Introduction
Gastric cancer is one of the most common malignant
tumours. Despite significant advances in the diagnosis and
treatment of gastric cancer, the mortality associated with gastric
cancer accounts for over 10% of the total cancer mortalities
worldwide and remains the second most common cause of
cancer-related mortality after lung cancer (1,2). At
present, the most commonly used treatment methods for gastric
cancer are surgery, radiotherapy and chemotherapy, and
comprehensive treatment depending on the patient-specific disease
manifestation. Of the available treatment methods for gastric
cancer, chemotherapy represents an extremely important and
irreplaceable option. However, since gastric cancer cells are prone
to the development of multidrug-resistance phenotypes, many
chemotherapeutic drugs are ineffective for gastric cancer.
With technological advances in molecular biology,
biological treatments such as gene therapy have become areas of
interest for the development of novel cancer therapeutics. The
introduction of RNA interference (RNAi) technology offers a way to
experimentally determine valid targets for gene therapy, as RNAi
can be used to elucidate the mechanisms underlying the occurrence
of multidrug-resistance and recurrence in gastric cancer.
Many molecular mechanisms associated with the
generation of multiple-drug resistance in gastric cancer exist, of
which the abnormal expression of the ATP-binding cassette subfamily
C member 4 (ABCC4) protein plays a key role. ABCC4 protein, also
known as the multi-drug resistance-associated protein, is an
important member of the ATP-binding cassette (ABC) transporter
family. ABCC4 was first identified on the basal membrane of
prostate epithelial cells (3).
ABCC4 controls the redistribution and excretion of many anti-viral
drugs, antibiotics, cytostatic drugs, and cardiovascular drugs and
plays key roles in the protection of cells and cellular signal
transduction pathways (4). In
addition, ABCC4 can transport intracellular drugs outside of cells,
which is often used by cancer cells as a drug resistance mechanism
(5). Recent studies have indicated
that ABCC4 expression is closely associated with the occurrence of
malignant tumours and drug resistance. Studies have also confirmed
that ABCC4 is overexpressed in gliomas, neuroblastomas,
retinoblastomas and melanomas as well as colon cancer and
colorectal cancer cells (6–9). In addition, ABCC4 expression is
correlated with drug resistance in leukaemia and ovarian cancer
cells (10,11).
The relationship between ABCC4 gene
expression and multidrug-resistance in gastric cancer has not been
reported. However, studies have shown that ABCC4 gene
expression is correlated with proliferation capacity and drug
resistance in leukaemia, ovarian cancer and colon cancer cells
(10–12). Therefore, we used RNAi to knock down
ABCC4 gene expression in multidrug-resistant gastric cancer
cells to study the role of ABCC4 gene expression in the
occurrence and progression of multiple-drug-resistant gastric
cancer.
Materials and methods
Cells, antibodies and reagents
Human gastric mucosal GES-1 cells, human gastric
cancer MGC-803 cells, and multidrug-resistant SGC-7901/Fu cells
were purchased from American Type Culture Collection (ATCC;
Rockefeller, MD, USA). ABCC4, P-gp, Bax, Bcl-2, CDK4 and cyclin D1
antibodies were purchased from Abcam (Santa Cruz, CA, USA). PI was
purchased from Sigma (Hercules, CA, USA), siRNA was synthesised by
GenePharma (Shanghai, China), and the RT-PCR reagent kit [Takara
RNA PCR kit (AMV) version 3.0] was purchased from Takara (Dalian,
China).
Cell culture
Human gastric mucosal GES-1 cells, human gastric
cancer MGC-803 cells, and human gastric cancer SGC-7901 cells were
cultured in RPMI-1640 culture medium (Carlsbad, CA, USA) containing
10% foetal bovine serum (FBS), 100 U/ml penicillin and 100 μg/ml
streptomycin at 37°C in the presence of 5% CO2.
SGC-7901/Fu cells were cultured in identical medium containing 20
μg/ml 5-fluorouracil (5-FU) to maintain 5-FU drug resistance. For
all experiments, cells were analysed at the logarithmic phase of
growth.
Cell viability assays
SGC-7901/Fu cells were seeded in 96-well culture
plates until the cells were at the logarithmic phase of growth.
Each well received 20 μl MTT (St. Louis, MO, USA) (0.5 mg/ml) and
was incubated at 37°C for 4 h. When the yellow solution appeared
with blue crystals, the culture medium was removed, and 150 μl of
DMSO was added to each well to dissolve the crystals. The
absorbance value (A490 nm) of each well was detected at 490 nm
using a microplate reader. The experiment included time points at
24, 48, 72, 96 and 120 h, and each group consisted of 8 replicate
wells.
Cell transfection
Cells were seeded in 6-well culture plates, and
transfection was performed when the cells reached 70% confluency.
Transfection experiments included a control, N-control, and RNAi
treatment groups. Culture medium was replaced with serum-free
RPMI-1640 culture medium containing both antibiotics. Cells were
inoculated with 4 μl of the appropriate virus solution
(concentration of 1.5×109 Tu/ml) for transfection in the
presence of Polybrene (the recombinant RNAi lentiviral vector
construct targeting the human ABCC4 gene, LV-shRNA-ABCC4,
was constructed previously in our laboratory). Infection was
conducted for 12 h, after which the culture medium was replaced
with RPMI-1640 culture medium containing 10% FBS. The efficiency of
RNA interference was determined by RT-PCR and western blotting.
RNA extraction and RT-PCR detection
Total RNA from the cells in each treatment group was
extracted. The extraction procedure was conducted according to the
manufacturer’s instructions included with the RNAiso™ Plus kit
(Takara, Japan). After the RNA concentrations were determined,
RT-PCR reactions were conducted using an RT-PCR reagent kit
(Takara) according to the manufacturer’s instructions. ABCC4, MDR1
and β-actin primers were synthesised by Invitrogen. The primers for
ABCC4 were as follows: upstream, 5′-CGCGTGTTCTTCTGGTGGCTC-3′ and
downstream, 5′-CTTTATCCCAGAACCCTTGC-3′. The primers for MDR1 were
as follows: upstream, 5′-CCCATCATTGCAATAGC AGG-3′ and downstream,
5′-GTTCAAACTTCTGCTCC TGA-3′. The primers for β-actin were as
follows: upstream, 5′-CTGGGACGACATGGAGAAAA-3′ and downstream,
5′-AAGGAAGGCTGGAAGAGTGC-3′. The PCR reaction contained 50 μl in
total volume, and the reaction conditions were as follows: 94°C for
2 min followed by 30 cycles of denaturation at 94°C for 30 sec,
annealing at 61°C for 30 sec, and extension at 72°C for 30 sec. The
PCR products were subjected to 1.2% agarose gel electrophoresis,
and the results were scanned and analysed using a gel documentation
system.
Detection of apoptosis
Cells from each treatment group were collected and
prepared as a single-cell suspension. Staining for apoptotic cells
was performed according to the instruction’s manual included in the
apoptosis reagent kit (Annexin V-FITC kit; BD Pharmingen, San
Diego, CA, USA). Briefly, Annexin V and PI were added separately
and stained at room temperature in the dark for 20 min. Detection
was performed using a flow cytometer (Becton-Dickinson, Franklin
Lakes, NJ, USA).
Cell cycle analysis
Cells from each treatment group were collected and
prepared as a single-cell suspension, after which the cells were
fixed overnight in ice-cold 70% ethanol at 4°C. Cells were stained
with 1 ml PI (containing 10 μg RNase and 5 μl Triton X-100) at 4°C
in the dark for 30 min. Cell cycle progression was analysed using a
flow cytometer (Becton-Dickinson).
Detection of caspase activity
Cells from each treatment group were collected, and
the activity of caspase-3, -8, and -9 was detected according to the
instruction’s manual included with the caspase activity detection
reagent kit (Beyotime, China). The analysis was performed using a
fluorescence spectrophotometer at an excitation wavelength of 400
nm and an emission wavelength of 505 nm.
Western blot analysis
After quantification using the bicin-choninic acid
(BCA) method, the samples were loaded on an SDS-PAGE gel and
separated. The proteins were transferred to a polyvinylidene
fluoride (PVDF) membrane using the semi-dry method. The membrane
was blocked overnight in 5% non-fat dry milk at 4°C. After washing
the membrane with TBST (Tris-buffered saline with Tween), the
primary antibodies were added followed by a 1-h hybridisation at
37°C and TBST washes. Secondary antibodies were then added followed
by a 1-h hybridisation at 37°C, a TBST wash, 5 min of the
chromogenic reaction, and autoradiography. The optical density
values were analysed and determined using the Quantity One
software, and the results are expressed as the ratio of the sample
optical density value to the optical density value of the internal
reference.
Inoculation of nude mice
Our animal experiment protocol was approved by the
Medical Ethics Committee of Guilin Medical University. A total of
20 male nude mice between 6 and 8 weeks of age at a body weight of
~25 g were purchased from the Experimental Animal Centre of Guilin
Medical University. Animals were randomly divided into 2 groups
with 10 animals in each group. Cells (5×105) from the
N-control and RNAi groups were injected subcutaneously into each
animal as appropriate. After 4 weeks of tumour growth, the mice
were sacrificed by cervical dislocation, and tumour tissues were
removed aseptically for further analysis.
Statistical analysis
Statistical analysis was performed using SPSS 16.0
software. All data are presented as the mean ± standard deviation.
Comparisons between groups were examined using one-way analysis of
variance (ANOVA) and LSD-t. p-values <0.05 were considered
statistically significant.
Results
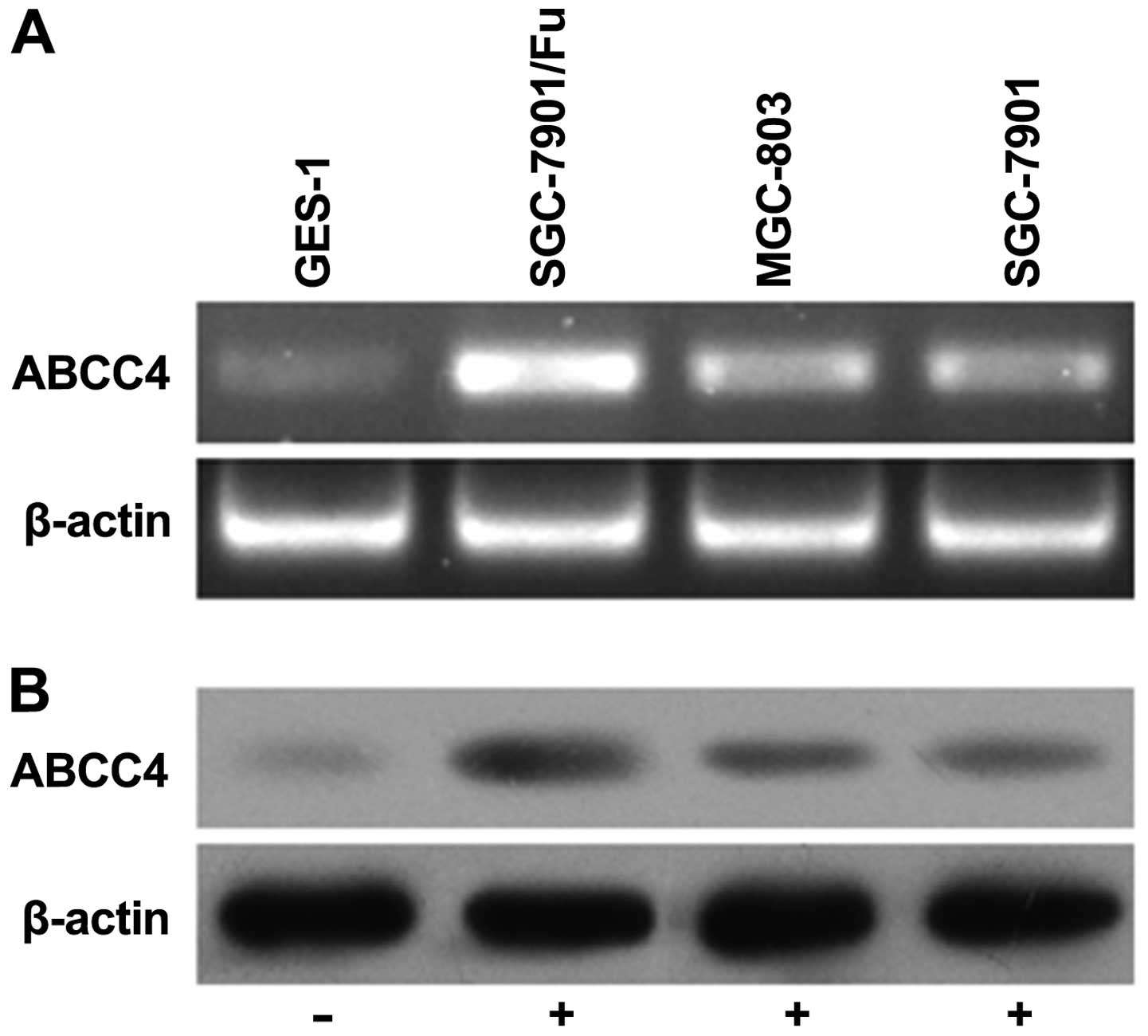
ABCC4 gene expression is increased in
multidrug-resistant gastric cancer SGC-7901/Fu cells
RT-PCR and western blotting were used to detect the
expression of ABCC4 mRNA and protein in the GES-1, MGC-803,
SGC-7901 and SGC-7901/Fu cells. Our results showed that the
expression levels of ABCC4 mRNA in the MGC-803, SGC-7901 and
SGC-7901/Fu cells were higher than the ABCC4 expression in
the GES-1 cells. Notably, the ABCC4 mRNA expression level
was highest in the SGC-7901/Fu cells. In addition, western blotting
results for ABCC4 protein expression levels in these 4 cell lines
were consistent with the mRNA expression level results. These
results revealed that the multidrug-resistant human gastric cancer
SGC-7901/Fu cells expressed the highest levels of the ABCC4
transcript and protein (Fig.
1).
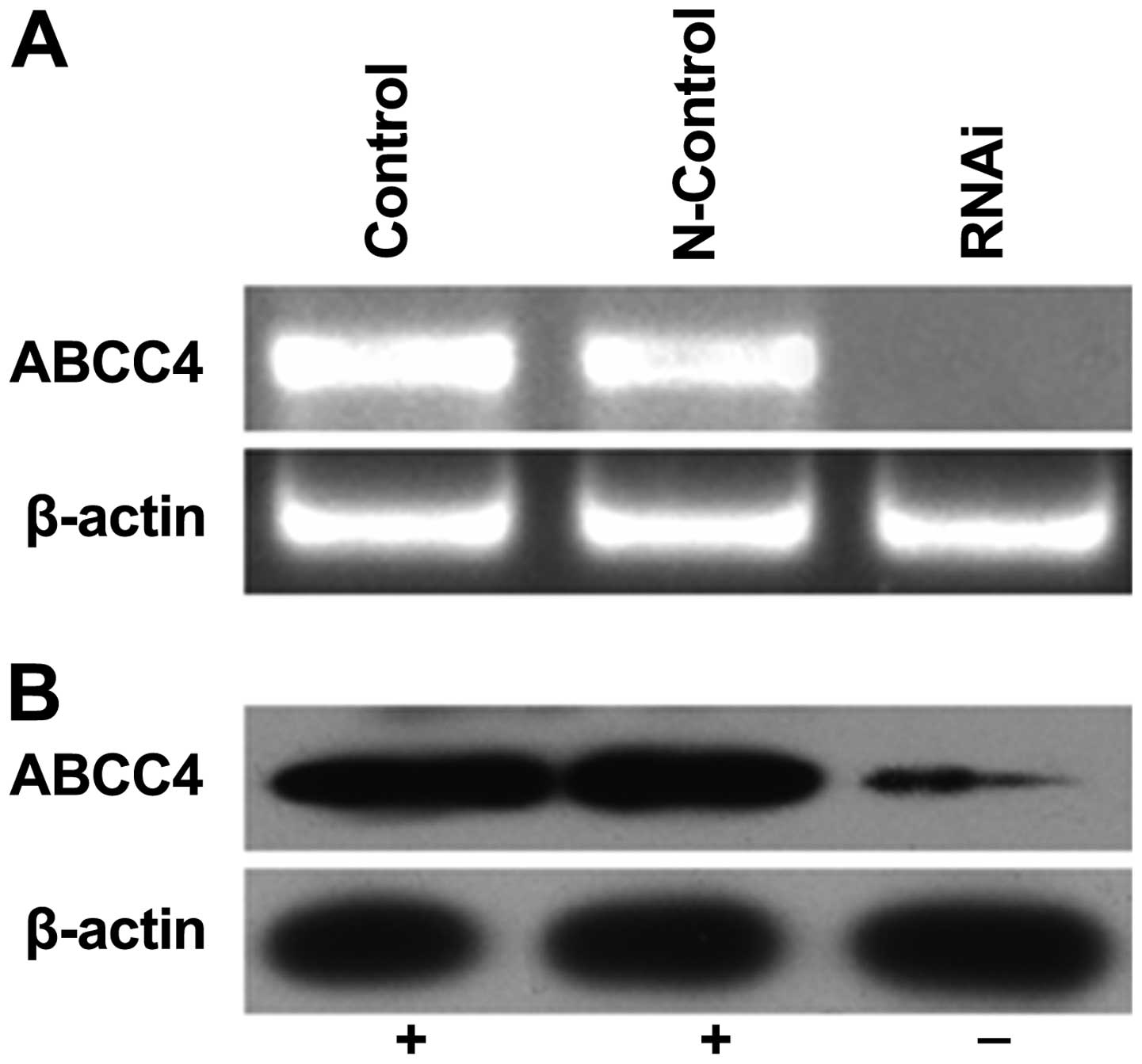
Knockdown of ABCC4 gene expression in
multidrug-resistant gastric cancer SGC-7901/Fu cells
RNAi was used to silence ABCC4 gene
expression in the SGC-7901/Fu cells. The cells were transfected
with the recombinant RNAi lentiviral vector targeting the human
ABCC4 gene (LV-shRNA-ABCC4) or with a negative control
construct, after which the expression levels of ABCC4 mRNA
and protein were analysed by RT-PCR and western blotting,
respectively. Four days after transfec-tion, the expression level
of ABCC4 mRNA was significantly downregulated relative to
the control and N-control groups. Consistently, the ABCC4 protein
expression level was also decreased in the cells receiving the
ABCC4 RNAi construct (Fig. 2).
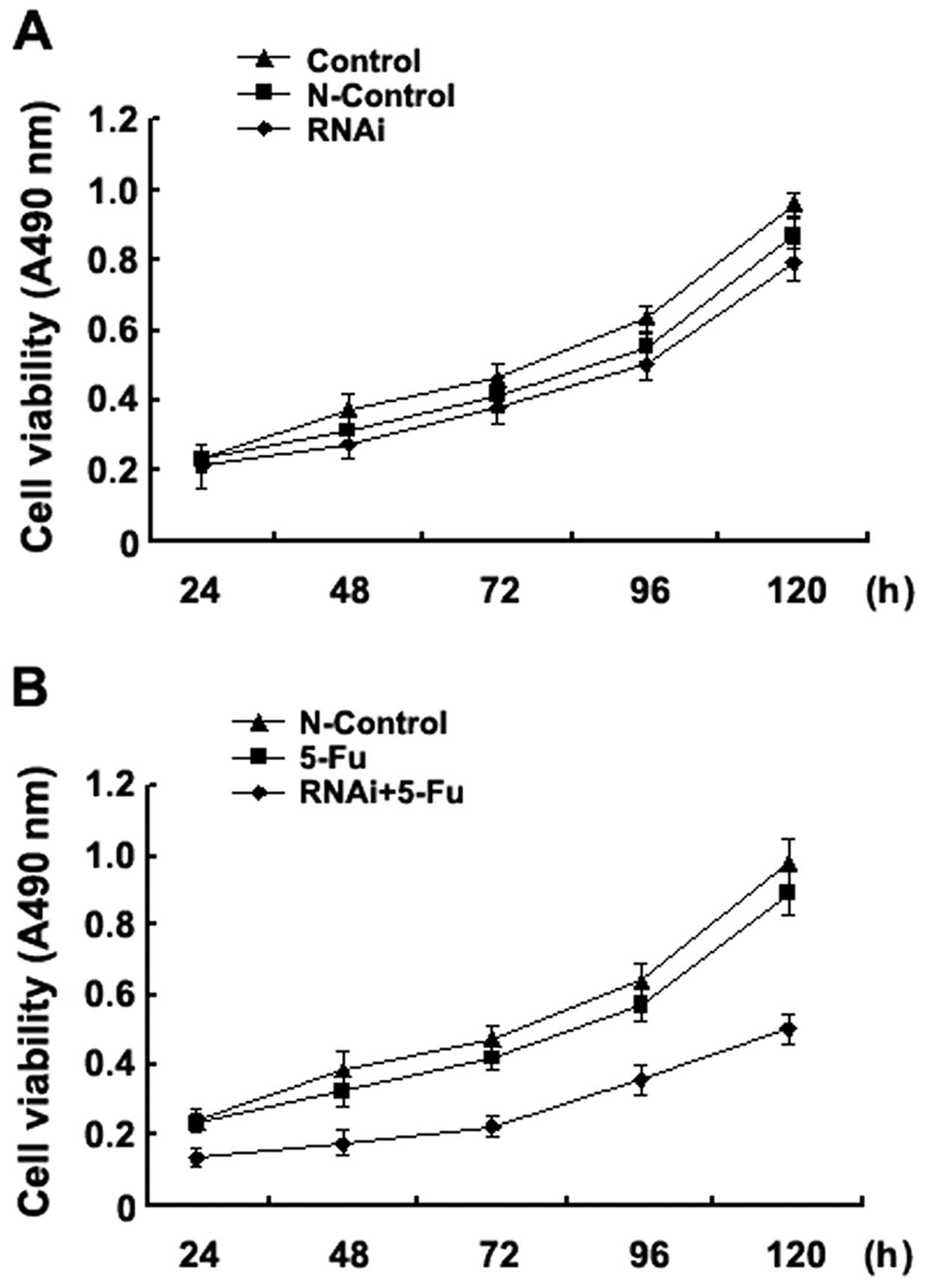
Silencing of the ABCC4 gene sensitises
SGC-7901/Fu cells to 5-FU
Next, we analysed the effect of the silencing of the
ABCC4 gene on the proliferation capacity of the
multidrug-resistant gastric cancer cell line SGC-7901/Fu. Our
results showed that when compared with the control and N-control
groups, at all time points (24, 48, 72, 96 and 120 h) after
transfection, the proliferation capacity of the SGC-7901/Fu cells
did not change (Fig. 3A). However,
upon treatment of the ABCC4-silenced cells with a
therapeutic dose of 5-FU, the proliferation capacity of the
SGC-7901/Fu cells significantly decreased relative to the controls
(Fig. 3B). Our results suggest that
silencing of the ABCC4 gene significantly enhanced the
inhibition of SGC-7901/Fu cell proliferation by 5-FU.
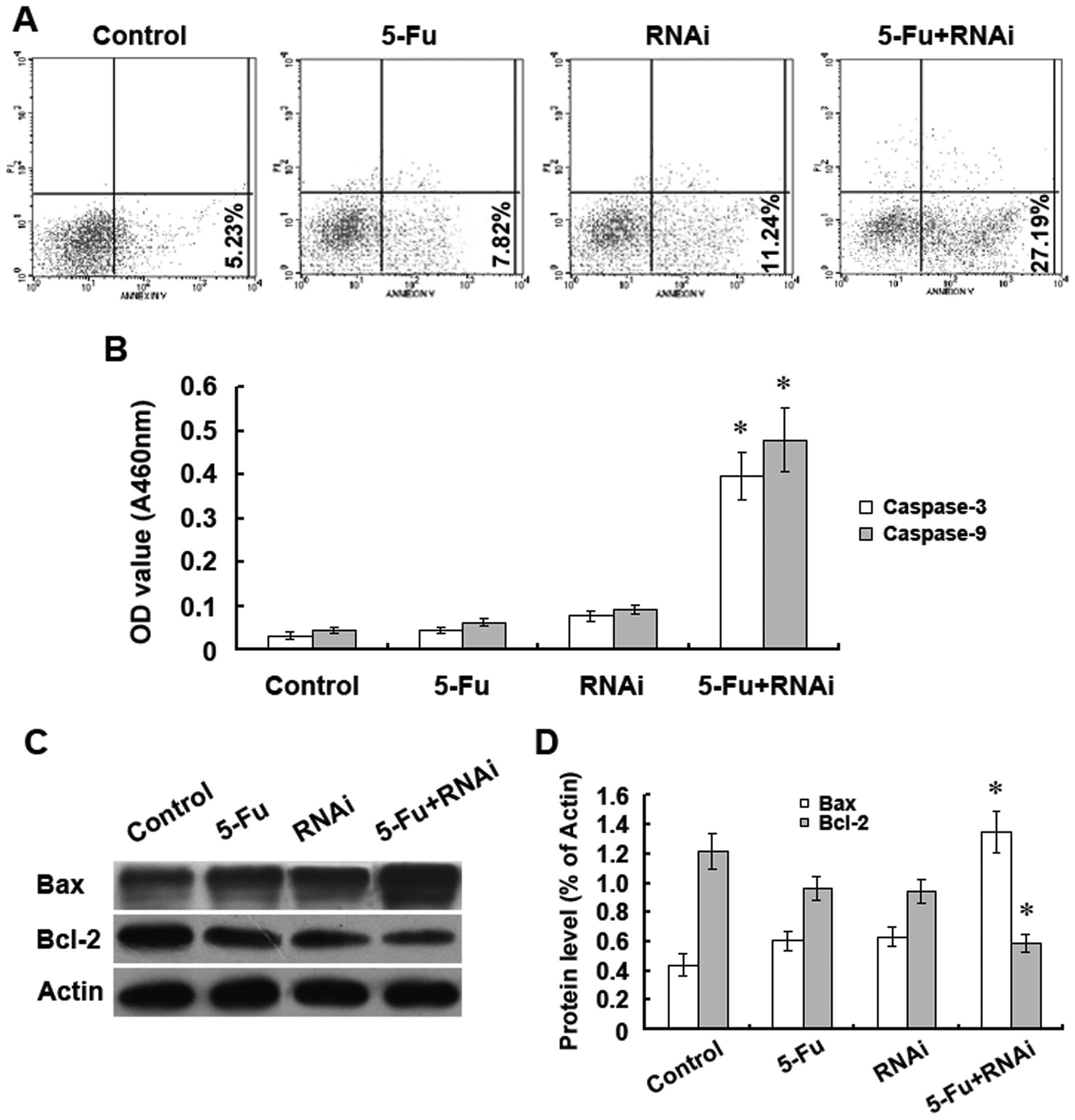
Silencing of the ABCC4 gene enhances
5-FU-induced apoptosis in SGC-7901/Fu cells
To investigate the molecular mechanisms underlying
the increase in 5-FU-induced inhibition of SGC-7901/Fu cell
proliferation upon ABCC4 knockdown, we analysed cells for
apoptosis by flow cytometry. Upon silencing of ABCC4
expression in the presence of 5-FU, the apoptosis rate in the
SGC-7901/Fu cells increased significantly relative to the other
treatment groups (Fig. 4A). The
expression levels of caspase-3 and -9 increased significantly in
the ABCC4-knockdown group (Fig.
4B). Furthermore, western blotting results showed that Bax
protein expression was significantly increased, whereas Bcl-2
protein expression was decreased (Fig.
4C and D). These results suggest that silencing of ABCC4
expression could enhance the ability of 5-FU to induce apoptosis in
SGC-7901/Fu cells. Furthermore, the increase in apoptosis appears
to be associated with the mitochondrial pathway.
ABCC4 knockdown sensitises SGC-7901/Fu
cells to 5-FU-mediated cell cycle arrest
To further investigate the molecular mechanisms
underlying the enhancement of 5-FU-induced inhibition of
SGC-7901/Fu cell proliferation upon ABCC4 knockdown, cell
cycle analysis was conducted by flow cytometry. Our results showed
that treatment with 5-FU or RNAi alone did not change the
distribution of the cell cycle. However, ABCC4 knockdown in
the presence of 5-FU resulted in a significant increase in the cell
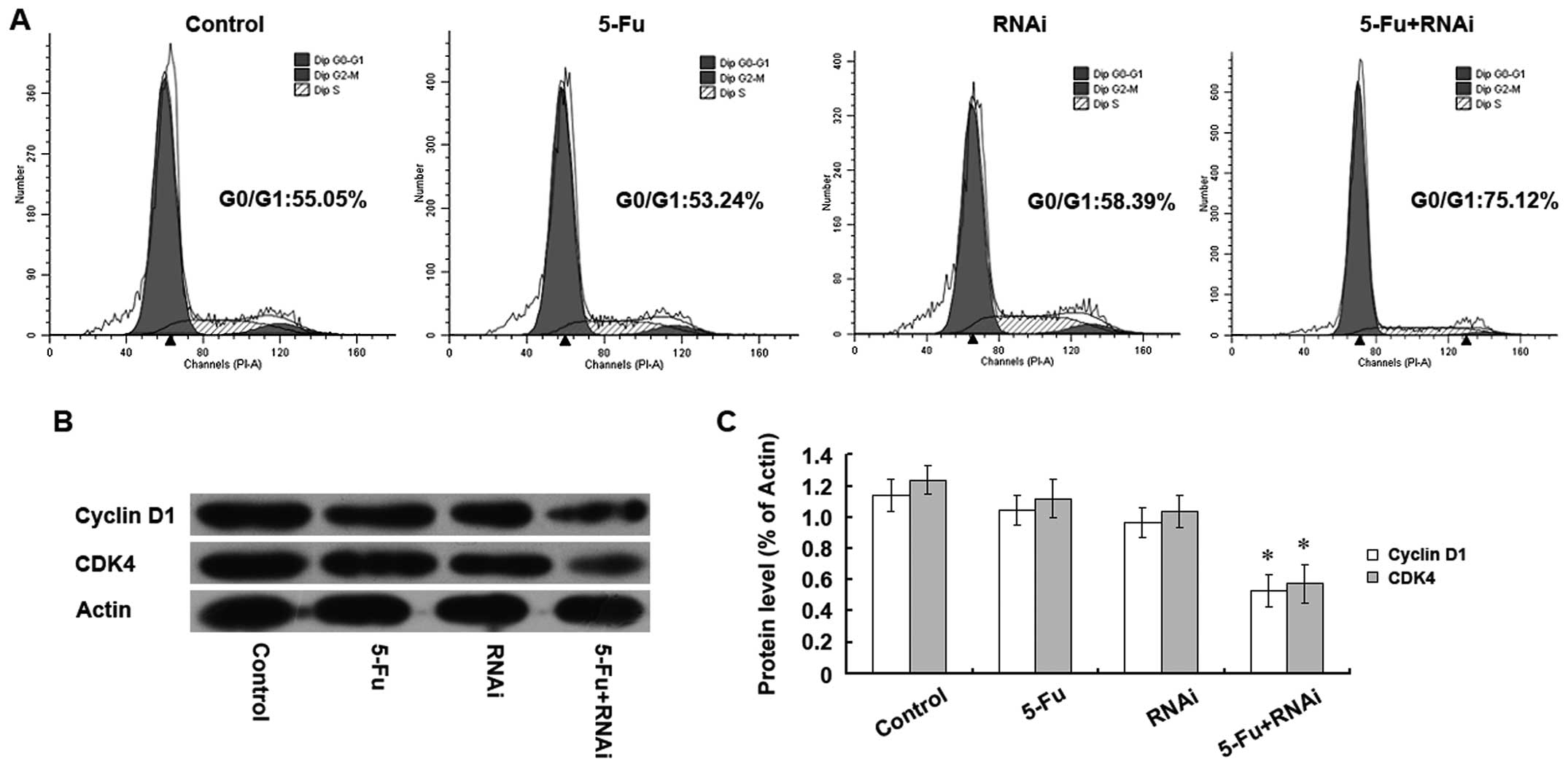
population arrested in the G1 phase (Fig. 5A). In addition, the protein
expression levels of the cell cycle proteins cyclin D1 and CDK4
were significantly decreased in the 5-FU+RNAi group (Fig. 5B and C). Since cyclin D1 and CDK4
proteins play indispensable roles in regulation of the G1 phase of
the cell cycle, our results indicate that the enhancement of
5-FU-induced inhibition of SGC-7901/Fu cell proliferation upon
ABCC4 knockdown was achieved by downregulating cyclin D1 and
CDK4 protein expression, thereby arresting the cell cycle in the G1
phase.
ABCC4 knockdown inhibits the growth of
tumour xeno-grafts
The animal experiment program was approved by the
Medical Ethics Committee of Guilin Medical University. The
subcutaneous tumour xenograft model in the nude mice was
conventionally established. Twenty nude mice were divided into
equal treatment groups according to the xenograft implanted. One
group received tumour implants that were subjected to ABCC4
knockdown, whereas the other group received tumour implants that
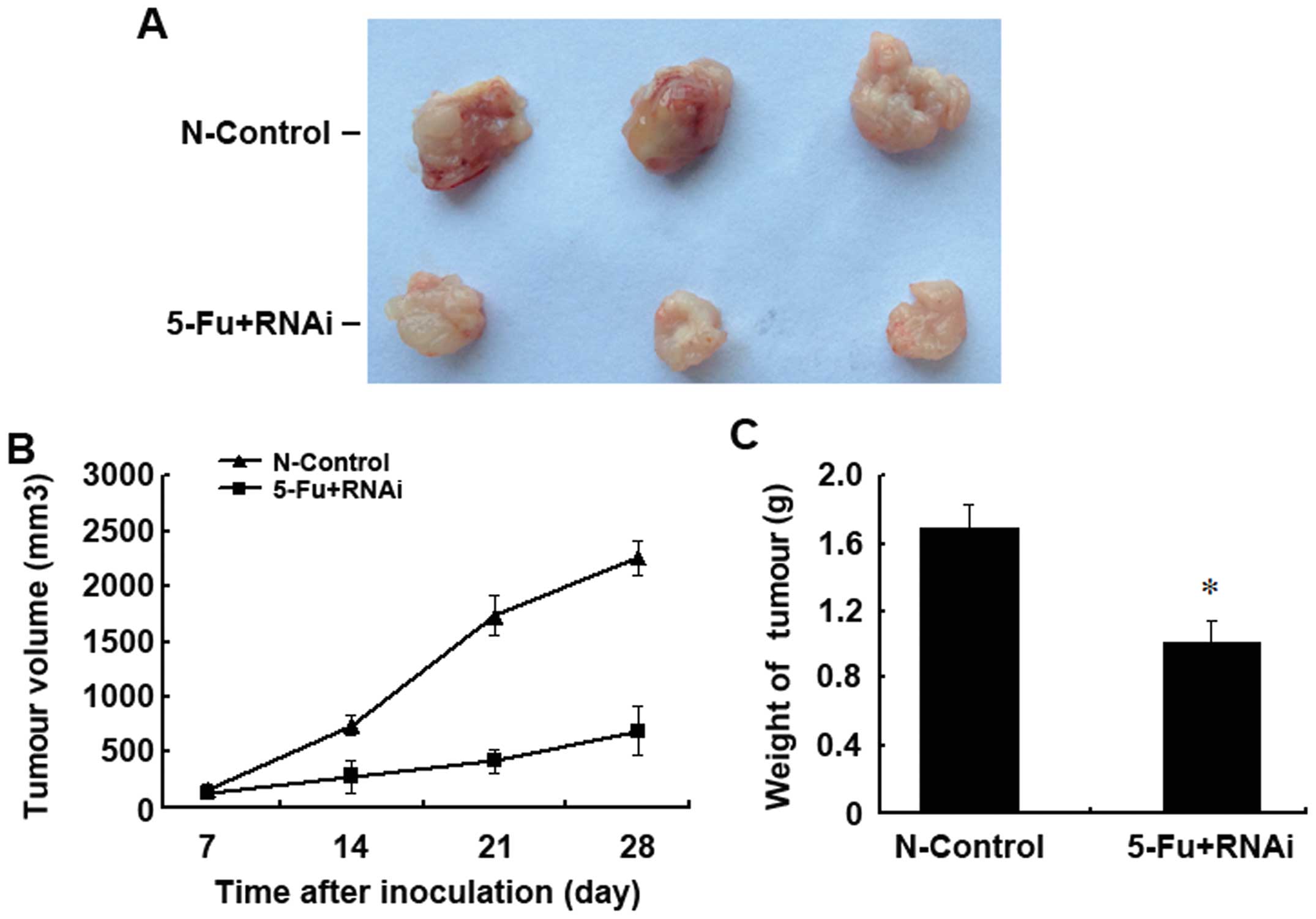
were subjected to non-specific control knockdown. Upon 5-FU
treatment, we found that at all time points, the tumour volume in
the 5-FU+RNAi group was significantly smaller than the tumour
volume in the N-control group. In addition, the weight of the
surgically removed tumours in the 5-FU+RNAi group was also
significantly lower than the tumour weight in the N-control group
(Fig. 6). Our results revealed that
ABCC4 knockdown in vivo significantly reversed the
multidrug-resistance in human gastric cancer.
ABCC4 knockdown restores the sensitivity
of SGC-7901/Fu cells to 5-FU through MDR1
The generation of multidrug resistance is closely
associated with the permeability glycopro-tein (P-gp) transport
protein (MDR1). To further investigate the mechanisms responsible
for the increase in the sensitivity of the drug-resistant gastric
cancer cell line SGC-7901/Fu to 5-FU upon ABCC4 knockdown,
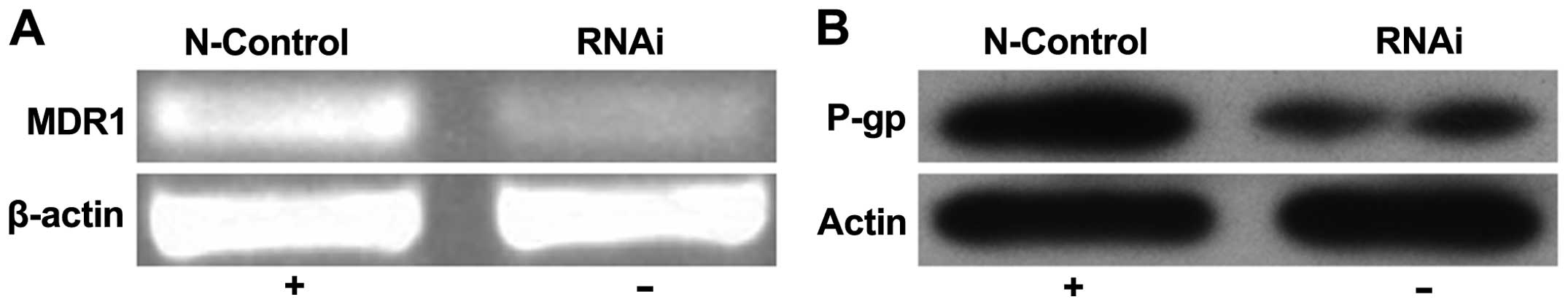
the expression levels of the P-gp (MDR1) transport protein were
analysed. Our results showed that upon ABCC4 knockdown, MDR1
transcript and protein expression also decreased (Fig. 7). These results indicate that the
observed increase in the sensitivity of SGC-7901/Fu cells to 5-FU
treatment upon ABCC4 knockdown could be associated with the
concomitant downregulation of P-gp (MDR1) expression.
Discussion
ABC transport protein family 4 (ABCC4) is a member
of the ABC transport protein family and is also known as
multidrug-resistance protein 4 (MRP4). ABCC4 was first identified
on the basal membrane of prostate epithelial cells (3). The ABCC4 locus is found on
human chromosome 13q32, and the full length of the gene is
approximately 312 kb. ABCC4 contains 31 exons and encodes
1325 amino acids (13). ABCC4 lacks
an N-terminal transmembrane domain and forms an MSD1-NBD1-MSD2-NBD2
structure. ABCC4 possesses the simplest structure of the proteins
in the ABC family (14). ABCC4 can
transport many endogenous and exogenous organic anions with various
structures outside of the cell. This feature of ABCC4 provides
cells with the ability to tolerate various cytotoxic compounds and
to protect important tissues from xenobiotic damage (15). In addition, ABCC4 also affects drug
metabolism in cells contributing to drug resistance.
The association between ABCC4 and cancer has become
an area of interest for many researchers in recent years. Dong
et al and Cai et al (16,17)
showed that ABCC4 was highly expressed in androgen-induced prostate
cancer cells, and subsequent studies further confirmed the presence
of a high copy number of ABCC4 mRNA in prostate cancer (18). The purpose of our study was to
investigate the expression of ABCC4 in drug-resistant human
gastric cancer cells and to assess the potential for ABCC4-targeted
inactivation in the treatment of drug-resistant gastric cancer. Our
results showed that ABCC4 transcript and protein levels were
increased in multiple human gastric cancer cell lines especially a
drug-resistant gastric cancer cell line. These results suggest that
ABCC4 expression and the development of drug resistance in human
gastric cancer are likely related hinting at the potential value of
ABCC4 as a therapeutic target for drug-resistant gastric
cancer.
To further elucidate whether ABCC4 is closely
associated with the occurrence and development of drug resistance
in human gastric cancer, we knocked down ABCC4 expression
levels in a drug-resistant human gastric cancer cell line and
observed the biological changes. Our results showed that upon
ABCC4 knockdown, proliferation of the drug-resistant human
gastric cancer cell line SGC-7901/Fu was inhibited. Furthermore,
these cells displayed an increase in apoptosis and G1 cell cycle
arrest. Knockdown of ABCC4 also inhibited the growth of
tumour xenografts in nude mice, suggesting that high expression
levels of ABCC4 in drug-resistant human gastric cancer cells
promote tumour cell proliferation. Previous studies have shown that
high ABCC4 expression levels could promote the development of
malignant tumours such as prostate cancer and ovarian cancer
(11,19,20).
Consistently, our results showed that ABCC4 plays an important
regulatory role in the proliferation of drug-resistant human
gastric cancer cells.
To further investigate the role of ABCC4 in the
regulation of the inhibition of drug-resistant human gastric cancer
cell proliferation, we analysed apoptosis and cell cycle
progression. The effect of ABCC4 expression on apoptosis has not
been thoroughly researched. Our results showed that downregulation
of ABCC4 increased apoptosis in drug-resistant human gastric
cancer cells. A study by Dai et al (21) showed that ABCC4 could regulate the
mTOR signalling pathway, thereby regulating apoptosis.
Consistently, our results showed that downregulation of
ABCC4 is closely associated with the inhibition of the
proliferation of drug-resistant human gastric cancer cells and the
occurrence of apoptosis. In addition, we also found that
downregulation of ABCC4 could arrest the cell cycle of
drug-resistant human gastric cancer cells at the G0/G1 phase. These
results collectively suggest that high ABCC4 expression levels
observed in drug-resistant human gastric cancer cells are necessary
for tumour cell proliferation. Furthermore, down-regulation of
ABCC4 results in the inhibition of proliferation of
drug-resistant human gastric cancer cells.
Several previous studies have shown that the
mitochondrial pathway and death receptor pathway (22) are the classical apoptotic pathways
in many tumour cells. Bcl-2 and Bax play important roles in the
mitochondrial apoptosis pathway (23,24).
If Bax translocates from the cytoplasm to the mitochondrial
membrane, permeability of the mitochondrial membrane changes to
promote cytochrome c release from the mitochondria into the
cytoplasm thereby activating the caspase cascade culminating in
apoptosis (25–27). In this study, we found that
downregulation of ABCC4 in drug-resistant human gastric
cancer cells caused translocation of Bax and Bcl-2 and
significantly increased caspase-3 and -9 expression levels. We
speculated that during the mitochondrial pathway of apoptosis,
release of cytochrome c into the cytoplasm may activate the
biological effects of caspase-9 and -3, thereby playing a key role
in the activation of apoptosis. These results suggest that
downregulation of ABCC4 can induce apoptosis in
drug-resistant human gastric cancer cells through the mitochondrial
pathway.
The occurrence of drug resistance in malignant
tumours is thought to be closely associated with the abnormal
expression of MDR1 and P-glycoprotein on the membrane of tumour
cells. Therefore, some researchers have proposed that
downregulation of these two proteins using a variety of methods
could reduce the occurrence of drug resistance in tumour cells
(28,29). Abnormal expression of MDR1 and
P-glycoprotein is usually closely associated with the generation of
drug resistance in tumour cells. Studies have shown that
downregulation of MDR1 and P-glycoprotein in drug-resistant tumour
cells could inhibit the generation of drug resistance in tumour
cells (30). The present study
showed that downregulation of ABCC4 in the presence of 5-FU
significantly inhibited the proliferation and cell cycle
progression of drug-resistant human gastric cancer cells and
inhibited tumour cell growth in nude mice. In addition, the
expression levels of MDR1 transcript and protein and P-glycoprotein
on the membrane of tumour cells also decreased significantly. These
results indicate that downregulation of ABCC4 could alter
the expression levels of MDR1 and P-glycoprotein on the cell
membrane thus inhibiting proliferation of drug-resistant human
gastric cancer cells.
In summary, the present study showed that ABCC4 is
highly expressed in drug-resistant human gastric cancer cells.
Furthermore, downregulation of ABCC4 increased apoptosis and
cell cycle arrest in drug-resistant human gastric cancer cells
through the regulation of Bcl-2/Bax in the mitochondrial pathway,
thereby restoring the sensitivity of the drug-resistant cancer
cells to 5-FU. Our study may contribute to making a case for the
targeting of ABCC4 for the clinical treatment of drug resistance in
gastric cancer.
Acknowledgements
This study was funded by the Guangxi Zhuang
Autonomous Region Health Department (Z2012403) and from
Guangxi.
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sun J, Song Y, Wang Z, Chen X, Gao P, Xu
Y, Zhou B and Xu H: Clinical significance of palliative gastrectomy
on the survival of patients with incurable advanced gastric cancer:
a systematic review and meta-analysis. BMC Cancer. 13:5772013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
van de Ven R, de Groot J, Reurs AW,
Wijnands PG, van de Wetering K, Schuetz JD, de Gruijl TD, Scheper
RJ and Scheffer GL: Unimpaired immune functions in the absence of
Mrp4 (Abcc4). Immunol Lett. 124:81–87. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Russel FG, Koenderink JB and Masereeuw R:
Multidrug resistance protein 4 (MRP4/ABCC4): a versatile efflux
transporter for drugs and signalling molecules. Trends Pharmacol
Sci. 29:200–207. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Keppler D: Multidrug resistance proteins
(MRPs, ABCCs): importance for pathophysiology and drug therapy.
Handb Exp Pharmacol. 201:299–323. 2011. View Article : Google Scholar
|
|
6
|
Norris MD, Smith J, Tanabe K, Tobin P,
Flemming C, Scheffer GL, Wielinga P, Cohn SL, London WB, Marshall
GM, Allen JD and Haber M: Expression of multidrug transporter
MRP4/ABCC4 is a marker of poor prognosis in neuroblastoma and
confers resistance to irinotecan in vitro. Mol Cancer Ther.
4:547–553. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hendig D, Langmann T, Zarbock R, Schmitz
G, Kleesiek K and Götting C: Characterization of the ATP-binding
cassette transporter gene expression profile in Y79: a
retinoblastoma cell line. Mol Cell Biochem. 328:85–92. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Heimerl S, Bosserhoff AK, Langmann T,
Ecker J and Schmitz G: Mapping ATP-binding cassette transporter
gene expression profiles in melanocytes and melanoma cells.
Melanoma Res. 17:265–273. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Holla VR, Backlund MG, Yang P, Newman RA
and DuBois RN: Regulation of prostaglandin transporters in
colorectal neoplasia. Cancer Prev Res. 1:93–99. 2008. View Article : Google Scholar
|
|
10
|
Gillet JP, Efferth T, Steinbach D, Hamels
J, de Longueville F, Bertholet V and Remacle J: Microarray-based
detection of multidrug resistance in human tumor cells by
expression profiling of ATP-binding cassette transporter genes.
Cancer Res. 64:8987–8993. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Beretta GL, Benedetti V, Cossa G, Assaraf
YG, Bram E, Gatti L, Corna E, Carenini N, Colangelo D, Howell SB,
Zunino F and Perego P: Increased levels and defective glycosylation
of MRPs in ovarian carcinoma cells resistant to oxaliplatin.
Biochem Pharmacol. 79:1108–1117. 2010. View Article : Google Scholar
|
|
12
|
Gradilone A, Pulcinelli FM, Lotti LV,
Trifirò E, Martino S, Gandini O, Gianni W, Frati L, Aglianò AM and
Gazzaniga P: Celecoxib upregulates multidrug resistance proteins in
colon cancer: lack of synergy with standard chemotherapy. Curr
Cancer Drug Targets. 8:414–420. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lamba JK, Adachi M, Sun D, Tammur J,
Schuetz EG, Allikmets R and Schuetz JD: Nonsense mediated decay
down-regulates conserved alternatively spliced ABCC4 transcripts
bearing nonsense codons. Hum Mol Genet. 12:99–109. 2003. View Article : Google Scholar
|
|
14
|
Ravna AW, Sylte I and Sager G: Binding
site of ABC transporter homology models confirmed by ABCB1 crystal
structure. Theor Biol Med Model. 6:202009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Adachi M, Sampath J, Lan LB, Sun D,
Hargrove P, Flatley R, Tatum A, Edwards MZ, Wezeman M, Matherly L,
Drake R and Schuetz J: Expression of MRP4 confers resistance to
ganciclovir and compromises bystander cell killing. J Biol Chem.
277:38998–39004. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dong Y, Zhang H, Gao AC, Marshall JR and
Ip C: Androgen receptor signaling intensity is a key factor in
determining the sensitivity of prostate cancer cells to selenium
inhibition of growth and cancer-specific biomarkers. Mol Cancer
Ther. 4:1047–1055. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cai C, Omwancha J, Hsieh CL and
Shemshedini L: Androgen induces expression of the multidrug
resistance protein gene MRP4 in prostate cancer cells. Prostate
Cancer Prostatic Dis. 10:39–45. 2007. View Article : Google Scholar
|
|
18
|
Ho LL, Kench JG, Handelsman DJ, Scheffer
GL, Stricker PD, Grygiel JG, Sutherland RL, Henshall SM, Allen JD
and Horvath LG: Androgen regulation of multidrug
resistance-associated protein 4 (MRP4/ABCC4) in prostate cancer.
Prostate. 68:1421–1429. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hagmann W, Jesnowski R, Faissner R, Guo C
and Löhr JM: ATP-binding cassette C transporters in human
pancreatic carcinoma cell lines. Upregulation in
5-fluorouracil-resistant cells. Pancreatology. 9:136–144. 2009.
View Article : Google Scholar
|
|
20
|
Bagnoli M, Beretta GL, Gatti L, Pilotti S,
Alberti P, Tarantino E, Barbareschi M, Canevari S, Mezzanzanica D
and Perego P: Clinicopathological impact of ABCC1/MRP1 and
ABCC4/MRP4 in epithelial ovarian carcinoma. Biomed Res Int.
2013:1432022013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dai ZJ, Gao J, Kang HF, Ma YG, Ma XB, Lu
WF, Lin S, Ma HB, Wang XJ and Wu WY: Targeted inhibition of
mammalian target of rapamycin (mTOR) enhances radiosensitivity in
pancreatic carcinoma cells. Drug Des Devel Ther. 7:149–159. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nieminen AI, Partanen JI and Klefstrom J:
c-Myc blazing a trail of death: coupling of the mitochondrial and
death receptor apoptosis pathways by c-Myc. Cell Cycle.
6:2464–2472. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Renault TT, Teijido O, Antonsson B, Dejean
LM and Manon S: Regulation of Bax mitochondrial localization by
Bcl-2 and Bcl-x(L): keep your friends close but your enemies
closer. Int J Biochem Cell Biol. 45:64–67. 2013. View Article : Google Scholar
|
|
24
|
Lindsay J, Esposti MD and Gilmore AP:
Bcl-2 proteins and mito-chondria - specificity in membrane
targeting for death. Biochim Biophys Acta. 1813:532–539. 2011.
View Article : Google Scholar
|
|
25
|
Saito M, Korsmeyer SJ and Schlesinger PH:
BAX-dependent transport of cytochrome c reconstituted in pure
liposomes. Nat Cell Biol. 2:553–555. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yuan S and Akey CW: Apoptosome structure,
assembly, and procaspase activation. Structure. 21:501–515. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
McIlwain DR, Berger T and Mak TW: Caspase
functions in cell death and disease. Cold Spring Harb Perspect
Biol. 5:a0086562013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhu CY, Lv YP, Yan DF and Gao FL:
Knockdown of MDR1 increases the sensitivity to adriamycin in drug
resistant gastric cancer cells. Asian Pac J Cancer Prev.
14:6757–6760. 2013. View Article : Google Scholar
|
|
29
|
Wang Y, Ma G, Wang Q, Wen M, Xu Y, He X,
Zhang P, Wang Y, Yang T, Zhan P and Wei G: Involvement of CUL4A in
regulation of multidrug resistance to P-gp substrate drugs in
breast cancer cells. Molecules. 19:159–176. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tang B, Du J, Wang J, Tan G, Gao Z, Wang Z
and Wang L: Alpinetin suppresses proliferation of human hepatoma
cells by the activation of MKK7 and elevates sensitization to
cis-diammined dichloridoplatium. Oncol Rep. 27:1090–1096. 2012.
|