Introduction
Prostate cancer is a commonly diagnosed malignant
disease in males worldwide, and there are no effective treatment
options once the cancer becomes metastatic and refractory to
hormonal therapy and chemotherapy. In prostatic tissues, including
both the normal prostate and prostate cancer, the expression of
several specific proteins, such as prostate-specific antigen (PSA),
prostate-specific membrane antigen (PSMA), prostatic acid
phosphatase (PAP) and prostate secretory protein-94 (PSP94) has
been described (1–3). By targeting these prostate-specific
proteins to establish anti-tumor vaccines, immunotherapy has been
developed, and has recently been suggested to have potential for
the treatment of prostate cancer (4–7).
PAP is a secretory prostate-specific protein that
consists of 354 amino acids with an estimated molecular mass of 41
kDa (1,2). Homologs of the human PAP gene have
been identified in rats and mice, and these proteins respectively
share 75 and 81% homology with the human protein at the amino acid
level (2). PAP is overexpressed in
95% of prostate cancer tissues (8),
and the tissue specificity of PAP makes it an attractive target
antigen for immunotherapy against prostatic malignancy (8,9).
Several vaccine candidates have been developed based on the PAP
protein using cell-based medicine (10,11),
DNA vaccines (12) or peptide
antigens (13,14).
Using the full-length PAP protein as a vaccine
target, sipuleucel-T (Provenge®; Dendreon Inc., Seattle,
WA, USA), an autologous active cellular immunotherapy, was
developed for the treatment of metastatic castration-resistant
prostate cancer (10). The cellular
agent is manufactured from individual peripheral monocytes enriched
by leukapheresis, and is an antigen-presenting cell vaccine loaded
ex vivo with a fusion protein linking PAP to
granulocyte-macrophage colony-stimulating factor (GMCSF) (15,16).
After incubation with the PAP-GMCSF fusion protein, the
immunologically activated cells are intravenously administered to
patients every two weeks for a total of three infusions. A
placebo-controlled randomized phase III trial demonstrated that the
personalized cell-based vaccine showed evidence of efficacy in
reducing the risk of death among patients with prostate cancer
refractory to hormonal therapy (10). In the patients treated with
sipuleucel-T, there was a relative reduction of 22% in the risk of
death compared with the placebo group, representing a 4.1-month
improvement in median survival (25.8 months in the sipuleucel-T
group vs. 21.7 months in the placebo group). Based on the results
of a phase III study, the US Food and Drug Administration (FDA)
approved sipuleucel-T for the treatment of prostate cancer in April
2010, and this was the first antigen-specific immunotherapy
officially approved in a developed country.
Although sipuleucel-T can prolong the overall
survival in patients with progressive prostate cancer, more robust
immunological effects seem to be possible that can further improve
survival. In order to enhance the therapeutic effects of the
PAP-GMCSF fusion protein, we experimentally tried to modify the
methods and to develop a novel vaccination strategy with the
additional use of multiple PAP-fused cytokines, including human
interleukin-2 (IL2), IL4 and IL7. The reason for adding these
interleukins is that these cytokines can potently activate
anticancer immune cells and upregulate the effects of the
therapeutic vaccination for cancer treatment (17–19).
The cost for sipuleucel-T is reported to be 93,000
US$ per course of treatment, thus making this therapeutic vaccine
an expensive treatment option (11). One of the ways to reduce the
pharmacological cost is to increase the production efficiency of
the PAP-GMCSF fusion protein. Since we previously established a
super gene expression (SGE) cassette to amplify the production of a
recombinant protein in 293-F cells (20,21),
we herein attempted to apply this mammalian expression system to
produce multiple PAP-fused cytokines. In the present study, we
validated the activity of the PAP-fused proteins as cytokines and
demonstrated the advantages of the combined use of multiple
PAP-fused cytokines in in vitro and in vivo
situations.
Materials and methods
Construction of the SGE plasmid vectors
encoding PAP-fused cytokines
The construction of a plasmid vector with the SGE
system was performed as previously described (20,21).
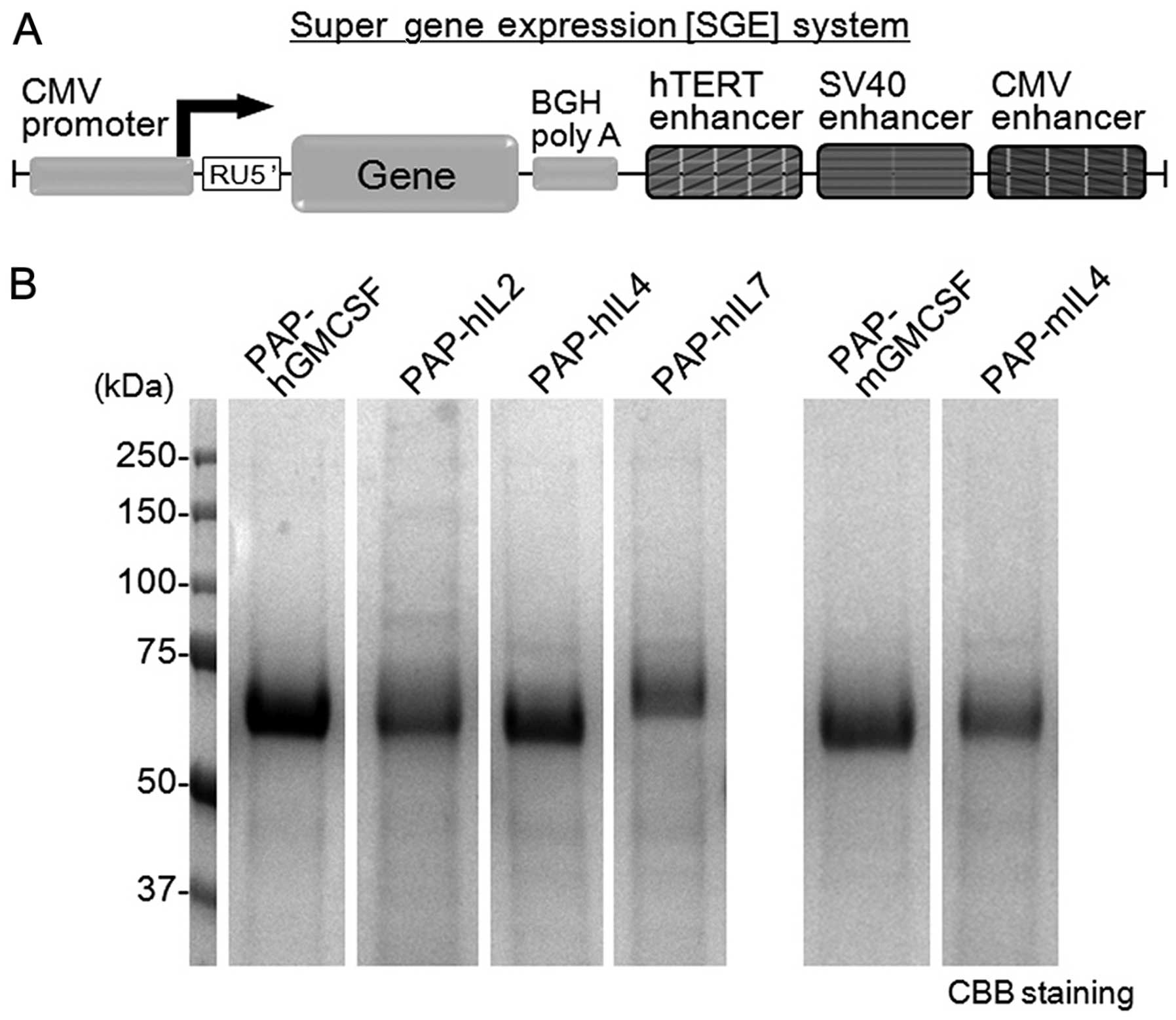
The gene expression cassette with the CMV promoter, sequences of
RU5′, bovine growth hormone polyadenine nucleotides (BGH polyA) and
tandem elements of triple translational enhancers hTERT, SV40 and
CMV was artificially synthesized and cloned into the pIDT-SMART
vector (Integrated DNA Technologies, Inc., Coralville, IA, USA)
(Fig. 1A). The RU5′ sequence [269
bp, accession no. J02029 (374–642)] was derived from the R segment
and part of the U5 sequence of the HTLV type 1 long terminal repeat
and was used to enhance the stability of DNA and RNA and increase
the translation efficiency (21).
The tandem element of the triple translational enhancers consists
of the hTERT enhancer [189 bp, accession no. DQ264729 (1618–1806)],
SV40 enhancer [319 bp, accession no. AY864928 (2156–2474)] and CMV
enhancer [479 bp, accession no. AJ318513 (159–637)]. The tandem
sequence of the hTERT, SV40 and CMV enhancers is the main element
of the SGE system. The SGE plasmid encoding a fusion protein of
full-length human PAP and one of the cytokines (human GMCSF, IL2,
IL4, IL7 or mouse GMCSF or IL4) were then constructed and used to
express the recombinant PAP-fused cytokines. The fusion cytokines
were designed to be histidine-tagged for purification.
Preparation of the recombinant PAP-fused
cytokines
The recombinant PAP-fused cytokines were expressed
by the FreeStyle 293 expression system (Invitrogen, Carlsbad, CA,
USA) with the respective SGE plasmids according to the
manufacturer’s instructions. The expression system was designed to
allow the transfection of suspended 293-F cells (derived from
HEK293 cells) in serum-free culture medium. Transient transfection
with the plasmid vector was performed, and the secreted recombinant
fusion protein in the culture supernatant was collected. The
PAP-fused cytokines were purified using the histidine tag included
in the fusion protein, as previously described (22). The recombinant PAP-fused cytokines
were stocked and maintained at −80°C until use. The PAP-fused human
and mouse cytokines were analyzed for the protein amount, purity
and concentration by examining the densitometry of the bands on
SDS-PAGE with Coomassie brilliant blue (CBB) staining.
Preparation and culture conditions of
blood mononuclear cells
Human peripheral blood mononuclear cells (PBMCs)
were prepared from the blood of healthy donors by the standard
procedure using Ficoll-Paque centrifugation (22). This research was carried out on
humans following the international and national regulations.
Written informed consent was obtained from the subjects. For some
experiments, human CD14-positive monocytes were purchased from
Lonza (Walkersville, MD, USA). Mouse blood was obtained from the
inferior vena cava, and the mononuclear cells were prepared by
Ficoll-Paque centrifugation. The blood mononuclear cells and
CD14-positive monocytes were cultured either in LGM-3 medium
(Lonza) alone or in the presence of recombinant PAP-fused cytokines
under the indicated conditions. As a positive control for the
differentiation of human dendritic cells, the PBMCs and
CD14-positive monocytes were cultured in LGM-3 medium supplemented
with human granulocyte-macrophage colony-stimulating factor
(hGMCSF; 2 ng/ml) and human interleukin-4 (hIL4; 2 ng/ml) (both
from R&D Systems, Minneapolis, MN, USA) (17,22).
The cells were cultured at 37°C in humidified incubators containing
air with 5% CO2.
Proliferation assay using TF-1 cells
TF-1, a human GMCSF-dependent proliferative cell
line (23,24), was used to analyze the cytokine
activity of PAP-hGMCSF. TF-1 cells (104 cells/well) were
plated on 96-well plates and cultured as previously reported
(23). The PAP-hGMCSF was added to
the culture medium at the indicated concentrations derived from a
3-fold serial dilution. hGMCSF was used as a positive control for
the GMCSF-dependent proliferation. After three days of incubation,
the proliferation of the TF-1 cells was analyzed with the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay, according to the manufacturer’s instructions.
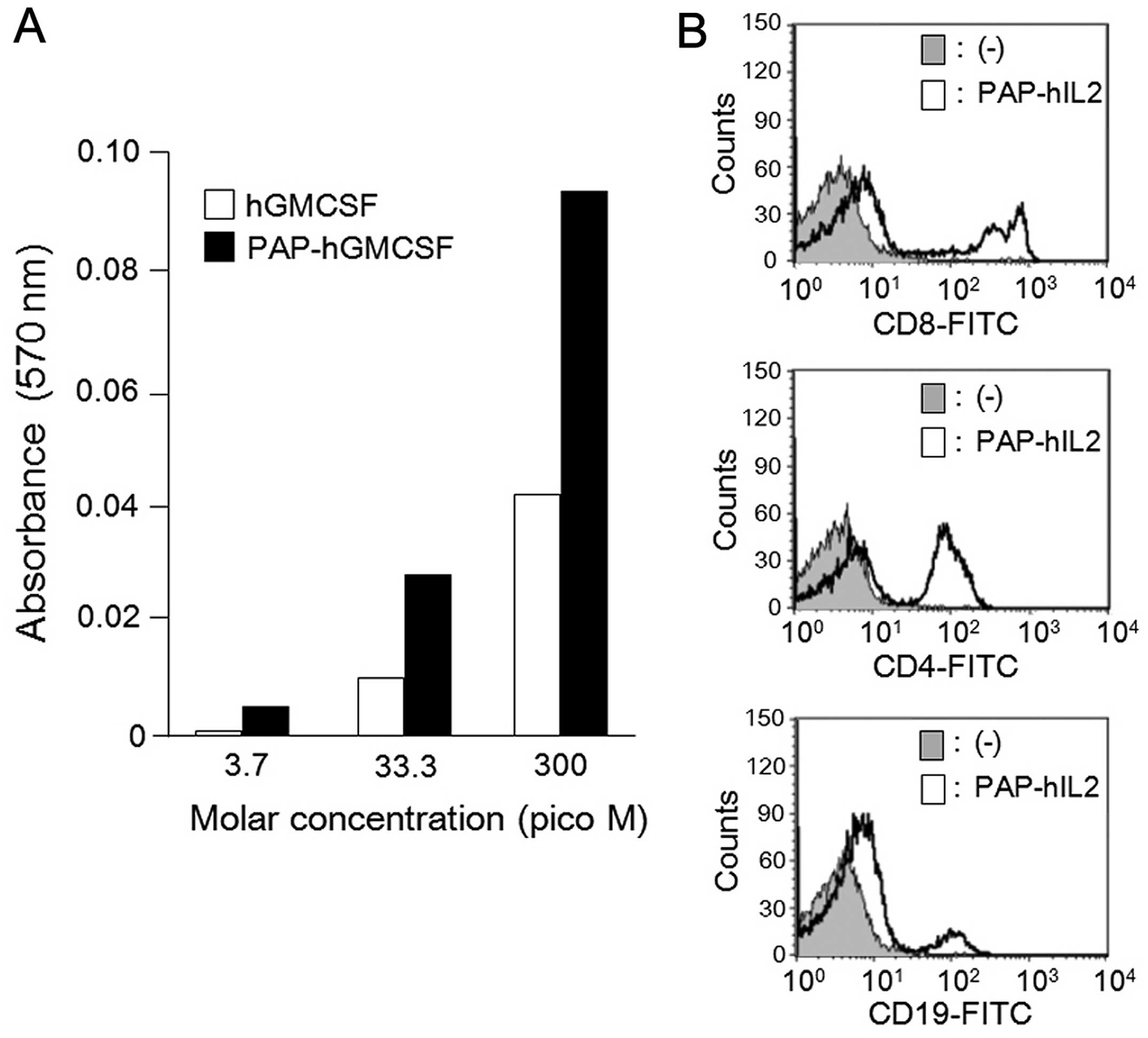
Flow cytometric analysis
To analyze the proliferation of CD8+,
CD4+ and CD19+ lymphocytes, flow cytometry
was performed as previously described (22). Human PBMCs (75×104
cells/well) were incubated with PAP-hIL2 (1 μg/ml) for four days on
6-well plates, and then the floating cells were examined for each
surface antigen. The cells were stained with the following
FITC-conjugated antibodies for 60 min on ice: CD8 (551347; BD
Pharmingen, San Diego, CA, USA) as a marker for cytotoxic T
lymphocytes, CD4 (555346) for helper T lymphocytes and CD19
(555412) for B lymphocytes. After staining, 2×104 cells
were acquired on a FACSCalibur flow cytometer and analyzed using
the CellQuest software program (both from Becton-Dickinson,
Franklin Lakes, NJ, USA).
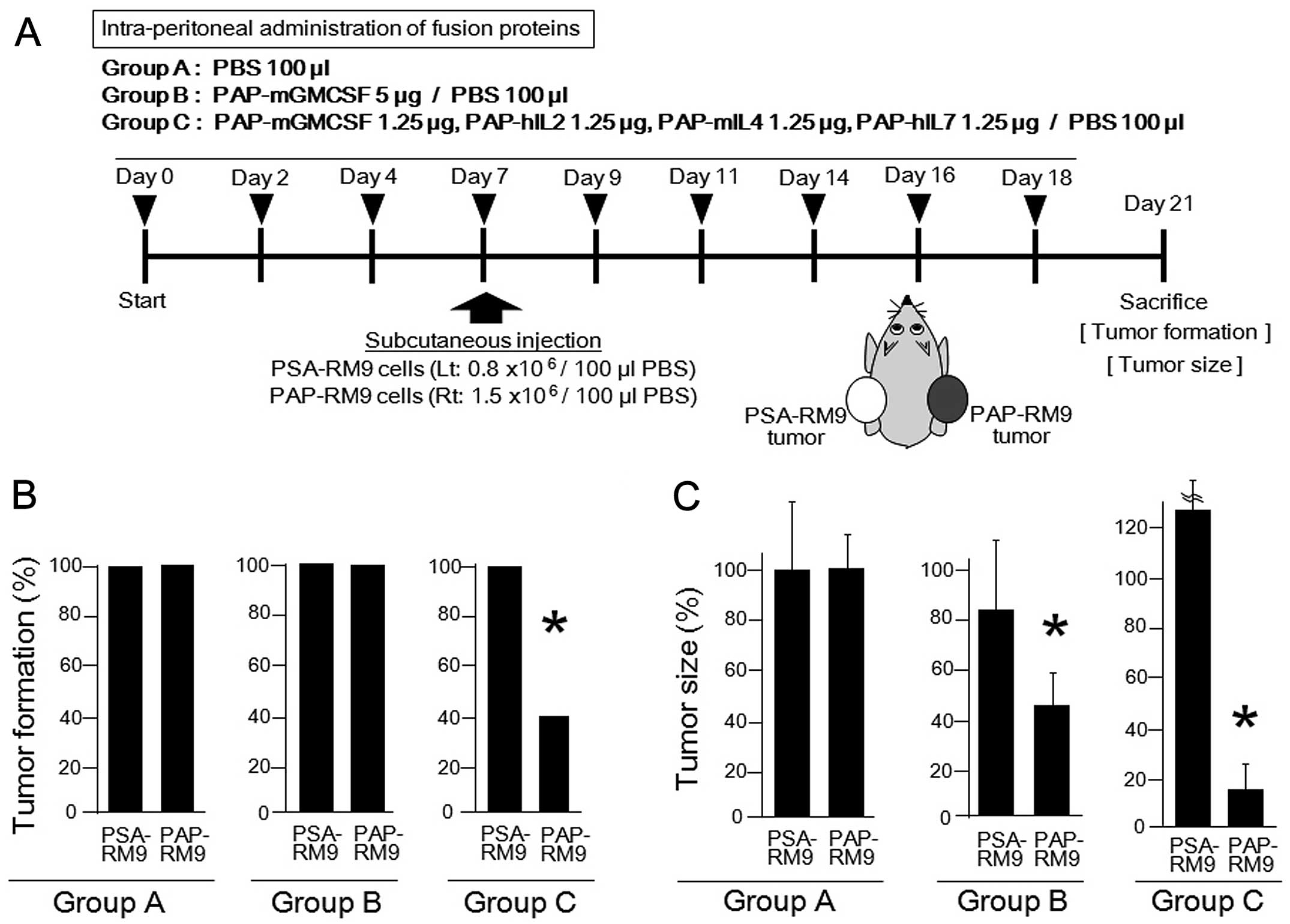
Animal experiments
The RM9 mouse prostate cancer cell line was kindly
provided by Dr T.C. Thompson (The University of Texas, Houston, TX,
USA) (25) and was used in the
animal experiments. In our experimental set-up, we prepared PSA-RM9
and PAP-RM9 cells stably expressing the human PSA or PAP protein,
respectively. The stable clones of RM9 cells were established by
the transfection of plasmids encoding the full-length human PSA or
PAP genes and a neomycin-resistance gene, as previously described
(20,21,26).
Using the PSA-RM9 and PAP-RM9 cells, we developed a mouse prostate
cancer model bearing both PSA- and PAP-expressing tumors. The in
vivo experimental schedule and treatments with the PAP-fused
cytokines are shown in Fig. 4A.
Since IL2 and IL7 possess cross-reactivity between human and mouse
species (27–29), we used PAP-human IL2 and PAP-human
IL7 instead of PAP-mouse IL2 and PAP-mouse IL7, respectively. A
total of nine intraperitoneal administrations of the PAP-fused
cytokines were performed. On day 7, the PSA-RM9 and PAP-RM9 cells
were subcutaneously inoculated into the left and right femurs,
respectively, of C57BL/6 adult male mice. All mice were examined
for the tumor formation and for the tumor size on day 21, and then
were sacrificed. Animal experiments using anticancer cytokines were
approved by the Animal Care and Use Committee, Okayama
University.
Statistical analysis
The data are expressed as the means ± standard
error. Student’s t-test or the Chi-square test was used to
determine the statistical significance of differences between the
two groups. Differences were considered to be statistically
significant for values of p<0.05.
Results
Production of recombinant multiple
PAP-fused cytokines using the SGE system
We previously developed the SGE system in order to
improve the protein production by conventional gene expression
systems (20,21). In the gene expression cassette with
the SGE system, triple translational enhancer sequences of hTERT,
SV40 and the CMV enhancer were inserted downstream of the BGH polyA
sequence (Fig. 1A). We recently
reported that the SGE system significantly enhanced adenoviral
vector-mediated gene expression (~2- to 5-fold) in human cancer
cell lines, as determined by western blot analysis) in comparison
to the system using conventional gene expression (20). Therefore, we herein attempted to
apply this mammalian expression system to produce the multiple
PAP-fused cytokines.
The amount of protein produced and the purity of the
recombinant PAP-fused cytokines produced by the SGE system were
analyzed by SDS-PAGE with CBB staining (Fig. 1B). As a result, a significant amount
of PAP-fused cytokines was obtained in the serum-free culture
medium of the 293-F cells on day 5 by the transient gene
expression. The recombinant fusion proteins were recognized as
single bands by gel electrophoresis. The concentration (mg/l) of
the fusion proteins in the medium was calculated: PAP-hGMCSF,
123.3; PAP-hIL2, 91.7; PAP-hIL4, 96.9; PAP-hIL7, 64.5; PAP-mGMCSF,
94.2; PAP-mIL4, 62.8.
Recombinant PAP-fused cytokines maintain
their cytokine activity
In order to confirm the cytokine activity of the
recombinant PAP-fused cytokines, we conducted in vitro
experiments with PAP-hGMCSF and PAP-hIL2. It is known that human
GMCSF stimulates the proliferation of TF-1 cells (23,24).
Therefore, we first analyzed the growth dependency of TF-1 cells by
adding PAP-hGMCSF in the culture medium. The cell proliferation at
the indicated concentrations was assessed by determining the
absorbance of the cells in the MTT assay. The results indicated
that proliferation of the TF-1 cells was increased in a
dose-dependent manner by PAP-hGMCSF (Fig. 2A). We next investigated the IL2
activity of PAP-hIL2 by evaluating the proliferation of lymphocyte
lineages. The cultivation of human PBMCs in the presence of
PAP-hIL2 resulted in the rapid growth of the CD8+,
CD4+ and CD19+ lymphocytes within four days,
as determined by flow cytometry (Fig.
2B). These results indicate that the recombinant PAP-fused
cytokines produced by the SGE system retain the usual cytokine
activity.
Differentiation of dendritic cells is
enhanced by the combined use of multiple PAP-fused cytokines,
including PAP-GMCSF
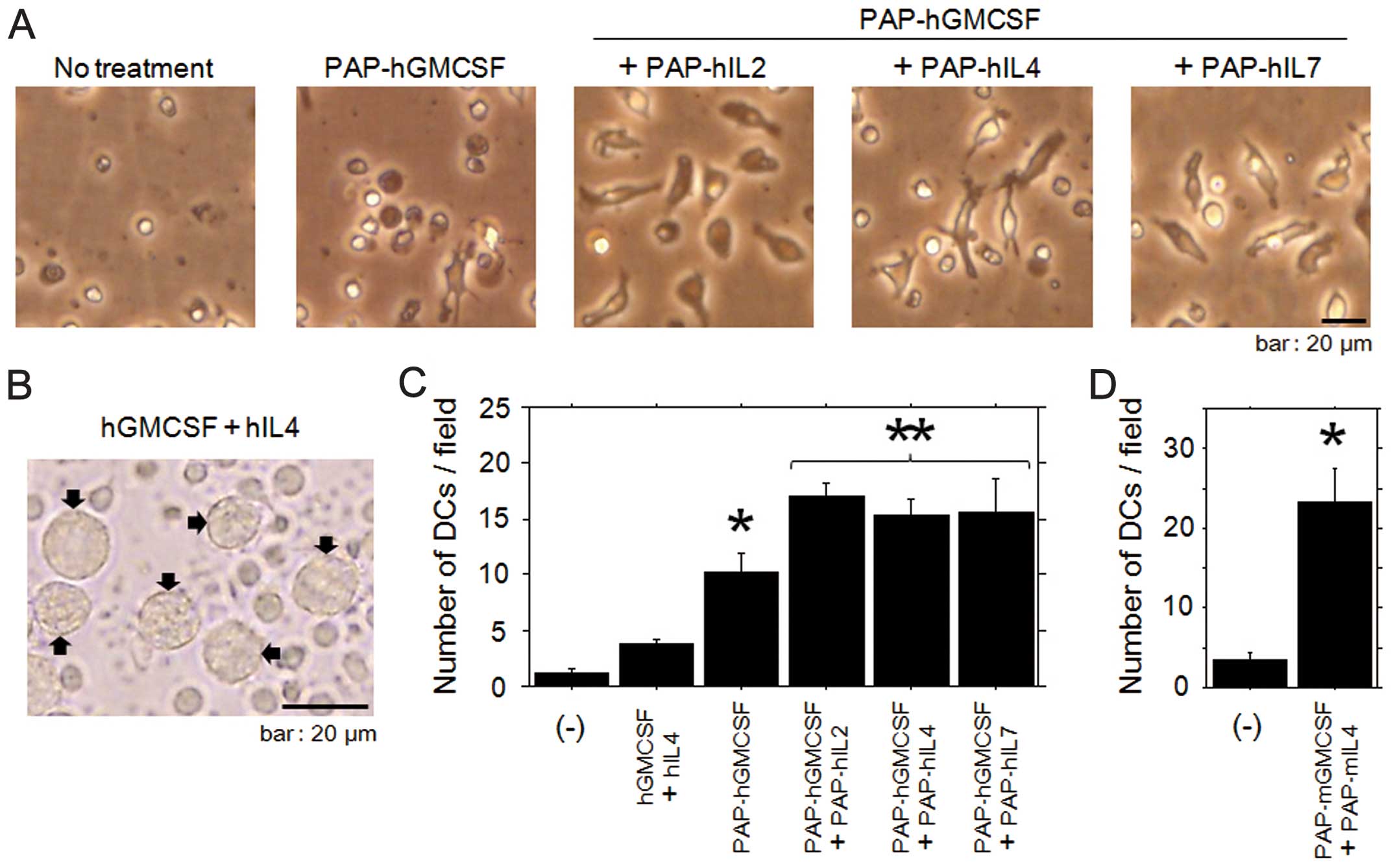
To confirm the ability of the multiple PAP-fused
cytokines to induce the differentiation of monocytes into dendritic
cells, purified monocytes from PBMCs were cultured in the presence
of recombinant fusion cytokines. Since GMCSF has been considered to
be an essential cytokine for the differentiation and maturation of
dendritic cells (17), we basically
used PAP-GMCSF and attempted to combine the other PAP-fused
cytokines with it in order to augment the immunological effects.
During the first two days of treatment with the multiple PAP-fused
cytokines (including PAP-hGMCSF), most of the monocytes displayed
cellular elongation (Fig. 3A). The
ratio of elongated monocytes in samples treated with the multiple
fusion cytokines was higher than that in the cells treated with
PAP-hGMCSF alone. With regard to the monocytes cultivated with
medium alone, there were only a few cells with dendritic-cell like
features.
We next quantified the dendritic cell
differentiation induced by the treatment with multiple PAP-fused
cytokines, including PAP-GMCSF. Since it has been well established
that dendritic cells can be differentiated by incubation with GMCSF
and IL-4 (17,22), we referred to the morphological
features of cells treated with these cytokines as a positive
control for dendritic cells (Fig.
3B). After the incubation with PAP-fused human cytokines, the
number of cells developing into human dendritic cells was counted
in several microscopic fields. When treated with the multiple fused
cytokines, the number of developing dendritic cells was
significantly increased in comparison to those treated with
PAP-GMCSF alone (Fig. 3C). The
enhanced differentiation of mouse dendritic cells was also
confirmed by cultivating the blood mononuclear cells with multiple
fusion cytokines, including PAP-mGMCSF and PAP-mIL4 (Fig. 3D). These results indicated that the
differentiation of dendritic cells was enhanced by the combined use
of multiple PAP-fused cytokines, including PAP-GMCSF.
In vivo therapeutic effects are enhanced
by the combined use of multiple PAP-fused cytokines, including
PAP-GMCSF
The in vitro results in the present study
indicated the possibility that a PAP-fused cytokine-based in
vivo increase in immune cells may upregulate the anticancer
immune response by targeting the PAP molecule. In order to
investigate the antitumor effects of PAP-fused cytokines, in
vivo experiments were performed using a mouse prostate cancer
model bearing both PSA- and PAP-expressing tumors (Fig. 4A). As a PAP-targeting vaccine, the
multiple PAP-fused cytokine treatment would be expected to be most
effective for use against the initial stages of prostate cancer or
for minimal disease. Therefore, the vaccine was started to be
administered before the cancer cell inoculation in our preclinical
mouse model.
In the preliminary experiments, in vitro and
in vivo stable expression of the PSA and PAP proteins was
confirmed for the PSA-RM9 and PAP-RM9 cells, respectively (data not
shown). Since IL2 and IL7 possess cross-reactivity between humans
and mice (27–29), we used PAP-human IL2 and PAP-human
IL7 instead of PAP-mouse IL2 and PAP-mouse IL7, respectively. We
first analyzed whether the PAP-fused cytokines could protect mice
against challenge with a PAP-expressing tumor. The incidence of
PSA-RM9 and PAP-RM9 tumor formation was analyzed on day 21 after
the subcutaneous inoculation of cancer cells. In the present study,
PSA-expressing tumors derived from PSA-RM9 cells were monitored for
the effects of the PAP-based immunological treatments. The
co-administration of PAP-GMCSF, -IL2, -IL4 and -IL7 significantly
prevented the tumor induction of PAP-RM9 cancer cells (Fig. 4B). On the other hand, the treatment
with PAP-GMCSF alone failed to prevent the tumor formation.
We next investigated whether PAP-fused cytokines
inhibits PAP-RM9 tumor growth in vivo. Significant
inhibition of the tumor growth was observed in the groups treated
with both PAP-GMCSF alone and with the co-administration of
PAP-GMCSF, -IL2, -IL4 and -IL7 (Fig.
4C). The in vivo therapeutic effects of the multiple
PAP-fused cytokines were superior to the effects observed with
PAP-GMCSF treatment alone. For the control PSA-expressing tumors
derived from PSA-RM9 cells, no significant therapeutic effect was
observed following the treatments with PAP-fused cytokines. Based
on the results of the tumor challenge and growth, it was concluded
that PAP-specific immune activation occurred in the mice treated
with the PAP-fused cytokines, and the antitumor effects were
significantly enhanced by the additional cytokine (IL2, IL4 and
IL7) fusion proteins.
Discussion
In the present study, we experimentally validated a
PAP-GMCSF-based vaccine strategy with multiple PAP-fused cytokines
for the immunotherapy of prostate cancer. We also demonstrated the
availability of the SGE system for the production of recombinant
PAP-fused cytokines. With regard to the SGE system, we originally
developed it as a gene expression system that allows the gene of
interest to be expressed with very high efficiency in 293-F cells
(20,21). In this SGE construct of the vectors,
the linkage of triple translational enhancer sequences of hTERT,
SV40 and CMV enhancers was inserted into a site downstream of the
sequence of the BGH polyA. The CMV promoter driving SGE system
robustly enhanced the gene expression of plasmid and adenoviral
vectors in comparison to the cassette with CMV promoter alone
(20). The superiority of the CMV
promoter-SGE system was also observed compared to the gene
expression cassette with EF-1α and CAG promoters alone (21). Using this SGE system, we succeeded
in producing a significant amount of the PAP-fused cytokines (human
GMCSF, IL2, IL4, IL7, and mouse GMCSF and IL4) in the medium of
293-F cells. The recombinant proteins were easily concentrated for
use in the experiments and demonstrated activity as cytokines.
We herein examined the activity of the fusion
proteins as cytokines in vitro, and significant upregulation
of dendritic cell differentiation from monocytes was achieved by
treatment with PAP-GMCSF when it was used with the other PAP-fused
cytokines. The PAP-fused human IL2 led to significantly increased
proliferation of T and B lymphocytes, as determined by flow
cytometry. We thus validated the cytokine functions of the
PAP-fused cytokines. Dendritic cells are antigen-presenting cells
that play important roles in anticancer immune responses. GMCSF has
been considered to be the main cytokine required for the
differentiation and maturation of dendritic cells (17). The combined use of GMCSF and IL4 is
the most extensively characterized and utilized combination for the
in vitro differentiation of dendritic cells from peripheral
blood monocytes (17,22). In terms of the effects of the
different cytokine combinations with GMCSF on the development of
dendritic cells, the addition of IL2 and IL7 also plays important
roles (17,30–32).
Moreover, previous reports indicate that IL2 induces the
differentiation and/or maturation toward a dendritic cell phenotype
without GMCSF (31), and could
enhance the motility of dendritic cells (30).
With regard to the other immune cells (other than
antigen-presenting cells), CD4+ and CD8+ T
lymphocytes, CD19+ B lymphocytes and natural killer (NK)
cells all play important roles in antitumor immunity. Interleukins,
including IL2, IL4 and IL7, are known to exhibit and enhance
antitumor effects, not only through the activation of these
antitumor immune cells, yet also via the activation of macrophages
and lymphokine-activated killer (LAK) cells (3,33–35).
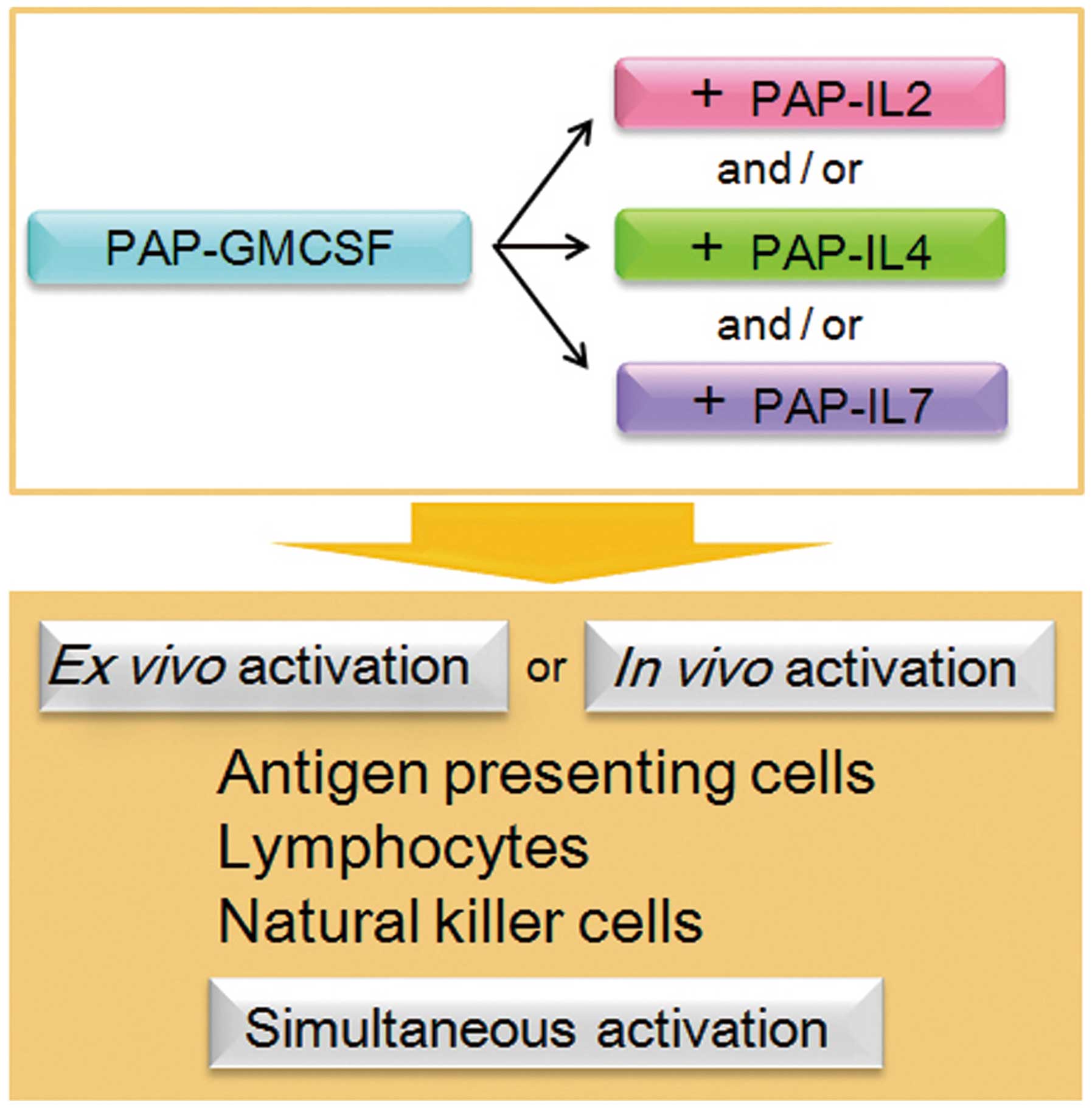
Thus, as a result of the enhanced cancer-specific immunity
following the simultaneous activation of these immune cells, the
combined use of multiple cytokines is a promising strategy
(Fig. 5). Our results also indicate
that this strategy could be adaptable for both ex vivo
activation and in vivo activation of the immune cells for
immunotherapy. Further studies are warranted toward the clinical
application of the PAP-GMCSF-based vaccine strategy with multiple
PAP-fused cytokines for prostate cancer treatment.
To further demonstrate the utility of the multiple
PAP-fused cytokines, we investigated the in vivo therapeutic
effects of the recombinant proteins in a mouse prostate cancer
model bearing both PSA- and PAP-expressing tumors. The
co-administration of PAP-GMCSF, -IL2, -IL4 and -IL7 significantly
prevented tumor induction and inhibited tumor growth in the
PAP-expressing tumors, yet not in the PSA-expressing tumors. The
finding indicates that PAP-specific immune activation occurred in
the mice treated with the PAP-fused cytokines. We also demonstrated
that the tumor growth suppression by the multiple PAP-fused
cytokines was superior to the effect with PAP-GMCSF alone.
Therefore, the vaccination against PAP was significantly
upregulated by the additional cytokine (IL2, IL4 and IL7) fusion
proteins. The in vivo results are consistent with our in
vitro findings showing the increased induction of dendritic
cells and lymphocytes by the fusion cytokines. It is conceivable
that the PAP-fused cytokines, including PAP-GMCSF, induced the
robust differentiation of the monocytes into antigen-presenting
dendritic cells, resulting in the activation of PAP-specific
CD4+ and CD8+ T lymphocytes in the treated
mice. These activated T lymphocytes are then thought to work
against the PAP-expressing tumor lesions in the mouse model,
targeting the antigen and mediating the anti-tumor responses. On
the other hand, based on the increased induction of
CD19+ B lymphocytes by the fusion cytokines in
vitro, the antitumor effects could also be partially due to the
humoral immunity of the PAP-specific antibodies induced in the
mouse model.
A question that remains to be answered is associated
with the peptide epitopes of the PAP antigen in the cellular arm of
the immune response in the current C57BL/6 mouse model. Notably,
other investigators recently reported that three human PAP epitopes
(114–128, 299–313 and 230–244) were immunologically processed for
vaccinations in mice (8). They
demonstrated that the PAP (114–128) epitope, which is identical
between human and mouse species at the amino acid level, elicits
CD4+ and CD8+ T lymphocyte-specific responses
in C57BL/6 mice. Furthermore, when administered to mice bearing
prostate cancer, the PAP (114–128) peptide prevented and reduced
the growth of tumors in the prophylactic and therapeutic settings.
These studies showed that the antitumor effects were associated
with the infiltration of CD8+ tumor-infiltrating
lymphocytes, and proposed that PAP (114–128) is a highly relevant
peptide on which to base vaccines for the treatment of prostate
cancer. Therefore, it is conceivable that the PAP epitopes,
including the peptide (114–128), played essential roles in the
anti-PAP vaccination processes in our mouse model after treatment.
Additionally, we herein adopted a vaccination strategy using the
full-length PAP protein as an immunogen. The use of entire proteins
brings about significant advantages, since it can allow the immune
cells to present multiple epitopes, including unknown epitopes
associated with different MHC class I molecules, in addition to
helper epitopes associated with MHC class II molecules (36).
The previously described therapeutic vaccine,
sipuleucel-T, is composed of autologous antigen-presenting cells
cultured with a fusion protein of PAP-GMCSF, and its administration
prolonged the overall survival among patients with metastatic
castration-resistant prostate cancer (10). Sipuleucel-T has opened a new era for
the treatment of prostate cancer (11), and immunotherapy has become one of
the attractive treatment strategies for various cancers. The immune
responses to the immunized PAP antigen were augmented in patients
who received sipuleucel-T (10),
indicating that strategies that can enhance the effects of
vaccination are attractive for the next step in immunotherapy. We
herein demonstrated the advantages of the in vitro and in
vivo combined use of multiple PAP-fused cytokines, including
PAP-GMCSF, and therefore propose that the combined use of these
fusion proteins could be promising for the enhancement of the
immunological effects in prostatic antigen-based vaccination
therapy. Moreover, if the super gene expression (SGE) system is
applied in the Good Manufacturing Practice (GMP) setting, it could
be used for the efficient production and cost-reduction of
PAP-fused cytokines for clinical use. Further examinations will be
required to apply the current strategy employing the PAP-fused
cytokines for ex vivo and in vivo human
immunotherapy.
In conclusion, we demonstrated the advantages of the
combined use of multiple PAP-fused cytokines, including PAP-GMCSF,
under in vitro and in vivo experimental conditions,
and propose that this prostatic antigen-based vaccination strategy
should be promising for future clinical development. The current
findings provide immunological insight for enhancing the
therapeutic effects of cytokine-based immunotherapy. It should also
be noted that the approach using multiple antigen-fused cytokines
is adaptable to other cancer types by changing the prostatic PAP
antigen to a different cancer-specific antigen.
Acknowledgements
This study was supported by scientific research
grants (KAKENHI: 24390368, 25462478, 25670683 and 26293352) from
the Ministry of Education, Culture, Sports, Science and Technology
of Japan. We thank Ms. Fusaka Oonari (Okayama University) for her
valuable assistance. Okayama University and Momotaro-Gene Inc. are
applying for patents on the SGE systems. Okayama University and Dr
M. Watanabe are applying for a patent regarding the cancer
antigen-fused cytokines. Dr M. Watanabe, Dr Y. Nasu and Dr H. Kumon
are the inventors of the patents and own stock in Momotaro-Gene
Inc. Dr Kumon is the chief science officer of the company.
References
|
1
|
Fong L, Ruegg CL, Brockstedt D, Engleman
EG and Laus R: Induction of tissue-specific autoimmune prostatitis
with prostatic acid phosphatase immunization: implications for
immunotherapy of prostate cancer. J Immunol. 159:3113–3117.
1997.PubMed/NCBI
|
|
2
|
Fong L, Brockstedt D, Benike C, Breen JK,
Strang G, Ruegg CL and Engleman EG: Dendritic cell-based
xenoantigen vaccination for prostate cancer immunotherapy. J
Immunol. 167:7150–7156. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Roos AK, Pavlenko M, Charo J, Egevad L and
Pisa P: Induction of PSA-specific CTLs and anti-tumor immunity by a
genetic prostate cancer vaccine. Prostate. 15:217–223. 2005.
View Article : Google Scholar
|
|
4
|
Saha A, Chatterjee SK, Mohanty K, Foon KA
and Bhattacharya-Chatterjee M: Dendritic cell based vaccines for
immunotherapy of cancer. Cancer Ther. 1:299–314. 2003.
|
|
5
|
Matera L: The choice of the antigen in the
dendritic cell-based vaccine therapy for prostate cancer. Cancer
Treat Rev. 36:131–141. 2010. View Article : Google Scholar
|
|
6
|
Drake CG: Prostate cancer as a model for
tumour immunotherapy. Nat Rev Immunol. 10:580–593. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Watanabe M, Nasu Y and Kumon H:
Adenovirus-mediated REIC/Dkk-3 gene therapy: Development of an
autologous cancer vaccination therapy (Review). Oncol Lett.
7:595–601. 2014.PubMed/NCBI
|
|
8
|
Saif JM, Vadakekolathu J, Rane SS,
McDonald D, Ahmad M, Mathieu M, Pockley AG, Durrant L, Metheringham
R, Rees RC and McArdle SE: Novel prostate acid phosphatase-based
peptide vaccination strategy induces antigen-specific T-cell
responses and limits tumour growth in mice. Eur J Immunol.
44:994–1004. 2014. View Article : Google Scholar
|
|
9
|
Peshwa MV, Shi JD, Ruegg C, Laus R and van
Schooten WC: Induction of prostate tumor-specific CD8+
cytotoxic T-lymphocytes in vitro using antigen-presenting cells
pulsed with prostatic acid phosphatase peptide. Prostate.
36:129–138. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kantoff PW, Higano CS, Shore ND, Berger
ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims
RB, Xu Y, Frohlich MW and Schellhammer PF; IMPACT Study
Investigators. Sipuleucel-T immunotherapy for castration-resistant
prostate cancer. N Engl J Med. 363:411–422. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Di Lorenzo G, Ferro M and Buonerba C:
Sipuleucel-T (Provenge®) for castration-resistant
prostate cancer. BJU Int. 110:E99–E104. 2012. View Article : Google Scholar
|
|
12
|
McNeel DG, Dunphy EJ, Davies JG, Frye TP,
Johnson LE, Staab MJ, Horvath DL, Straus J, Alberti D, Marnocha R,
Liu G, Eickhoff JC and Wilding G: Safety and immunological efficacy
of a DNA vaccine encoding prostatic acid phosphatase in patients
with stage D0 prostate cancer. J Clin Oncol. 27:4047–4054. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Machlenkin A, Paz A, Bar Haim E,
Goldberger O, Finkel E, Tirosh B, Volovitz I, Vadai E, Lugassy G,
Cytron S, Lemonnier F, Tzehoval E and Eisenbach L: Human CTL
epitopes prostatic acid phosphatase-3 and six-transmembrane
epithelial antigen of prostate-3 as candidates for prostate cancer
immunotherapy. Cancer Res. 65:6435–6442. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Matsueda S, Takedatsu H, Yao A, Tanaka M,
Noguchi M, Itoh K and Harada M: Identification of peptide vaccine
candidates for prostate cancer patients with HLA-A3 supertype
alleles. Clin Cancer Res. 11:6933–6943. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Burch PA, Breen JK, Buckner JC, Gastineau
DA, Kaur JA, Laus RL, Padley DJ, Peshwa MV, Pitot HC, Richardson
RL, Smits BJ, Sopapan P, Strang G, Valone FH and Vuk-Pavlović S:
Priming tissue-specific cellular immunity in a phase I trial of
autologous dendritic cells for prostate cancer. Clin Cancer Res.
6:2175–2182. 2000.PubMed/NCBI
|
|
16
|
Small EJ, Fratesi P, Reese DM, Strang G,
Laus R, Peshwa MV and Valone FH: Immunotherapy of
hormone-refractory prostate cancer with antigen-loaded dendritic
cells. J Clin Oncol. 18:3894–3903. 2000.PubMed/NCBI
|
|
17
|
Zou GM and Tam YK: Cytokines in the
generation and maturation of dendritic cells: recent advances. Eur
Cytokine Netw. 13:186–199. 2002.PubMed/NCBI
|
|
18
|
Liao W, Lin JX and Leonard WJ: IL-2 family
cytokines: new insights into the complex roles of IL-2 as a broad
regulator of T helper cell differentiation. Curr Opin Immunol.
23:598–604. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Marçais A, Viel S, Grau M, Henry T, Marvel
J and Walzer T: Regulation of mouse NK cell development and
function by cytokines. Front Immunol. 4:4502013. View Article : Google Scholar :
|
|
20
|
Watanabe M, Sakaguchi M, Kinoshita R, Kaku
H, Ariyoshi Y, Ueki H, Tanimoto R, Ebara S, Ochiai K, Futami J, Li
SA, Huang P, Nasu Y, Huh NH and Kumon H: A novel gene expression
system strongly enhances the anticancer effects of a
REIC/Dkk-3-encoding adenoviral vector. Oncol Rep. 31:1089–1095.
2014.PubMed/NCBI
|
|
21
|
Sakaguchi M, Watanabe M, Kinoshita R, Kaku
H, Ueki H, Futami J, Murata H, Inoue Y, Li SA, Huang P, Putranto
EW, Ruma IM, Nasu Y, Kumon H and Huh NH: Dramatic increase in
expression of a transgene by insertion of promoters downstream of
the cargo gene. Mol Biotechnol. 56:621–630. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Watanabe M, Kashiwakura Y, Huang P, Ochiai
K, Futami J, Li SA, Takaoka M, Nasu Y, Sakaguchi M, Huh NH and
Kumon H: Immunological aspects of REIC/Dkk-3 in monocyte
differentiation and tumor regression. Int J Oncol. 34:657–663.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kitamura T, Tojo A, Kuwaki T, Chiba S,
Miyazono K, Urabe A and Takaku F: Identification and analysis of
human erythropoietin receptors on a factor-dependent cell line,
TF-1. Blood. 73:375–380. 1989.PubMed/NCBI
|
|
24
|
Klampfer L, Zhang J and Nimer SD: GM-CSF
rescues TF-1 cells from growth factor withdrawal-induced, but not
differentiation-induced apoptosis: the role of BCL-2 and MCL-1.
Cytokine. 11:849–855. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lu X, Park SH, Thompson TC and Lane DP:
ras-Induced hyperplasia occurs with mutation of p53, but activated
ras and myc together can induce carcinoma without p53 mutation.
Cell. 70:153–161. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen J, Watanabe M, Huang P, Sakaguchi M,
Ochiai K, Nasu Y, Ouchida M, Huh NH, Shimizu K, Kashiwakura Y, Kaku
H and Kumon H: REIC/Dkk-3 stable transfection reduces the malignant
phenotype of mouse prostate cancer RM9 cells. Int J Mol Med.
24:789–794. 2009.PubMed/NCBI
|
|
27
|
Charley B, Petit E, Leclerc C and Stefanos
S: Production of porcine interleukin-2 and its biological and
antigenic relationships with human interleukin-2. Immunol Lett.
10:121–126. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chazen GD, Pereira GM, LeGros G, Gillis S
and Shevach EM: Interleukin 7 is a T-cell growth factor. Proc Natl
Acad Sci USA. 86:5923–5927. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Barata JT, Silva A, Abecasis M, Carlesso
N, Cumano A and Cardoso AA: Molecular and functional evidence for
activity of murine IL-7 on human lymphocytes. Exp Hematol.
34:1133–1142. 2006.PubMed/NCBI
|
|
30
|
Kradin RL, Xia W, Pike M, Byers HR and
Pinto C: Interleukin-2 promotes the motility of dendritic cells and
their accumulation in lung and skin. Pathobiology. 64:180–186.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bykovskaja SN, Buffo MJ, Bunker M, Zhang
H, Majors A, Herbert M, Lokshin A, Levitt ML, Jaja A, Scalise D,
Kosiban D, Evans C, Marks S and Shogan J: Interleukin-2 induces
development of denditric cells from cord blood CD34+
cells. J Leukoc Biol. 63:620–630. 1998.PubMed/NCBI
|
|
32
|
Civallero M, Barni S, Nano R and Capelli
E: Dendritic cells and interleukin-2: cytochemical and
ultrastructural study. Histol Histopathol. 15:1077–1085.
2000.PubMed/NCBI
|
|
33
|
Lynch DH, Namen AE and Miller RE: In vivo
evaluation of the effects of interleukins 2, 4 and 7 on enhancing
the immunotherapeutic efficacy of anti-tumor cytotoxic T
lymphocytes. Eur J Immunol. 21:2977–2985. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mackensen A, Lindemann A and Mertelsmann
R: Immunostimulatory cytokines in somatic cells and gene therapy of
cancer. Cytokine Growth Factor Rev. 8:119–128. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sakaguchi M, Kataoka K, Abarzua F,
Tanimoto R, Watanabe M, Murata H, Than SS, Kurose K, Kashiwakura Y,
Ochiai K, Nasu Y, Kumon H and Huh NH: Overexpression of REIC/Dkk-3
in normal fibroblasts suppresses tumor growth via induction of
interleukin-7. J Biol Chem. 284:14236–14244. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Iwamoto H, Ojima T, Hayata K, Katsuda M,
Miyazawa M, Iida T, Nakamura M, Nakamori M, Iwahashi M and Yamaue
H: Antitumor immune response of dendritic cells (DCs) expressing
tumor-associated antigens derived from induced pluripotent stem
cells: in comparison to bone marrow-derived DCs. Int J Cancer.
134:332–341. 2014. View Article : Google Scholar
|