngeal cancer cell invasion and migration. Through stable
transfection for laryngeal cells SNU-46 to overexpress DJ-1
protein, the apoptosis rate decreased, proliferation rate
increased, invasion and migration capabilities also improved. This
suggests that the DJ-1 gene may play a role in laryngeal cancer
genes similar to oncogenes, and laryngeal DJ-1 gene high expression
may lead to increased laryngeal malignancy, reducing the prognosis.
Zhu et al used immunohistochemistry to detect tumor tissues
of 7l postoperative patients with laryngeal squamous cell carcinoma
and laryngeal mucosa of 9 patients with non-laryngeal who were
treated between January 1995 and January 2006. After analyzing the
relationship between DJ-1 protein expression and
clinicopathological features, they found DJ-l protein expression
levels in laryngeal squamous cell carcinoma were higher than in
normal laryngeal mucosa, and survival rates may be lower for
laryngeal cancer patients with higher levels of DJ-1 protein
expression (23). In 2012, Zhu
et al also found that the DJ-l protein overexpression in
tumor tissue of supraglottic laryngeal squamous cell carcinoma was
associated with lymph node metastasis. Lymph node metastasis has
higher incidence for patients with high DJ-1 protein expression.
The five-year survival rate of patients with high DJ-1 protein
expression in supraglottic laryngeal squamous cell carcinoma is
also lower (53.9 vs. 88.0%; p=0.007; log-rank test) (24). The data on DJ-1 expression levels
and clinical tumor malignancy and prognosis are consistent with our
conclusion regarding the impact of DJ-1 protein on laryngeal
carcinoma cell SNU-46 functions. The DJ-1 gene may play a role
similar to oncogenes in laryngeal carcinoma, and can inhibit
apoptosis. The DJ-1 gene can extend the survival time of laryngeal
cancer cells, and provide conditions for laryngeal cancer cell
growth. At the same time, the DJ-1 gene can promote the
proliferation of laryngeal cancer cells, resulting in the rapid
expansion of laryngeal cancer cells and accelerated tumor growth.
More importantly, the DJ-1 gene can enhance the ability of
laryngeal cancer cell invasion and migration. It may lead to tumor
lymph node metastasis and distant metastasis, resulting in an
adverse impact on the prognosis of patients.
Through stable transfection of DJ-1 gene to SNU-46
cells and detection of changes in the biological characteristics of
the tumor cells, this study preliminarily showed the DJ-1 gene can
inhibit tumor cell apoptosis and promote proliferation, migration
and invasion of tumor cells. Regarding the molecular pathway
mechanism which leads to changes in the biological characteristics
of these tumors, current studies support that DJ-1 protein affects
biological properties of the cells mainly through negative
regulation of PTEN (phosphatase and tensin homolog) expression. Kim
et al found DJ-1 expression in breast cancer specimens was
negatively correlated with PTEN and positively correlated with
PKB/Akt (6). Zhu et al found
that the DJ-l protein overexpression in tumor tissue of
supraglottic laryngeal squamous cell carcinoma was accompanied by
low expression of PTEN (24). PTEN
is a tumor suppressor that can inhibit the phosphorylation of PIP2
to reduce the PIP3 generation and ultimately reduce PKB/Akt
activation and inhibit cell growth (4.) DJ-1 protein overexpression
can lead to reduced expression of PTEN, thereby removing the
inhibition of PTEN on cell growth, and promoting cell growth and
proliferation. Studies have also suggested that the DJ-1 gene can
promote the PI3K/AKT/mTOR pathway in lung cancer cells by
regulating PTEN gene, thereby enabling increased expression of a
variety of detoxifying enzymes and promoting cell proliferation
(11). Regarding the approach of
DJ-1 gene regulation on PTEN, some researchers believe the DJ-1
gene may be involved in the regulation of PTEN gene through the
PI3K-AKT/PKB signaling pathway to act on c-myc (25). Other researchers consider that DJ-1
may regulate PTEN through their redox-sensitive molecular chaperone
activity (26). In addition,
studies have also found that DJ-1 can activate hypoxia-inducible
factor l (HIF1) in the absence of oxygen, and has a crucial role in
maintaining the stability of HIF1, therefore DJ-1 is resistant to
hypoxia-mediated apoptosis, protecting cells under hypoxia.
Meanwhile DJ-1 can also regulate activity of AMP protein kinase
(AMPK) which controls cell metabolism. This regulation is more
obvious under a hypoxic environment (27). These studies suggest that the
laryngeal apoptosis rate decreased after the overexpression of DJ-1
most likely since DJ-1 can help tumor cells to evade apoptosis
incurred by an anoxic environment.
Our previous studies showed Akt and mTOR
overexpression in laryngeal cancer are correlated with prognosis,
and are negatively correlated with tumor differentiation. The
recurrent rate of patients with overexpression of downstream gene
EIF4E on the signaling pathway in laryngeal tissue and
overexpression of eIF4E on surgical margins is high, and the
prognosis is poor. mTOR inhibitor rapamycin can stop Hep-2 cells in
the G1 phase, inhibiting their proliferation and promoting
apoptosis. Joint application of rapamycin and cisplatin can have a
synergistic effect (28). LSCC DJ-1
protein overexpression and Akt/mTOR overexpression often occur
simultaneously. Previous studies have shown DJ-1 may mediate the
PI3K/AKt/mTOR signaling pathway to affect the survival of a variety
of tumor cells (6,27). Through the construction of SNU-46
laryngeal squamous cell line of stably overexpressed DJ-1 protein,
and detection of expression level changes of proteins associated
with the PI3K/AKt/mTOR signaling pathway between this cell and
normal SNU-46 cell line, this study provided preliminary validation
that DJ-1 may affect the biological characteristics of laryngeal
cancer cells through the PI3K/AKt/mTOR signaling pathway.
Through the construction of stable overexpression of
DJ-1 protein in laryngeal carcinoma cell lines, the present study
established a foundation for subsequent in vivo experiments
in nude mice. We will carry out more comprehensive studies on
SNU-46-D1 cell lines with stable overexpression of the DJ-1 gene to
gain more in-depth understanding of the function of DJ-1 gene in
the occurrence and development of laryngeal cancer and cell
signaling pathways mediated by DJ-1 gene, thereby laying the
foundations for the assessment of the feasibility and efficacy of
the DJ-1 gene as a new therapeutic target for laryngeal cancer.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (81072224), the Young Faculty
Cultivation Project of Sun Yat-sen University (10ykpy10), and the
Science and Technology Planning Project of Guangdong Province
(2009B030801109).
References
|
1
|
Nagakubo D, Taira T, Kitaura H, et al:
DJ-1, a novel oncogene which transforms mouse NIH3T3 cells in
cooperation with ras. Biochem Biophys Res Commun.
231:509–513. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Annesi G, Savettieri G, Pugliese P, et al:
DJ-1 mutations and parkinsonism-dementia-amyotrophic lateral
sclerosis complex. Ann Neurol. 58:803–807. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Miller DW, Ahmad R, Hague S, et al: L166P
mutant DJ-1, causative for recessive Parkinson’s disease, is
degraded through the ubiquitin-proteasome system. J Biol Chem.
278:36588–36595. 2003.
|
|
4
|
Martinat C, Shendelman S, Jonason A, et
al: Sensitivity to oxidative stress in DJ-1-deficient dopamine
neurons: an ES-derived cell model of primary Parkinsonism. PLoS
Biol. 2:e3272004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Moore DJ, Zhang L, Troncoso J, et al:
Association of DJ-1 and parkin mediated by pathogenic DJ-1
mutations and oxidative stress. Hum Mol Genet. 14:71–84. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim RH, Peters M, Jang Y, et al: DJ-1, a
novel regulator of the tumor suppressor PTEN. Cancer Cell.
7:263–273. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
MacKeigan JP, Clements CM, Lich JD, et al:
Proteomic profiling drug-induced apoptosis in non-small cell lung
carcinoma: identification of RS/DJ-1 and RhoGDIα. Cancer Res.
63:6928–6934. 2003.PubMed/NCBI
|
|
8
|
Hod Y: Differential control of apoptosis
by DJ-1 in prostate benign and cancer cells. J Cell Biochem.
92:1221–1233. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Le Naour F, Misek DE, Krause MC, et al:
Proteomics-based identification of RS/DJ-1 as a novel circulating
tumor antigen in breast cancer. Clin Cancer Res. 7:3328–3335.
2001.PubMed/NCBI
|
|
10
|
Yoshino T, Shiina H, Urakami S, et al:
Bcl-2 expression as a predictive marker of hormone-refractory
prostate cancer treated with taxane-based chemotherapy. Clin Cancer
Res. 12:6116–6124. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shi W, Zhang X, Pintilie M, et al:
Dysregulated PTEN-PKB and negative receptor status in human breast
cancer. Int J Cancer. 104:195–203. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Davidson B, Hadar R, Schlossberg A, et al:
Expression and clinical role of DJ-1, a negative regulator of PTEN,
in ovarian carcinoma. Hum Pathol. 39:87–95. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chu EA and Kim YJ: Laryngeal cancer:
diagnosis and preoperative work-up. Otolaryngol Clin North Am.
41:673–695. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar
|
|
15
|
Adelstein DJ, Li Y, Adams GL, et al: An
intergroup phase III comparison of standard radiation therapy and
two schedules of concurrent chemoradiotherapy in patients with
unresectable squamous cell head and neck cancer. J Clin Oncol.
21:92–98. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hoffman HT, Porter K, Karnell LH, et al:
Laryngeal cancer in the United States: changes in demographics,
patterns of care, and survival. Laryngoscope. 116:1–13. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Denis F, Garaud P, Bardet E, et al: Final
results of the 94-01 French Head and Neck Oncology and Radiotherapy
Group randomized trial comparing radiotherapy alone with
concomitant radiochemotherapy in advanced-stage oropharynx
carcinoma. J Clin Oncol. 22:69–76. 2004. View Article : Google Scholar
|
|
18
|
Forastiere AA, Maor M, Weber RS, et al:
Long-term results of intergroup RTOG 91-11: a phase III trial to
preserve the larynx-induction cisplatin/5-FU and radiation therapy
versus concurrent cisplatin and radiation therapy versus radiation
therapy. J Clin Oncol. 24(Suppl 18): 55172006.
|
|
19
|
Seiwert TY and Cohen EE: State-of-the-art
management of locally advanced head and neck cancer. Br J Cancer.
92:1341–1348. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Brockstein B, Haraf DJ, Rademaker AW, et
al: Patterns of failure, prognostic factors and survival in
locoregionally advanced head and neck cancer treated with
concomitant chemoradiotherapy: a 9-year, 337-patient,
multi-institutional experience. Ann Oncol. 15:1179–1186. 2004.
|
|
21
|
Kutter J, Ozsahin M, Monnier P and Stupp
R: Combined modality treatment with full-dose chemotherapy and
concomitant boost radiotherapy for advanced head and neck
carcinoma. Eur Arch Otorhinolaryngol. 262:1–7. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kerr JF, Wyllie AH and Currie AR:
Apoptosis: a basic biological phenomenon with wide-ranging
implications in tissue kinetics. Br J Cancer. 26:239–257. 1972.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhu XL, Wang ZF, Lei WB, et al: DJ-1: a
novel independent prognostic marker for survival in glottic
squamous cell carcinoma. Cancer Sci. 101:1320–1325. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhu XL, Wang ZF, Lei WB, et al:
Tumorigenesis role and clinical significance of DJ-1, a negative
regulator of PTEN, in supraglottic squamous cell carcinoma. J Exp
Clin Cancer Res. 31:942012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sitaram RT, Cairney CJ, Grabowski P, et
al: The PTEN regulator DJ-1 is associated with hTERT expression in
clear cell renal cell carcinoma. Int J Cancer. 125:783–790. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shendelman S, Jonason A, Martinat C, et
al: DJ-1 is a redox-dependent molecular chaperone that inhibits
α-synuclein aggregate formation. PLoS Biol. 2:e3622004.PubMed/NCBI
|
|
27
|
Vasseur S, Afzal S, Tardivel-Lacombe J, et
al: DJ-1/PARK7 is an important mediator of hypoxia-induced cellular
responses. Proc Natl Acad Sci USA. 106:1111–1116. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lei W, Jia T, Su Z, et al: Combined effect
of rapamycin and cisplatin on survival of Hep-2 cells in vitro.
Oncol Res. 18:73–81. 2009. View Article : Google Scholar : PubMed/NCBI
|