1. Introduction
Leukemia is a type of cancer of the blood or bone
marrow characterized by an abnormal increase in immature leukocytes
called ‘blasts’ which are arrested in the early phases of
differentiation. Most leukemias show non-random chromosomal
abnormalities, of which the majority are chromosomal
translocations. These genetic lesions cause activation of
proto-oncogenes and inactivation of tumor-suppressor genes,
ultimately resulting in leukemogenesis. The molecular pathological
alterations in the disease impair the regulation of normal cellular
processes, such as cell differentiation, cell proliferation, cell
cycle progression and cell death. Leukemia is a common malignant
disease and can occur in individuals at any age. Thousands of
people died from leukemia every year around the world. There are
four major types of leukemia, including acute myeloid leukemia
(AML), chronic myeloid leukemia (CML), acute lymphoblastic leukemia
(ALL) and chronic lymphocytic leukemia (CLL). Acute leukemia is
characterized by a rapid clinical progression and accumulation of
malignant blood cells, whereas chronic leukemia is characterized by
a slow clinical progression and an increase in relatively mature
but abnormal leukocytes. Lymphoblastic and lymphocytic leukemias
involve lymphocytes. Myeloid leukemia affects red blood cells, some
leukocytes and platelets. Epidemiologic, genotypic and animal model
data all indicate a multistep and complicated oncogenic process of
leukemia. Notably, the diversifying genetic and molecular
alterations that drive malignant transformation of blood cells
critically contribute to the pathogenesis of leukemia and the
heterogeneity of the disease. Therefore, understanding the genetic
basis and molecular events of various leukemias may provide new
insights into leukemia diagnoses, prognoses and therapies.
Forkhead box (FOX) proteins are a superfamily of
evolutionarily conserved transcriptional factors which play
significant roles in a wide variety of cellular processes, such as
differentiation, proliferation, cell cycle progression, apoptosis,
metabolism and migration. They are characterized by a forkhead or
winged helix DNA-binding domain. Fifty human FOX proteins, which
are further divided into 19 subfamilies (FOXA to FOXS) according to
their sequence homology inside and outside the DNA-binding domain,
have been identified (1). Human FOX
proteins are a large family, displaying a remarkable functional
diversity and regulation complexity. Besides being transcription
factors, FOX proteins are also pioneer factors, modulators of other
transcription factors and epigenetic effectors (1). They are also components of a number of
important signaling pathways in embryonic development, such as the
mitogen-activated protein kinase (MAPK), AKT1 (AKT) and Hedgehog
pathways (2).
The deregulation of FOX proteins can change cell
fate, which is closely related to developmental genetic diseases
and malignancies and plays a key role in many cancers including
leukemia. Based on this, FOX proteins provide potential targets for
diagnosis and treatment of a multitude of human cancers.
Accumulating evidence suggests that FOXM1 and FOXOs are correlated
with various biological processes in leukemia development, such as
leukemia initiation, progression and drug resistance after
chemotherapy. This review highlights the complex regulatory
mechanisms of FOXM1 and FOXOs in leukemia, their critical roles in
the pathogenesis of leukemia and questions remaining to be
addressed concerning these issues. This review also summarizes the
clinical relevance of these FOX proteins and discusses their
potential as therapeutic targets and prognostic markers in
leukemia.
2. Aberrant expression of FOXM1 and its
oncogenic roles in leukemia
FOXM1 is previously known as HFH-11, MPP-2, WIN and
Trident. The locus of the human FOXM1 gene is situated at
chromosome 12p13-3. It consists of 10 exons, including an exon Va
(A1) and an exon VIIa (A2) which are alternatively spliced,
producing 3 isoforms: FOXM1A, B and C (3). The FOXM1A isoform is transcriptionally
inactive, whereas both FOXM1B and FOXM1C are transcriptionally
active. FOXM1 is expressed in proliferating cells, but not in
quiescent or terminally differentiated cells (3). FOXM1 stimulates expression of CCNA2
(cyclin A2), CCNB1 (cyclin B1) and CDC25B phosphatase as well as
degrades CDK2 inhibitors CDKN1A (P21CIP1) and CDKN1B (P27KIP1),
which is decisive for cell proliferation (3). FOXM1 regulates cell cycle progression
and genomic stability by activating expression of many genes, such
as AURKA (Aurora-A), AURKB (Aurora-B), PLK1, SKP2, CKS1BP7 (CKS1),
BIRC5 (survivin) and CENPA, B, F isoforms (3). Upregulation of FOXM1 expression is
found in different human carcinomas (1). FOXM1 is a pleiotropic player in tumors
and its deregulation is associated with tumorigenesis and cancer
progression. First, the involvement of FOXM1 in cancer initiation
correlates with its roles in cell cycle progression and
proliferation. Second, FOXM1 initiates angiogenesis by stimulating
VEGFA expression in solid tumors (1). Third, epithelial-mesenchymal
transition (EMT) induced by FOXM1 overexpression through activation
of CAV1 is related to the invasiveness and aggressiveness of cancer
(1). Furthermore, upregulation of
FOXM1 expression triggers cancer invasion and metastasis through
activation of MMP2 and MMP9 expression (1). Finally, overexpression of FOXM1
enhances OCT4 transcription and induces stem cell phenotypes of
cancer cells, leading to cancer progression and relapse (1). FOXM1 is regulated by various oncogenic
signaling pathways and oncogenes or tumor-suppressor pathways and
tumor suppressors in cancer (Table
I). FOXM1 is a downstream effector of not only oncogenic KRAS
(RAS)- MAPK1, 3 (MAPK), Sonic Hedgehog, NFKB1 (NF-κB) and EGFR
signaling pathways, but also the TP53 (P53)-CDKN1A and CDKN2A
(P16)-RB1 (pRB) tumor suppressor pathways in different carcinoma
cells (3–10). Upstream oncogenes, such as E7, CCND1
(cyclin D1)/CDK4, CDK6 and NPM1 (nucleophosmin) and tumor
suppressors, such as FOXO3, CDKN2A, CHEK2 (CHK2) interact with
FOXM1 and mediate its expression (11–17).
Emerging evidence has shown that microRNAs (miRNAs) such as
miR-370, miR-134, miR-31 and miR-149 downregulate FOXM1 expression
directly and their deregulation plays a part in cancer initiation
and progression (Table I) (18–21).
 | Table IImportant regulators interacting
upstream of FOXM1 in cancer. |
Table I
Important regulators interacting
upstream of FOXM1 in cancer.
| Regulator | Effect | Identified cancer
type(s) | Authorss
(ref.) |
|---|
| Signaling
pathways |
| KRAS-MAPK1, 3 | + | Osteosarcoma | Major et al
(4) |
| Sonic
Hedgehog | + | Basal cell
carcinoma | Teh et al
(5) |
| NFKB-1 | + | Laryngeal squamous
cell carcinoma | Penzo et al
(6); Jiang et al (7) |
| EGFR | + | Breast cancer | Bektas et al
(8) |
| TP53-CDKN1A | − | Hepatocellular
carcinoma, colorectal carcinoma | Barsotti et
al (9); Rovillain et al
(10) |
| CDKN2A-RB1 | − | Osteosarcoma | Barsotti et
al (9); Rovillain et al
(10) |
| Molecules |
| E7 | + | Cervical squamous
carcinomas | Luscher-Firzlaff
et al (11); McMurray et
al (12) |
| CCND1/CDK4, 6 | + | Melanoma,
osteosarcoma | Anders et al
(13) |
| NPM1 | + | Osteosarcoma,
breast cancer, pancreatic cancer | Bhat et al
(14) |
| STAT3 | + | Leukemia | Mencalha et
al (25) |
| FOXO3 | − | Breast cancer | McGovern et
al (15) |
| CDKN2A | − | Hepatocellular
carcinomas | Kalinichenko et
al (16) |
| CHEK2 | − | Osteosarcoma | Tan et al
(17) |
| miR-370 | − | Gastric cancer,
leukemia | Feng et al
(18); Zhang et al (23) |
Several lines of evidence that FOXM1 contributes to
the pathogenesis of leukemia have been reported even though they
are not as comprehensive as those of other cancers. FOXM1 is
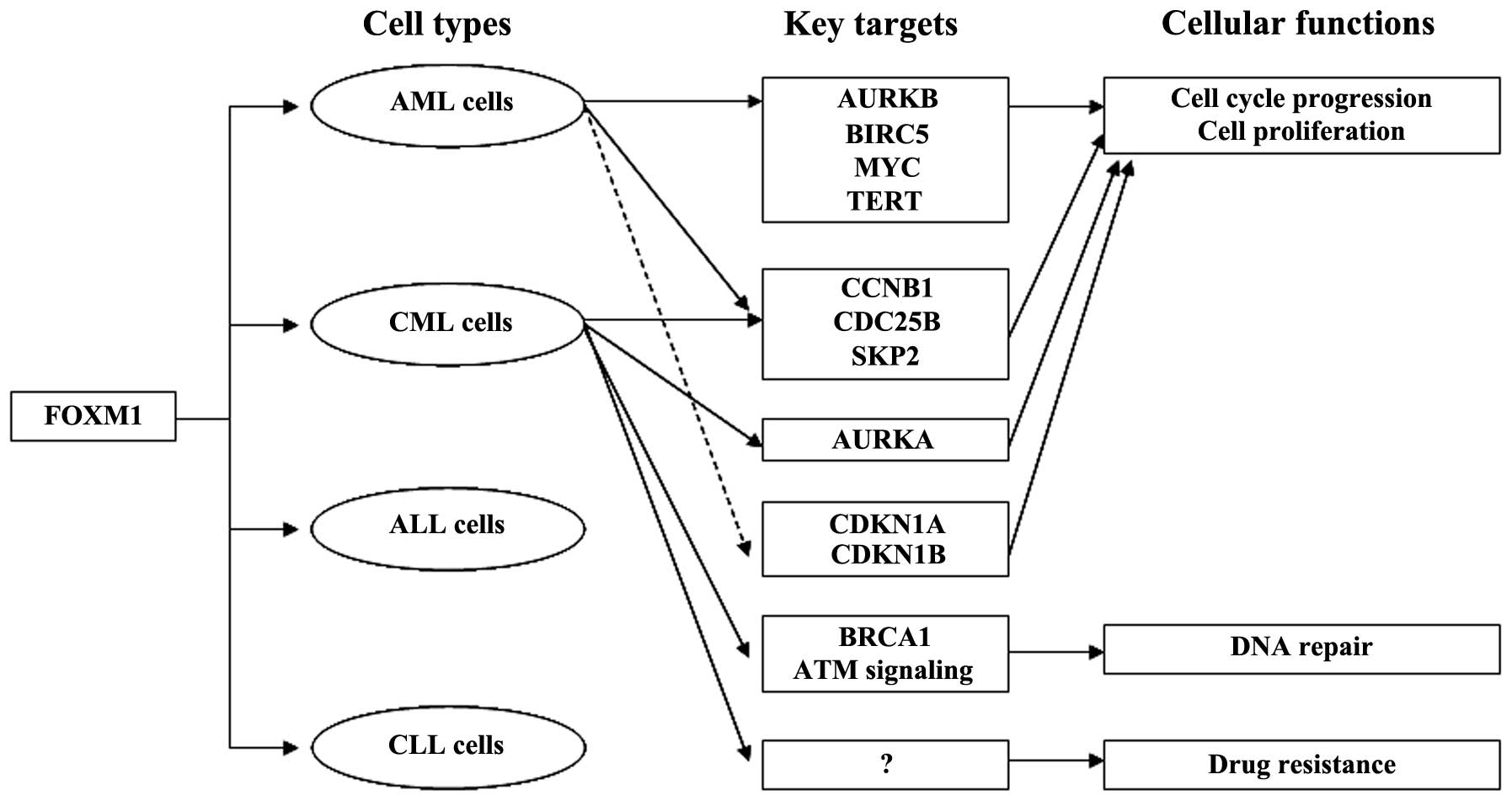
overexpressed in both AML cell lines and primary AML cells
(22,23). In AML cells, FOXM1 is a key mediator
of cell proliferation and governs cell cycle progression through
modulation of the expression of cell cycle-related proteins
(22). FOXM1 knockdown with FOXM1
siRNA was found to reduce cell proliferation and tumorigenicity by
suppressing the expression of AURKB, BIRC5, CCNB1 and CDC25B and
increasing the expression of CDKN1A and CDKN1B in AML cells
(22). Previous studies have shown
that overexpression of FOXM1 initiated by downregulation of
tumor-suppressor miR-370 expression is involved in AML and CML
development (23,24). FOXM1 was found to target MYC
(c-myc), SKP2 and TERT (hTERT) as well as CDKN1B, and its aberrant
expression is essential for the proliferation of AML cells
(23). A recent study revealed that
FOXM1 is a target of STAT3, and aberrant FOXM1 expression depends
on constitutively activated STAT3, which is essential for the
proliferation, survival and drug resistance of CML cells (25). Upregulated FOXM1 mediates multiple
genes and signaling pathways involved in other significant cellular
processes such as CCNB1, AURKA, CDC25B and SKP2 in cell cycle
progression, BRCA1 and ATM signaling in DNA repair in CML cells,
which correlates closely with leukemia development and progression
(25). Overexpression of FOXM1 is
associated with existence of FLT3-ITD receptors in AML cells
(26). Up to 30% of AML patients
have internal tandem duplications (ITD) within the FLT3 gene.
FLT3-ITD receptors maintain constitutive tyrosine kinase activity
even though no FLT3 ligand binds. It has been demonstrated that
inhibition of FLT3-ITD by FLT3 receptor tyrosine kinase inhibitor
AC220 hinders FOXM1 expression in MV4-11 AML cells (26). Taken together, the reported studies
suggest that upregulation of FOXM1 is crucial for myeloid leukemia
development and regulates many genes and cellular processes in
myeloid leukemia (Fig. 1).
3. Deregulation of FOXO transcription
factors and the regulatory mechanisms in leukemia
FOXOs constitute one of the largest subgroups of
forkhead family members. There are four members in this subfamily:
FOXO1, FOXO3, FOXO4 and FOXO6. They govern a wide variety of
cellular processes including cell cycle arrest, apoptosis, DNA
damage repair, stress response, angiogenesis and metabolism. They
activate or repress multiple genes involved in these cellular
processes such as BAD and FASLG (FASL) in apoptosis, CDKN1A, CDKN1B
and PLK1 in cell cycle progression, GADD45A in DNA damage repair,
and CAT and SOD2 in oxidative stress (1,27).
Post-translational modifications, such as acetylation, methylation
and ubiquitination, are required for normal functioning of FOXOs
(27). Accumulating data support
that FOXOs are bona fide tumor suppressors. Inactivation of FOXO
proteins has been discovered in a number of malignancies including
breast cancer, prostate cancer, glioblastoma and leukemia (27). AKT1, IKBKB (IKK) and MAPK1 (ERK) are
three commonly activated oncogenic kinases and target FOXO3
(FOXO3A) in human cancers (Table
II) (28–30). Oncogenic kinases AKT1, IKBKB and
MAPK1 phosphorylate FOXO3 at different phosphorylation sites in
response to external stimulation, resulting in FOXO3 nuclear
exclusion, degradation and final transcriptional suppression.
Additionally FOXOs are regulated by miRNAs, such as miR-155,
miR-182, miR-224, miR-9 and miR-421, which is essential for many
cellular processes in terms of cell survival, cell proliferation
and tumorigenesis. Deregulation of these miRNAs is one of the
underlying mechanisms of cancer development including leukemia
development (Table II) (31–35).
 | Table IIImportant regulators interacting
upstream of FOXO transcription factors in cancer. |
Table II
Important regulators interacting
upstream of FOXO transcription factors in cancer.
| FOXO factor | Regulator | Effect | Identified cancer
type | Authors (ref.) |
|---|
| FOXO3 | Signaling
pathways |
| PIK3CA-AKT1 | − | Leukemia | Brunet et al
(28) |
| NFKB1-IKBKB | − | Breast cancer | Hu et al
(29) |
| MAPK1 | − | Breast cancer | Yang et al
(30) |
| TGFB1-AKT1 | + | Leukemia | Naka et al
(51) |
| MAPK8-JUN | + | Leukemia | Sykes et al
(54) |
| Molecules |
| miR-182 | − | Lymphoblastic
malignancies | Yang et al
(32) |
| FOXO1 | Signaling
pathways |
| PIK3CA-AKT1 | − | Leukemia | Hussain et
al (47) |
| Molecules |
| miR-9 | − | Leukemia | Senyuk et al
(34) |
| FOXO4 | Signaling
pathways |
| PIK3CA-AKT1 | − | Leukemia | Oteiza et al
(48) |
| Molecules |
| tax | − | Leukemia | Oteiza et al
(48) |
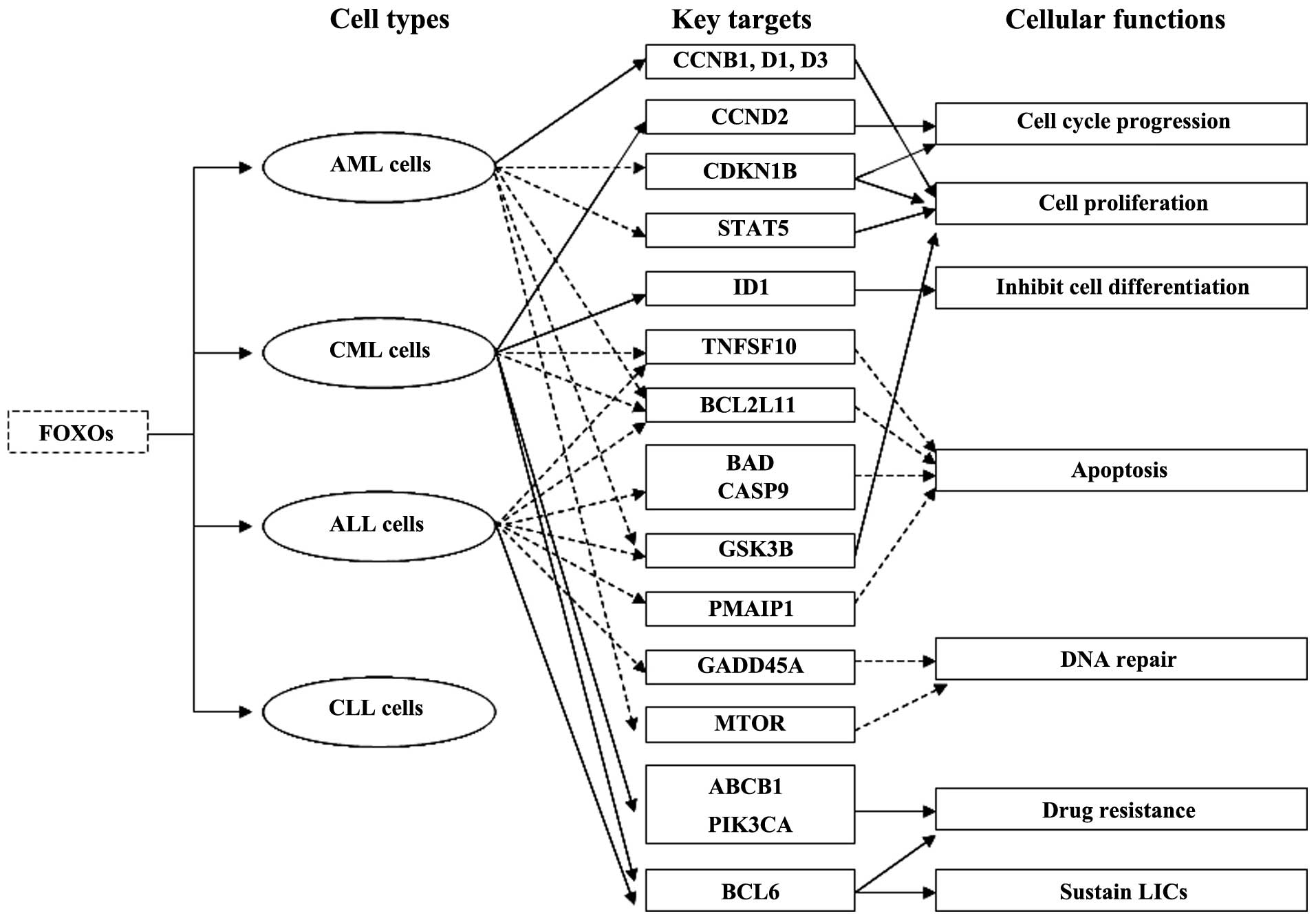
Aberrant expression of FOXOs plays an important role
in leukemogenesis (Fig. 2). At
times chromosomal translocations in leukemia create FOXO fusion
genes. The fusion proteins KMT2A (MLL)-FOXO3 and KMT2A-FOXO4 have
been demonstrated to immortalize myeloid progenitors and induce AML
as the result of oncogenic activation of KMT2A by CR2 and CR3 which
are transcriptional effector domains of FOXO3 and FOXO4 and
subsequent KMT2A-mediated cellular transformation (36).
To date, several findings have concluded that the
inhibition of FOXO3 is involved in the signaling cascade by which
the BCR-ABL1 (ABL) fusion gene initiates oncogenic transformation
in leukemic cells. A previous study showed that conditional
inhibition of BCR-ABL1 by STI571 (imatinib, Gleevec) activated
FOXO3 expression and downregulated CCND2 (cyclin D2) expression in
BCR-ABL1-positive CML cells. This finding also suggests that
BCR-ABL1 induces FOXO3 and BCL6 inactivation, CCND2 upregulation
and cell cycle progression may be responsible for the oncogenic
transformation of CML (37).
Another study revealed that overexpression of ID1 (inhibitor of DNA
binding 1) mediated by AKT1 activation/ FOXO3 inhibition is
required for leukemic transformation in BCR-ABL1-positive CML cells
(38). Constitutively active ID1,
which is a transcriptional target of FOXO3, inhibits cell
differentiation and generates a differentiation block, leading to
leukemic transformation of hematopoietic cells (38). The BCR-ABL1 oncoprotein induces
leukemogenesis through stimulating PIK3CA (PI3K)-AKT1-FOXO3
signaling, which promotes cell proliferation and prevents apoptosis
(39–41). Active PIK3CA-AKT1 signaling causes
phosphorylation of FOXO3 and its retention in the cytoplasm,
leading to inhibition of FOXO3 expression and downregulation of
FOXO3 targeting cell cycle inhibitory genes and pro-apoptotic
genes, such as TNFSF10 (TRAIL) and BCL2L11 (BIM) in
BCR-ABL1-positive CML and acute lymphoblastic leukemia (ALL)
(41). In addition, the
proteasome-dependent degradation and suppression of FOXO3 protein,
which results in inhibition of the FOXO3 targets, is responsible
for evasion of apoptosis and leukemogenesis in BCR-ABL1-driven CML
and ALL cells (41).
Inactivation of FOXO3 function mediated by oncogenic
tyrosine kinase FLT3 also plays a part in leukemogenesis.
Experiments performed in a Tet-On Ba/F3 cell line, which expresses
FLT3-ITD depending on doxycycline, have shown that FLT3-ITD
receptors inhibit apoptosis and promote cell proliferation through
phosphorylation of FOXO3 and suppression of its target genes CDKN1B
and BCL2L11 (42). These data
suggest that FLT3-ITD expression induced suppression of FOXO3 and
its target genes may be the underlying mechanism of oncogenic
transformation in hematopoietic malignancies such as human AML
(42). Furthermore, studies in
vitro and in vivo as well as in samples from AML
patients have revealed that constitutive AKT1 activation and FOXO3
inactivation induced by FLT3-ITD lead to cell proliferation and
leukemic transformation of myeloid cells in AML (43).
FOXO3 suppression triggered by its upstream
oncogenic signaling pathways is crucial for the uncontrolled
proliferation and survival of leukemic cells. FOXO3 is known to be
regulated by the PIK3CA-AKT1, MAPK1 (ERK-MAPK) and IKBKB signaling
pathways in AML cells (44). More
recently, an in-depth study revealed that the nuclear exclusion of
FOXO3 and its inactivation are not due to the deregulation of the
PIK3CA-AKT1 or the MAPK1 signaling pathway but the constitutive
active IKBKB activity in AML cells (44). These findings indicate that rescuing
FOXO3 activity by IKBKB inhibitors is necessary for AML therapy
because of IKBKB-dependent FOXO3 regulation in AML.
In T-cell acute lymphoblastic leukemia (T-ALL)
cells, PIK3CA-AKT1-FOXO1 signaling is constitutively active because
of mutated PTEN phosphatase which is a tumor suppressor and
negative modulator of the PIK3CA-AKT1 pathway (45–47).
Mutations in PTEN phosphatase generate an anti-apoptotic effect and
promote cell survival through activation of PIK3CA-AKT1-FOXO1
signaling. Specifically, active PIK3CA-AKT1-FOXO1 signaling causes
phosphorylation of BAD (Bad), GSK3B (GSK3) and CASP9 (caspase-9)
and prevents apoptosis, resulting in leukemogenesis (45–47).
FOXO4 inactivation contributes to adult T-cell
leukemia (ATL) induced by human T-cell leukemia virus type 1
(HTLV-1). The HTLV-1 tax oncoprotein downregulates FOXO4
transcriptional activity and is a master regulator in HTLV-1
initiated oncogenic transformation of infected T cells (48). Tax oncoprotein activates the
PIK3CA-AKT1 pathway, in turn stimulates FOXO4 phosphorylation and
nuclear exclusion and mediates ubiquitination, proteasomal
degradation and inhibition of FOXO4 and its target genes such as
GADD45A in HTLV-1-transformed cells (48).
Although accumulating data highlight that FOXOs
function as tumor suppressors in leukemia; paradoxically, FOXOs
also have been shown to be important for maintenance of leukemia
stem cells and responsible for drug resistance in leukemia. FOXO3
plays a dual role of sensitivity and resistance in response to
chemotherapeutic drugs in leukemia. Following doxorubicin
treatment, FOXO3 initially induces cell cycle arrest and apoptosis;
thereafter, continued activation of FOXO3 leads to drug resistance
by stimulating ABCB1 (MDR1) and PIK3CA expression in CML cells
(49,50). Specifically, FOXO3 regulates PIK3CA
catalytic subunit p110α directly, enhances PIK3CA/AKT1 activity,
causes phosphorylation of FOXOs and their nuclear exclusion,
thereby inhibiting or activating FOXO target genes that are
important for cell proliferation, apoptosis and differentiation
(50). These findings suggest that
FOXO3 may be an ideal target with which to prevent MDR (multidrug
resistance) in CML. FOXO3 has been confirmed to be required for
maintenance of CML stem cells which trigger the recurrence of CML
after tyrosine kinase inhibitor (TKI), imatinib therapy and the
TGFB1 (TGF-β)-AKT1-FOXO3 pathway is not only essential for the
survival of imatinib-resistant CML stem cells but also contributes
to tumorigenicity of CML cells (51). Leukemia-initiating cells (LICs) are
characterized by suppressed AKT1 phosphorylation and nuclear
localization of FOXO3. TGFB1 regulates the AKT1-FOXO3 pathway and
promotes nuclear localization of FOXO3 in LICs (51). Recently, another study demonstrated
that FOXO3-BCL6-CDKN2A (ARF)/TP53 signaling pathways are involved
in sustaining LICs after TKI treatment in CML and Philadelphia
chromosome-positive (Ph+) ALL and prolonged treatment with a
combination of AKT1-FOXO3 pathway inhibitor imatinib and BCL6
peptide inhibitor RI-BPI could eradicate LICs efficiently (52,53).
Proto-oncogene BCL6 sustains leukemia stem cells and induces drug
resistance by suppressing the CDKN2A-TP53 pathway after TKI
treatment in leukemia driven by BCR-ABL1 fusion genes (52,53).
Moreover, a recent study discovered that FOXOs play pivotal roles
in the maintenance of LICs by preventing their differentiation and
apoptosis and are involved in leuke-mogenesis (54). The MAPK8 (JNK)-JUN (c-JUN) pathway
stimulates FOXO nuclear localization and antagonizes the effect
caused by FOXO inhibition in LICs in AML (54). Different from AML and CML cells,
evasion of apoptosis and drug resistance are linked to both mutated
tumor-suppressor CDKN2A (P16INK4A) and elevated AKT1 (PKB)-FOXO3
signaling, which decreases TNFSF10 and PMAIP1 (Noxa) expression in
pediatric T-ALL cells (55).
4. Leukemia therapeutics targeting FOXM1 and
FOXOs
Several lines of evidence suggest that chemical
inhibitors targeting FOXM1 may be developed as novel antileukemic
agents which may supplement present remedies. FOXM1
inhibitors/thiazole antibiotics siomycin A and thiostrepton, which
are potent inhibitors of FOXM1 transcriptional activity and
FOXM1-dependent transcription, efficiently inhibit cell growth and
induce apoptosis in MV4-11, THP1, CEM, HL60 and U937 leukemia cell
lines (26,56). Proteasome inhibitors MG115, MG132
and bortezomib have been demonstrated to induce apoptosis by
inhibiting FOXM1 transcriptional activity and expression in the
HL-60 leukemia cell line (57).
Moreover, two phospha sugar derivatives,
2,3,4-tribromo-3-methyl-1-phenylphospholane 1-oxide (TMPP) and
2,3-dibromo-3-methyl-1-phenylphospholane 1-oxide (DMPP) have been
discovered to inhibit FOXM1 expression, inducing G2/M cell cycle
blockage at low concentrations and apoptosis at high concentrations
in leukemic cells (58). They are
promising agents in targeted antileukemic therapy.
Homoharringtonine (HHT), which is a traditional Chinese medicine
used for AML and CML treatment, has been shown to upregulate the
level of miR-370 directly and inhibit FOXM1 expression, inducing
apoptosis in CML cells (24).
Addition of miR-370 mimics enhanced the efficacy of HHT through
suppressing FOXM1 expression (24).
In addition, CDKN2A, an upstream tumor suppressor of FOXM1, has
been confirmed to be successfully used as a therapeutic
intervention target in vitro and in vivo in liver
cancer (59). These data suggest
that developing agents which target upstream interactive oncogenes,
tumor suppressors and signaling pathways of FOXM1 may be effective
approaches for leukemia therapy (Table
I).
Imatinib, a regularly used antileukemic agent, has
been confirmed to inhibit BCR-ABL1 by activating FOXO3 and inducing
BCL2L11-dependent apoptosis in CML (40). Due to the potent tumor-suppressing
function of FOXO3, drugs that activate FOXO3 and its downstream
genes similar to imatinib are potential antileukemic agents.
Proteasome inhibitor bortezomib (Velcade) was found to induce
apoptosis by activating the expression of FOXO3 and its downstream
pro-apoptotic genes TNFSF10 (TRAIL) and BCL2L11 in both
imatinib-sensitive and imatinib-resistant leukemic cells from
patients with BCR-ABL1-positive CML or ALL (41). Bortezomib has been proven to be a
promising chemotherapeutic agent with which to treat BCR-ABL1-
induced leukemia (41). Cell
penetrating TAT-FOXO3 fusion proteins have been reported to induce
apoptotic cell death in Jurkat, K562 leukemic cells and primary
cells from chronic lymphocytic leukemia (CLL) patients (60). They are probably developed as
effective antileukemic agents. FOXO3 activation has been shown to
be essential for the efficacy of the alisertib/cytarabine
combination therapy in AML (61).
Either single agent or the combination of both agents induced
expression of FOXO3 and its pro-apoptotic targets, CDKN1B and
BCL2L11, therefore leading to apoptosis in AML cells (61).
Besides targeting FOXO3, activating other FOXO
transcription factors may be valid for leukemia treatment.
AKT1-FOXO1 signaling is constitutively active in T-ALL cells and
suppressing the pathway by curcumin leads to cell growth inhibition
by GSK3B inactivation (47).
Curcumin inhibits cell proliferation and promotes apoptosis through
dephosphorylation of FOXO1 and successive activation of apoptotic
signaling in T-ALL cells (47).
5. Additional clinical relevance of FOXM1
and FOXOs in leukemia
Upregulation of FOXM1 is linked to FLT3-ITD
expression and adverse prognosis in AML (26). Inhibition of FLT3-ITD decreases
FOXM1 expression and stimulation of FLT3 by the FLT3 ligand
increases FOXM1 expression in AML cells, suggesting that FOXM1
could be a suitable prognostic marker for AML patients (26). In addition, it has been reported
that FOXM1 target genes, which mediate several cellular processes
including cell cycle, differentiation, ageing, genomic stability,
epigenetic and stem cell renewal, can be used to diagnose and
evaluate aggressiveness of early squamous carcinoma (62). These data suggest that FOXM1 and its
target genes are potential biomarkers for leukemia diagnosis and
prognosis.
Phospho-FOXO1 is detected in most AML patients
because of phosphorylation of AKT1 signaling (63). The presence of phospho-FOXO1 was
found to be correlated with reduced survival and an unfavorable
outcome in AML patients, suggesting that phospho-FOXO1 is a
valuable molecular marker for AML prognosis (63). High levels of phosphorylation of
FOXO3 have also been found in AML patients and were confirmed to be
an adverse prognostic factor in AML, which is relevant to increased
proliferation, resistance to therapy and reduced survival (64). Higher levels of phospho- FOXO3
increased levels of CCNB1, D1 and D3, pGSK3B, pMTOR, and pSTAT5 and
promoted cell proliferation, which was associated with higher WBCs,
percent marrow and blood blasts in AML patients (64). Taken together, phospho-FOXOs are
useful indicators with which to estimate the prognosis of AML
patients.
Clinical evidence has shown that FOXO3 is an
adequate theranostic marker for treatment with bortezomib in
Ph+ ALL as FOXO3 is a key mediator in the pathogenesis
of the disease and FOXO3 expression is suppressed after treatment
with bortezomib (65). Moreover,
comparing human bone marrow samples of Ph+ ALL,
Ph− ALL and normal controls has revealed that FOXO3
downregulation is specific to Ph+ ALL (65).
6. Conclusion and future perspectives
Deregulation of FOXM1 and FOXOs is involved in
leukemogenesis and leukemia development. The collective data
support that overexpressed FOXM1 is a key player in cell
proliferation, cell survival, cell cycle progression, drug
resistance and DNA repair in leukemic cells, but they are not
perfect answers to the pathogenesis of leukemia in contrast to
studies conducted in other cancers. The molecular mechanisms by
which FOXM1 governs leukemia initiation, invasion, drug resistance
and other cellular processes require further clarification.
Moreover, the significance of FOXM1 in other types of leukemia such
as ALL and CLL development is unclear and remains to be determined.
In addition, it will be interesting and worthwhile to identify
additional miRNAs which target FOXM1 and mediate its expression in
leukemic cells. Targeting FOXM1 with therapeutic inhibitors may be
an effective strategy for leukemia therapy. In addition, FOXM1
could be a valuable biomarker for leukemia diagnosis and prognosis
due to its aberrant expression and cellular functions in leukemic
cells, but further investigations needs to be carried out. The
abnormal expression of FOXOs plays a prominent part in the
proliferation and survival of leukemic cells and their evasion of
apoptosis. Activation of FOXOs is responsible for not only the
initial cytotoxic response to some antileukemic drugs but also
subsequent resistance in leukemia. FOXO3-enriched leukemia stem
cells and induced MDR genes contribute to the acquisition of drug
resistance in leukemia. Developing therapeutic agents that restore
the function of tumor-suppressor FOXOs may be rational and well
suitable for leukemia treatment. But while developing therapeutic
agents targeting FOXOs, we should consider the dual effects of
FOXOs in response to chemotherapy. Additionally, FOXOs are
potential diagnostic and prognostic tools for leukemia in the
clinic.
Overall, therapeutic intervention of the upstream
interactive oncogenes and tumor suppressors of FOXM1 and FOXOs
including miRNAs and oncogenic and tumor-suppressor signaling
pathways converging on FOXM1 and FOXOs as well as the FOX proteins
themselves in leukemia may be reasonable strategies with which to
treat the disease (Tables I and
II). Therefore, identifying novel
regulatory signaling pathways and molecules interacting upstream of
FOXM1 and FOXOs in leukemic cells is critically important but very
challenging.
Finally, it will be of value to clarify how aberrant
expression of FOXM1 and FOXOs relates to various chromosomal
translocations in leukemia, which will provide novel insights into
leukemia pathogenesis, and may facilitate the development of novel
therapeutics and prognostic markers.
Acknowledgements
The present study was supported by a grant from the
Natural Science Foundation of Hunan Province in China (grant no.
10JJ2015) and a grant from the Natural Science Foundation of China
(grant no. 30771096).
Notes
[1] Gene
nomenclature: The gene name in parenthesis after every official
HUGO Gene Nomenclature Committee (HGNC) symbol is the name used in
the cited references.
References
|
1
|
Lam EW, Brosens JJ, Gomes AR and Koo CY:
Forkhead box proteins: tuning forks for transcriptional harmony.
Nat Rev Cancer. 13:482–495. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Carlsson P and Mahlapuu M: Forkhead
transcription factors: key players in development and metabolism.
Dev Biol. 250:1–23. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Laoukili J, Stahl M and Medema RH: FoxM1:
at the crossroads of ageing and cancer. Biochim Biophys Acta.
1775:92–102. 2007.PubMed/NCBI
|
|
4
|
Major ML, Lepe R and Costa RH: Forkhead
box M1B transcriptional activity requires binding of Cdk-cyclin
complexes for phosphorylation-dependent recruitment of p300/CBP
co-activators. Mol Cell Biol. 24:2649–2661. 2004. View Article : Google Scholar
|
|
5
|
Teh MT, Wong ST, Neill GW, Ghali LR,
Philpott MP and Quinn AG: FOXM1 is a downstream target of Gli1 in
basal cell carcinomas. Cancer Res. 62:4773–4780. 2002.PubMed/NCBI
|
|
6
|
Penzo M, Massa PE, Olivotto E, et al:
Sustained NF-κB activation produces a short-term cell proliferation
block in conjunction with repressing effectors of cell cycle
progression controlled by E2F or FoxM1. J Cell Physiol.
218:215–227. 2009.
|
|
7
|
Jiang LZ, Wang P, Deng B, Huang C, Tang
WX, Lu HY and Chen HY: Overexpression of Forkhead Box M1
transcription factor and nuclear factor-κB in laryngeal squamous
cell carcinoma: a potential indicator for poor prognosis. Hum
Pathol. 42:1185–1193. 2011.
|
|
8
|
Bektas N, Haaf At, Veeck J, et al: Tight
correlation between expression of the Forkhead transcription factor
FOXM1 and HER2 in human breast cancer. BMC Cancer. 8:422008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Barsotti A and Prives C: Pro-proliferative
FoxM1 is a target of p53-mediated repression. Oncogene.
28:4295–4305. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rovillain E, Mansfield L, Caetano C, et
al: Activation of nuclear factor-kappa B signaling promotes
cellular senescence. Oncogene. 30:2356–2366. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Luscher-Firzlaff JM, Westendorf JM,
Zwicker J, et al: Interaction of the fork head domain transcription
factor MPP2 with the human papilloma virus 16 E7 protein:
enhancement of transformation and transactivation. Oncogene.
18:5620–5630. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
McMurray HR, Nguyen D, Westbrook TF and
McAnce DJ: Biology of human papillomaviruses. Int J Exp Pathol.
82:15–33. 2001. View Article : Google Scholar
|
|
13
|
Anders L, Ke N, Hydbring P, et al: A
systematic screen for CDK4/6 substrates links FOXM1 phosphorylation
to senescence suppression in cancer cells. Cancer Cell. 20:620–634.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bhat UG, Jagadeeswaran R, Halasi M and
Gartel AL: Nucleophosmin interacts with FOXM1 and modulates the
level and localization of FOXM1 in human cancer cells. J Biol Chem.
286:41425–41433. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
McGovern UB, Francis RE, Peck B, et al:
Gefitinib (Iressa) represses FOXM1 expression via FOXO3a in breast
cancer. Mol Cancer Ther. 8:582–591. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kalinichenko VV, Major ML, Wang X, et al:
Foxm1b transcription factor is essential for development of
hepatocellular carcinomas and is negatively regulated by the p19ARF
tumor suppressor. Genes Dev. 18:830–850. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tan Y, Raychaudhuri P and Costa RH: Chk2
mediates stabilization of the FoxM1 transcription factor to
stimulate expression of DNA repair genes. Mol Cell Biol.
27:1007–1016. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Feng Y, Wang L, Zeng J, et al: FoxM1 is
overexpressed in Helicobacter pylori-induced gastric
carcinogenesis and is negatively regulated by miR-370. Mol Cancer
Res. 11:834–844. 2013.
|
|
19
|
Li J, Wang Y, Luo J, Fu Z, Ying J, Yu Y
and Yu W: miR-134 inhibits epithelial to mesenchymal transition by
targeting FOXM1 in non-small cell lung cancer cells. FEBS Lett.
586:3761–3765. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lin PC, Chiu YL, Banerjee S, et al:
Epigenetic repression of miR-31 disrupts androgen receptor
homeostasis and contributes to prostate cancer progression. Cancer
Res. 73:1232–1244. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ke Y, Zhao W, Xiong J and Cao R: miR-149
inhibits non-small-cell lung cancer cells EMT by targeting FOXM1.
Biochem Res Int. 2013:5067312013.PubMed/NCBI
|
|
22
|
Nakamura S, Hirano I, Okinaka K, et al:
The FOXM1 transcriptional factor promotes the proliferation of
leukemia cells through modulation of cell cycle progression in
acute myeloid leukemia. Carcinogenesis. 31:2012–2021. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang X, Zeng J, Zhou M, et al: The tumor
suppressive role of miRNA-370 by targeting FoxM1 in acute myeloid
leukemia. Mol Cancer. 11:562012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhou M, Zeng J, Wang X, et al: MiR-370
sensitizes chronic myeloid leukemia K562 cells to homoharringtonine
by targeting Forkhead box M1. J Transl Med. 11:2652013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mencalha AL, Binato R, Ferreira GM, Du
Rocher B and Abdelhay E: Forkhead box M1 (FoxM1) gene is a new
STAT3 transcriptional factor target and is essential for
proliferation, survival and DNA repair of K562 cell line. PLoS One.
7:e481602012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu LL, Zhang DH, Mao X, Zhang XH and
Zhang B: Over-expression of FoxM1 is associated with adverse
prognosis and FLT3-ITD in acute myeloid leukemia. Biochem Biophys
Res Commun. 446:280–285. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang J and Hung MC: A new fork for
clinical application: targeting forkhead transcription factors in
cancer. Clin Cancer Res. 15:752–757. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Brunet A, Bonni A, Zigmond MJ, et al: Akt
promotes cell survival by phosphorylating and inhibiting a Forkhead
transcription factor. Cell. 96:857–868. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hu MC, Lee DF, Xia W, et al: IκB kinase
promotes tumorigenesis through inhibition of forkhead FOXO3a. Cell.
117:225–237. 2004.
|
|
30
|
Yang JY, Zong CS, Xia W, et al: ERK
promotes tumorigenesis by inhibiting FOXO3a via MDM2-mediated
degradation. Nat Cell Biol. 10:138–148. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kong W, He L, Coppola M, Guo J, Esposito
NN, Coppola D and Cheng JQ: MicroRNA-155 regulates cell survival,
growth, and chemosensitivity by targeting FOXO3a in breast cancer.
J Biol Chem. 285:17869–17879. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang A, Ma J, Wu M, et al: Aberrant
microRNA-182 expression is associated with glucocorticoid
resistance in lymphoblastic malignancies. Leuk Lymphoma.
53:2465–2473. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liao WT, Li TT, Wang ZG, et al:
microRNA-224 promotes cell proliferation and tumor growth in human
colorectal cancer by repressing PHLPP1 and PHLPP2. Clin Cancer Res.
19:4662–4672. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Senyuk V, Zhang Y, Liu Y, et al: Critical
role of miR-9 in myelopoiesis and EVI1-induced leukemogenesis. Proc
Natl Acad Sci USA. 110:5594–5599. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen L, Tang Y, Wang J, Yan Z and Xu R:
miR-421 induces cell proliferation and apoptosis resistance in
human nasopharyngeal carcinoma via downregulation of FOXO4. Biochem
Biophys Res Commun. 435:745–750. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
So CW and Cleary ML: Common mechanism for
oncogenic activation of MLL by forkhead family proteins. Blood.
101:633–639. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fernández de Mattos S, Essafi A, Soeiro I,
et al: FoxO3a and BCR-ABL regulate cyclin D2 transcription through
a STAT5/BCL6-dependent mechanism. Mol Cell Biol. 24:10058–10071.
2004.PubMed/NCBI
|
|
38
|
Birkenkamp KU, Essafi A, van der Vos KE,
et al: FOXO3a induces differentiation of Bcr-Abl-transformed cells
through transcriptional down-regulation of Id1. J Biol Chem.
282:2211–2220. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ghaffari S, Jagani Z, Kitidis C, Lodish HF
and Khosravi-Far R: Cytokines and BCR-ABL mediate suppression of
TRAIL-induced apoptosis through inhibition of forkhead FOXO3a
transcription factor. Proc Natl Acad Sci USA. 100:6523–6528. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Essafi A, Fernandez de Mattos S, Hassen
YA, et al: Direct transcriptional regulation of Bim by FoxO3a
mediates STI571-induced apoptosis in Bcr-Abl-expressing cells.
Oncogene. 24:2317–2329. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jagani Z, Song K, Kutok JL, et al:
Proteasome inhibition causes regression of leukemia and abrogates
BCR-ABL-induced evasion of apoptosis in part through regulation of
forkhead tumor suppressors. Cancer Res. 69:6546–6555. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Scheijen B, Ngo HT, Kang H and Griffin JD:
FLT3 receptors with internal tandem duplications promote cell
viability and proliferation by signaling through Foxo proteins.
Oncogene. 23:3338–3349. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Brandts CH, Sargin B, Rode M, et al:
Constitutive activation of Akt by Flt3 internal tandem duplications
is necessary for increased survival, proliferation, and myeloid
transformation. Cancer Res. 65:9643–9650. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chapuis N, Park S, Leotoing L, et al: IκB
kinase overcomes PI3K/Akt and ERK/MAPK to control FOXO3a activity
in acute myeloid leukemia. Blood. 116:4240–4250. 2010.
|
|
45
|
Dahia PL, Aguiar RC, Alberta J, et al:
PTEN is inversely correlated with the cell survival factor Akt/PKB
and is inactivated via multiple mechanisms in haematological
malignancies. Hum Mol Genet. 8:185–193. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Vazquez F and Sellers WR: The PTEN tumor
suppressor protein: an antagonist of phosphoinositide 3-kinase
signaling. Biochim Biophys Acta. 1470:M21–M35. 2000.PubMed/NCBI
|
|
47
|
Hussain AR, Al-Rasheed M, Manogaran PS,
Al-Hussein KA, Platanias LC, Al Kuraya K and Uddin S: Curcumin
induces apoptosis via inhibition of PI3′-kinase/AKT pathway in
acute T cell leukemias. Apoptosis. 11:245–254. 2006.PubMed/NCBI
|
|
48
|
Oteiza A and Mechti N: The human T-cell
leukemia virus type 1 oncoprotein tax controls forkhead box O4
activity through degradation by the proteasome. J Virol.
85:6480–6491. 2011. View Article : Google Scholar
|
|
49
|
Hui RC, Francis RE, Guest SK, et al:
Doxorubicin activates FOXO3a to induce the expression of multidrug
resistance gene ABCB1 (MDR1) in K562 leukemic cells. Mol Cancer
Ther. 7:670–678. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Hui RC, Gomes AR, Constantinidou D, et al:
The forkhead transcription factor FOXO3a increases
phosphoinositide-3 kinase/Akt activity in drug-resistant leukemic
cells through induction of PIK3CA expression. Mol Cell Biol.
28:5886–5898. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Naka K, Hoshii T, Muraguchi T, et al:
TGF-β-FOXO signaling maintains leukaemia-initiating cells in
chronic myeloid leukaemia. Nature. 463:676–680. 2010.
|
|
52
|
Duy C, Hurtz C, Shojaee S, et al:
BCL6 enables Ph+ acute lymphoblastic leukaemia
cells to survive BCR-ABL1 kinase inhibition. Nature.
473:384–388. 2011. View Article : Google Scholar
|
|
53
|
Hurtz C, Hatzi K, Cerchietti L, et al:
BCL6-mediated repression of p53 is critical for leukemia stem cell
survival in chronic myeloid leukemia. J Exp Med. 208:2163–2174.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Sykes SM, Lane SW, Bullinger L, et al:
AKT/FOXO signaling enforces reversible differentiation blockade in
myeloid leukemias. Cell. 146:697–708. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Ausserlechner MJ, Salvador C, Deutschmann
A, et al: Therapy-resistant acute lymphoblastic leukemia (ALL)
cells inactivate FOXO3 to escape apoptosis induction by TRAIL and
Noxa. Oncotarget. 4:995–1007. 2013.PubMed/NCBI
|
|
56
|
Bhat UG, Halasi M and Gartel AL: Thiazole
antibiotics target FoxM1 and induce apoptosis in human cancer
cells. PLoS One. 4:e55922009. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Bhat UG, Halasi M and Gartel AL: FoxM1 is
a general target for proteasome inhibitors. PLoS One. 4:e65932009.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Nakamura S, Yamashita M, Yokota D, et al:
Development and pharmacologic characterization of deoxybromophospha
sugar derivatives with antileukemic activity. Invest New Drugs.
28:381–391. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Gusarova GA, Wang IC, Major ML,
Kalinichenko VV, Ackerson T, Petrovic V and Costa RH: A
cell-penetrating ARF peptide inhibitor of FoxM1 in mouse
hepatocellular carcinoma treatment. J Clin Invest. 117:99–111.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Essafi M, Baudot AD, Mouska X, Cassuto JP,
Ticchioni M and Deckert M: Cell-penetrating TAT-FOXO3 fusion
proteins induce apoptotic cell death in leukemic cells. Mol Cancer
Ther. 10:37–46. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Kelly KR, Nawrocki ST, Espitia CM, et al:
Targeting Aurora A kinase activity with the investigational agent
alisertib increases the efficacy of cytarabine through a
FOXO-dependent mechanism. Int J Cancer. 131:2693–2703. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Teh MT, Hutchison IL, Costea DE, et al:
Exploiting FOXM1-orchestrated molecular network for early squamous
cell carcinoma diagnosis and prognosis. Int J Cancer.
132:2095–2106. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Cheong JW, Eom JI, Maeng HY, Lee ST, Hahn
JS, Ko YW and Min YH: Constitutive phosphorylation of FKHR
transcription factor as a prognostic variable in acute myeloid
leukemia. Leuk Res. 27:1159–1162. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Kornblau SM, Singh N, Qiu Y, Chen W, Zhang
N and Coombes KR: Highly phosphorylated FOXO3A is an adverse
prognostic factor in acute myeloid leukemia. Clin Cancer Res.
16:1865–1874. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Dewar R, Chen ST, Yeckes-Rodin H, Miller K
and Khosravi-Far R: Bortezomib treatment causes remission in a Ph+
ALL patient and reveals FoxO as a theranostic marker. Cancer Biol
Ther. 11:552–558. 2011. View Article : Google Scholar : PubMed/NCBI
|