Introduction
Gastric cancer is a major global health concern,
with an estimated 989,600 new cases and more than 738,000
attributable deaths in 2011 (1).
Peritoneal carcinomatosis (PC) is a characteristic feature of
gastric cancer and is a critical factor underlying its poor
prognosis (2–4).
PC is relatively resistant to systemic chemotherapy
due to the poor blood supply and oxygenation of cancer cells in the
peritoneum (5). A large body of
data has shown that systemic administration of S-1 or taxanes may
be efficacious in treating PC. These compounds have a high
sensitivity in targeting poorly differentiated adenocarcinoma,
which is a common microscopic type of peritoneal tumor (6,7).
Furthermore, when administered intravenously, some of these
compounds are transported into the peritoneal cavity (8,9).
Therefore, combining S-1 with intraperitoneal (i.p.) administration
of taxanes has been proposed as an effective and feasible treatment
strategy for PC. Ishigami et al established a protocol of
i.p. paclitaxel with S-1 plus intravenous paclitaxel, in which the
median survival time (MST) was 22.5 months and the 1-year survival
rate was 78% (10). S-1 plus i.p.
docetaxel also demonstrated a promising 1-year survival rate of
70%, with an MST of 16.2 months in patients with severe PC
(11). However, these clinical
outcomes have been largely unsatisfactory since most patients die
of recurrence within 5 years. PC is characterized by cancer cell
infiltration and proliferation, which is accompanied by extensive
stromal fibrosis. This results in the development of
chemoresistance and obstructive disorders such as ileus,
obstructive jaundice and hydronephrosis (12). Therefore, it is necessary to develop
new treatment strategies to target the fibrosis in PC.
The fibrous tissue found in organs during PC is
produced by cancer-associated fibroblasts (CAFs), which are
recruited from orthotopic fibroblast pools (13), bone marrow-derived fibrocytes
(14), and human peritoneal
mesothelial cells (HPMCs) (15).
These cells can undergo epithelial-mesenchymal transition (EMT) to
differentiate into an extracellular matrix-producing
myofibroblastic phenotype in the presence of TGF-β released from
gastric cancer cells (16).
Therefore, TGF-β signaling represents a promising potential target
for tumor fibrosis in PC.
Protein-bound polysaccharide K (PSK;
Krestin®) is isolated and purified from the cultured
mycelium of the Basidiomycete Coriolus versicolor (17). PSK is considered a biological
response modifier, and has been approved for use in combination
with chemotherapy to prolong the survival of patients with gastric
cancer or colorectal cancer.
PSK also appears to inhibit TGF-β signaling through
suppression of TGF-β production, direct binding with TGF-β, and
through acting on TGF-β receptors (18–20).
Ono et al further reported that PSK can suppress Smad2
phosphorylation, resulting in the inhibition of EMT in the
colorectal cancer SW837 cell line (21). Here, we investigated whether PSK
could inhibit both the EMT-like change of HPMCs in response to
TGF-β signaling in vitro, and the subsequent induction of
tumor fibrosis by co-inoculum of gastric cancer OCUM-2MD3 cells and
HPMCs in vivo.
Materials and methods
Cell lines and cell culture
HPMCs were isolated from surgical specimens of the
human omentum, as previously described (22). Written informed consent for use of
these specimens, as required by the Institutional Review Board at
Kanazawa University, Japan, was obtained from patients undergoing
elective abdominal surgery. Small pieces of omentum were
immediately washed extensively in phosphate-buffered saline (PBS)
and were incubated in pre-warmed PBS containing 0.125% trypsin/EDTA
(Gibco/Invitrogen, USA) for 30 min at 37°C. The suspension was then
passed through a 100-μm pore nylon mesh (Becton-Dickinson, Japan)
to remove undigested fragments and centrifuged at 1,500 rpm for 5
min. The collected cells were cultured in RPMI-1640 medium
(Gibco/Invitrogen) supplemented with 20% heat-inactivated fetal
bovine serum (FBS; Nichirei Bioscience Inc., Japan), 100 IU/ml
penicillin, 100 mg/ml streptomycin (Gibco/Invitrogen), and 2 mM
glutamine (Nissui Pharmaceutical Co. Ltd., Japan). The cells were
seeded in gelatin-coated 75-cm2 flasks (BD BioCoat, USA)
and cultured in 10 ml of medium at 37°C in a humidified atmosphere
of 5% CO2 in air. Subconfluent HPMCs were trypsinized
with 0.125% trypsin/EDTA before use. HPMCs were used from passage 1
to 3 in all experiments.
OCUM-2MD3, a cell line derived from a human
scirrhous gastric cancer and with high peritoneal-seeding activity,
was kindly provided by the Department of Surgical Oncology of Osaka
City University of Medicine. OCUM-2MD3 cells were seeded in
75-cm2 dishes (Becton Dickinson, Tokyo, Japan) and
cultured in 10 ml Dulbecco’s modified Eagle’s medium (Life
Technologies, Tokyo, Japan) supplemented with 10% heat-inactivated
FBS, 100 IU/ml penicillin, 100 mg/ml streptomycin, 2 mM glutamine,
and 0.5 mM sodium pyruvate, at 37°C in a humidified atmosphere of
5% CO2 in air.
Reagents
Protein-bound polysaccharide was kindly provided by
the Kureha Chemical Ind. Co. (Japan) and TGF-β was purchased from
Sigma-Aldrich, Inc. (USA).
Phase contrast microscopy
HPMCs in cultures were treated with TGF-β1 (10
ng/ml) or both TGF-β1 and PSK (100, 500 μg/ml) for 72 h and
morphological changes were visualized by phase contrast microscopy.
The images were captured using a Nikon inverted microscope (Nikon
Corp., Japan).
Immunofluorescence
For visualization of E-cadherin and α-SMA in the
HPMCs, the cells were grown on 4-well, collagen type I-coated
culture slides (BD BioCoat) and then fixed in a 1:1 mixture of
methanol and acetone for 10 min. Briefly, the slides were immersed
in methanol containing 0.3% H2O2 for 30 min,
blocked with 3.3% normal goat serum in PBS, and incubated with the
E-cadherin antibody (H-108, rabbit polyclonal IgG; diluted 1:100;
Santa Cruz Biotechnology, Inc., USA) and α-SMA (1A4, mouse
monoclonal IgG; diluted 1:100; DakoCytomation, Denmark) at 4°C
overnight. Following three PBS washes, immunoreactivity was
visualized by incubating the sections with an anti-mouse IgG
antibody conjugated with Alexa Fluor® 488 and an
anti-rabbit IgG antibody conjugated with Alexa Fluor®
546 (1:400; Molecular Probes/Invitrogen, USA) for 1 h at room
temperature. Cells were then incubated with Hoechst 33258 for
nuclear staining for 5 min and mounted with propyl gallate
containing phenylenediamine under glass coverslips. Slides were
observed with an immunofluorescence microscope (BX50/BX-FLA;
Olympus, Japan).
Mouse xenograft model
All animal experiments were performed according to
Kanazawa University’s standard guidelines. Female immunocompromised
BALB/c-nu/nu mice (4–6 weeks old; Charles River Laboratories
Inc., Japan) with an average body weight of 20 g were maintained
under sterile conditions and used for all in vivo
experiments. OCUM-2MD3 cells were co-cultured with an equivalent
number of HPMCs, and a total of 5×106 cells in 100 μl of
RPMI-1640 was then subcutaneously injected into the dorsal side of
each mouse on day 0. Mice were then divided into two groups: i)
mice given normal chow (control, n=10) and ii) mice given chow
mixed with 1% PSK from day 1 (PSK, n=10). The PSK concentration
within the chow was adjusted to be at a dose of approximately 1
g/kg body weight/day, which was 1.5-fold higher than the clinical
dose of PSK (3 g/day) estimated by the surface area normalization
method (23). Mice were allowed
unrestricted access to water and to the standard or mixed chow. On
day 15, the animals were sacrificed, and the tumors were harvested.
Tumor specimens were collected for immunohistochemical
examination.
Histological and immunohistochemical
examination
Tumor specimens were fixed in 10% neutral-buffered
formalin and embedded in paraffin. Sections were stained with
hematoxylin and eosin and Azan. To analyze fibrosis, Azan
(blue)-stained areas were measured on a video display
(magnification, ×200) in a blinded manner using a QuickGrain
digital image analyzer (Inotech, Hiroshima, Japan). Two sections
were selected randomly from each sample and three fields from each
section were evaluated; samples from 10 mice in each group were
examined. To evaluate α-SMA expression, the sections were
immunostained with an α-SMA antibody (1A4, mouse monoclonal IgG,
diluted 1:100; DakoCytomation, Japan) at 4°C overnight, and treated
with EnVision reagent (Dako Co., Japan) for visualization.
Statistical analysis
All data are expressed as mean ± SD. Statistical
analyses were conducted using SPSS statistical software, version
11.0 (SPSS, Inc., USA). Comparisons of drug effects were carried
out using the Student’s t-test. A p-value of <0.05 was
considered to indicate a statistically significant difference.
Results
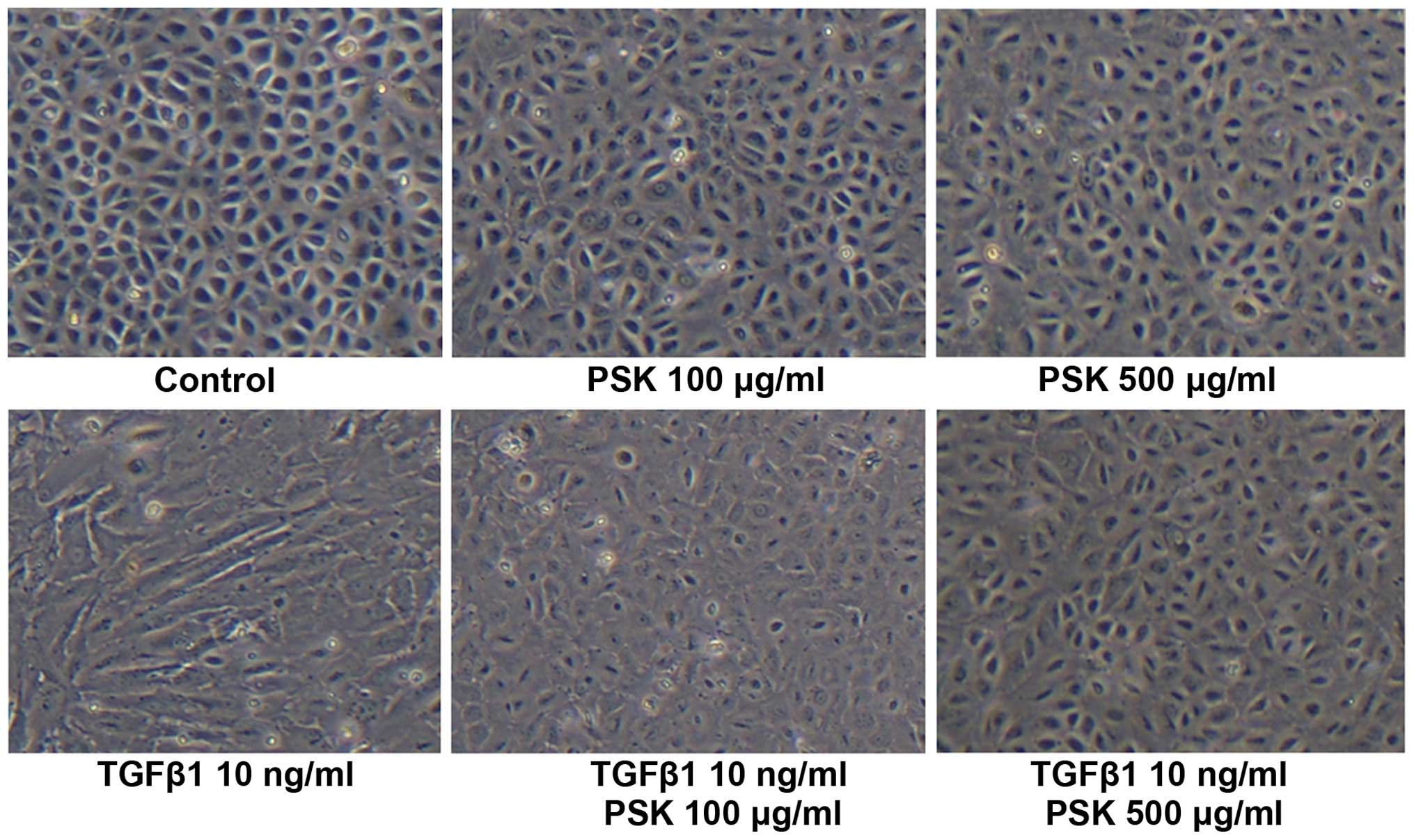
Effect of PSK on the morphological change
in HPMCs following treatment with TGF-β1
Control HPMCs exhibited a polygonal and
cobblestone-like growth pattern, whereas HPMCs treated with TGF-β1
adopted the spindle-shaped morphological characteristic of
fibroblasts. Pretreatment with PSK blocked these morphological
changes induced by TGF-β1 in a concentration-dependent manner
(Fig. 1).
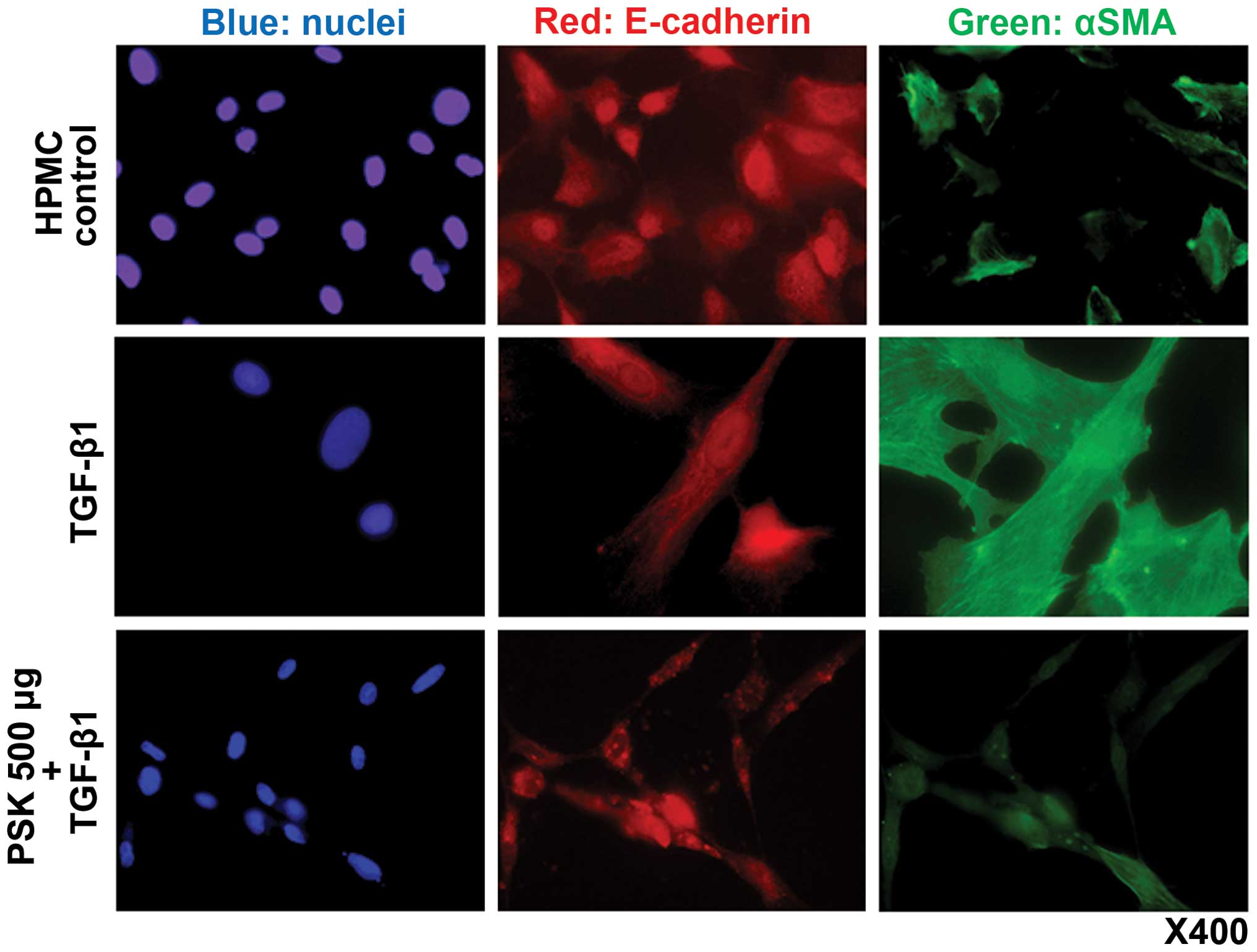
Immunofluorescence examination
Expression of E-cadherin and α-SMA were evaluated by
indirect immunostaining and confocal microscopy. In the absence of
TGF-β1, HPMCs did not express α-SMA in the cytoplasm. Treatment
with TGF-β1 induced cytoplasmic α-SMA expression, a recognized
component of EMT change. Pretreatment of HPMCs with PSK prior to
TGF-β1 administration prevented this EMT-like change (Fig. 2).
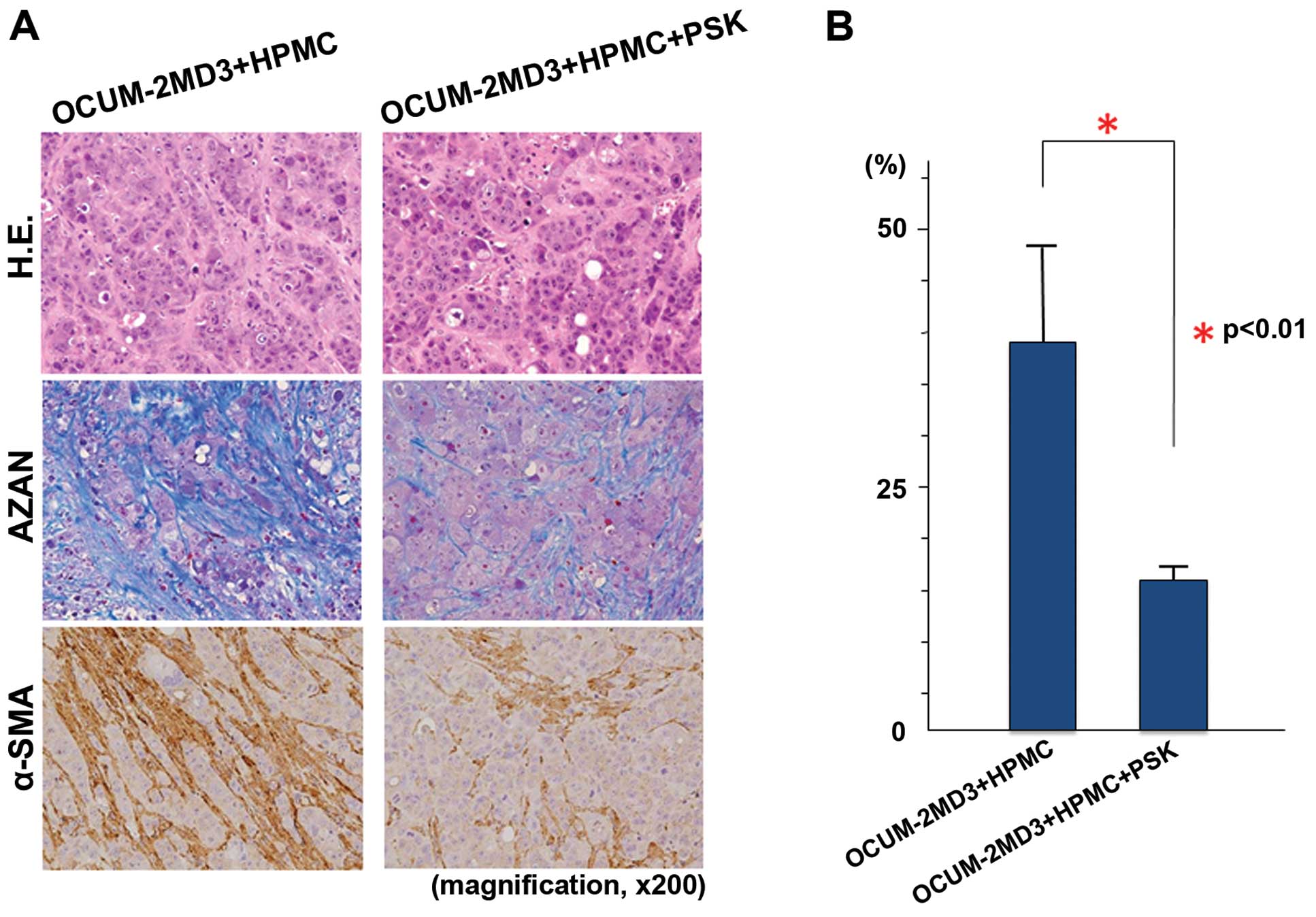
Histological and immunohistochemical
examination of the xenograft tumors
Xenograft tumors resulted from the inoculation of
BALB/c-nu/nu mice with a suspension of OCUM-2MD3 cells and
HPMCs contained many α-SMA-positive cells and a large amount of
collagen fibers. Administration of PSK to these mice yielded a
reduction in the number of α-SMA-positive cells and a decreased
amount of collagen fibers (Fig.
3A). To semi-quantitatively evaluate the degree of tumor
fibrosis, we examined the fibrotic areas in each tumor sample. Azan
staining revealed a significant difference in the degree of
fibrosis in the PSK group compared with the control (p<0.01;
Fig. 3B).
Discussion
The source of CAFs has not been fully elucidated.
However, orthotopic fibroblasts and bone marrow-derived cells may
function as myofibroblasts during EMT. In line with these findings,
we previously reported that bone marrow-derived fibrocytes can
contribute to tumor proliferation and fibrosis in gastric cancer
(14).
In this present study, we demonstrated that HPMCs
transformed into myofibroblast-like cells following exposure to
TGF-β, and that these cells contributed to tumor-associated
fibrosis. We previously demonstrated that HPMCs promoted fibrosis
in a mouse xenograft model when co-inoculated with MKN45 cells
(15). Together, these results
suggest that HPMCs have a latent ability to function as CAFs and
induce fibrosis in the tumor microenvironment in multiple cancer
cell types.
Although orthotopic cancer-associated fibroblasts
cross-talk with gastric cancer cells to enhance tumor progression,
free cancer cells in the intraperitoneal cavity could produce
peritoneal fibrous tumors in the absence of orthotopic fibroblasts.
In PC of gastric cancer, seeded cancer cells attach to HPMCs and
transform them into myofibroblast-like cells by releasing TGF-β.
Spindle-shaped HPMCs can then facilitate adhesion of cancer cells
to the basement membrane and subsequently infiltrate the basement
membrane as CAFs together with cancer cells. This concept is in
agreement with the fact that PC can develop in any organ covered by
HPMCs.
TGF-β is regarded as one of the key molecules
responsible for the differentiation of a variety of precursor cells
to a myofibroblastic phenotype (24). Our results revealed that HPMCs also
adopt an elongated spindle-shaped morphology from their normal
cobblestone-like growth pattern, and exhibit overexpression of
α-SMA when exposed to TGF-β. This supports the notion that TGF-β
secreted from OCUM-2MD3 cells transformed HPMCs into myofibroblasts
within the microenvironment of our co-inoculated mouse model,
leading to the promotion of tumors with a fibrous stroma.
The control of TGF-β signaling is required for the
inhibition of organ fibrosis during PC, and for decreasing cancer
invasiveness and metastasis. PSK is known to suppress TGF-β signal
transduction through inhibition of Smad2 phosphorylation (21). We now demonstrated that PSK
inhibited TGF-β-induced EMT-like change in HPMCs, and that fibrosis
in xenograft tumors of PSK-treated mice was significantly reduced.
Previous reports have shown that a TGF-β neutralizing antibody and
a TGF-β receptor kinase inhibitor can both suppress EMT and reduce
stromal fibrosis (25,26). However, the long-term clinical use
of these agents may lead to major complications due to the
likelihood of adverse effects from interference with the many
important roles of TGF-β in normal tissues (27).
Previously, PSK has been thought to contribute to
the maintenance of nutrition and immune strength in cancer patients
during chemotherapy. As such, PSK has been approved for use in
combination with chemotherapy to prolong the survival of patients
with gastric or colorectal cancer. The effectiveness of PSK as a
postoperative adjuvant for immunochemotherapy has been demonstrated
by a meta-analysis (28). Here, the
hazard ratio of 5-year survival was 0.88 (95% confidence interval:
0.79–0.98, p=0.018), verifying that chemotherapy + PSK enhanced the
survival of patients with curatively resected gastric cancer. These
randomized clinical trials were conducted to examine the
effectiveness of postoperative adjuvant immunochemotherapy compared
with chemotherapy alone.
At present, S-1 is the standard agent for adjuvant
chemotherapy for stage II and III patients with curative resected
gastric cancer in Japan (29,30).
The Hokuriku-Kinki Immunochemotherapy Study Group on Gastric Cancer
is undertaking a clinical trial of S-1 alone versus S-1 plus PSK
for curatively resected stage II and IIIA gastric cancer (31). It is expected that the S-1 plus PSK
group will demonstrate longer survival times than the S-1 alone
group in those patients with peritoneal recurrence.
To date, PSK has been widely used clinically in
combination with chemotherapy without the observation of any
serious side-effects. Therefore, it may hold promise as an
anti-fibrotic agent for the treatment of gastric cancer patients
with PC.
Acknowledgements
This study was supported in part by Grants-in-Aid
for Young Scientists (B; 24791402 to J.K.) from the Japan Society
for the Promotion of Science.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
14:69–90. 2011. View Article : Google Scholar
|
|
2
|
Yamazaki H, Oshima A, Murakami R, Endoh S
and Ubukata T: A long-term follow-up study of patients with gastric
cancer detected by mass screening. Cancer. 63:613–617. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen CY, Wu CW, Lo SS, Hsieh MC, Lui WY
and Shen KH: Peritoneal carcinomatosis and lymph node metastasis
are prognostic indicators in patients with Borrmann type IV gastric
carcinoma. Hepatogastroenterology. 49:874–877. 2002.PubMed/NCBI
|
|
4
|
Maruyama K, Kaminishi M, Hayashi K, et al:
Gastric cancer treated in 1991 in Japan: data analysis of
nationwide registry. Gastric Cancer. 9:51–66. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fushida S, Oyama K, Kinoshita J, et al:
Intraperitoneal chemotherapy as a multimodal treatment for gastric
cancer patients with peritoneal metastasis. J Cancer Ther. 4:6–15.
2013. View Article : Google Scholar
|
|
6
|
Osugi H, Takada N, Takemura M, et al: Oral
fluoropyrimidine anticancer drug TS-1 for gastric cancer patients
with peritoneal dissemination. Oncol Rep. 9:811–815.
2002.PubMed/NCBI
|
|
7
|
Mai M, Sakata Y, Kanamaru R, et al: A late
phase II clinical study of RP56976 (docetaxel) in patients with
advanced or recurrent gastric cancer: a cooperative study group
trial (group B). Gan To Kagaku Ryoho. 26:487–496. 1999.PubMed/NCBI
|
|
8
|
Ohshima T, Yamada R, Hatori S, et al:
Pharmacokinetics of S-1 in patients with peritoneal dissemination
of gastric cancer. Oncol Rep. 16:361–366. 2006.
|
|
9
|
Naitoh H, Kawaguchi A, Yamamoto H, et al:
Measurement of docetaxel concentration in blood and ascites after
drip infusion into each vessel and intraperitoneal cavity of
gastric cancer. Gan To Kagaku Ryoho. 31:2031–2034. 2004.(In
Japanese). PubMed/NCBI
|
|
10
|
Ishigami H, Kitayama J, Kaisaki S, et al:
Phase II study of weekly intravenous and intraperitoneal paclitaxel
combined with S-1 for advanced gastric cancer with peritoneal
metastasis. Ann Oncol. 21:67–70. 2010. View Article : Google Scholar
|
|
11
|
Fushida S, Kinoshita J, Kaji M, et al:
Phase I/II study of intraperitoneal docetaxel plus S-1 for the
gastric cancer patients with peritoneal carcinomatosis. Cancer
Chemother Pharmacol. 71:1265–1272. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tsukada T, Fushida S, Harada S, et al:
Low-dose paclitaxel modulates tumour fibrosis in gastric cancer.
Int J Oncol. 42:1167–1174. 2013.PubMed/NCBI
|
|
13
|
Fuyuhiro Y, Yashiro M, Noda S, et al:
Cancer-associated orthotopic myofibroblasts stimulate the motility
of gastric cartinoma cells. Cancer Sci. 103:797–805. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Terai S, Fushida S, Tsukada T, et al: Bone
marrow derived ‘fibrocytes’ contribute to tumor proliferation and
fibrosis in gastric cancer. Gastric Cancer. May 4–2014.(Epub ahead
of print). View Article : Google Scholar
|
|
15
|
Tsukada T, Fushida S, Harada S, et al: The
role of human peritoneal mesothelial cells in the fibrosis and
progression of gastric cancer. Int J Oncol. 41:476–482.
2012.PubMed/NCBI
|
|
16
|
Coussens LM and Werb Z: Inflammation and
cancer. Nature. 420:860–867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tsukagoshi S, Hashimoto Y, Fujii G,
Kobayashi H, Nomoto K and Orita K: Krestin (PSK). Cancer Treat Rev.
11:131–155. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Matsunaga K, Hosokawa A, Oohara M, Sugita
N, Harada M and Nomoto K: Direct action of a protein-bound
polysaccharide, PSK, on transforming growth factor-beta.
Immunopharmacology. 40:219–230. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yamaguchi Y, Minami K, Ohshita A,
Kawabuchi Y, Noma K and Toge T: Enhancing effect of PS-K on
IL-2-induced lymphocyte activation: possible involvement of
antagonistic action against TGF-beta. Anticancer Res. 24:639–647.
2004.PubMed/NCBI
|
|
20
|
Zhang H, Morisaki T, Matsunaga H, et al:
Protein-bound polysaccharide PSK inhibits tumor invasiveness by
down-regulation of TGF-beta1 and MMPs. Ciin Exp Metastasis.
18:343–352. 2000.
|
|
21
|
Ono Y, Hayashida T, Konagai A, et al:
Direct inhibition of the transforming growth factor-β pathway by
protein-bound polysaccharide through inactivation of Smad2
signaling. Cancer Sci. 103:317–324. 2012. View Article : Google Scholar
|
|
22
|
Yung S, Li FK and Chan TM: Peritoneal
mesothelial cell culture and biology. Perit Dial Int. 26:162–173.
2006.PubMed/NCBI
|
|
23
|
Reagan-Shaw S, Nihal M and Ahmad N: Dose
translation from animal to human studies revisited. FASEB J.
22:659–661. 2008. View Article : Google Scholar
|
|
24
|
Evans RA, Tian YC, Steadman R and Philips
AO: TGF-beta1-mediated fibroblast-myofibroblast terminal
differentiation - the role of Smad proteins. Exp Cell Res.
282:90–100. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shinto O, Yashiro M, Kawajiri H, et al:
Inhibitory effect of a TGFbeta receptor type-I inhibitor, Ki26894,
on invasiveness of scirrhous gastric cancer cells. Br J Cancer.
102:844–851. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ly ZD, Wang HB, Dong Q, et al: Mesothelial
cells differentiate into fibroblast-like cells under the scirrhous
gastric cancer microenvironment and promote peritoneal
carcinomatosis in vitro and in vivo. Mol Cell Biochem. 377:177–185.
2013. View Article : Google Scholar
|
|
27
|
Roberts AB and Sporn MB: The transforming
growth factor-βs. Peptides, Growth Factors and Their Receptors Part
I. Sporn MB and Roberts AB: Springer-Verlag; Berlin: pp. 419–472.
1990, View Article : Google Scholar
|
|
28
|
Oba K, Teramukai S, Kobayashi M, Matsui T,
Kodera Y and Sakamoto J: Efficacy of adjuvant immunochemotherapy
with polysaccharide K for patients with curative resections of
gastric cancer. Cancer Immunol Immunother. 56:905–911. 2007.
View Article : Google Scholar
|
|
29
|
Sakuramoto S, Sasako M, Yamaguchi T, et
al: Adjuvant chemotherapy for gastric cancer with S-1, an oral
fluoropyrimidine. N Engl J Med. 357:1810–1820. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fujii M, Kochi M and Takayama T: Recent
advances in chemotherapy for advanced gastric cancer in Japan. Surg
Today. 40:295–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ueda Y, Fujimura T, Kinami S, et al: A
randomized phase III trial of postoperative adjuvant therapy with
S-1 alone versus S-1 plus PSK for stage II/IIIA gastric cancer:
Hokuriku-Kinki Immunochemotherapy Study Group-Gastric Cancer
(HKIT-GC). Jpn J Clin Oncol. 36:519–522. 2006. View Article : Google Scholar : PubMed/NCBI
|