Introduction
Epithelial ovarian cancer (EOC) is the second most
common gynecologic cancer and the most common cause of mortality
from gynecologic malignancies. It is highly metastatic, and most
patients are diagnosed at an advanced stage (1–3).
Despite recent gains in survival, most patients experience
recurrence and metastasis, and the 5-year survival rate for
patients with advanced-stage ovarian cancer has remained unchanged
(20–30%) over the past 20 years (4,5).
Multiple genetic alterations are involved in the pathogenesis of
ovarian cancer, and many of the signaling pathways and proteins
involved have been characterized (6).
Metastasis is a complex process involving
restructuring of the extracellular matrix and resulting in tumor
cell migration, invasion, and establishment at new sites (7–9). Cell
migration is a key factor in the invasion and metastasis of cancers
(10). In addition, hypoxia is a
common characteristic of growing solid tumors such as those of
ovarian cancer (11,12). Preliminary studies from our group
have shown that drug resistance and metastasis are significantly
enhanced in ovarian cancer cells under hypoxic conditions induced
by physical or chemical methods (unpublished data).
Microtubule-associated protein 1 light chain 3 (LC3)
is localized to the membrane bilayer of autophagosomes (13–16).
It is converted from the cytosolic form (LC3-I) to the
autophagosome membrane-associated form (LC3-II) and is degraded by
lysosomal enzymes within autolysosomes during non-selective
autophagy (17). LC3 was first
identified as 1 of 3 light chains associated with purified
microtubule-associated protein (MAP)1A and MAP1B and is considered
to be involved in microtubule assembly/disassembly. It has been
reported to bind to the Rab7 GTPase effector FYVE and coiled-coil
domain 1 (FYCO1) to mediate microtubule plus-end-directed vesicle
transport (18,19). It has also been identified as a
homolog of mammalian autophagy-related protein 8 (ATG8) (20).
LC3 expression has been reported to be common in the
metastasis of various types of human cancer. Lazova et al
(21) reported the common
expression of LC3B in malignancies, supporting emerging evidence
that autophagy plays a role in cancer progression. High levels of
LC3B expression were associated with proliferation, invasion and
metastasis, high nuclear grade, high mitotic rate, and worse
outcome. Normal melanocytes adjacent to the tumor and melanoma
cells of early in situ melanomas showed little or no LC3B
expression. However, in primary invasive melanoma, punctate LC3B
staining was widespread in nests of florid in situ tumor
cells and in 100% of invasive tumors in the dermis (21). Sato et al (22) reported that 90% of primary
colorectal tumors and 100% of lymphnode and liver metastases were
positive for punctate LC3 expression. Normal mucosal epithelium
adjacent to the tumors was negative. Yoshioka et al
(23) reported strong LC3
expression in 53% of esophageal, 58% of gastric, and 63% of
colorectal cancer. Han et al (24) reported that overexpression of LC3
was associated with metastasis, poor clinical prognosis, and
vasculogenic mimicry in patients with melanoma. Fujii et al
(25) reported that 87% of their
patients with pancreatic cancer were LC3B-positive, correlating
with shorter disease-free periods and poor patient outcome.
The mechanism(s) underlying cytoskeletal changes and
cell migration and invasion in EOC metastasis remains to be
elucidated. The aim of the present study was to assess whether LC3
was involved in this process. We hypothesized that LC3 promotes
tumor cell migration and invasion under hypoxic conditions by
altering the cytoskeleton.
Materials and methods
Cell culture and reagents
Human HO8910PM and HO8910 EOC cell lines were
obtained from the Chinese Academy of Sciences Cell Bank. The
parental HO8910 cell line was established from ascites of a patient
with malignant papillary serous adenocarcinoma of the ovary.
HO8910PM is a highly metastatic clone obtained by limiting-dilution
cloning of the HO8910 line and has been used in many studies, owing
to its highly metastatic activity. Cells were grown in Roswell Park
Memorial Institute (RPMI)-1640 medium (Thermo Scientific Hyclone,
Logan, UT, USA) plus 10% fetal bovine serum (FBS; Gibco/Life
Technologies, Carlsbad, CA, USA) at 37°C in a humidified atmosphere
at normoxic conditions of 20% O2, 5% CO2 and
75% N2. Experiments were performed in 6-well culture
plates at 80% cell confluence.
3-Methyladenine (3-MA), a specific inhibitor of
autophagy and inhibitor of the conversion of LC3-I to LC3-II
(26–29), was purchased from Sigma-Aldrich (St.
Louis, MO, USA). Rabbit polyclonal anti-LC3B antibody (no. 2775)
and rabbit monoclonal anti-Rho-associated, coiled-coil-containing
protein kinase 1 (ROCK1) antibody (no. 4035) were purchased from
Cell Signaling Technology (Beverly, MA, USA). Mouse monoclonal
anti-β-actin antibody (mAb 8226) was purchased from Abcam
(Cambridge, England, UK). Mouse monoclonal anti-ras homolog gene
family, member A (RhoA) antibody (no. ARH03) and rhodamine
phalloidin (no. PHDR1) were purchased from Cytoskeleton Inc.
(Denver, CO, USA). The Rho Assay Reagent (Rhotekin RBD, agarose;
recombinant protein expressed in Escherichia coli; no.
14-383, lot no. 2013105) was purchased from EMD Millipore
(Billerica, MA, USA). Goat anti-rabbit IgG secondary antibody
[AlexaFluor 488 goat anti-rabbit IgG (H+L); no. A11008] was
purchased from Invitrogen/Life Technologies (Grand Island, NY,
USA). Horseradish peroxidase (HRP)-conjugated secondary antibodies
were purchased from Millipore.
Small-interfering RNA transfection for
LC3B knockdown
LC3B target-specific small-interfering RNA (siRNA)
plasmid vector and a scrambled siRNA control plasmid were purchased
from Shanghai GenePharma, Ltd. (Shanghai, China). The siRNA
sequences used were: LC3B, sense: 5′-GCGAGU UGGUCAAGAUCAUTT-3′ and
antisense: 5′-AUGAUC UUGACCAACUCGCTT-3′; negative control, sense:
5′-UUC UCCGAACGUGUCACGUTT-3′ and antisense: 5′-ACGUGA
CACGUUCGGAGAATT-3′. The cells were transfected with siRNA using
Lipofectamine 2000 (Invitrogen/Life Technologies) according to the
manufacturer’s instructions. Cells (2×105 per well) were
seeded in 6-well culture plates at 30–50% confluence. Each plate
was transfected with 100 pmol plasmid containing LC3B siRNA or
negative control siRNA and 5 μl Lipofectamine 2000. Transfection
efficiency averaged 60–70% as measured by green fluorescent protein
expression. The cells were allowed to recover in RPMI-1640 medium
for 6 h after transfection. Western blotting was performed to
validate the knockdown efficiency, and the cells were divided for
different assays.
Induction of hypoxia
Hypoxic environmental conditions were achieved by
culturing cells in an airtight humidified chamber with a gas
mixture containing 1% O2, 5% CO2 and 94%
N2. After 48 h, transfection with siRNA was performed or
3-MA was dissolved in phosphate-buffered saline, pH 7.4 (PBS) and
added to the medium at a final concentration of 1.25, 2.5, or 5 mM.
The flasks were exposed to hypoxic conditions or maintained in a
normoxic environment for an additional 24 h, and the assays were
performed.
Western blotting
Cells were collected for western blotting to
determine the protein expression and trypan blue exclusion to
determine cell viability. Briefly, proteins from the total cell
lysates were separated by 10–15% sodium dodecyl sulfate
polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred
to a nitrocellulose membrane (Bio-Rad Laboratories, Hercules, CA,
USA). The membrane was blocked in 5% non-fat dried milk, washed in
Tris-buffered saline containing 0.1% Tween-20, and incubated at 4°C
overnight with specific primary antibodies: Rabbit polyclonal
anti-LC3B antibody (1:500 dilution), mouse monoclonal anti-RhoA
antibody (1:1000 dilution), rabbit monoclonal anti-ROCK1 antibody
(1:1000 dilution), and mouse monoclonal anti-β-actin antibody
(1:5000 dilution) as a loading control. This was followed by
incubation with HRP-conjugated secondary antibodies. The proteins
were detected with an enhanced chemiluminescence detection system
(GE Healthcare, Little Chalfont, Buckinghamshire, UK).
Cell migration, invasion and adhesion
assays
Cell migration was assessed with a Transwell chamber
system (Corning Inc., Life Sciences, Tewksbury, MA, USA). A total
of 2.0×105 cells in RPMI-1640 medium were added to the
upper chamber under normoxic or hypoxic conditions. RPMI-1640
medium containing 10% FBS was added to the lower chamber. Migration
assays were conducted for 6 h at 37°C. The insert was then washed
with 1X PBS, and the non-migrated cells were removed with a cotton
swab. The cells were fixed in 4% paraformaldehyde and stained with
0.1% crystal violet. Excess staining was washed away with water.
The cells were identified under a microscope, and a cell count of
five different fields was performed.
Cell invasion was assessed with Matrigel-coated
24-well chambers. A total of 1.0×105 cells in RPMI-1640
medium were added to the insert. RPMI-1640 medium containing 10%
FBS was added to the lower chamber. Invasion assays were conducted
for 24 h at 37°C under normoxic or hypoxic conditions, and the
cells were fixed and counted as described for cell migration
assays. For the invasion assays involving siRNA, the cells were
transfected with LC3B siRNA for 48 h prior to invasion assays being
performed.
Cell adhesion was assessed with Matrigel-coated
96-well plates [dry-coated with Matrigel and blocked with 1% bovine
serum albumin (BSA) for 1 h]. A total of 4×104 cells
were allowed to adhere to Matrigel-coated wells for 2 h at 37°C
under hypoxic conditions. The wells were washed three times with
PBS. A volume of 10 μl
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
substrate was added to each well, and incubation was continued for
an additional 4 h. The number of adherent cells in each well was
quantified by staining with dimethyl sulfoxide and absorbance was
measured at 490 nm.
Staining of cytoskeletal components
Control cells or cells subjected to LC3B knockdown
were washed with PBS and blocked with 1% BSA. The cells were then
incubated with rabbit polyclonal anti-LC3B antibody (1:500
dilution) at 4°C overnight. The cells were washed with PBS,
incubated with AlexaFluor 488 goat anti-rabbit IgG (H+L) (1:300
dilution) for 2 h, and rhodamine phalloidin (1:1000 dilution) was
added for the last 30 min. Cell nuclei were stained during the last
8 min with 20 μl 4′,6-diamidino-2-phenylindole (DAPI). Images were
captured by laser confocal microscopy (Zeiss LSM 710), and image
analysis was performed with ZEN microscopic image analysis software
(Zeiss).
RhoA pull-down assay
Activated RhoA in cells was detected with Rho Assay
Reagent according to the manufacturer’s instructions. Activated
GTP-RhoA was detected by western blotting and mouse monoclonal
anti-RhoA antibody (1:1000 dilution).
Statistical analysis
Experiments were repeated at least three times.
Continuous data are presented as mean ± standard deviation. To
assess differences among groups, One-way analysis of variance was
performed, followed by the Tukey and Dunnett T3 post-hoc tests.
P<0.05 was considered statistically significant. All the
statistical analyses were two-sided and performed with SPSS
software (version 15.0; SPSS Inc., Chicago, IL, USA).
Results
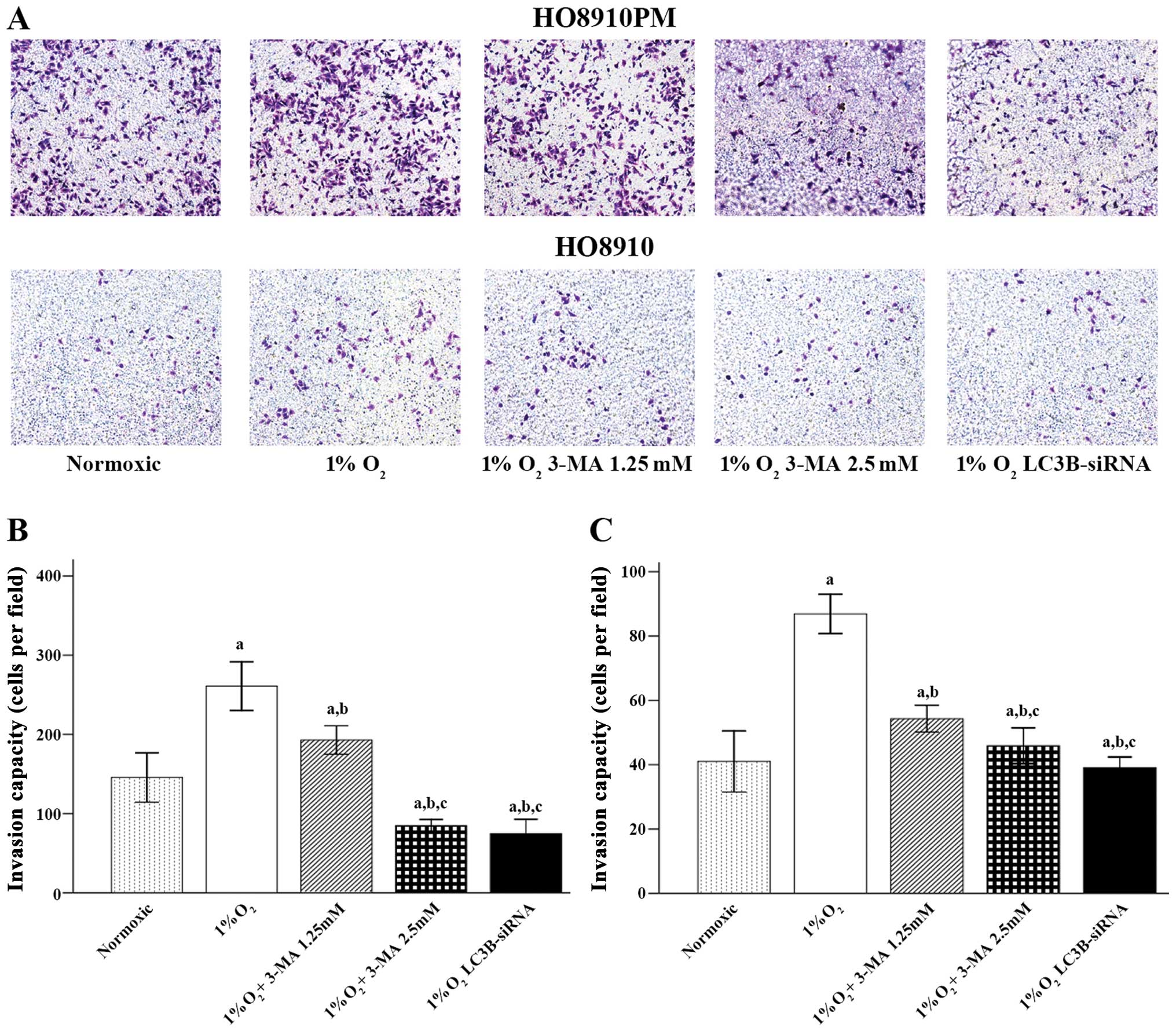
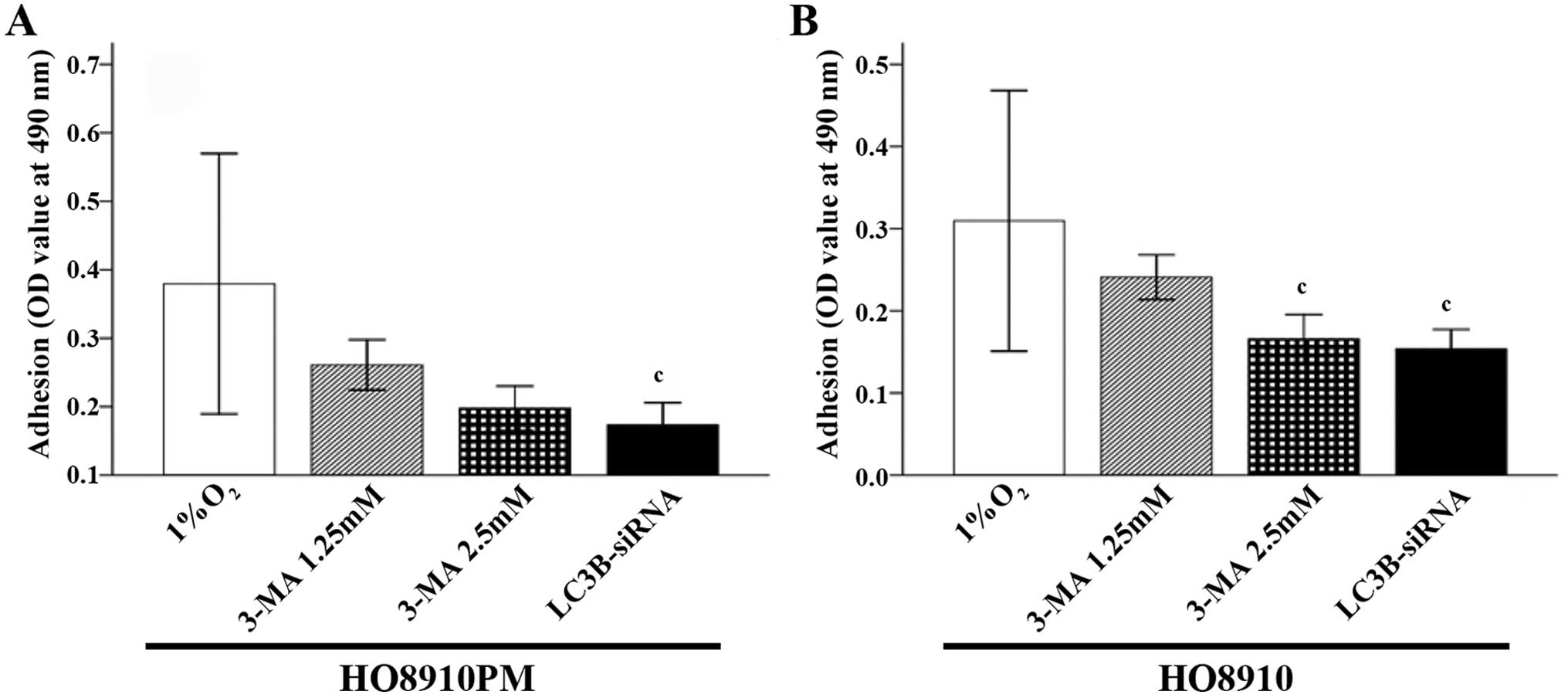
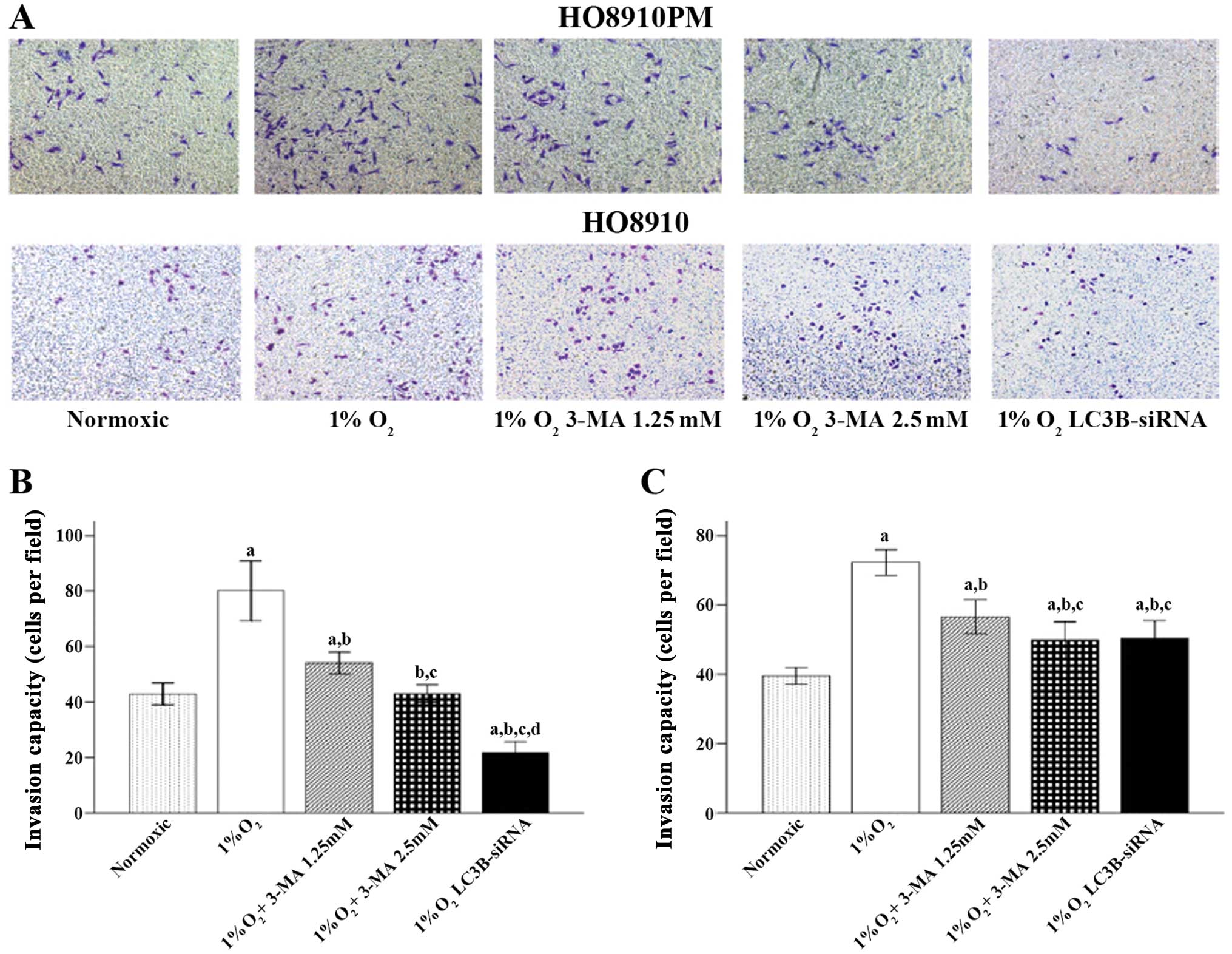
EOC migration and invasion are decreased
by LC3B siRNA or 3-MA treatment under hypoxic conditions
The results showed that hypoxic conditions promoted
HO8910PM and HO8910 cell migration, invasion and adhesion (Figs. 1–3,
respectively). When LC3B was blocked with 3-MA or LC3B siRNA
treatment, cell migration and invasion were significantly decreased
compared to that in control cells (1% O2/hypoxia alone;
all p<0.001 for both HO8910PM and HO8910 cells). In addition,
the cells treated with LC3B siRNA showed significantly less
migration (p=0.021 in HO8910PM cells and p<0.001 in HO8910
cells) (Fig. 1) and invasion
(p<0.001 for the two cell types) (Fig. 2) compared to that in 1.25 mM
3-MA-treated cells. Adhesion was significantly reduced in cells
treated with LC3B siRNA compared to that in 1.25 mM 3-MA-treated
cells (p=0.021 in HO8910PM cells and p=0.004 in HO8910 cells)
(Fig. 3).
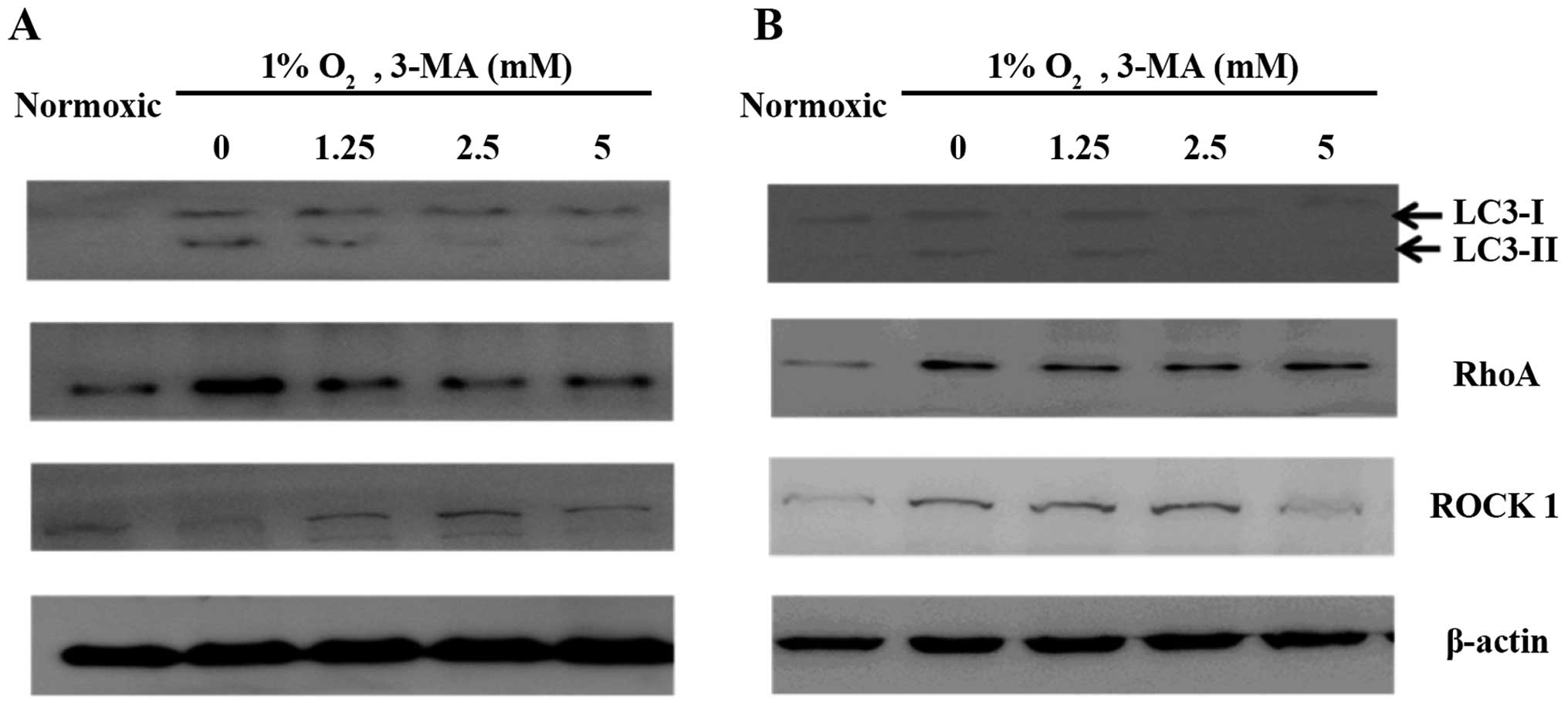
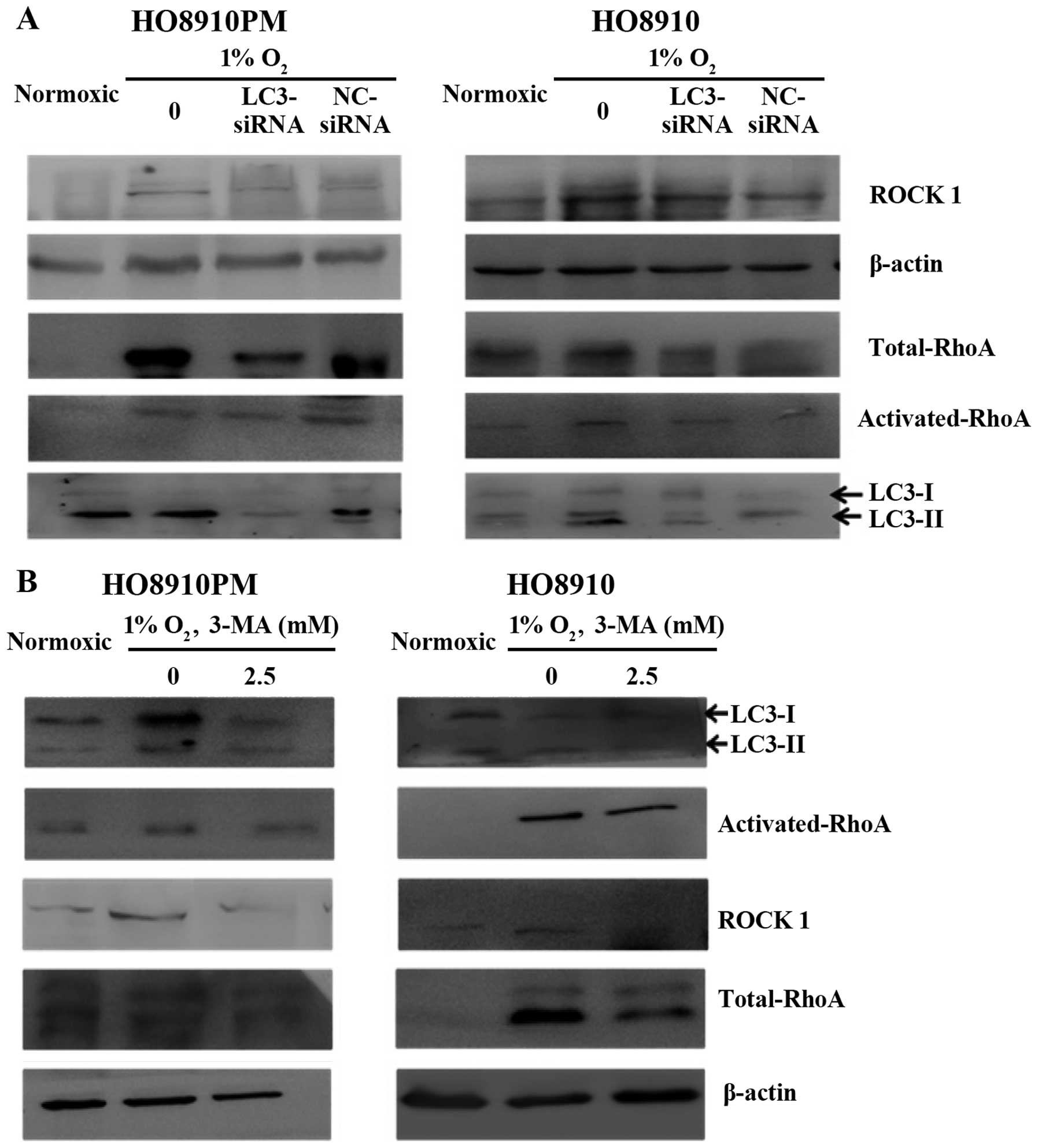
Effect of LC3B siRNA or 3-MA treatment on
LC3B and RhoA expression in HO8910PM and HO8910 cells under hypoxic
conditions
LC3B expression and conversion of LC3B-I to LC3B-II
was observed in the two cell lines under hypoxic conditions
(Figs. 4 and 5). In addition, RhoA expression was
clearly increased under hypoxic conditions. LC3B siRNA (Fig. 5) and 24-h 3-MA treatment (Figs. 4 and 5) each decreased LC3B expression under
hypoxic conditions. Control siRNA had no effect on LC3B expression.
We also found that 3-MA treatment decreased activated RhoA
expression and expression of the downstream effector molecule ROCK1
in HO8910 cells, and LC3B siRNA decreased activated RhoA and ROCK1
expression in HO8910PM cells. We also confirmed hypoxia-induced
RhoA expression, which was inhibited via LC3B siRNA in the two cell
lines, though it appeared that control siRNA had some interference
effect on RhoA expression in HO8910 cells. These results suggested
that RhoA may be associated with LC3B and that the mechanism by
which LC3B promotes metastasis may involve the RhoA pathway in EOC
cells.
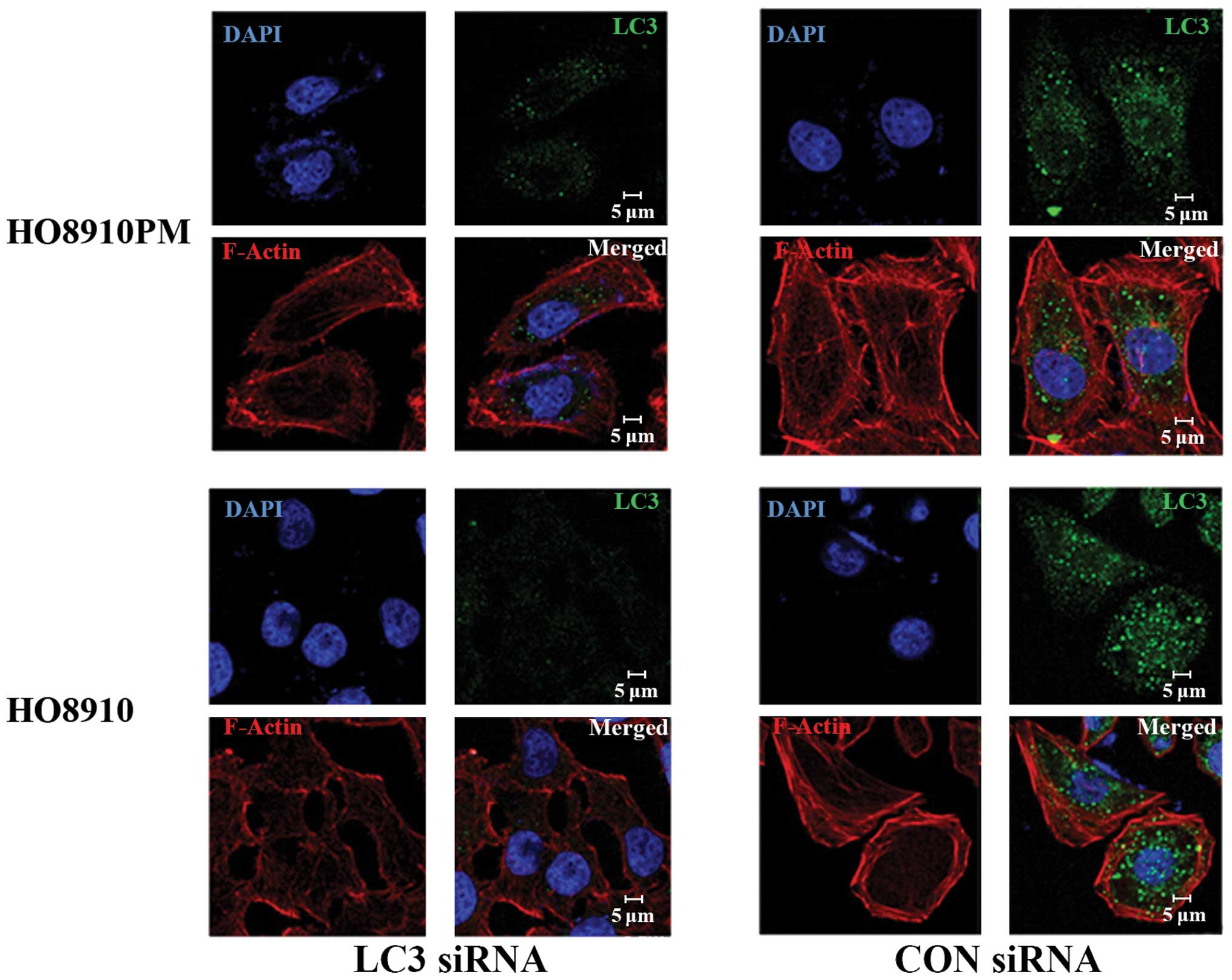
Effect of LC3B siRNA on filamentous
actin
Filamentous actin (F-actin) and LC3B in EOCs under
hypoxic conditions were detected by laser scanning confocal
microscopy (Fig. 6). The cells
transfected with LC3 siRNA showed F-actin fibers that lacked
tension and direction and showed a disordered arrangement. The
cells transfected with control siRNA showed F-actin fibers with
good tension and direction and filament bundles, suggestive of good
invasion ability.
Discussion
The aim of the present study was to determine
whether LC3B is involved in the migration and invasion of EOC
cells. We hypothesized that LC3B expression is advantageous for
tumor development and that inhibition of LC3B is potentially
effective in the treatment or prevention of metastasis, potentially
by depriving tumor cells of sources of energy. Using an in
vitro Transwell migration assay, we showed that inhibition of
LC3B via LC3B siRNA or 3-MA treatment decreased EOC cell migration
and invasion.
HO8910PM and HO8910 EOC cells showed a weak LC3B
expression under normoxic conditions and increased LC3B expression
under hypoxic conditions. Inhibition of LC3B expression via siRNA
or 24-h 3-MA treatment reduced the expression of hypoxia-induced
LC3B to levels similar to those shown under normoxic conditions.
These results support the potential utility of LC3B as an
endogenous marker of tumor hypoxia.
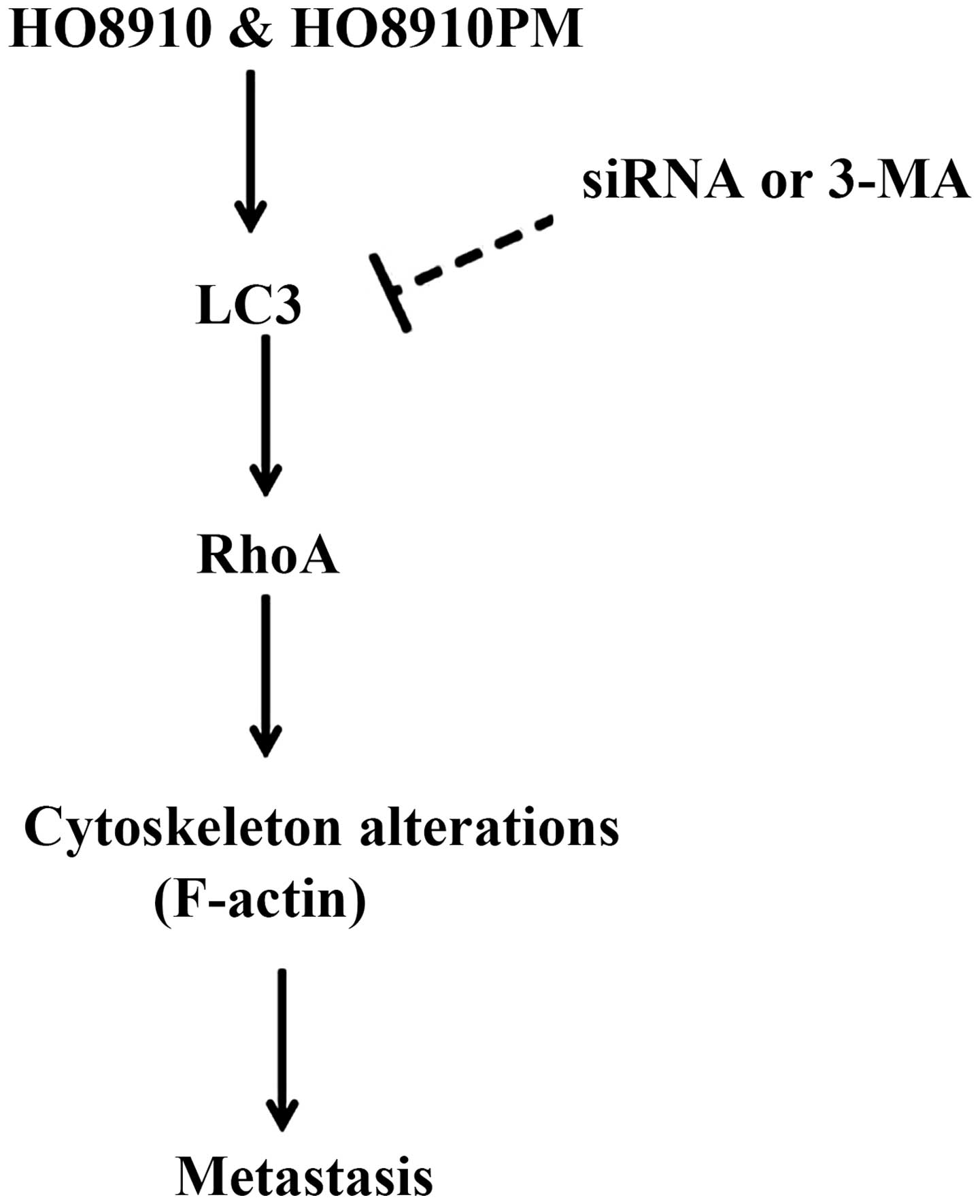
Notably, the downregulation of LC3B decreased the
expression of RhoA and its downstream effector ROCK1, suggesting an
association between hypoxia-induced LC3B and RhoA. The cytoskeleton
is important for maintaining cell shape for motility. Members of
the Rho GTPase family regulate the organization and stability of
F-actin including in actin cytoskeleton remodeling (membrane
protrusion, cell adhesion, and motility) (18). Cell protrusion and spreading are
associated with integrin-matrix interactions for surface attachment
and focal adhesion function (30).
RhoA regulates the formation of stress fibers and the contractile
ring via the stimulation of actin polymerization and activation of
myosin (18). LC3B
siRNA-transfected cells showed broken and disorganized F-actin
fibers, with a lack of tension and direction. This was associated
with reduced cell migration and invasion. LC3B siRNA thus had an
effect on actin filaments, potentially by modulating RhoA activity
(Fig. 7). The specific mechanism(s)
whereby LC3B affects RhoA GTPase activity and leads to changes in
the cytoskeleton remains to be elucidated.
In conclusion, the present results emphasize the
potential of LC3 as a marker of hypoxia and/or metastasis and
demonstrate that the inhibition of LC3 may be a promising strategy
for reducing metastasis of human malignant EOC cells. In
vivo studies may prove useful in assessing this potential.
Acknowledgements
We thank Dr Yi Jing (Department of Biochemistry and
Molecular Cell Biology, Shanghai Jiao Tong University School of
Medicine, Shanghai, China) for technical assistance. This study was
supported by the National Natural Science Foundation of China
(grant no. 81101972).
References
|
1
|
Davidson B, Reich R, Trope CG, Wang TL and
Shih IeM: New determinates of disease progression and outcome in
metastatic ovarian carcinoma. Histol Histopathol. 25:1591–1609.
2010.PubMed/NCBI
|
|
2
|
Bagnato A, Spinella F and Rosanò L:
Emerging role of the endothelin axis in ovarian tumor progression.
Endocr Relat Cancer. 12:761–772. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Itamochi H: Targeted therapies in
epithelial ovarian cancer: molecular mechanisms of action. World J
Biol Chem. 1:209–220. 2010. View Article : Google Scholar
|
|
4
|
Opipari AW Jr, Tan L, Boitano AE, Sorenson
DR, Aurora A and Liu JR: Resveratrol-induced autophagocytosis in
ovarian cancer cells. Cancer Res. 64:696–703. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Simonin K, Brotin E, Dufort S, Dutoit S,
Goux D, N’diaye M, Denoyelle C, Gauduchon P and Poulain L: Mcl-1 is
an important determinant of the apoptotic response to the
BH3-mimetic molecule HA14-1 in cisplatin-resistant ovarian
carcinoma cells. Mol Cancer Ther. 8:3162–3170. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Saad AF, Hu W and Sood AK:
Microenvironment and pathogenesis of epithelial ovarian cancer.
Horm Cancer. 1:277–290. 2010. View Article : Google Scholar
|
|
7
|
Zhang J, Yang Z, Xie L, Xu L, Xu D and Liu
X: Statins, autophagy and cancer metastasis. Int J Biochem Cell
Biol. 45:745–752. 2013. View Article : Google Scholar
|
|
8
|
Bhoopathi P, Gondi CS, Gujrati M, Dinh DH
and Lakka SS: SPARC mediates Src-induced disruption of actin
cytoskeleton via inactivation of small GTPases Rho-Rac-Cdc42. Cell
Signal. 23:978–1987. 2011. View Article : Google Scholar
|
|
9
|
Du H, Yang W, Chen L, Shen B, Peng C, Li
H, Ann DK, Yen Y and Qiu W: Emerging role of autophagy during
ischemia-hypoxia and reperfusion in hepatocellular carcinoma. Int J
Oncol. 40:2049–2057. 2012.PubMed/NCBI
|
|
10
|
Sun Y, Liu JH, Sui YX, Jin L, Yang Y, Lin
SM and Shi H: Beclin1 overexpression inhibits proliferation,
invasion and migration of CaSki cervical cancer cells. Asian Pac J
Cancer Prev. 12:1269–1273. 2011.
|
|
11
|
Rouschop KM and Wouters BG: Regulation of
autophagy through multiple independent hypoxic signaling pathways.
Curr Mol Med. 9:417–424. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schlie K, Spowart JE, Hughson LR, Townsend
KN and Lum JJ: When cells suffocate: autophagy in cancer and immune
cells under low oxygen. Int J Cell Biol. 2011:470–597. 2011.
View Article : Google Scholar
|
|
13
|
Indelicato M, Pucci B, Schito L, Reali V,
Aventaggiato M, Mazzarino MC, Stivala F, Fini M, Russo MA and
Tafani M: Role of hypoxia and autophagy in MDA-MB-231 invasiveness.
J Cell Physiol. 223:359–368. 2010.PubMed/NCBI
|
|
14
|
Tanida I, Ueno T and Kominami E: LC3
conjugation system in mammalian autophagy. Int J Biochem Cell Biol.
36:2503–2518. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Martinet W, De Meyer GR, Andries L, Herman
AG and Kockx MM: In situ detection of starvation-induced autophagy.
J Histochem Cytochem. 54:85–96. 2006. View Article : Google Scholar
|
|
16
|
Peracchio C, Alabiso O, Valente G and
Isidoro C: Involvement of autophagy in ovarian cancer: a working
hypothesis. J Ovarian Res. 5:222012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hu YL, DeLay M, Jahangiri A, Molinaro AM,
Rose SD, Carbonell WS and Aghi MK: Hypoxia-induced autophagy
promotes tumor cell survival and adaptation to antiangiogenic
treatment in glioblastoma. Cancer Res. 72:1773–1783. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Aguilera MO, Berón W and Colombo MI: The
actin cytoskeleton participates in the early events of
autophagosome formation upon starvation induced autophagy.
Autophagy. 8:1590–1603. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pankiv S, Alemu EA, Brech A, Bruun JA,
Lamark T, Overvatn A, Bjørkøy G and Johansen T: FYCO1 is a Rab7
effector that binds to LC3 and PI3P to mediate microtubule plus
end-directed vesicle transport. J Cell Biol. 188:253–269. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rapisarda A, Uranchimeg B, Scudiero DA,
Selby M, Sausville EA, Shoemaker RH and Melillo G: Identification
of small molecule inhibitors of hypoxia-inducible factor 1
transcriptional activation pathway. Cancer Res. 62:4316–324.
2002.PubMed/NCBI
|
|
21
|
Lazova R, Camp RL, Klump V, Siddiqui SF,
Amaravadi RK and Pawelek JM: Punctate LC3B expression is a common
feature of solid tumors and associated with proliferation,
metastasis, and poor outcome. Clin Cancer Res. 18:370–379. 2012.
View Article : Google Scholar
|
|
22
|
Sato K, Tsuchihara K, Fujii S, Sugiyama M,
Goya T, Atomi Y, Ueno T, Ochiai A and Esumi H: Autophagy is
activated in colorectal cancer cells and contributes to the
tolerance to nutrient deprivation. Cancer Res. 67:9677–9684. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yoshioka A, Miyata H, Doki Y, Yamasaki M,
Sohma I, Gotoh K, Takiguchi S, Fujiwara Y, Uchiyama Y and Monden M:
LC3, an autophagosome marker, is highly expressed in
gastrointestinal cancers. Int J Oncol. 33:461–468. 2008.PubMed/NCBI
|
|
24
|
Han C, Sun B, Wang W, Cai W, Lou D, Sun Y
and Zhao X: Overexpression of microtubule-associated protein-1
light chain 3 is associated with melanoma metastasis and
vasculogenic mimicry. Tohoku J Exp Med. 223:243–251. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fujii S, Mitsunaga S, Yamazaki M, Hasebe
T, Ishii G, Kojima M, Kinoshita T, Ueno T, Esumi H and Ochiai A:
Autophagy is activated in pancreatic cancer cells and correlates
with poor patient outcome. Cancer Sci. 99:1813–1819.
2008.PubMed/NCBI
|
|
26
|
Qin AP, Liu CF, Qin YY, Hong LZ, Xu M,
Yang L, Liu J, Qin ZH and Zhang HL: Autophagy was activated in
injured astrocytes and mildly decreased cell survival following
glucose and oxygen deprivation and focal cerebral ischemia.
Autophagy. 6:738–753. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Codogno P and Meijer AJ: Autophagy and
signaling: their role in cell survival and cell death. Cell Death
Differ. 12(Suppl 2): 1509–1518. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Myeku N and Figueiredo-Pereira ME:
Dynamics of the degradation of ubiquitinated proteins by
proteasomes and autophagy: association with sequestosome 1/p62. J
Biol Chem. 286:22426–22440. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Song J, Qu Z, Guo X, Zhao Q, Zhao X, Gao
L, Sun K, Shen F, Wu M and Wei L: Hypoxia-induced autophagy
contributes to the chemoresistance of hepatocellular carcinoma
cells. Autophagy. 5:1131–1144. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kadandale P, Stender JD, Glass CK and
Kiger AA: Conserved role for autophagy in Rho1-mediated cortical
remodeling and blood cell recruitment. Proc Natl Acad Sci USA.
107:10502–10507. 2010. View Article : Google Scholar : PubMed/NCBI
|