Introduction
Pancreatic cancer (PC) is the fourth leading cause
of cancer-associated mortality in the European Union, being the
only major cancer site for which no improvement in mortality rates
is predicted for both genders. By contrast, a slight increase in
mortality rates is expected (1).
Surgical resection is the only curative therapeutic
modality with a 5-year postoperative survival rate of ~10–15%.
However, only ~20% of patients are diagnosed with surgically
resectable PC, 40% of patients have metastatic disease and the
remaining 40% have locally advanced PC in the borderline resectable
or unresectable advanced PC form.
Therefore, PC poses a challenge to oncologists and,
in particular, controversy surrounds radiation oncology (RO) in the
management of PC. Advances in the field of RO have led to
improvements in imaging and targeting as well as radiation
treatment delivery. Although these advances have the potential to
increase tumour control and decrease toxicity, to the best of our
knowledge, randomized clinical evidence supporting their widespread
application does not exist and there is still a great variety in
patterns of practice in different regions worldwide.
Patterns-of-care studies, initially developed in the
US in the mid-1970s, are a reliable retrospective study design used
to establish the national practice for cancer patients during a
specific study period (2,3). Patterns-of-care surveys on PC were
conducted in Germany (4), Japan
(5,6) and the USA (7). However, to the best of our knowledge,
in Italy, no information is available on national RO approach to
pancreatic neoplasm.
In the present study, the Italian Society of
Radiation Oncology Gastrointestinal Cancer Study Group (AIRO-GI)
conducted a nationwide survey among radiation oncologists
investigating the clinical practice of PC diagnosis and treatment
in Italian university and community hospitals with the aim to
subsequently be, proactive in suggesting collaborative multicentre
trials. To the best of our knowledge, this is the first report to
establish the manner in which RT is used in the treatment of PC in
Italy.
Materials and methods
Survey design and questionnaire
The study questionnaire was designed by AIRO-GI
members in September 2013. The main goal of the questionnaire was
to enquire into the local standards for PC diagnosis and RT
treatment at the participating centres. Data selected and assessed
were from 2012.
The questionnaire, consisting of 40 items, was
grouped in four sessions: i) data of the participating physicians:
professional site (e.g., university or community hospital, private
practice), number and type of PC patients treated per year (2012),
work methods (presence or absence of multidisciplinary group),
indications for RT and applied techniques [3D conformal RT (3DCRT),
intensity-modulated RT (IMRT), image-guided RT (IGRT),
intraoperative RT (IORT), stereotactic RT (SBRT), brachytherapy
(BRT)]; ii) questions on the local standard diagnostic procedures
for PC and issues concerning the histological type; iii) queries
concerning the RT treatment standard for resectable (including
neoadjuvant and adjuvant therapy) and advanced PC, as well as dose
and fractionation details according to treatment modality; iv)
questions on RT technical aspect (immobilization systems, use of
contrast, gating systems, image fusion), and intention of the
individual centres to join multicentre trials. The multiple-choice
and open questions were part of the survey.
Participating physicians
The participating centres were not pre-selected.
From November 2013 to January 2014 the questionnaire was proposed
by the AIRO-GI to all 140 Directors of the Italian Radiation
Oncology Institutions as per AIRO website (www.radioterapiaitalia.it: update as per November
2013).
The study methodology was focused on the division of
the Italian territory in major geographic areas (North East, North
West, North Central, South Central, South and Islands) and the
identification for each of them of a radiation oncologist
responsible for soliciting and collecting questionnaires via
e-mail.
Statistical analysis
Returned questionnaires were collected centrally at
the Fondazione ʻGiovanni Paolo IIʼ-UCSC, Campobasso and data were
entered into an electronic database. The data processing in
collaboration with the Institute of Statistics of Aviano occurred
in the first six months of 2014. Study data were analyzed by SAS
statistical software (version 9.3; SAS Institute Inc., Cary, NC,
USA).
Results
Eighty questionnaires, accounting for 57% of the 140
Italian centers available on the AIRO website were completed and
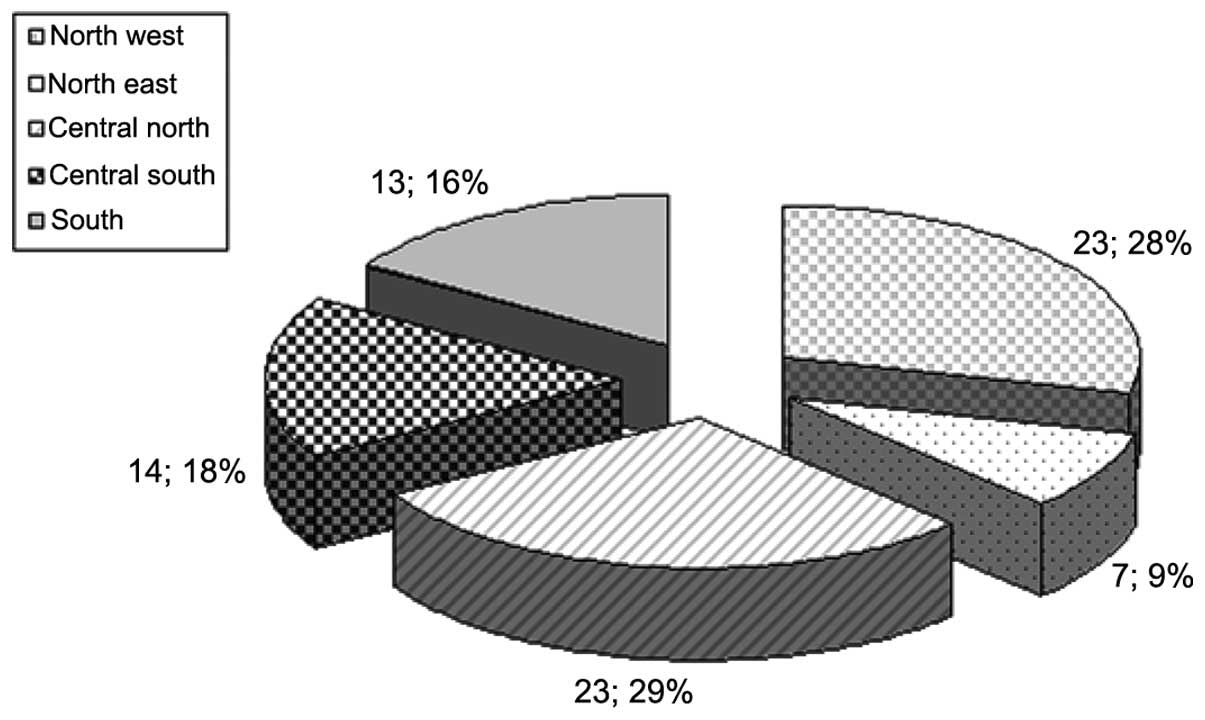
returned. The responding centres were evenly distributed in five
major areas identified with a predominance of the north-central
part and reflected the distribution of RT centres throughout the
country (Fig. 1).
Session 1
Most of the participating physicians came from
community hospitals (N=58), followed by private (N=12) and
university hospitals (N=10).
Only 5% of RT services did not treat PC patients,
due to the following reasons: i) patients were referred to a
reference centre for pancreatic neoplasm; ii) patients were
recorded into the Hospital (Surgery or Medical Oncology services)
but were not addressed to a radiation oncologist.
The majority (95%) of radiation oncologists
evaluated PC cases. The main causes of no RT indication were poor
general condition/co-morbidities (45%), lack of guidelines (25%),
inadequate timing (e.g., time elapsed from surgery) (10%),
excessive waiting list (4%), internal policy (4%), inadequacies of
the centre (e.g., lack of day hospital or inpatient treatment for
the management of acute complications) (4%), patient’s age (4%) and
prior systemic therapy (4%). The absolute figure of PC patients
treated in 2012 was 568, although this number did not reflect the
reality as only 57% of Italian centres replied to the
questionnaire, and of those only 69% of centres participating in
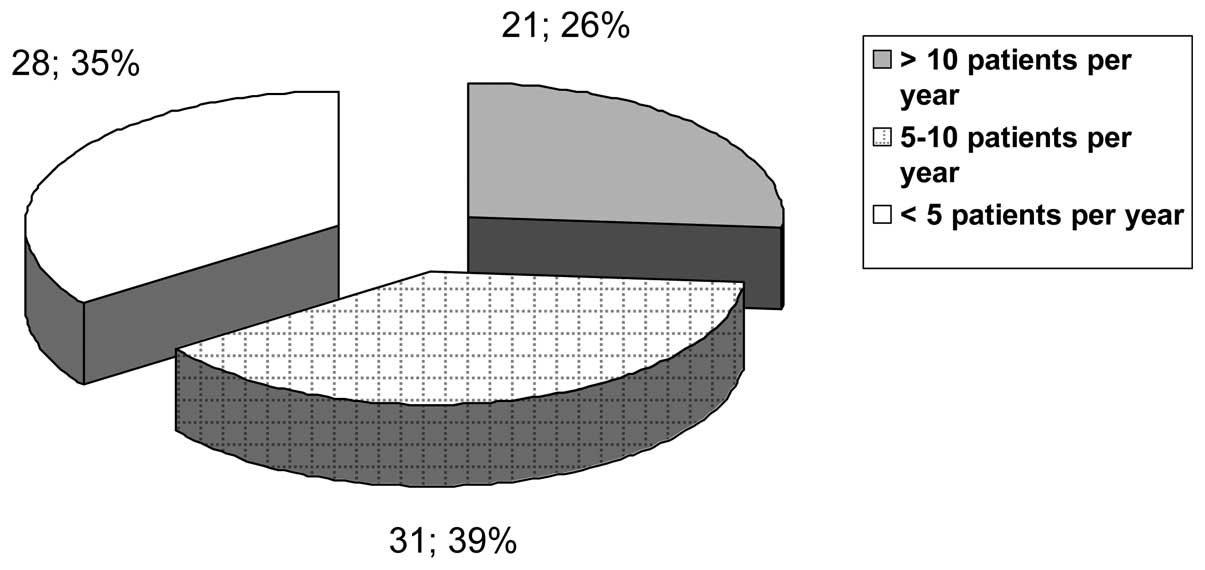
the survey provided the absolute number of irradiated patients.
Stratification in groups according to number of treated patients
per year (2012) is shown in Fig. 2.
A tumour board for PC was reported by 63% of participating centres,
including ‘always’ a surgeon, a radiation and a medical oncologist
(100% of cases), often a radiologist (80%) and an endoscopyst
(80%), sometimes a nuclear medicine physicist (36%), a pathologist
(40%), a physician nutrition specialist (32%) and a pain therapist
(26%). The tumour board meeting was periodic in approximately two
third of cases, while upon request in the the remaining third of
cases.
Radical pancreatectomy procedure was mostly
performed in the same centre in 59% of cases, while in other
neighbouring health facilities or in Italian referral centres in 23
and 18% of cases, respectively. IORT was performed exclusively in
six centres with <5 patients/year in 2012.
Session 2
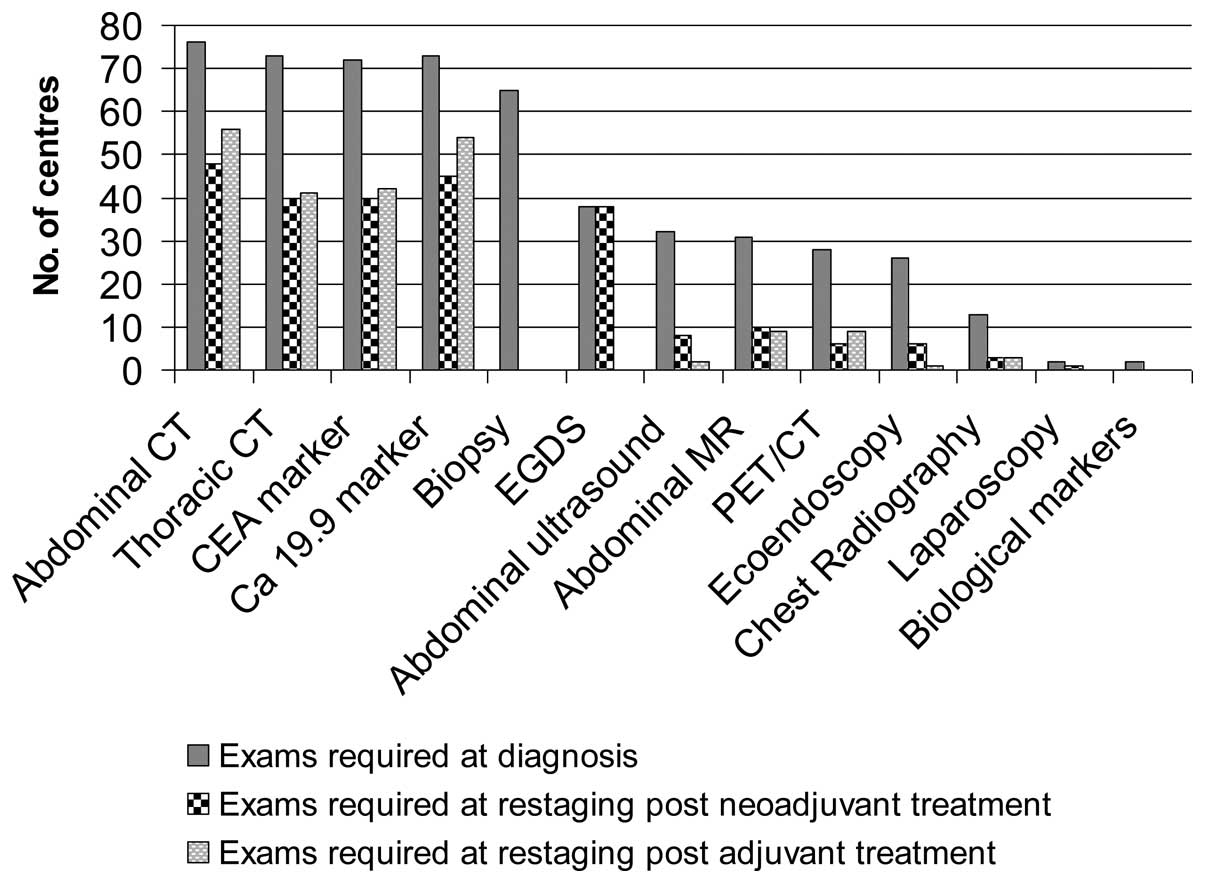
Fig. 3 shows the
diagnostic examinations as required by the different RO units for
PC staging and restaging following neoadjuvant or adjuvant
treatment. In particular, at the time of diagnosis, thoracic and
abdominal CT (91 and 95%, respectively) as well as biopsy and
marker determination (81 and 91%, respectively) were performed in
the majority of patients. Esophageal-gastroduodenoscopy (EGDS),
abdominal ultrasound, abdominal magnetic resonance (MR), PET/CT,
ultrasound endoscopy and laparoscopy (LPS) were considered less
mandatory and use of these techniques was restricted to selected
cases. Chest X-rays and biological markers for examination of
polymorphism were not deemed necessary by 79 and 94% of responding
centres, respectively. For the PC treatment, the majority of the
physicians (63%) needed histological confirmation, although were
prompt to perform the treatment only on clinical diagnosis
(imaging-markers-symptoms) after a single (63%) or at least two
(54%) biopsy attempts.
Session 3
This session investigated the core of RO approaches
in terms of indication criteria, dose, fractionation and
chemotherapy schedules. For convenience, this section was divided
into three parts while taking into account the three potential
approaches (neoadjuvant, adjuvant, exclusive and/or palliative) to
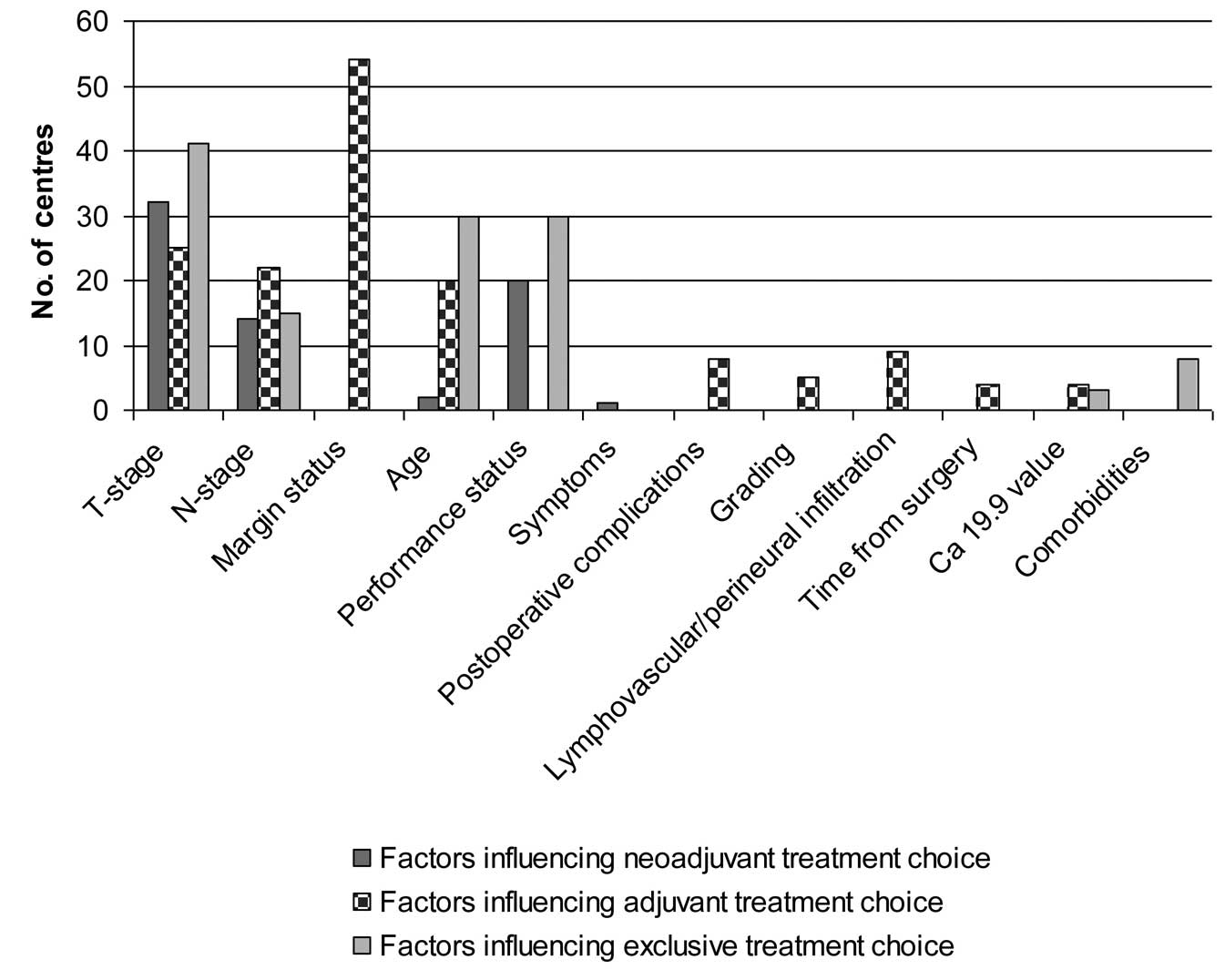
the PC from the point of view of the radiooncologist. Clinical
factors influencing the treatment approach for the three different
settings (neoadjuvant, adjuvant and exclusive) are detailed in
Fig. 4.
i) Neoadjuvant setting
This setting account for neoadjuvant RT (nRT),
neoadjuvant chemoradiation (nCT/RT) and induction chemotherapy
(iCT).
Thirty-eight percent of centres commonly stated the
use of nCT/RT, while only 7.5% in selected cases. Clinical factors
influencing the neoadjuvant choice are shown in Fig. 4. PC plus nodal drainage were the
contoured target volumes and 45–55 Gy was the range of prescribed
doses. Only eight RO services commonly used >50 Gy as a total
dose. Mostly, fractionation was conventional with 1.8 and 2 Gy in
70 and 22%, respectively. The drugs used for radiosensitizing were
gemcitabine (63.4%), 5-fuorouracil/capecitabine (26.8%) and others
(9.8%).
Induction chemotherapy was performed routinely in
38.7% of cases and again 7.5% RT units selected iCT in certain
cases. Results of the questionnaire suggested it was possible to
indicate>1iCT schedule per centre. The schedules most frequently
used were gemcitabine-oxaliplatin (45%), gemcitabine alone (32.5%)
or 5-fluorouracil-irinotecan-oxaliplatin (i.e., folfirinox) (12.5%)
mostly with 3 or 4 cycles prior to RT (range, 1–12).
The specialist prescribing and administering
chemotherapy drugs was the medical oncologist in 71% of cases and
the radiation oncologist in the remaining 29%.
During a period of 2–8 weeks from the completion of
neoadjuvant treatment about two third of centres (62.5%) performed
clinical instrumental restaging (Fig.
3), mostly after 4 or 6 weeks.
ii) Adjuvant setting
This setting accounted for adjuvant RT (aRT)
followed by chemotherapy, adjuvant chemoradiation (aCT/RT) and
adjuvant chemotherapy (aCT).
Sixty-two percent of centres declared the use of
aCT-RT or aRT followed or not by chemotherapy. The aRT was
prescribed to PC bed at doses ranging from 45 to 54 Gy in 91% of
cases. Doses >50 Gy were applied in 19 centres. Nodal drainage
in the same dose range was irradiated by 97% of responders. Almost
always conventional fractionation was applied. The drugs most
frequently used for radiosensitizing were gemcitabine (58%) or
5-fuorouracil/capecitabine (i.v. or per os) (40%), although (2%) a
two-drug schedule (gemcitabine plus oxaliplatin) was rarely used.
After aRT, 2-4 chemotherapy cycles were added and administered by
the majority of centres.
Adjuvant CT was the treatment declared by 47 (58.7%)
of the centres, while 15% of responders stated use of this
technique in selected cases and 26% of RT units did not perform it
in order not to compromise concomitant chemoradiation treatment or
due to patient performance status or the decision of the medical
oncologist.
The most frequently used drugs were gemcitabine
alone (58.6%), gemcitabine-oxaliplatin (20.7%) or
5-fluorouracil/capecitabine (13.8%) with a number of scheduled
cycles ranging from 1 and 10 before RT, although 2, 3 or 6 cycles
were the most performed.
Again, the specialist prescribing and administering
concomitant chemotherapy was the medical oncologist in 74% of cases
and the radiation oncologist in the remaining 18%. A minority of
centres (8%) did not reply to this question. In the period between
8 and 12 weeks from adjuvant treatment completion, 65% of centres
performed frst follow-up (Fig.
3).
iii) Exclusive and palliative
setting
This section account for exclusive radiation or
chemoradiation (eCT/RT), exclusive chemotherapy (eCT), and
palliative radiotherapy (pRT) for the treatment of locally advanced
PC unfit for surgery.
Forty-six (57.5%) and 10 (12.5%) of centres used
eCT/RT always or in selected cases, respectively. The dose
prescribed to PC ranged between 24 and 66 Gy in 10–34 fractions,
and 57 RO units performed nodal irradiation. The drugs most
frequently used as radio sensitizing were gemcitabine alone (56.3%)
or 5-fuorouracil/capecitabine (41.8%).
Forty-five (56.25%) and 8 (10%) of RO units declared
the use of eCT in unresectable patients always or in selected
cases, respectively. The schemes most frequently used were
gemcitabine-oxaliplatin (44.2%), gemcitabine alone (36.5%),
folfirinox (13.5%) or 5-fluorouracil/capecitabine (5.8%) with a
number of scheduled cycles ranging from 2 to 12, although 3, 4 or 6
were the more frequent numbers of cycles administered.
Forty percent of investigated centres declared the
use of pRT, being treated either loco-regional disease (tumour
and/or lymph nodes) as well metastases.
Session 4
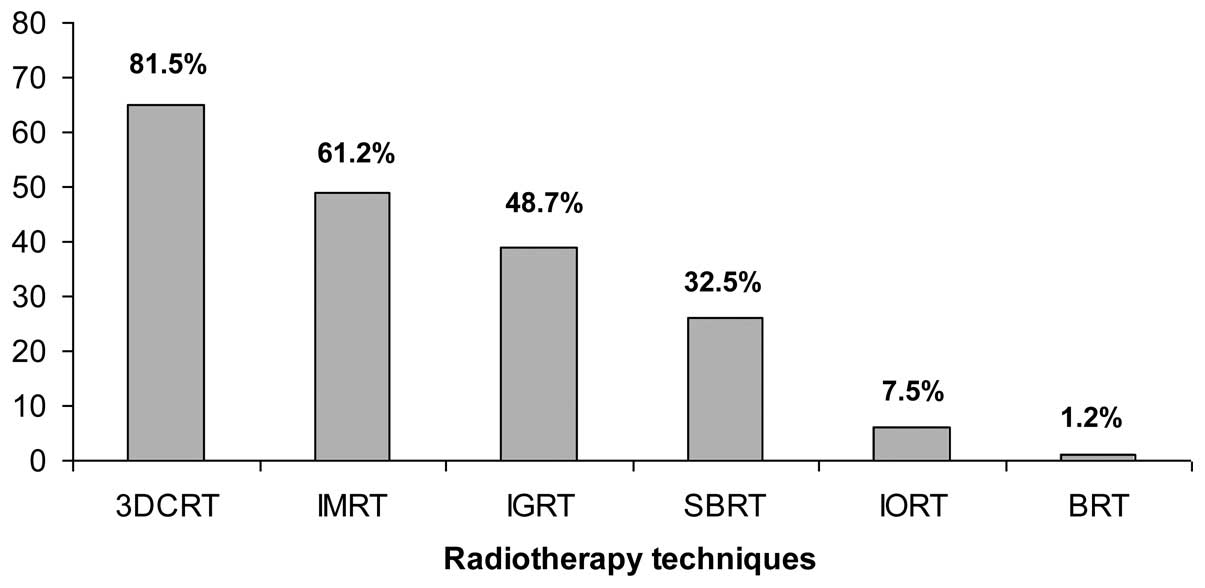
Treatment techniques for PC irradiation available at
censored centres are reported in Fig.
5. The vast majority (81.5%) had the possibility to use a
3D-conformal radiotherapy technique. Intensity-modulated
radiotherapy and image-guided radiotherapy were available in 61.2
and 48.7% of cases, respectively. SBRT was an emerging technique
and was applied in 32.5% of RO units.
Immobilization systems were used in 84% of centres,
while a set-up CT scan with intravenous contrast medium was used
(always or in selected cases) in 44% of cases against 56% not
employing it. Six RO units stated the routine use of gating systems
against 55 that did not use it, while 15 RO services used gating
when the clinical situation required it specifically. Forty-four
centres performed routine image fusion in the treatment planning
process, while 23 only when the clinical situation specifically
required it.
As regards the last question: ʻYour centre may be
willing to participate in a national study on treatment of PCʼ,
most clinicians answered ʻyes, of courseʼ (97%), while a small
number (3%) refused.
Discussion
To the best of our knowledge, few studies on PC
patterns of RT practice are available (4–7)
worldwide and no information is available regarding the Italian
reality.
In the present survey, we have focused on the
Italian PC radiotherapy practice in 2012, collecting data from 80
(57%) RO units distributed throughout the country. In comparison
with other RO surveys conducted in Italy in 2012 (lung cancer) and
2008 (breast and head/neck cancer), the number of responding
centres was encouraging, being 45, 48 and 50%, as reported by
Ramella et al (8), Aristei
et al (9) and Frata et
al (10), respectively. This
finding reveals an increased sense of membership of radiation
oncologists in the RO community and a willingness to deepen their
knowledge of the Italian reality in order to improve the quality of
their investigation.
The survey attempted to address important issues
such as: the main causes of non-indication to radiation treatment,
the actual number of patients treated by Italian RO, the preferred
or ʻmostly chosenʼ treatment setting and the most applied RT
techniques.
Concerning the main causes of non-indication to
radiation treatment, our results indicated that slightly less than
half of the patients (45%) were referred to RT services in poor
general condition or carrying severe co-morbidities. This finding
should be compared to previous intensive systemic treatments that
heavily impacted on patient performance status, and is a bit
discordant with the fact that 63% of the centres participating in
the survey reported a tumour board with periodic meeting.
A second cause was the perception of lack of
guidelines that was impacted by 25% of the non-indication to
radiation treatment. In fact, the clinical target volume contouring
proposal in preoperative/definitive and postoperative PC settings
was suggested (11,12). Moreover, AIRO-GI groups published a
handy, pocket-sized manual that summarizes the main
gastrointestinal guidelines [(13)
available on line at www.radioterapiaitalia.it/Linee guida AIRO)]. It is
likely, however, that the time between these publications and the
administration of the survey was not sufficient for their spreading
and validation.
The absolute figure of PC patients (N=568) treated
by RO services in 2012 was low when compared with 11,400 PC
esteemed cases in 2012 (14).
However, it is to be considered that ~40% of patients exhibited
metastatic disease at diagnosis and underwent chemotherapy or best
supportive care. Moreover, a potential bias may lie in the fact
that only 57% of the 140 Italian centres replied to the
questionnaire, and of these, only 69% of centres declared the
absolute number of irradiated patients. This figure reflects the
changing scenario of international guidelines and the results of
randomized trials that have raised questions regarding the optimal
treatment of PC. Nevertheless, radiation oncologists have
considerable room for improvement in terms of treatment strategies
and patterns of RT as well as in terms of combined efforts in
prospective studies investigating new combinations of chemotherapy
and/or biologic agents with RT.
The present study also showed the current treatment
of patients with PC in Italian hospitals. In a previously conducted
meta-analysis the efficacy of neoadjuvant chemoradiation in
patients with PC was investigated (15). The authors of that metaanalysis
concluded that for patients with resectable tumours, the current
data did not suggest an obvious advantage of neoadjuvant therapy.
By contrast, patients presenting locally advanced/unresectable
tumours were offered neoadjuvant therapy and then re-evaluated for
resection (15). In the Japanese
survey, there was no mention of a neoadjuvant approach, although
authors reported that 20.8% of patients were treated using an
investigational protocol (6). Our
data showed that ~38% of centres reported the use of nCT/RT or nCT
approach. These data are also higher than the German ones. Boeck
et al reported a 21% proportion of neoadjuvant treatment
considering the latter unjustified, outside of clinical trials, due
to the lack of evidence based rationale for this therapy (4). The discrepancy with international data
may be owing to different national guidelines: in Italy, AIOM
guidelines suggest that the evaluation of neoadjuvant approach for
borderline resectable PC patients (16) should be performed by a tumour board.
A second explanation for the relatively high percentage of
neoadjuvant treatments is selection bias. PC observed by RO was
pre-selected by surgeons and clinical oncologists as potential
candidates to irradiation, and this upstream selection justified
the high rate of nCT/RT subsequently performed. Furthermore, an
incorrect understanding of the so-called term ʻneoadjuvant
treatmentʼ including operable patients, border-line resectable and
locally advanced PC was considered a third possible
explanation.
Evidence suggests gemcitabine-oxaliplatin followed
by gemcitabine alone were the most employed nCT schedules,
administered mostly by 3–4 scheduled cycles (range, 1–12) prior to
RT (15). The drug most used for
radiosensitizing was gemcitabine (63.4%), followed by
5-fluorouracil/capecitabine (26.8%). Andriulli et al
(17) assessed the benefit of
neoadjuvant/preoperative gemcitabine chemotherapy used alone or in
combination with other agents and/or concurrent radiotherapy in
patients with localised PC. The results from that meta-analysis
provided marginal support regarding the benefits of
neoadjuvant/preoperative gemcitabine chemotherapy for patients with
localised PC, but indicated a potential advantage for only a
minority of those with unresectable lesions. Gemcitabine-based
neoadjuvant/preoperative therapies showed a promising rate of
tumour response, although at the expense of considerable toxicity
(17).
No definite standard has been established in the
adjuvant treatment of PC. The available data from randomized phase
III trials (ESPAC-1 and CONKO-001) indicate that adjuvant
chemotherapy may substantially prolong DFS and cause a moderate
increase of overall survival (18–21).
However, an optimal chemotherapy regimen remains to be defined.
Notably, in Italy as well as in Germany (4), gemcitabine chemotherapy [according to
the CONKO-001 trial (20,21)] was the preferred schema, while aCT
according to the ʻMayo regimenʼ [bolus 5-FU plus folinic acid,
ESPAC-1 study (19)] was selected
by few physicians. A reduction in toxicity was cited as the
explanation, based on the ESPAC-3 trial, whereas gemcitabine was
not superior to the Mayo regimen with respect to the primary
end-point of overall survival, although the authors reported a 50%
reduction of treatment-associated serious adverse events using
gemcitabine (22). In our survey
approximately two third of centres reported the use of aCT/RT or
aCT approach, with a slight predominance of aCT/RT that again could
be explained, considering that centres that adhere to the survey
are potentially the most active in the research field and/or the
aforementioned upstream patient selection.
Although the optimal RT dose has yet to be defined,
the NCCN guidelines have recommended a dose of 50–60 Gy and of
45–54 Gy at conventional fractionation for primary definitive
chemoradiation and postoperative RT, respectively (23). Moreover, large outcomes-based
analysis for patients treated with adjuvant radiotherapy in
resected PC showed that the optimal dose appears to decrease
between 50 and 55 Gy and patients treated within this range had the
longest median overall survival (24). In the present survey, most patients
were treated with a total dose of 50–54.9 Gy in the exclusive and
postoperative groups. Major deviations from these doses were
probably associated with the SBRT technique used in 26 centres.
Therefore, Italian radiation oncology centres as well as the
Japanese ones (6) appear to have
appropriatedly utilized the literature guideline recommendations.
Of note, no dose data have been reported by German authors
(4).
Concerning the RT volumes, in the majority of
patients, the primary neoplasm (or tumour bed) plus local drainage
were irradiated, irrespective of the treatment setting. Although
there is no consensus concerning the elective nodal irradiation
(ENI) in PC, it may be justified in a treatment with curative
intent due to the high frequency of lymphatic spread (60–80%)
reported in head PC (25–27) and the high rate of local and nodal
failure (up to 75%) (28,29). Therefore, NCCN practice guidelines
have recommended that when 5-FU-based chemoradiotherapy is used,
the treatment volumes should include the primary tumour location
and the regional lymph nodes (23).
Previous reports have indicated that the rate of severe toxicity is
greater in patients treated with gemcitabine-based
chemoradiotherapy than in those treated with 5-FU-based
chemoradiotherapy (17,30). Additional studies investigating the
optimal radiation field when using chemotherapy drugs, such as
gemcitabine, should be conducted. In particular, it is essential in
the Italian scenario whereas the drug most frequently used for
radiosensitizing was gemcitabine alone (58%) or in combination with
oxaliplatin (2%).
With regard to the RT treatment technique, computed
tomography-based treatment planning and 3DCRT were used for >80%
of patients, respectively. This finding is in accordance with the
NCCN guidelines (23) and reveal an
high quality level of RO procedures.
Moreover, 61% of PC may be treated by IMRT and 49%
by IGRT. These percentages are noteworthy and suggest that probably
PC patients are more likely to be irradiated in technology-advanced
structures as compared to less equipped hospitals. Notably, SBRT
with or without 3DCRT was frequently reported in Italy. A growing
body of literature has contributed to the spread of this latter
technique (31–34), which can improve local control by
increasing doses without impairing normal tissue. Additional
prospective studies are required to assess the efficacy of SBRT in
PC.
A main drawback of the present study was the number
of responding centres that, although higher than in previous
surveys (8–10), limited the analysis. Moreover, we
cannot deny heterogeneity in our analysis due to different
interpretations of survey queries.
In conclusion, the present study has shown that
extensive variation exists with regard to treatment strategies and
the patterns of RT. Nevertheless, a favourable attitude towards
cooperative studies emerged from the national survey that provided
evidence for improved PC treatment in Italy. In the future,
repeated surveys and point-by-point comparisons with the results
from other countries may demonstrate how RO for PC has been
developed and optimized in adhering to the international standard
of care.
Acknowledgments
We are grateful to Professor Massimo Falconi,
Professor of ʻVita-Salute San Raffaeleʼ University, Milano, for
helping with the drafting of the questionnaire and providential
suggestions. Moreover, we are particularly grateful to the 80
leading Italian institutions that kindly participated in the
survey. The order of these institutions are listed in alphabetic
order: 1. Paola Franzone Radioterapia ASO SSS Antonio e
Biagio-Alessandria; 2. Giovanna Mantellao, Radioterapia, State
Hospital, Ancona; 3. Fernanda Migliaccio, Radioterapia Oncologica,
Ospedale Regionale ʻU. Pariniʼ, Aosta; 4. Simona Borghesi,
Radioterapia, Az.USL 8 Arezzo; 5. Fasano Carla, Radioterapia,
Ospedale C. e G. Mazzoni, Ascoli Piceno; 6. Maria Tessa,
Radioterapia, Ospedale Cardinal Massaia, Asti; 7. Giovanni Boz,
Radioterapia, Centro di Riferimento Oncologico, Aviano; 8. Lisa
Paoletti, Radioterapia, Ospedale Santa Maria Annunziata, Bagno a
Ripoli (FI); 9. Marco Lioce, Radioterapia, Istituto Tumori
ʻGiovanni Paolo IIʼ, Bari; 10. Francesco Romeo Filippone,
Radioterapia, Osp. Papa Giovanni XXIII, Bergamo; 11. Gregorio Moro,
Radioterapia, Ospedale degli Infermi, Biella; 12. Renzo Mazzarotto,
Radioterapia, Policlinico S. Orsola Malpighi, Bologna; 13. Giovanni
Piero Frezza, Radioterapia, Ospedale Bellaria, Bologna; 14. Marco
Possanzini, Radioterapia, Ospedale Oncologico Regionale A. Businco,
Cagliari; 15. Vincenzo Picardi, Radioterapia, Fondazione ʻGiovanni
Paolo IIʼ, UCSC, Campobasso; 16. Angelo Tagliagambe, Radioterapia,
Ospedale Civico, Carrara; 17. Corrado Spatola, Radioterapia,
Policlinico ʻG. Rodolicoʼ, Catania; 18. Alfio Di Grazia,
Radioterapia, REM, Catania; 19. Domenico Genovesi, Radioterapia,
Ospedale ʻSS Annunziataʼ, Univ. ʻG. D’Annunzioʼ, Chieti; 20.
Francesca Corazzi, Radioterapia, Città di Castello; 21. Luciano
Scandolaro, Radioterapia, Ospedale ʻS. Annaʼ, San Fermo della
Battaglia (Como); 22. Pierpaolo Ziccarelli, Radioterapia, Ospedale
Mariano Santo, Cosenza; 23. Riccardo Vigna-Taglianti, Radioterapia,
A.S.O.S. Croce e Carle, Cuneo; 24. Franco Casamassima, Centro di
Radioterapia-Ecomedica, Empoli; 25. Giampaolo Zini, Radioterapia,
Arcispedale S. Anna, Ferrara; 26. Pierluigi Bonomo, Radioterapia,
Ospedale Careggi, Firenze; 27 Luca Cionini, Radioterapia, Centro
Oncologico Fiorentino, Firenze; 28. Lorenzo Livi, Radioterapia,
Casa di Cura S Chiara, Firenze; 29 Almalina Bacigalupo,
Radioterapia, AOU IRCCS San Martino, Istituto Nazionale per la
Ricerca sul Cancro, Genova; 30. Filippo Grillo Ruggieri,
Radioterapia, Ospedale Galliera Genova; 31. Piera Sciacero,
Radioterapia, ASL TO4 Ospedale Civile, Ivrea (TO); 32. Ernesto Di
Cesare Radioterapia, Ospedale S Salvatore, L’Aquila; 33. Giancarlo
Arcangeli, Radioterapia, Ospedale S. Maria Goretti, Latina; 34.
Donatella Russo, Radioterapia, Ospedale ʻV. Fazziʼ, Lecce; 35. Rita
Bagnoli, Radioterapia, Ospedale Campo Marte, Lucca; 36. Massimo
Giannini, Radioterapia, Ospedale Generale Provinciale, Macerata; 37
Antonino Romeo, Radioterapia, IRCCS-IRST Meldola (Forlì-Cesena);
38. Carmela Palazzolo, Radioterapia, AOOR Papardo-Piemonte di
Messina, Messina; 39. Mauro Palazzi, Radioterapia, Azienda
Ospedaliera Niguarda Ca Granda, Milano; 40. Rita Marina Niespolo,
Radioterapia, Ospedale S. Gerardo, Monza; 41. Radioterapia, Centro
Aktis Marano di Napoli, Napoli; 42. Paola Murino, Radioterapia,
Ospedale Ascalesi Napoli; 43. Milena Di Genesio Pagliuca,
Radioterapia, Ospedale Maggiore della Carità, Novara; 44. Luciana
Caravatta, Radioterapia, Ospedale S. Francesco, Nuoro; 45. Ornella
Lora, Radioterapia, Istituto Oncologico Veneto, Padova; 46. Teresa
Cucchiara, Radioterapia, Ospedale Civico, Palermo; 47. Giovanni
Battista Ivaldi, Radioterapia, Fondazione Salvatore Maugeri, Pavia;
48. Fabio Arcidiacono, Radioterapia, Ospedale S. Maria della
Misericordia, Perugia; 49. Francesca Maurizi, Radioterapia, A.O.
Ospedali Riuniti Marche Nord, Pesaro; 50. Aldo Sainato e Luna
Valentina Cernusco, Radioterapia, Azienda Ospedaliera Universitaria
Pisana, Pisa; 51. Marzano Salvino, Radioterapia, Azienda
Ospedaliera ASL 4, Prato; 52. Cinzia Iotti, Radioterapia,
Arcispedale di S.M. Nuova, Reggio Emilia; 53. Rossella Maglio,
Radioterapia, Ospedale S. Camillo De Lellis, Rieti; 54. Michele
Fiore, Radioterapia, Campus Biomedico, Roma; 55. Gian Carlo
Mattiucci, Radioterapia, Università Cattolica del Sacro Cuore,
Roma; 56. Maria Vittoria Leone, Radioterapia, Ospedale S Giovanni
Calibita-Fatebenefratelli, Roma; 57 Antonella Ciabattoni,
Radioterapia, Ospedale S. Filippo Neri, Roma; 58. Mattia F. Osti,
Radioterapia, Ospedale S. Andrea, Roma; 59. Pier Carlo Gentile,
Radioterapia, Ospedale Villa S. Pietro FBF, Roma; 60. Nadia
Bulzonetti, Radioterapia, Ospedale Umberto I, Roma; 61. Carlo
Capirci, Radioterapia, Ospedale Santa Maria della Misericordia,
Rovigo; 62. Salvatore Sandro Parisi, Radioterapia, Casa Sollievo
della Sofferenza, S Giovanni Rotondo; 63. Marco Orsatti,
Radioterapia, Ospedale Civile, Sanremo; 64. Corrado Marziano,
Radioterapia, Ospedale San Paolo, Savona; 65. Pieromaria Bianchi,
Radioterapia, Ospedale Santissima Trinità, Sora; 66. Tindaro
Scolaro, Radioterapia, Ospedale Felettino ASL5, La Spezia; 67.
Fabrizio Fusconi, Radioterapia, Ospedale Spoleto; 68. Giovanni
Silvano, Radioterapia, Ospedale S. Giuseppe Moscati, Taranto; 69.
Ernesto Maranzano, Radioterapia, Ospedale S. Maria, Terni; 70.
Elena Del Mastro, Radioterapia, IRCCS Candiolo, Torino; 71. Claudia
Airaldi, Radioterapia, Ospedale Mauriziano Umberto I°, Torino; 72.
Paolo Rovea, Radioterapia, Ospedale Molinette, Torino; 73. Maria
Grazia Ruo Redda, Radioterapia, Ospedale San Luigi Gonzaga,
Orbassano (TO); 74. Giuseppe Pani, Radioterapia, Ospedale Santa
Chiara, Trento; 75. Vittorio Baggio, Radioterapia, Ospedale S.
Maria di Ca’ Foncello, Treviso; 76. Sandro Fongione, Radioterapia,
Ospedale Santa Maria della Misericordia, Udine; 77. Paolo
Antognoni, Radioterapia, Ospedale di Circolo e Fond. Macchi Varese;
78. Maria Chiara Bassi, Radioterapia, Ospedale Castelli, Verbania;
79. Maria Silvia Favretto, Radioterapia, Ospedale San Bortolo,
Vicenza; 80. Maria Elena Rosetto, Radioterapia, Ospedale BelColle,
Viterbo. The authors would like to thank the two radiation therapy
technologists, Angela Palmieri and Laura Palumbo, for their
valuable support in collecting and entering data into the
database.
References
|
1
|
Malvezzi M, Bertuccio P, Levi F, La
Vecchia C and Negri E: European cancer mortality predictions for
the year 2013. Ann Oncol. 24:792–800. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hanks GE, Coia LR and Curry J: Patterns of
care studies: past, present, and future. Semin Radiat Oncol.
7:97–100. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Owen JB, Sedransk J and Pajak TF: National
averages for process and outcome in radiation oncology: methodology
of the patterns of care study. Semin Radiat Oncol. 7:101–107. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Boeck S, Bruns CJ, Sargent M, Schäfer C,
Seufferlein T, Jauch KW and Heinemann V: Current oncological
treatment of patients with pancreatic cancer in Germany: results
from a national survey on behalf of the Arbeitsgemeinschaft
Internistische Onkologie and the Chirurgische Arbeitsgemeinschaft
Onkologie of the Germany Cancer Society. Oncology. 77:40–48. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kato H, Usui M, Isaji S, Nagakawa T, Wada
K, Unno M, Nakao A, Miyakawa S and Ohta T: Clinical features and
treatment outcome of borderline resectable pancreatic head/body
cancer: a multi-institutional survey by the Japanese Society of
Pancreatic Surgery. J Hepatobiliary Pancreat Sci. Mar 14–2013.Epub
ahead of print. View Article : Google Scholar
|
|
6
|
Ogawa K, Ito Y, Karasawa K, Ogawa Y,
Onishi H, Kazumoto T, Shibuya K, Shibuya H, Okuno Y, Nishino S, Ogo
E, Uchida N, Karasawa K, Nemoto K and Nishimura Y: JROSG Working
Subgroup of Gastrointestinal Cancers: Patterns of radiotherapy
practice for pancreatic cancer in Japan: results of the Japanese
Radiation Oncology Study Group (JROSG) survey. Int J Radiat Oncol
Biol Phys. 77:743–750. 2010. View Article : Google Scholar
|
|
7
|
Wang Y, Schrag D, Brooks GA and Dominici
F: National trends in pancreatic cancer outcomes and pattern of
care among Medicare beneficiaries, 2000 through 2010. Cancer.
120:1050–1058. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ramella S, Maranzano E, Frata P, Mantovani
C, Lazzari G, Menichelli C, Navarria P, Pergolizzi S and Salvi F:
Radiotherapy in Italy for non-small cell lung cancer: patterns of
care survey. Tumori. 98:66–78. 2012.PubMed/NCBI
|
|
9
|
Aristei C, Amichetti M, Ciocca M, Nardone
L, Bertoni F and Vidali C; Italian Society of Radiation Oncology:
Radiotherapy in Italy after conservative treatment of early breast
cancer. A survey by the Italian Society of Radiation Oncology
(AIRO). Tumori. 94:333–334. 2008.PubMed/NCBI
|
|
10
|
Frata P, Ponticelli P, Cosentino D,
Buffoli A, Di Pilla A, Morrica B and Palazzi M: Radiotherapy
resources for the care of head and neck patients in Italy. A survey
by the head and neck group of the Italian Association for Radiation
Oncology (AIRO). Tumori. 94:59–64. 2008.PubMed/NCBI
|
|
11
|
Caravatta L, Sallustio G, Pacelli F,
Padula GD, Deodato F, Macchia G, Massaccesi M, Picardi V, Cilla S,
Marinelli A, Cellini N, Valentini V and Morganti AG: Clinical
target volume delineation including elective nodal irradiation in
preoperative and definitive radiotherapy of pancreatic cancer.
Radiat Oncol. 7:862012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Goodman KA, Regine WF, Dawson LA,
Ben-Josef E, Haustermans K, Bosch WR, Turian J and Abrams RA:
Radiation Therapy Oncology Group consensus panel guidelines for the
delineation of the clinical target volume in the postoperative
treatment of pancreatic head cancer. Int J Radiation Oncol Biol
Phys. 83:901–908. 2012. View Article : Google Scholar
|
|
13
|
Sainato A, Coppola M, Macchia G,
Bacigalupo A, Boz G, Sciacero P and Caravatta L: Pancreas -
Capitolo 3. La Radioterapia dei Tumori Gastrointestinali -
Indicazioni e Criteri Guida. Genovesi D, De Paoli A, Macchia G,
Sainato A, Lupattelli M, Osti MF, Friso ML, Gambacorta MA, Valvo F,
Mantello G, Niespolo R, Dionisi F and Genovesi D: referenti per
patologia. Associazione Italiana di Radioterapia Oncologica; pp.
69–118. 2012
|
|
14
|
AIRTUM working group: I numeri del cancro
in Italia 2012. Pancreas esocrino. 88–91. 2012.
|
|
15
|
Gillen S, Schuster T, Meyer Zum
Büschenfelde C, Friess H and Kleeff J: Preoperative/neoadjuvant
therapy in pancreatic cancer: a systematic review and meta-analysis
of response and resection percentages. PLoS Med. 7:e10002672010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Colucci G, Di Costanzo F, Falconi M,
Trodella L, Reni M and Scarpa A: AIOM linee guida, carcinoma del
pancreas esocrino. 2013, http://www.aiom.ithttps://www.aiom.it. Last access
June 20, 2014.
|
|
17
|
Andriulli A, Festa V, Botteri E, Valvano
MR, Koch M, Bassi C, Maisonneuve P and Sebastiano PD:
Neoadjuvant/preoperative gemcitabine for patients with localized
pancreatic cancer: a meta-analysis of prospective studies. Ann Surg
Oncol. 19:1644–1662. 2012. View Article : Google Scholar
|
|
18
|
Heinemann V and Boeck S: Perioperative
management of pancreatic cancer. Ann Oncol. 19(Suppl 7):
vii273–vii278. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Neoptolemos JP, Stocken DD, Friess H,
Bassi C, Dunn JA, Hickey H, Beger H, Fernandez-Cruz L, Dervenis C,
Lacaine F, Falconi M, Pederzoli P, Pap A, Spooner D, Kerr DJ and
Büchler MW; European Study Group for Pancreatic Cancer: A
randomized trial of chemoradiotherapy and chemotherapy after
resection of pancreatic cancer. N Engl J Med. 350:1200–1210. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Oettle H, Post S, Neuhaus P, Gellert K,
Langrehr J, Ridwelski K, Schramm H, Fahlk J, Zuelke C, Burkart C,
Gutberlet K, Kettner E, Schmalenberg H, Weigang-Koehler K,
Bechstein WO, Niedergethmann M, Schmidt-Wolf I, Roll L, Doerken S
and Riess H: Adjuvant chemotherapy with gemcitabine vs observation
in patients undergoing curative-intent resection of pancreatic
cancer: a randomized controlled trial. JAMA. 297:267–277. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Neuhaus P, Riess H, Post S, Gellert K,
Ridwelski K, Schramm H, Zuelke C, Fahlke J, Langrehr J and Oettle
H: CONKO-001: final results of the randomized, prospective,
multicenter phase III trial of adjuvant chemotherapy with
gemcitabine vs. observation in patients with resected pancreatic
cancer (PC). J Clin Oncol. 26(Suppl): abs. LBA4504. 2008.
|
|
22
|
Neoptolemos JP, Stocken DD, Bassi C,
Ghaneh P, Cunningham D, Goldstein D, Padbury R, Moore MJ, Gallinger
S, Mariette C, Wente MN, Izbicki JR, Friess H, Lerch MM, Dervenis
C, Oláh A, Butturini G, Doi R, Lind PA, Smith D, Valle JW, Palmer
DH, Buckels JA, Thompson J, McKay CJ, Rawcliffe CL and Büchler MW;
European Study Group for Pancreatic Cancer: Adjuvant chemotherapy
with fuorouracil plus folinic acid vs gemcitabine following
pancreatic cancer resection: a randomized controlled trial. JAMA.
304:1073–1081. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pancreatic Adenocarcinoma. v. 2: 2011,
National Comprehensive Cancer Network Practice Guidelines.
http://www.nccn.orghttps://www.nccn.org. Last access
June 20, 2014.
|
|
24
|
Hall WA, Colbert LE, Liu Y, Gillespie T,
Lipscomb J, Hardy C, Kooby DA, Prabhu RS, Kauh J and Landry JC: The
influence of adjuvant radiotherapy dose on overall survival in
patients with resected pancreatic adenocarcinoma. Cancer.
119:2350–2357. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sohn TA, Yeo CJ, Cameron JL, Koniaris L,
Kaushal S, Abrams RA, Sauter PK, Coleman J, Hruban RH and Lillemoe
KD: Resected adenocarcinoma of the pancreas-616 patients: results,
outcomes, and prognostic indicators. J Gastrointest Surg.
4:567–579. 2000. View Article : Google Scholar
|
|
26
|
Yoshida T, Matsumoto T, Sasaki A, Shibata
K, Aramaki M and Kitano S: Outcome of paraaortic node-positive
pancreatic head and bile duct adenocarcinoma. Am J Surg.
187:736–740. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Brunner TB, Merkel S, Grabenbauer GG,
Meyer T, Baum U, Papadopoulos T, Sauer R and Hohenberger W:
Definition of elective lymphatic target volume in ductal carcinoma
of the pancreatic head based on histopathologic analysis. Int J
Radiat Oncol Biol Phys. 62:1021–1029. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kayahara M, Nagakawa T, Ueno K, Ohta T,
Takeda T and Miyazaki I: Lymphatic flow in carcinoma of the distal
bile duct based on a clinicopathologic study. Cancer. 72:2112–2117.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hishinuma S, Ogata Y, Tomikawa M, Ozawa I,
Hirabayashi K and Igarashi S: Patterns of recurrence after curative
resection of pancreatic cancer, based on autopsy findings. J
Gastrointest Surg. 10:511–518. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Crane CH, Abbruzzese JL, Evans DB, Wolff
RA, Ballo MT, Delclos M, Milas L, Mason K, Charnsangavej C, Pisters
PW, Lee JE, Lenzi R, Vauthey JN, Wong AB, Phan T, Nguyen Q and
Janjan NA: Is the therapeutic index better with gemcitabine-based
chemoradiation than with 5-fluorouracil-based chemoradiation in
locally advanced pancreatic cancer? Int J Radiat Oncol Biol Phys.
52:1293–1302. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chang DT, Schellenberg D, Shen J, Kim J,
Goodman KA, Fisher GA, Ford JM, Desser T, Quon A and Koong AC:
Stereotactic radiotherapy for unresectable adenocarcinoma of the
pancreas. Cancer. 115:665–672. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Koong AC, Christofferson E, Le QT, Goodman
KA, Ho A, Kuo T, Ford JM, Fisher GA, Greco R, Norton J and Yang GP:
Phase II study to assess the efficacy of conventionally
fractionated radiotherapy followed by a stereotactic radiosurgery
boost in patients with locally advanced pancreatic cancer. Int J
Radiat Oncol Biol Phys. 63:320–323. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rwigema JC, Parikh SD, Heron DE, Howell M,
Zeh H, Moser AJ, Bahary N, Quinn A and Burton SA: Stereotactic body
radiotherapy in the treatment of advanced adenocarcinoma of the
pancreas. Am J Clin Oncol. 34:63–69. 2011. View Article : Google Scholar
|
|
34
|
Macchia G, Morganti AG, Cilla S, Ippolito
E, Massaccesi M, Picardi V, Mattiucci GC, Bonomo P, Tambaro R,
Pacelli F, Piermattei A, De Spirito M, Valentini V, Cellini N and
Deodato F: Quality of life and toxicity of stereotactic
radiotherapy in pancreatic tumours: a case series. Cancer Invest.
30:149–155. 2012. View Article : Google Scholar : PubMed/NCBI
|