Introduction
Nasopharyngeal carcinoma (NPC) is a leading cause of
cancer-related mortality in Southeastern Asia, particularly in
Southern China (1,2). Radiotherapy and concurrent
chemoradiotherapy are standard modalities for NPC at the early
stage and advanced stage, respectively (3). With the development of radiation
techniques and chemotherapy modalities, the local control rate of
patients in the early stage of the disease has risen to 70–90% over
the past decade. However, in patients in the late stages of the
disease (stages III–IV), the local control rate is only 50%, with a
5-year overall survival rate of 40–70% (4). The main causes of treatment failure
are local recurrence and distant metastasis; the key factor is the
presence of tumor cells possessing a resistance to radiation
(5). Therefore, it is urgent to
find novel, less toxic agents in combination with radiation to
decrease radiation resistance and increase the radiation effect, as
potential clinical candidates for this disease.
Metformin (1,1-dimethylbiguanide hydrochloride) is a
widely used anti-diabetic drug (6).
Recently, epidemiological analyses revealed that metformin reduced
the cancer risk in diabetic patients (7–9). A
number of preclinical studies have confirmed the anticancer
activity of metformin with both in vitro and in vivo
models (10). In addition,
metformin has been reported to enhance the effects of chemotherapy
(11,12) and radiation (13–15).
However, the effects of metformin combined with radiotherapy on NPC
cells remain unknown.
In the present study, we investigated the actions of
metformin on the radiosensitivity of NPC cells and explored the
underlying mechanisms. Our results may contribute to the
understanding of the mechanisms of action of the conventional drug
and highlight potential implications of the combination of
metformin with radiotherapy as an anticancer strategy.
Materials and methods
Cell culture and treatments
Three undifferentiated human NPC cell lines, CNE-2,
HONE-1 and SUNE-1, were maintained by our laboratory and cultured
in RPMI-1640 medium (HyClone, Logan, UT, USA) supplemented with 10%
fetal bovine serum (FBS) (Biological Industries), 100 U/ml
penicillin and 100 U/ml streptomycin in a humid atmosphere of 5%
CO2 at 37°C.
Irradiation condition
The NPC cells were exposed to 4 MV of X-rays with
various doses of irradiation (0–8 Gy) using a linear accelerator
(Eleketa, Stockholm, Sweden) with the source-skin-distance
technique (SSD=100 cm). The depth was set at 1 cm to the bottom of
the 6-well plate or 6-cm dishes.
Cell proliferation assay
Cells in the early log phase were trypsinized and
plated in a 96-well plate at a density of 1×104 cells
per well. Twenty-four hours later, the medium was removed and
replaced with fresh medium with metformin at the indicated
concentrations (0, 4, 8,16, 25, 32, 50 and 64 mM) for 24 or 48 h in
the presence of 1% FBS. Cell density was measured using the CCK-8
(Dojindo Molecular Technologies, Japan) assay following the
manufacturer’s instructions. The absorbance of each well was
determined at 450 nm using a microplate reader. The percentage of
surviving cells from each group relative to the control were
defined as the proliferation rate. For these studies, all
experiments were repeated at least three times.
Colony formation assay
Cells in early log phase were trypsinized and plated
in 6-well plates at 200, 400, 1,000, 2,000, and 4,000 cells per
well and cultured overnight to allow for cell attachment. Then
cells were treated with or without metformin for 24 h prior to
administration of irradiation with the exposure dose corresponding
to 0, 2, 4, 6, and 8 Gy. The cells were incubated for 10 days to
allow for the formation of colonies. Cells were fixed and stained
with 0.5% crystal violet (Sigma-Aldrich, St. Louis, MO, USA), and
colonies containing >50 cells were counted. Survival curves were
fitted using the multi-target click model in Graph Pad Prism 5.0
(GraphPad Software Inc., La Jolla, CA, USA). Each point on the
survival curve represents the mean surviving fraction from at least
three independent experiments.
Hoechst 33342 staining
Cells were grown on coverslips in 6-well plates and
treated with or without metformin for 24 h prior to administration
of 6 Gy or sham radiation. The treated cells were cultured for
another 12, 24 and 48 h, fixed, and then stained with Hoechst 33342
(Beyotime Biotech, China) at a final concentration of 10 μg/ml for
15 min, and scanned on a confocal microscope (magnification, ×600
for the nuclear and morphologic analyses; Leica TCS SP5, Wetzlar,
Germany). Apoptotic cells were identified by morphology and
condensation and fragmentation of their nuclei. The percentage of
apoptotic cells was calculated as the ratio of apoptotic cells to
total cells counted, multiplied by 100. Three independent
experiments were conducted, and at least 300 cells were counted for
each experiment.
Immunofluorescent staining
Cells (2.5×105/dish) were plated onto
sterile coverslips, and the following day were treated with or
without metformin. Twelve hours later, cells were irradiated at a
total dose of 6 Gy. Cells were collected at the indicated time
points (1, 4 and 12 h), washed, fixed and then blocked with 5% BSA
before incubation in rabbit monoclonal anti-γ-H2AX antibody
overnight at 4°C. After rinsing with PBS three times, the
coverslips were incubated in secondary anti-rabbit Alexa Fluor 488
antibody (1:500; Invitrogen, Camarillo, CA, USA) for 1 h at room
temperature. Then cells were stained with DAPI (Sigma-Aldrich) for
15 min. Coverslips were then mounted onto slides with anti-fade
mounting medium (Solarbio, China). The images were visualized, and
representative views of the cells were recorded by a confocal
microscope (Leica TCS SP5). For each treatment condition, the
numbers of γ-H2AX foci were counted for 50 cells at least. For the
negative-control staining, the primary antibodies were omitted.
Western blot analysis
After treatment with metformin and/ or irradiation
(6 Gy), sample preparation for immunoblotting was carried out as
previously described (16). The
membrane was then incubated with the appropriate primary antibody,
anti-caspase-9, anti-caspase-3, anti-phospho-histone H2AX(Ser139),
anti-ATM, anti-phospho-ATM(Ser1981), anti-ATR,
anti-phospho-ATR(Ser428), anti-DNA-PK, anti-Ku70, anti-Ku80,
anti-Rad50 and anti-p95/NBS1 (1:1,000; CST, Danvers, MA, USA), and
anti-β-actin (1:1,500; Beyotime Biotech, China). The protein of
interest was detected with goat anti-rabbit or anti-mouse
IgG-horseradish peroxidase-conjugated secondary antibody (1:1,500;
Beyotime Biotech, China). The band intensities were measured using
Image J 1.41 software (NIH, Bethesda, MD, USA). Data are presented
as the relative protein levels normalized to β-actin, and the ratio
of the control samples was taken as 1.0.
Statistical analysis
All data are expressed as mean values ± SD. For
two-group comparison, the Student’s t-test method was used. For
more than a two-group comparison, one-way ANOVA was used. SPSS 13.0
software was used for all statistical analyses (SPSS, Chicago, IL,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Metformin inhibits the proliferation of
NPC cells in a dose-and time-dependent manner
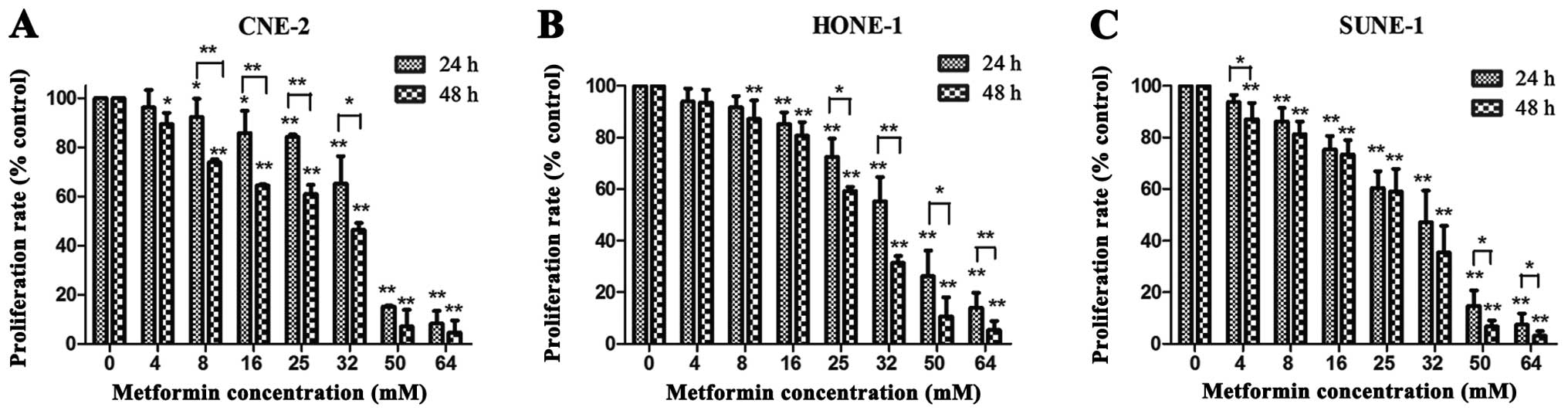
CCK-8 assay was performed to evaluate the effects of
metformin on the proliferation of NPC cell lines, CNE-2, HONE-1,
and SUNE-1. Increasing concentrations of metformin and prolonged
time from 24 to 48 h resulted in greater reduction in the cell
viability of the three cell lines. It was revealed that metformin
inhibited NPC cell proliferation in a dose- and time-dependent
manner (Fig. 1A–C). The 50%
inhibitory concentrations (IC50) of metformin in the
CNE-2, HONE-1 and SUNE-1 cells were 23.39±0.06, 26.12±0.02 and
24.75±0.04 mM, respectively. Metformin inhibited the proliferation
of the three NPC cell lines slightly when at concentrations <8
mM. Consequently, the metformin concentration of 5 mM was thought
to be a mild dose and was chosen to examine its radiosensitization
effect in the following studies.
Metformin pretreatment followed by
irradiation reduces the colony forming ability of NPC cells
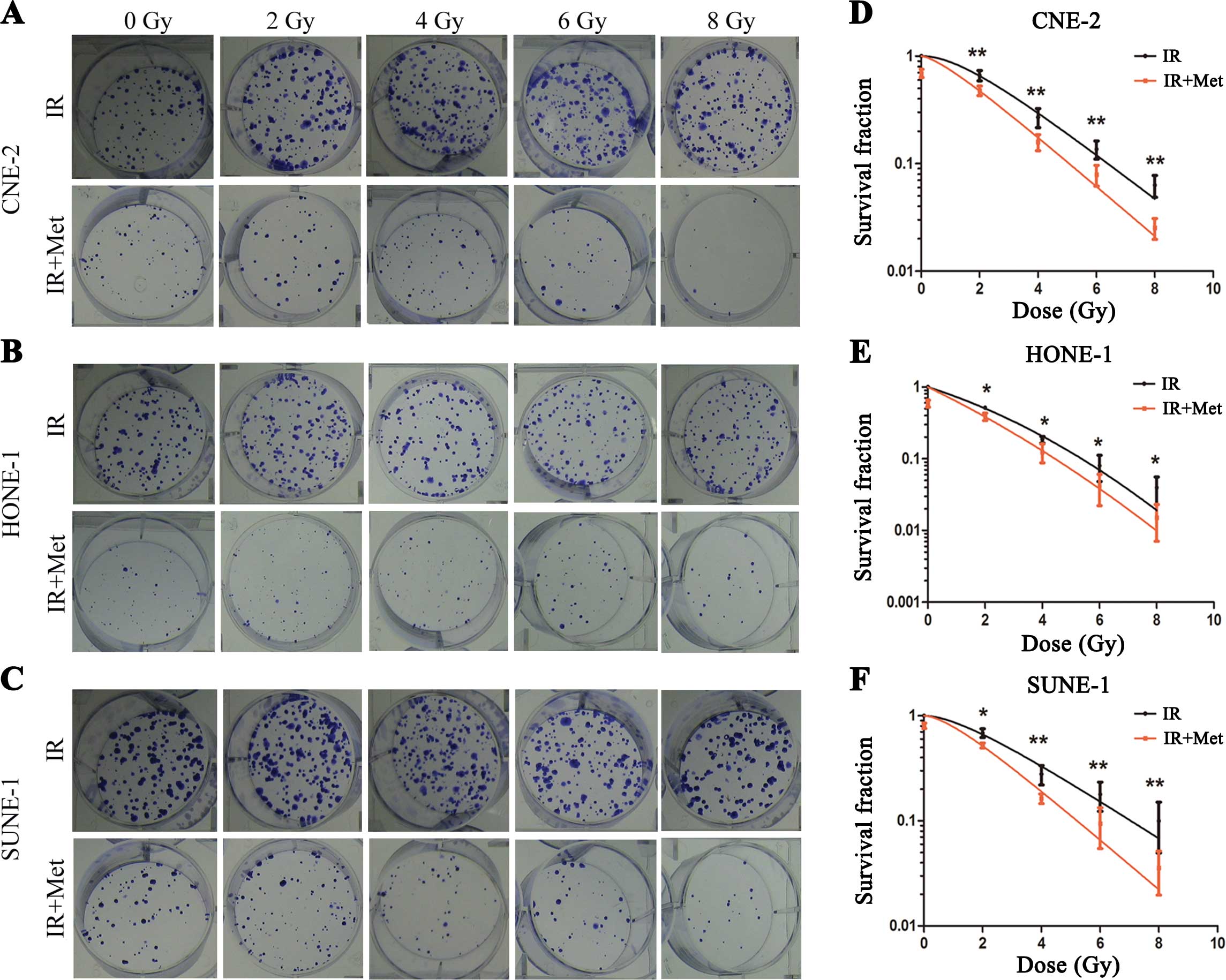
A clonogenic assay was used to determine whether
metformin enhances the radio-sensitivity of NPC cells. Irradiation
caused a dose-dependent reduction in clonogenic survival in the NPC
cell lines, and we found significant variation in intrinsic
radiosensitivity (Fig. 2). The
radiation sensitivity was expressed as the surviving fraction at a
clinically relevant dose of 2 Gy (SF2), and sensitization
enhancement ratios (SER) were calculated. As shown in Fig. 2, the SF2 for the CNE-2 cells
following IR and IR+Met was 0.663±0.145 and 0.478±0.097,
respectively (P=0.003). The SF2 for the HONE-1 cells following IR
and IR+Met was 0.514±0.044 and 0.387±0.079, respectively (P=0.012).
The SF2 for the SUNE-1 cells following IR and IR+Met was
0.686±0.132 and 0.522±0.057, respectively (P=0.012). When the dose
increased the differences in the surviving fraction between the
three cell populations became wider. Consistently, metformin
markedly enhanced the radiosensitivity of NPC cell lines with SERs
of 1.12 for CNE-2, 1.20 for HONE-1 and 1.22 for SUNE-1 cells.
Metformin pretreatment followed by
irradiation increases cell apoptosis
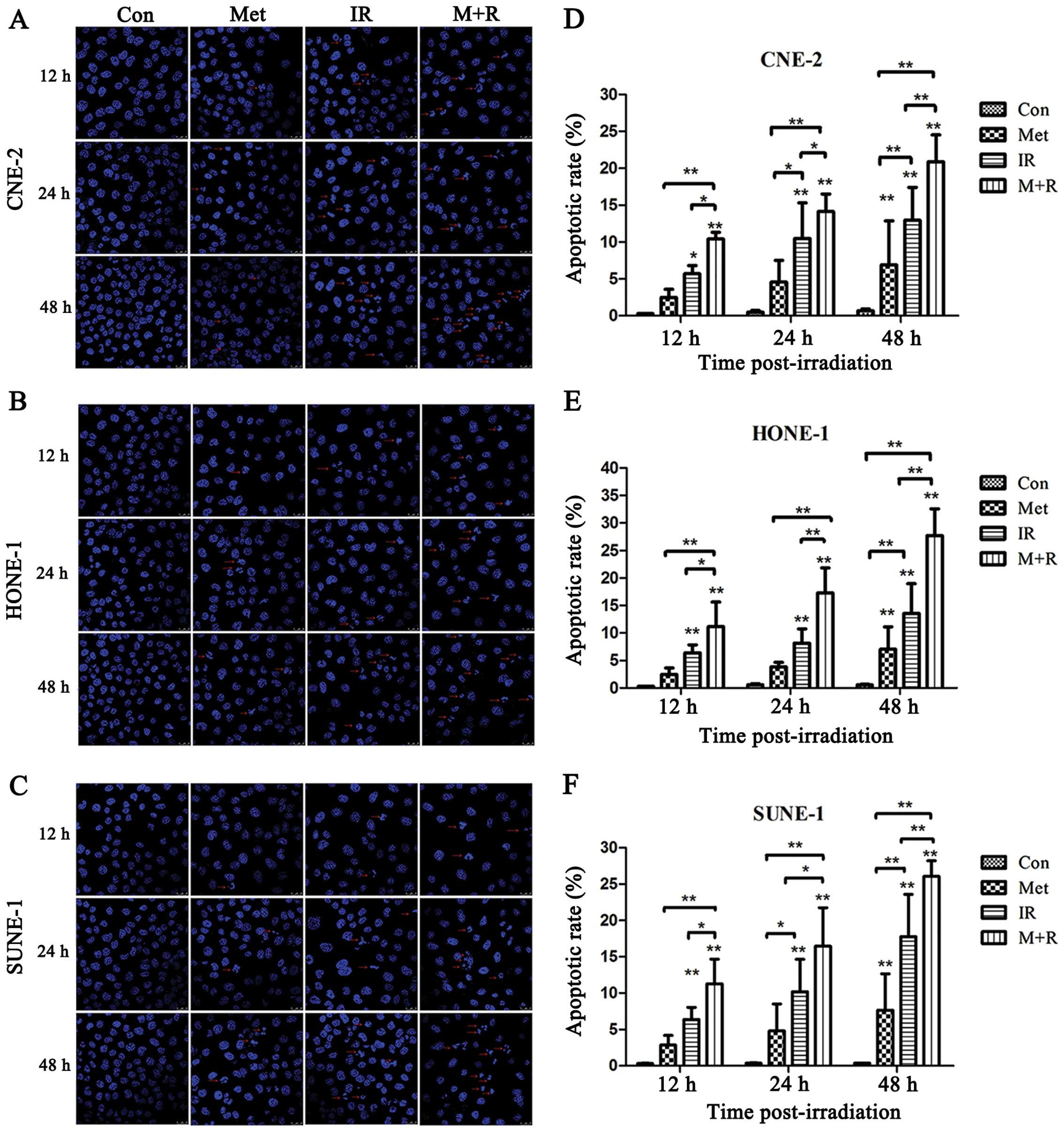
To investigate whether the radiosensitization effect
of metformin is associated with cell apoptosis, the apoptosis of
NPC cells was examined by examining morphological changes of nuclei
by confocal microscopy at 12, 24 and 48 h post-irradiation, and
determining the levels of the caspase-3 and caspase-9 by western
blot assay. The apoptotic cells, which were characterized by
condensed and fragmented nuclei, are shown in Fig. 3A–C. Further analysis revealed that
metformin plus irradiation significantly increased cell apoptosis
in all of the three NPC cell lines compared with cells exposed to
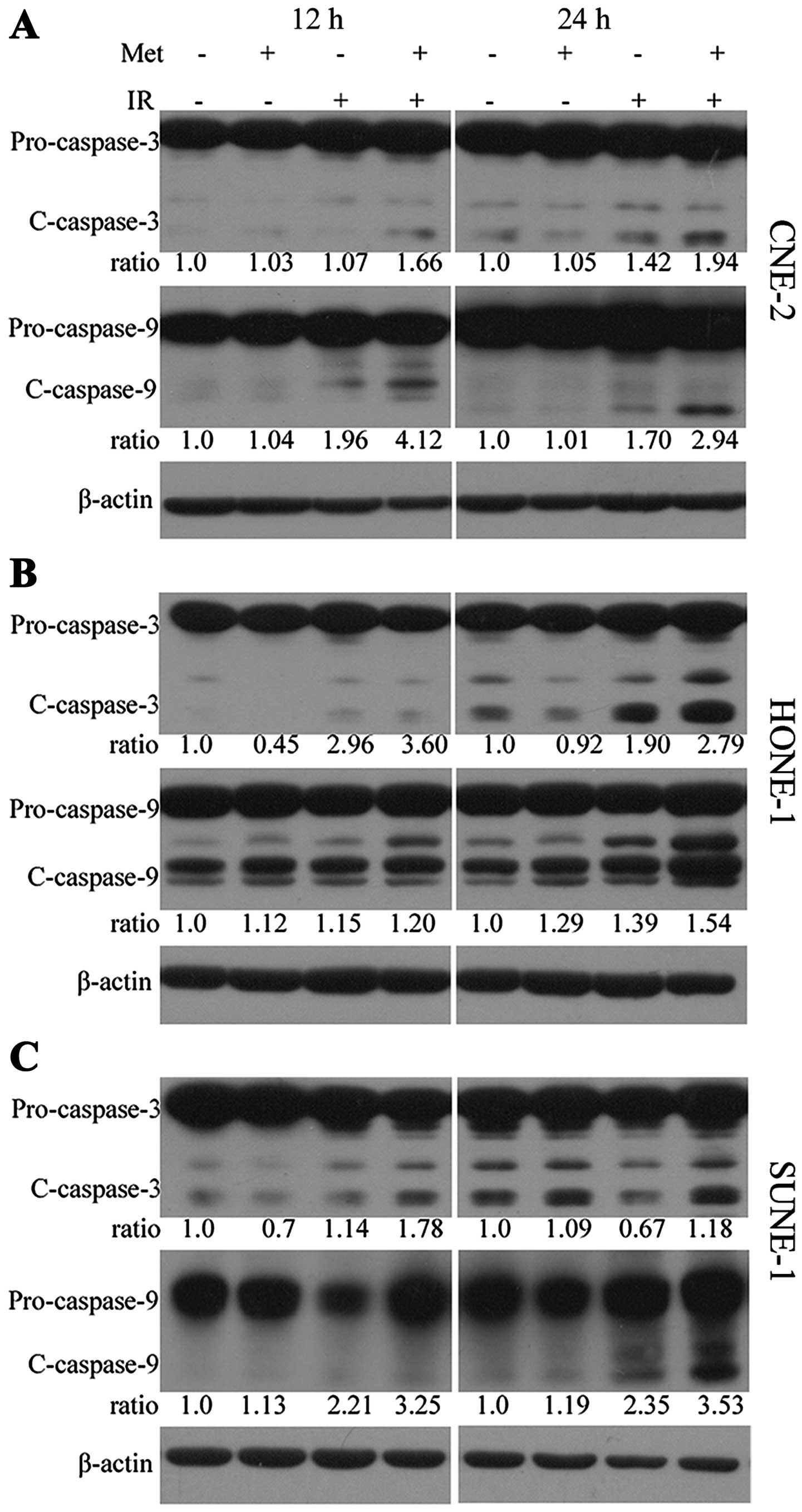
irradiation alone over a certain time-period (Fig. 3D–F). The cleavage of caspase-3 and
caspase-9 proteins were markedly increased in the cells treated
with the combination of irradiation and metformin compared with the
cells exposed to irradiation or metformin alone (Fig. 4A–C).
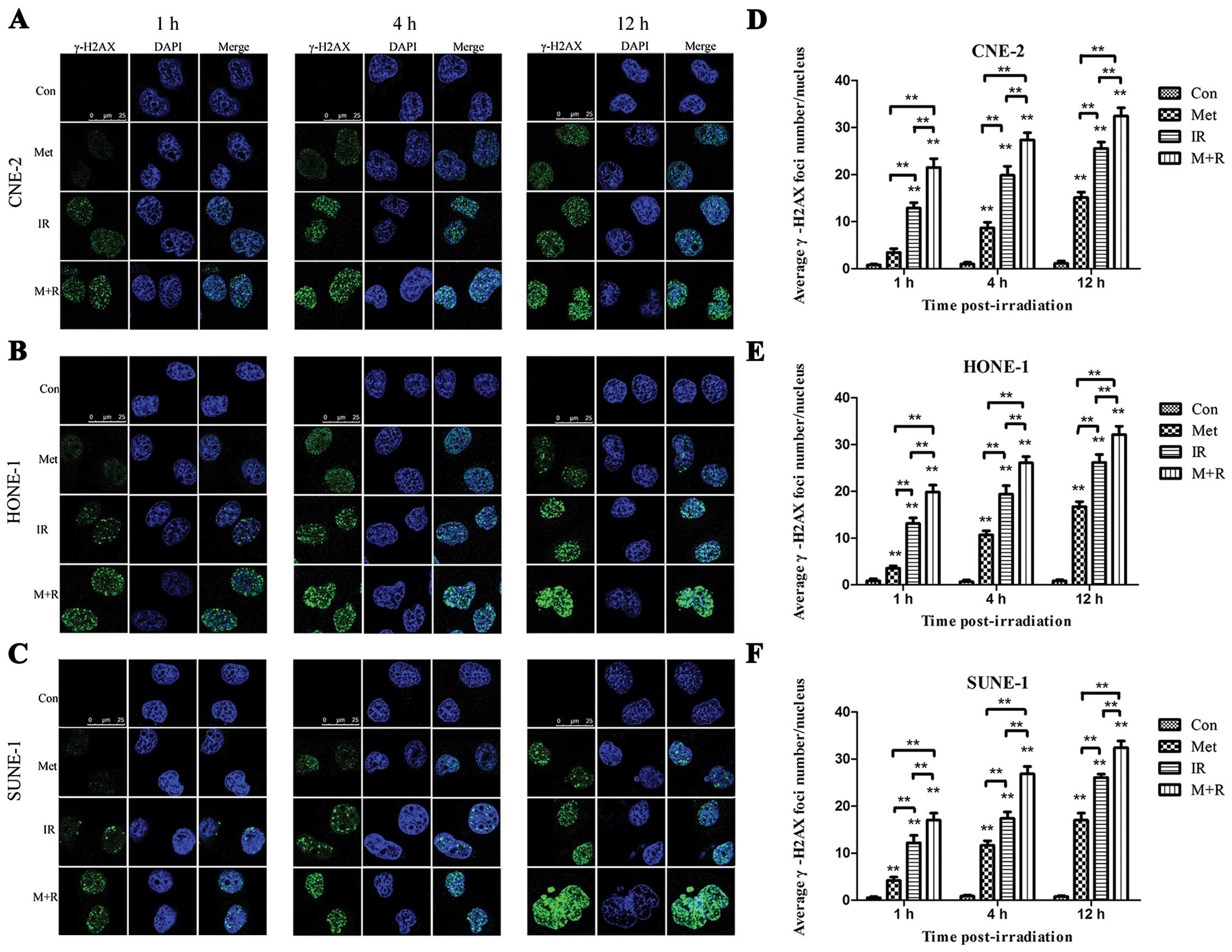
Metformin pretreatment followed by
irradiation induces γ-H2AX focus formation and increases the
expression of γ-H2AX
γ-H2AX has been identified as a marker of DNA
double-strand breaks (DSBs). Immunocytochemical analysis using
anti-γ-H2AX antibodies was conducted in order to determine the
effects of metformin on DNA repair. As shown in representative
micrographs in Fig. 5A–C, the
number of γ-H2AX foci was clearly distinguished after the different
treatments. The mean number of γ-H2AX foci per cell treated with
irradiation or metformin alone was compared with the mean number of
focu in cells treated with a combination of metformin and
irradiation. The mean number of γ-H2AX foci was shown to be
increased in the cells treated with irradiation alone over time,
while γ-H2AX foci in the cells exposed to metformin (5 mM, 12 h)
prior to irradiation were dramatically increased over a 1-, 4- and
12-h time course in the CNE-2 (Fig.
5D), HONE-1 (Fig. 5E), and
SUNE-1 (Fig. 5F) cells.
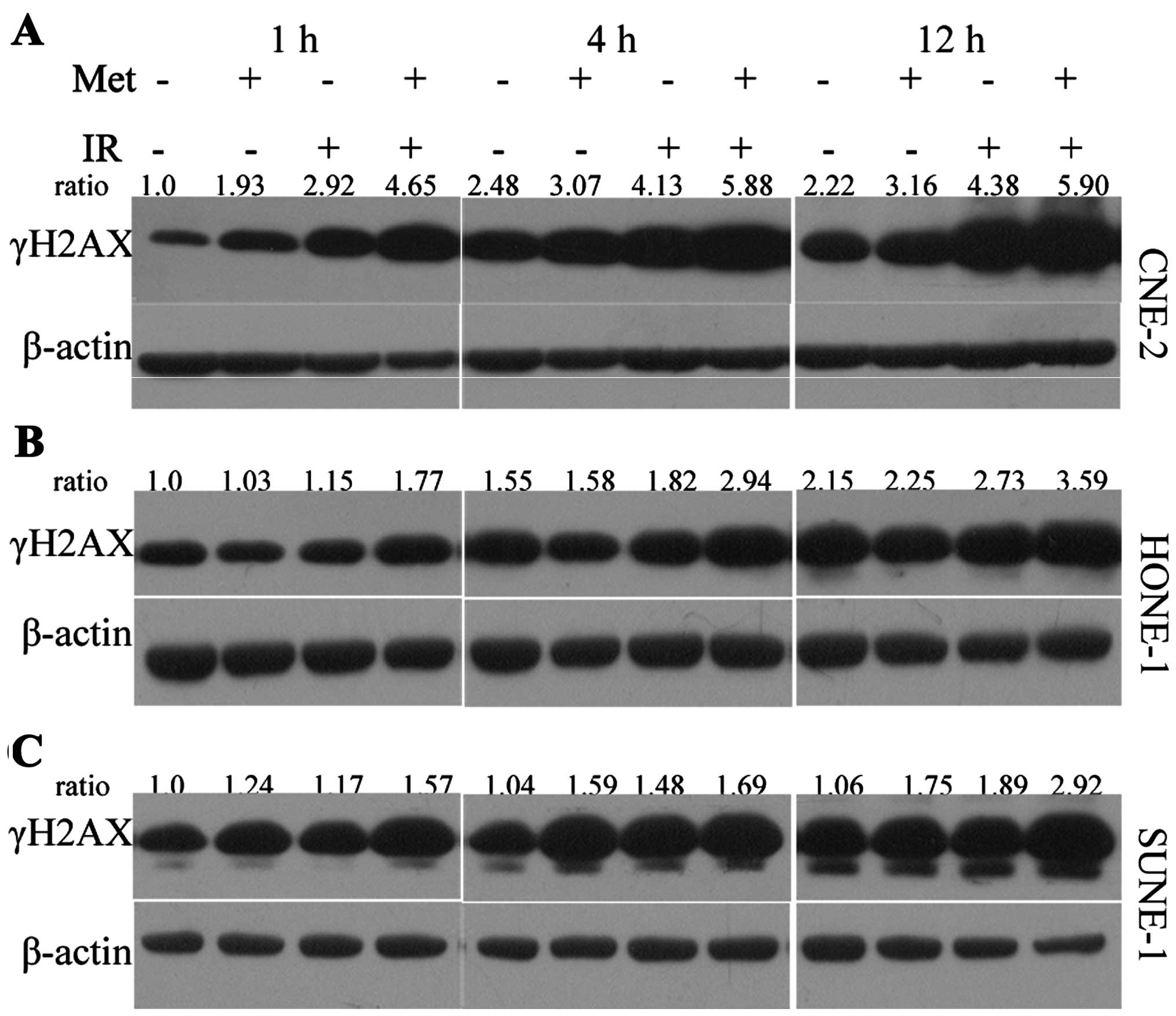
Consistently, metformin plus irradiation significantly increased
the expression of γ-H2AX protein compared with the expression in
cells treated with metformin or irradiation alone (Fig. 6), as detected by western
blotting.
Metformin pretreatment followed by
irradiation affects the expression of DNA damage repair-associated
proteins
Next, we investigated the effects of metformin on
DNA damage repair proteins by western blotting. The protein levels
of DNA damage repair-associated proteins in the CNE-2, HONE-1 and
SUNE-1 cells under various conditions are shown in Fig. 7A. Clearly, in all cell lines, the
expression levels of p-ATM(Ser1981) and p-ATR(Ser428) were
upregulated, and the expression levels of ATM and ATR were
downregulated, which indicated that DNA damage repair was
inactivated over time. Furthermore, the expression level of DNA-PK
was also significantly gradually downregulated over time (Fig. 7B). However, only a modest effect on
the expression levels of Ku70, Ku80, Rad50, p95/NBS1 proteins was
noted (Fig. 7). The combination of
metformin with irradiation was far more effective than metformin or
irradiation alone.
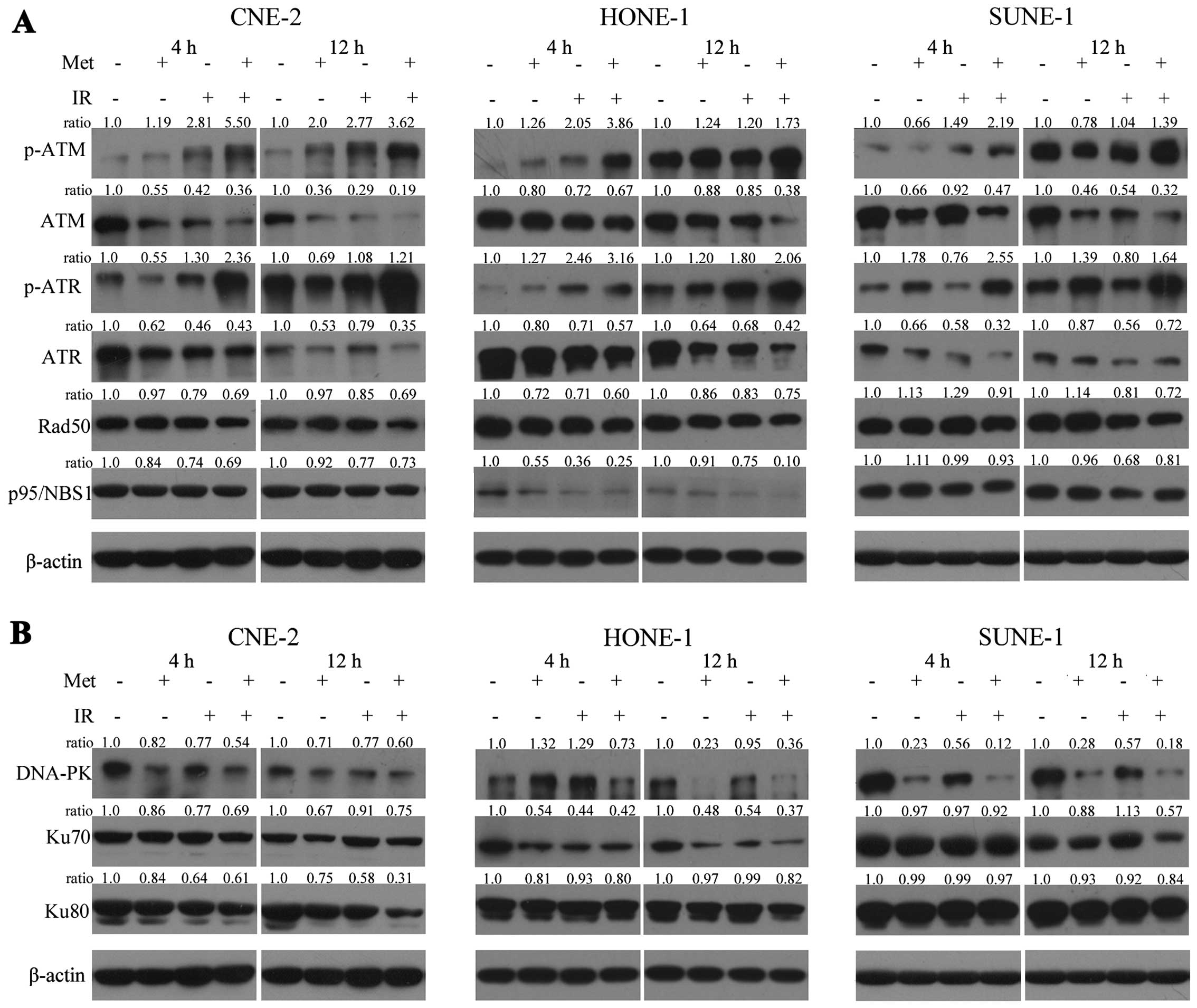 | Figure 7Effects of metformin combined with
irradiation on the signals associated with the DNA damage repair
pathway in NPC cells. Whole cell lysates were prepared and western
blot analysis was performed using (A) anti-ATM, -p-ATM, -ATR,
-p-ATR, -Rad50, and -p95/NBS1 antibodies for the HR pathway, and
(B) anti-DNA-PK, -Ku70, and -Ku80 for the NHEJ pathway. In all cell
lines, the expression levels of p-ATM(Ser1981) and p-ATR(Ser428)
were upregulated, and the expression levels of ATM, ATR and DNA-PK
were downregulated. However, only a modest effect on the expression
levels of Ku70, Ku80, Rad50, p95/ NBS1 proteins over time was
noted. The combination of metformin with irradiation was far more
effective than metformin or irradiation alone. Experiments were
repeated at least 3 times, and the representative results are
shown. The ratios above each blot are expressed as the intensity of
the blot relative to that of the untreated control. |
Discussion
In addition to the direct anticancer effects,
metformin has also been reported to enhance the response of
irradiation in several types of tumors, including fibrosarcoma
(14), hepatoma (15), head and neck cancer (17), lung cancer and prostate cancer
(13). Similarly, the present study
reported for the first time that metformin markedly suppressed the
proliferation of NPC cells (Fig.
1). Further investigation demonstrated that metformin combined
with irradiation significantly decreased the clonogenic survival
abilities and enhanced the radiosensitivity of NPC cells, with a
sensitizing enhancement ratio (SER) of 1.12, 1.20 and 1.22 in
CNE-2, HONE-1 and SUNE-1 cells, respectively (Fig. 2). Interestingly, metformin has also
been evaluated in combination with radiotherapy or
chemoradiotherapy in several clinical trials, and survival benefits
were observed in metformin-treated patients compared to the
non-metformin-treated patients. Skinner et al investigated
the impacts of metformin on the outcome of radiation therapy for
head and neck cancer. Their data showed that metformin use was
significantly associated with decreased local recurrence rates
(LRR) as well as improved overall survival (OS), with 5-year OS
rates of 87% and 41%, respectively, for the patients receiving
metformin and the remaining patients (17). This was further verified by another
study, which found that metformin use was associated with a
dose-dependent increased response to concurrent chemoradiotherapy
in esophageal cancer with a higher pathologic complete response and
a decrease in field locoregional failure (18). Given the above findings, the
addition of metformin to radiotherapy might benefit the treatment
of malignancies, including nasopharyngeal carcinoma.
Apoptosis has previously been regarded as a
potential mechanism for radiosensitization. Lin et al showed
that a molecularly targeted aurora kinase inhibitor, VE-465,
significantly enhanced radiation-induced tumor growth suppression
by a mechanism involving increased apoptosis (19). Grosse et al found that
sunitinib combined with radiation induced apoptosis in follicular
thyroid cancer cells via the intrinsic pathway of apoptosis
(20). In addition, metformin has
been reported to enhance irradiation-induced apoptosis and activate
caspase-3 in lung cancer cells (21). In the present study, we additionally
observed that metformin in combination with irradiation
significantly increased the apoptotic rates (Fig. 3) and potentiated the cleavage of
caspase-9/-3 proteins (Fig. 4)
compared to either radiation or metformin treatment alone in NPC
cells, which suggests that metformin may sensitize NPC cells to
irradiation by promoting apoptosis.
Radiotherapy is one of the major therapeutic
strategies for cancer. Irradiation results in DNA damage and thus
initiates a variety of signaling events in cancer cells (22). Double-strand breaks (DSBs) are one
of the most important DNA damages caused by irradiation. In
response to DSBs, histone H2AX is rapidly activated and
phosphorylated. This phosphorylated form of H2AX is named γ-H2AX.
The expression of γ-H2AX is a sensitive indicator of
irradiation-induced DSBs, and γ-H2AX foci are used to identify the
number and location of DSBs and are widely used to evaluate
cellular radiosensitivity (23,24).
To investigate whether metformin altered DSB repair, we monitored
the formation of γ-H2AX foci in cells treated with metformin or
radiation. Our data indicated that the number of γ-H2AX foci and
the level of γ-H2AX protein were significantly increased over time
(1, 4, 12 h) following irradiation. Notably, although irradiation
or metformin alone induced nuclear γ-H2AX focus formation,
metformin plus irradiation markedly induced an increased number of
nuclear γ-H2AX foci compared to either irradiation or metformin
treatment (Fig. 5A–F). Similarly,
increased expression of γ-H2AX protein was observed in the NPC
cells treated with metformin plus irradiation compared to either
irradiation or metformin treatment alone as detected by
immunoblotting (Fig. 6). Therefore,
we concluded that metformin in combination with irradiation
markedly induced DNA damage in NPC cells, suggesting that the
radiosensitization effect of metformin might result from the
augmentation of irradiation-induced DNA damage.
Recent studies have explored the potential
mechanisms by which metformin enhances the response of irradiation
in several tumors. It was reported that metformin sensitized cancer
cells to irradiation by potentiating IR-induced AMPK activation
(13,14,21).
Other studies found that following the combination of metformin and
IR, enhancement of cytotoxic effects was noted by reducing ATP
production (15), potentiating
intracellular ROS levels induced by irradiation (25), and inducing senescence (17). To elucidate how metformin suppressed
the repair of irradiation-induced DSBs, we investigated the effects
of metformin on molecules involved in the non-homologous
end-joining (NHEJ) and homologous recombination (HR) pathways by
western blot assay. Our data demonstrated that metformin plus IR
significantly induced time-dependent upregulation of p-ATM and
downregulation of ATM thereby inhibiting DNA DSB repair pathways.
These results implied that metformin induced radiosensitivity by
decreasing the level of ATM expression. Furthermore, we examined a
range of protein substrates, including ATR, NBS1, and Rad50 after
DSBs were formed. Our findings showed that metformin pretreatment
significantly reduced the level of ATR kinase, the phosphorylated
form of ATR was strongly elevated, while the expression levels of
Rad50 and NBS1 proteins were only modestly downregulated after
exposure to irradiation (Fig. 7A).
DNA-PK, Ku70 and Ku80 are critical proteins involved in
non-homologous end-joining repair (NHEJ) of DNA DSBs. In the
present study, we found that the expression levels of DNA-PK, Ku70
and Ku80 proteins were much lower following the combination
treatment compared to metformin or irradiation alone, suggesting
that metformin may sensitize radiation via interfering with the
NHEJ pathway thus weakening DSB repair ability in NPC cells
(Fig. 7B). Taken together, these
results indicate that metformin might be a dual inhibitor of both
DNA-PKcs and ATM kinase, thus sensitizing NPC cells to irradiation
by induction of more DNA damage and inhibition of repair of
irradiation-induced DNA DSBs through diminishing NHEJ and HR
pathways. For decades, researches have focused on the development
of potent DNA-PKcs, and ATM inhibitors have yielded specific
compounds, some of which have been found to be extremely useful in
preclinical studies. Truman et al found downregulation of
ATM protein resulting in an increase in radiation-induced apoptosis
in human prostate cancer cells (26). Biddlestone-Thorpe et al
demonstrated that ATM kinase inhibitor KU-60019 radio-sensitized
human glioma and mouse glioma stem cells via inhibition of the
expression of ATM thereby inhibiting DNA repair capacity (27). In addition, Gil et al found
that the dual PI3K/mTOR inhibitor NVP-BEZ235 potently inhibited
both DNA-PKcs and ATM kinases and attenuated the repair of
IR-induced DNA damage in human glioblastoma, resulting in striking
tumor radiosensitization (28).
However, further exploration is required to clarify how metformin
regulates ATM and DNA-PK.
In summary, this study demonstrated that metformin
has a strong radiosensitizing potential in NPC cells. This
radio-sensitizing effect was associated with inhibition of DNA DSB
repair processes through HR repair and NHEJ repair signaling
pathways, thereby enhancing radiation-induced cell apoptosis. In
addition, our data suggest that metformin is a potent
radiation-sensitizing agent and may be a promising candidate for
clinical evaluation as part of a combined regimen for the treatment
of NPC.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (no. 81201736); the Science and
Technology Planning Project of Guangdong Province, China (nos.
KZ0710, 11401S009015, 11401S010013); and the Science and Technology
Innovation Project of Guangdong Medical College, China (no.
TD1124).
References
|
1
|
Yu MC and Yuan JM: Epidemiology of
nasopharyngeal carcinoma. Semin Cancer Biol. 12:421–429. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wei WI and Sham JS: Nasopharyngeal
carcinoma. Lancet. 365:2041–2054. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen L, Hu CS, Chen XZ, et al: Concurrent
chemoradiotherapy plus adjuvant chemotherapy versus concurrent
chemoradiotherapy alone in patients with locoregionally advanced
nasopharyngeal carcinoma: a phase 3 multicentre randomised
controlled trial. Lancet Oncol. 13:163–171. 2012. View Article : Google Scholar
|
|
4
|
Zhou J, Wang L, Xu X, Tu Y, Qin S and Yin
Y: Antitumor activity of Endostar combined with radiation against
human nasopharyngeal carcinoma in mouse xenograft models. Oncol
Lett. 4:976–980. 2012.PubMed/NCBI
|
|
5
|
Luftig M: Heavy LIFting: tumor promotion
and radioresistance in NPC. J Clin Invest. 123:4999–5001. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shaw RJ: Metformin trims fats to restore
insulin sensitivity. Nat Med. 19:1570–1572. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Evans JM, Donnelly LA, Emslie-Smith AM,
Alessi DR and Morris AD: Metformin and reduced risk of cancer in
diabetic patients. BMJ. 330:1304–1305. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Landman GW, Kleefstra N, van Hateren KJ,
Groenier KH, Gans RO and Bilo HJ: Metformin associated with lower
cancer mortality in type 2 diabetes: ZODIAC-16. Diabetes Care.
33:322–326. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Singh S, Singh PP, Singh AG, Murad MH and
Sanchez W: Antidiabetic medications and the risk of hepatocellular
cancer: a systematic review and meta-analysis. Am J Gastroenterol.
108:881–891. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ben SI, Le Marchand-Brustel Y, Tanti JF
and Bost F: Metformin in cancer therapy: a new perspective for an
old antidiabetic drug? Mol Cancer Ther. 9:1092–1099. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jiralerspong S, Palla SL, Giordano SH, et
al: Metformin and pathologic complete responses to neoadjuvant
chemotherapy in diabetic patients with breast cancer. J Clin Oncol.
27:3297–3302. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Iliopoulos D, Hirsch HA and Struhl K:
Metformin decreases the dose of chemotherapy for prolonging tumor
remission in mouse xenografts involving multiple cancer cell types.
Cancer Res. 71:3196–3201. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sanli T, Rashid A, Liu C, et al: Ionizing
radiation activates AMP-activated kinase (AMPK): a target for
radiosensitization of human cancer cells. Int J Radiat Oncol Biol
Phys. 78:221–229. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Song CW, Lee H, Dings RP, et al: Metformin
kills and radio-sensitizes cancer cells and preferentially kills
cancer stem cells. Sci Rep. 2:3622012.PubMed/NCBI
|
|
15
|
Liu J, Hou M, Yuan T, et al: Enhanced
cytotoxic effect of low doses of metformin combined with ionizing
radiation on hepatoma cells via ATP deprivation and inhibition of
DNA repair. Oncol Rep. 28:1406–1412. 2012.PubMed/NCBI
|
|
16
|
Xu Z, Fang S, Zuo Y, et al: Combination of
pigment epithelium-derived factor with radiotherapy enhances the
antitumor effects on nasopharyngeal carcinoma by downregulating
vascular endothelial growth factor expression and angiogenesis.
Cancer Sci. 102:1789–1798. 2011. View Article : Google Scholar
|
|
17
|
Skinner HD, Sandulache VC, Ow TJ, et al:
TP53 disruptive mutations lead to head and neck cancer treatment
failure through inhibition of radiation-induced senescence. Clin
Cancer Res. 18:290–300. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Skinner HD, McCurdy MR, Echeverria AE, et
al: Metformin use and improved response to therapy in esophageal
adenocarcinoma. Acta Oncol. 52:1002–1009. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lin ZZ, Chou CH, Cheng AL, Liu WL and
Chia-Hsien Cheng J: Radiosensitization by combining an aurora
kinase inhibitor with radiotherapy in hepatocellular carcinoma
through cell cycle interruption. Int J Cancer. 135:492–501. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Grosse J, Warnke E, Wehland M, et al:
Mechanisms of apoptosis in irradiated and sunitinib-treated
follicular thyroid cancer cells. Apoptosis. 19:480–490. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Storozhuk Y, Hopmans SN, Sanli T, et al:
Metformin inhibits growth and enhances radiation response of
non-small cell lung cancer (NSCLC) through ATM and AMPK. Br J
Cancer. 108:2021–2032. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jackson SP: Sensing and repairing DNA
double-strand breaks. Carcinogenesis. 23:687–696. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bonner WM, Redon CE, Dickey JS, et al:
GammaH2AX and cancer. Nat Rev Cancer. 8:957–967. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bourton EC, Plowman PN, Smith D, Arlett CF
and Parris CN: Prolonged expression of the γ-H2AX DNA repair
biomarker correlates with excess acute and chronic toxicity from
radiotherapy treatment. Int J Cancer. 129:2928–2934. 2011.
|
|
25
|
Sandulache VC, Skinner HD, Ow TJ, et al:
Individualizing anti-metabolic treatment strategies for head and
neck squamous cell carcinoma based on TP53 mutational status.
Cancer. 118:711–721. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Truman JP, Gueven N, Lavin M, et al:
Down-regulation of ATM protein sensitizes human prostate cancer
cells to radiation-induced apoptosis. J Biol Chem. 280:23262–23272.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Biddlestone-Thorpe L, Sajjad M, Rosenberg
E, et al: ATM kinase inhibition preferentially sensitizes
p53-mutant glioma to ionizing radiation. Clin Cancer Res.
19:3189–3200. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gil del Alcazar CR, Hardebeck MC,
Mukherjee B, et al: Inhibition of DNA double-strand break repair by
the dual PI3K/ mTOR inhibitor NVP-BEZ235 as a strategy for
radiosensitization of glioblastoma. Clin Cancer Res. 20:1235–1248.
2013.PubMed/NCBI
|