Introduction
Although advances have been made in decreasing the
mortality rates, the prognosis of lung cancer remains poor. With an
overall 5-year survival rate of <18%, the poor prognosis of lung
cancer is mainly due to lack of understanding of the mechanisms
involved in oncogenesis and drug resistance to chemotherapy
(1). Recent findings suggest that
cancer stem cells (CSCs) may be responsible for tumorigenesis and
contribute to resistance to chemotherapy (2,3). In
those studies, it was found that tumors are organized in
hierarchical heterogeneous cell populations, which constitute a
spectrum of phenotypically different cell types. Among these cell
subsets, CSCs have the ability to sustain tumor growth in a very
small fraction of tumor cells and possess the characteristics of
longevity, self-renewal, multilineage differentiation capacity and
tumorigenicity. In addition, CSCs express high levels of the
adenosine-triphosphate-binding cassette (ABC) transporter that is
responsible for pumping out chemotherapeutic drugs (4). Thus, identification of CSCs may be
useful to understand the mechanism of generation of lung cancer and
identify more effective therapeutic methods.
A useful method for the isolation of CSCs is
dependent on their ability to discharge lipophilic and fluorescent
dyes such as Hoechst 33342 by the ABC membrane transporter. Based
on the capacity to efflux Hoechst 33342 dye, side population (SP)
cells are considered to be enriched for CSCs in most types of human
tumors, such as glioma (5),
pancreatic cancer (6), renal cell
carcinoma (7), breast cancer
(8), and nasopharyngeal carcinoma
(9). Moreover, accumulating
evidence shows that SP cells were described to possess cancer stem
cell-like properties and may be a useful tool to identify cancer
stem cell populations (10,11). However, to the best of our
knowledge, few studies have examined the role of SP cells in lung
cancer.
Autophagy is a highly conserved catabolic process
among all eukaryotes involving the degradation of cellular
organelles and proteins. In a previous study, we found that
autophagy acted as a potential survival mechanism when the cancer
cells were treated with chemotherapy, and inhibition of autophagy
enhanced the lethal effect of cisplatin (12). In addition, recent evidence has
indicated that several types of tumor are dependent on autophagy
for growth under normal conditions (13). However, the exact role of autophagy
in the survival of SP cells of lung cancer to chemotherapy has yet
to be determined.
In the present study, we isolated SP cells from an
A549 lung cancer cell line using a fluorescence-activated cell
sorter (FACS). The cancer stem cell-like properties of SP cells
were verified through confocal fluorescence imaging, sphere
formation and cell proliferation and colony formation assays, and
gene expression in vitro and tumor formation in vivo.
We also investigated whether autophagy is involved in the survival
of SP cells in lung cancer cell lines to cisplatin.
Materials and methods
Cell culture
A549 lung cancer cells were obtained from the Key
Laboratory of The First Affiliated Hospital of Zhengzhou
University, and were cultured in RPMI-1640 supplemented with 100
ml/l fetal bovine serum (FBS) and 100 U/ml penicillin/streptomycin
at 37°C in a humidified atmosphere of 95% air and 5%
CO2.
FACS analysis and purification of SP
cells
A549 cells were eluted with 2.5 g/l trypsin and 0.5
g/l ethylenediaminetetraacetic acid, centrifuged, washed and
resuspended at 1×106 cells/ml in pre-warmed RPMi-1640
containing 2% FBS. The cells were then incubated for 120 min at
37°C with 5 µg/ml Hoechst 33342 alone or in combination with
50 µg/ml verapamil which inhibited ABC transporters. After
the incubation process, the cells were washed in phosphate-buffered
saline (PBS), centrifuged at 4°C and resuspended in PBS solution
supplemented with 2% FBS and 1 mM HEPES. The cells were filtered
through a 40 µm mesh filter and reserved at 4°C for flow
cytometry analysis. The SP cells were selected and sorted by FACS
(BD Biosciences, San Jose, CA, USA). The Hoechst dye was excited
with a UV laser at 346 nm and its fluorescence was measured with
630/22 (Hoechst 33342 Red) and 424/44 filters (Hoechst Blue).
Confocal fluorescence imaging
A549 cells were washed, resuspended and incubated
with 5 µg/ml Hoechst 33342 in the dark at 37°C for 30 min.
The cells were washed again in PBS three times, and 400 µl
PBS was added. Confocal microscopy was used to capture the images
of SP cells.
Sphere formation assays
SP cells were seeded at a density of
1×102 cells/well in 6-well plates. The cells were
cultured in Dulbecco’s modified eagle’s medium (DMEM)/F12 medium
containing 10 mg/l insulin, 20 µg/l epidermal growth factor
(EGF) and 10 µg/l basic fibroblast growth factor (bFGF).
Insulin, EGF and bFGF were added every 3 days. After culture for
10–14 days, the formation of floating spheres was visually assayed.
The spheres containing >30 cells were selected, and then
trypsinized to gain the single cells. After suspension of these
cells, the cells were added into 6-well plates at a density of
1×102 cells/well. The secondary sphere formation was
assayed after culture for 10–14 days. The number of spheres was
counted under the dissecting microscope.
Cell proliferation assay
SP and non-SP cells were counted and added into
96-well plates at a density of 104 cells/well. The cells
were cultured in RPMI-1640 supplemented with 100 ml/l FBS for 5
days. A Cell Counting kit-8 (CCK-8) analysis was used to detect and
compare the growth of these cells each day. The growth curve was
plotted according to the absorbance of each well.
Colony formation assay
SP and non-SP cells were cultured in RPMi-1640
supplemented with 100 ml/l FBS at a density of 1×102
cells/well for 10–14 days. The number of clones that contained
>50 cells were counted and recorded.
Gene expression
Total RNA was extracted from SP and non-SP cells
separately using TRIzol reagent according to the manufacturer’s
instructions. RNA was reverse-transcribed into cDNA using the
SuperScrit First-Strand Synthesis system (Invitrogen, Carlsbad, CA,
USA) as described in instructions. The RT-PCR was carried out using
the SuperScript One-Step kit (Invitrogen). The primers used were:
MDR1, 5′-CCCATCATTGCAATAGCAGG-3′ and 5′-GTTCAAACTTCTGCTCCTGA-5′ for
a 157-bp fragment; ABCG2, 5′-CTGAGATCCTGAGCCTTTGG-3′ and
5′-TGCCCATCACAACATCATCT-3′ for a 380-bp fragment; OCT4,
5′-CTGTAACCGGCGCCAGAA-3′ and 5′-TGCATGGGAGAGCCCAGA-3′ for a 218-bp
fragment; GAPDH, 5′-ACCACAGTCCATGCCATCAC-3′ and
5′-TCCACCACCCTGTTGCTGTA-3′ for a 249-bp fragment. The PCR products
were separated by electrophoresis in 2% agarose gel.
Tumor formation
SP and non-SP cells were sorted and resuspended at a
density ranging from 104 to 103 cells. The
cells were mixed with 50 µl Matrigel to prevent cell
dispersion and loss. The cells were subcutaneously injected into 6-
to 7-week-old nude mice, obtained from the Experimental Animal
Center of Zhengzhou University, China. The mice were monitored to
assess tumor formation for 8 weeks. The experiments were approved
by the Laboratory Animal Ethics Committee of Zhengzhou
University.
Autophagy expression
Cells were stained with 0.05 mol/l of
monodansylcadaverine (MDC) solution for 15 min and observed using a
confocal laser microscope to detect the autophagolysosomes. The
cells were collected and lysed in RIPA buffer containing protease
inhibitor and phosphatase inhibitor. Lysated proteins were
centrifuged at 14,000 rpm for 10 min and quantified. Proteins were
separated by a 4–20% gradient SDS/PAGE gel and transferred to
polyvinylidene fluoride (PVDF) membranes. The membrane was placed
in TBST solution containing 5% non-fat skim milk at room
temperature for 1 h. The membranes were incubated with diluted
primary antibodies overnight at 4°C and washed with TBST three
times for 10 min. Subsequently, the membrane was reacted with
secondary antibodies in 2.5% non-fat skim milk for 1 h at room
temperature, followed by washing with TBST three times for 10 min.
Blots were stripped and re-blotted with anti-β-actin, and the
membrane was examined for the band.
Apoptosis assay
SP cells were treated with the chemotherapeutic drug
cisplatin for 24 h and then incubated with or without
3-methyladenine (3-MA), which is an inhibitor of autophagy.
Autophagy expression in cells treated with chemotherapeutic agent
was measured as mentioned above. The level of apoptosis of SP cells
with or without 3-MA was determined by flow cytometric analysis.
The cells were then incubated in 5 ml binding buffer containing 5
ml Annexin V and 5 ml propidium iodide. The cells were gently
vortexed and assayed with an Annexin V-FITC apoptosis detection kit
according to the manufacturer’s instructions.
Statistical analysis
Data were presented as the mean ± SD. Statistical
significance (P<0.05) was evaluated by the Student’s t-test.
Results
Identification of SP cells in lung
cancer
SP cells were separated from A549 lung cancer cells
by their fluorescence profiles in a dual wavelength analysis by
FACS. The characteristic tails isolated from the complete
population were identified.
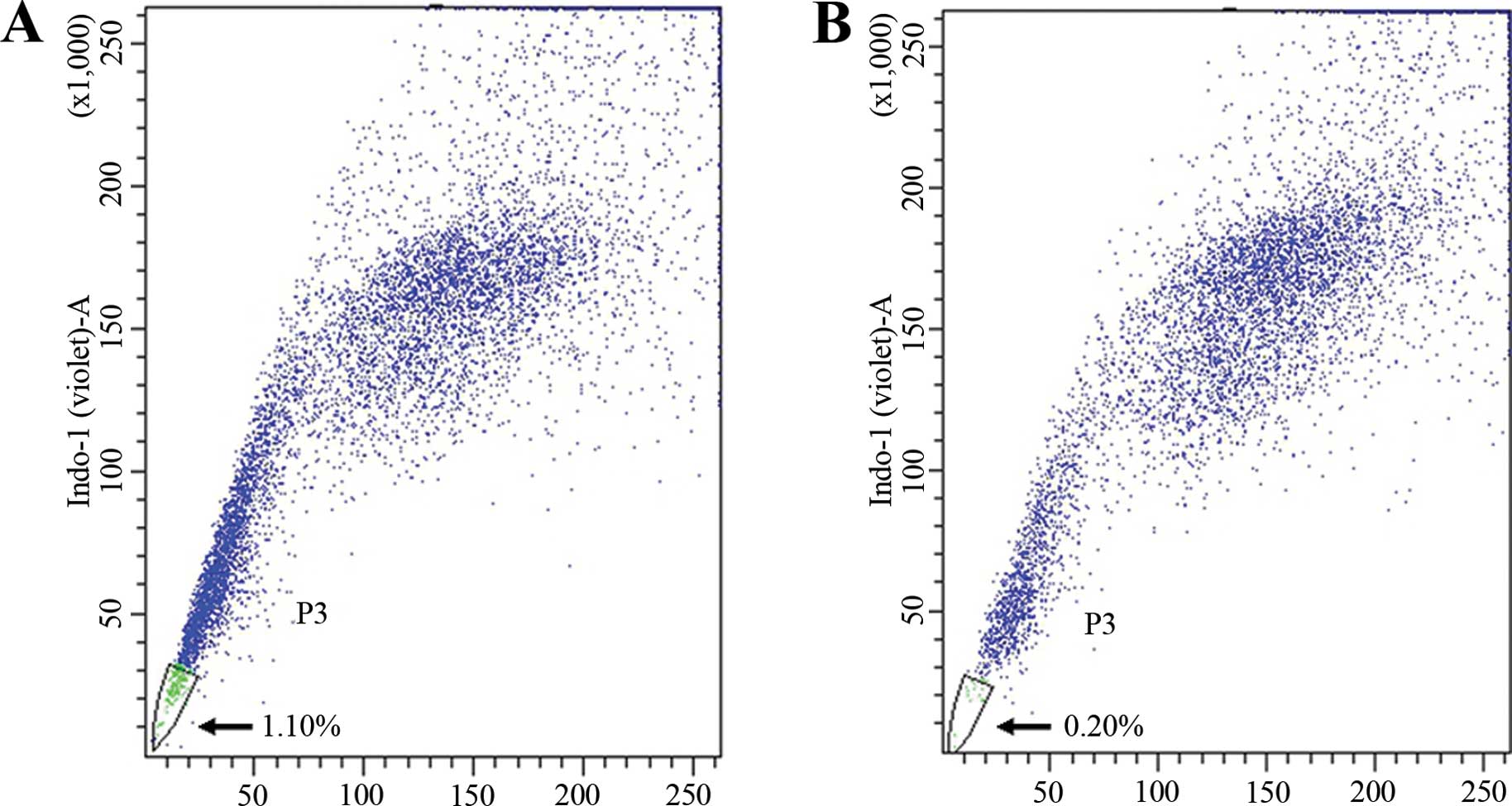
The percentage of SP cells analyzed by FACS was
1.10% (Fig. 1A). After
preincubation with verapamil which inhibited ABC transporters for
90 min, the percentage of SP cells decreased to 0.20% (Fig. 1B). This result showed that Hoechst
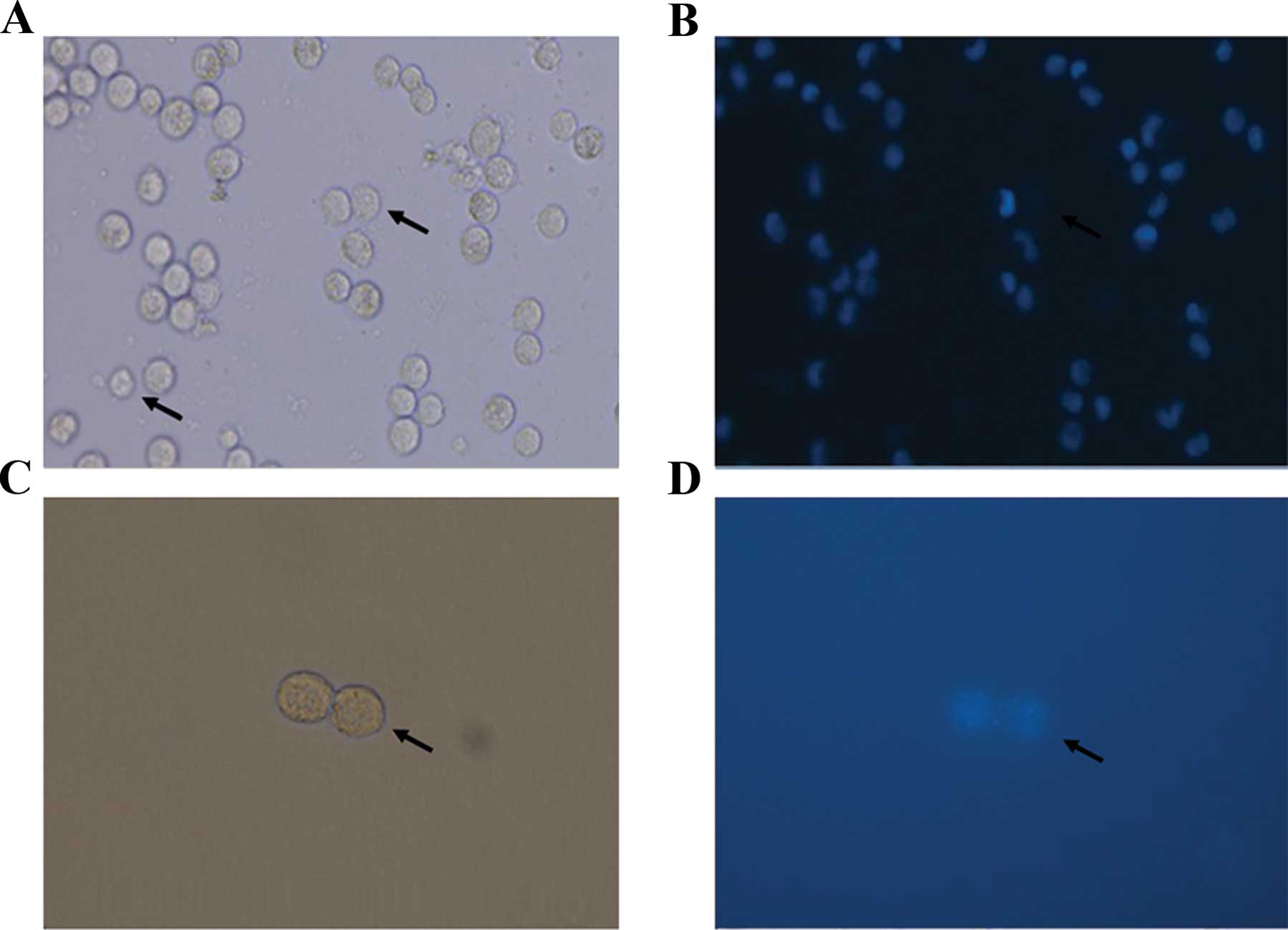
33342 exclusion was verapamil-sensitive. The images of SP cells
were captured by confocal microscopy (Fig. 2).
Sphere formation
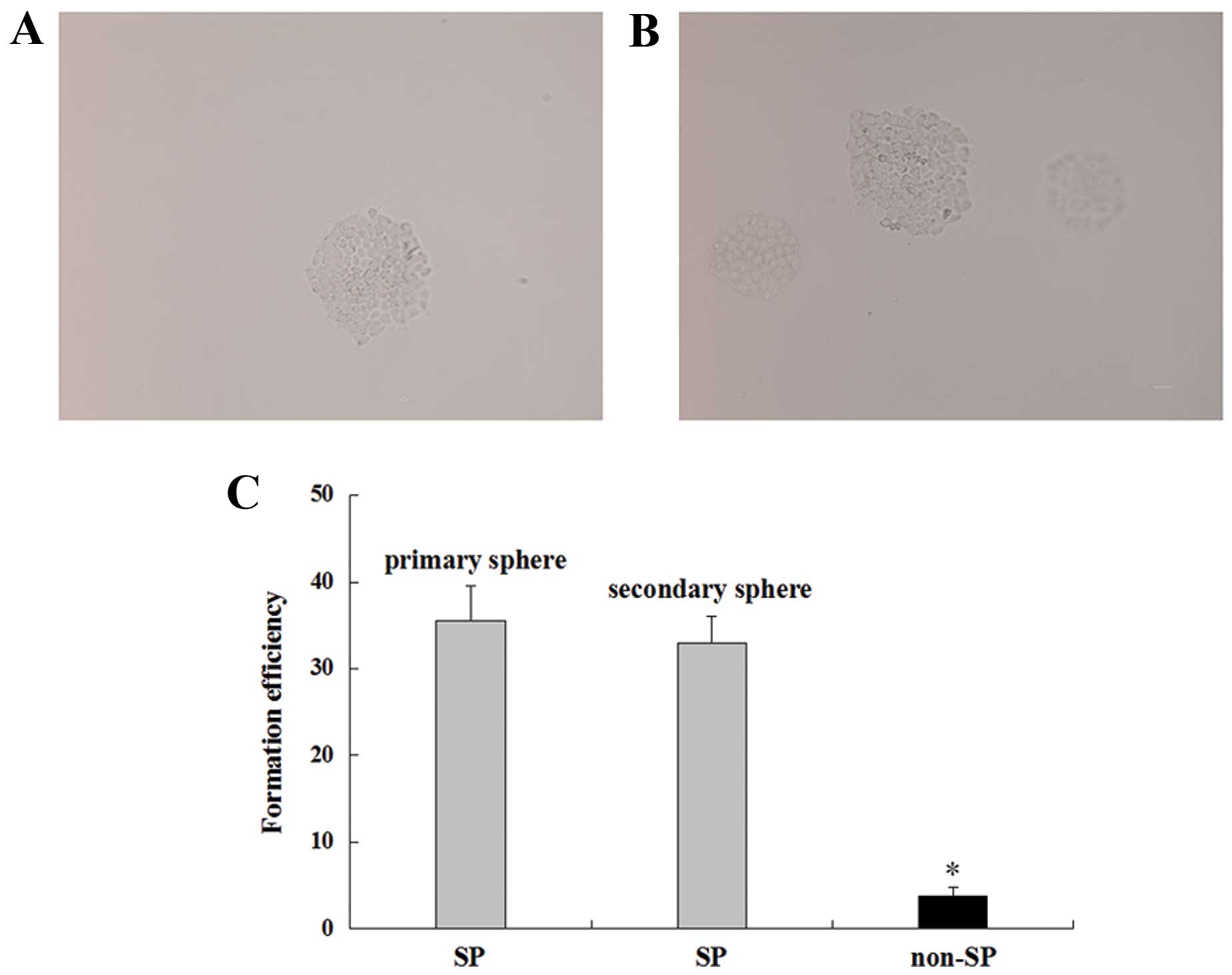
The floating spheres of SP cells were observed after
3–4 days of seeding, and the primary spheres containing >30
cells were observed after 10–14 days (Fig. 3A and B). The amount and formation
speed of the secondary sphere were similar to those of the primary
sphere (Fig. 3C), suggesting that
SP cells have self-renewal ability. However, non-SP cells could not
be propagated under the same conditions.
Cell proliferation and colony
formation
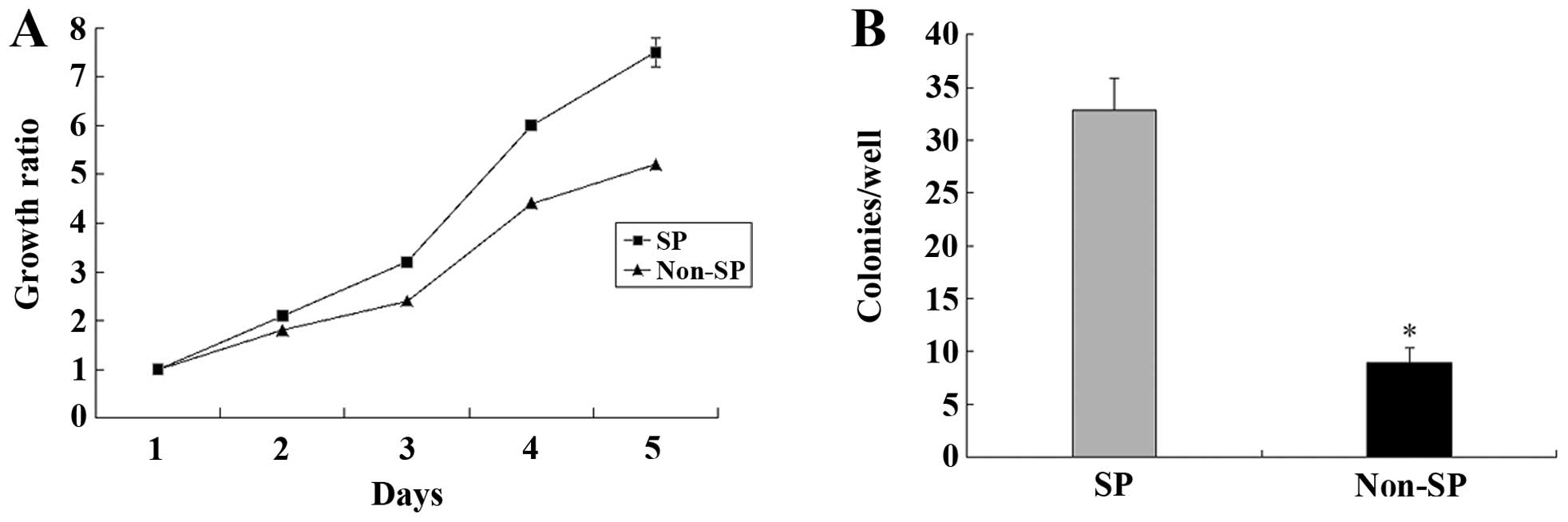
We performed CCK-8 analysis to compare the cell
proliferation of SP with non-SP cells. A significant difference in
cell proliferation between SP and non-SP cells was observed after 5
days of culture (P<0.05) (Fig.
4A). In addition, in the colony formation assay, there was a
statistically significant difference between SP and non-SP cells
over 10–14 days (P<0.05) (Fig.
4B).
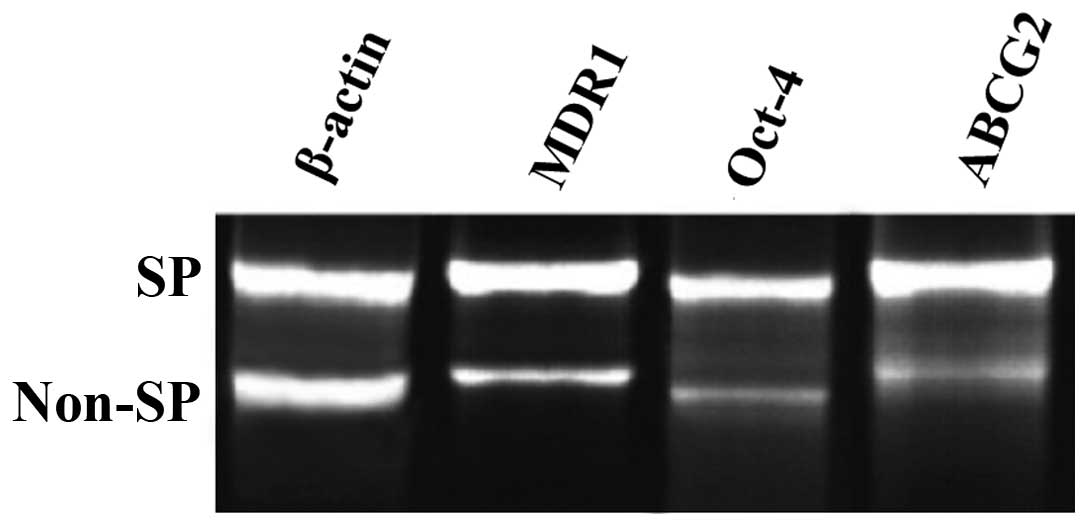
Expression of stem cell genes
MDR1 and ABCG2, which belong to ABC transporters,
contribute to the chemoresistance of SP cells. Compared to non-SP
cells, we found that the expression of MDR1 and ABCG2 in SP cells
was significantly upregulated. OCT-4, an embryonic stem cell
biomarker, was also highly expressed in SP compared with non-SP
cells. The results indicated that SP cells from A549 lung cancer
cells had some features of cancer stem cells (Fig. 5).
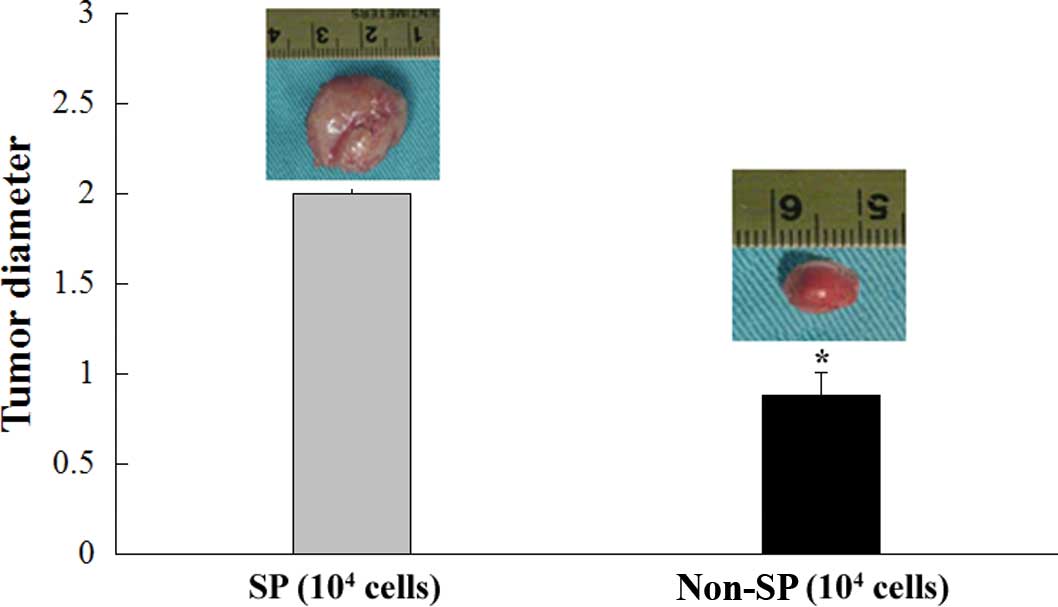
Tumor formation
To determine the tumorigenic potential of lung
cancer SP cells in vivo, SP and non-SP cells were
subcutaneously injected into 6- to 7-week-old nude mice. Each mouse
was injected with different density of SP and non-SP cells
individually. After 8 weeks, almost all of the mice injected with
SP cells at densities of 104–103 cells formed
tumors, whereas a smaller number of mice injected with non-SP cells
at a density of 104 cells formed tumors. Additionally,
the tumor diameters were apparently smaller than those in SP cells
with an equal cell number (Fig.
6).
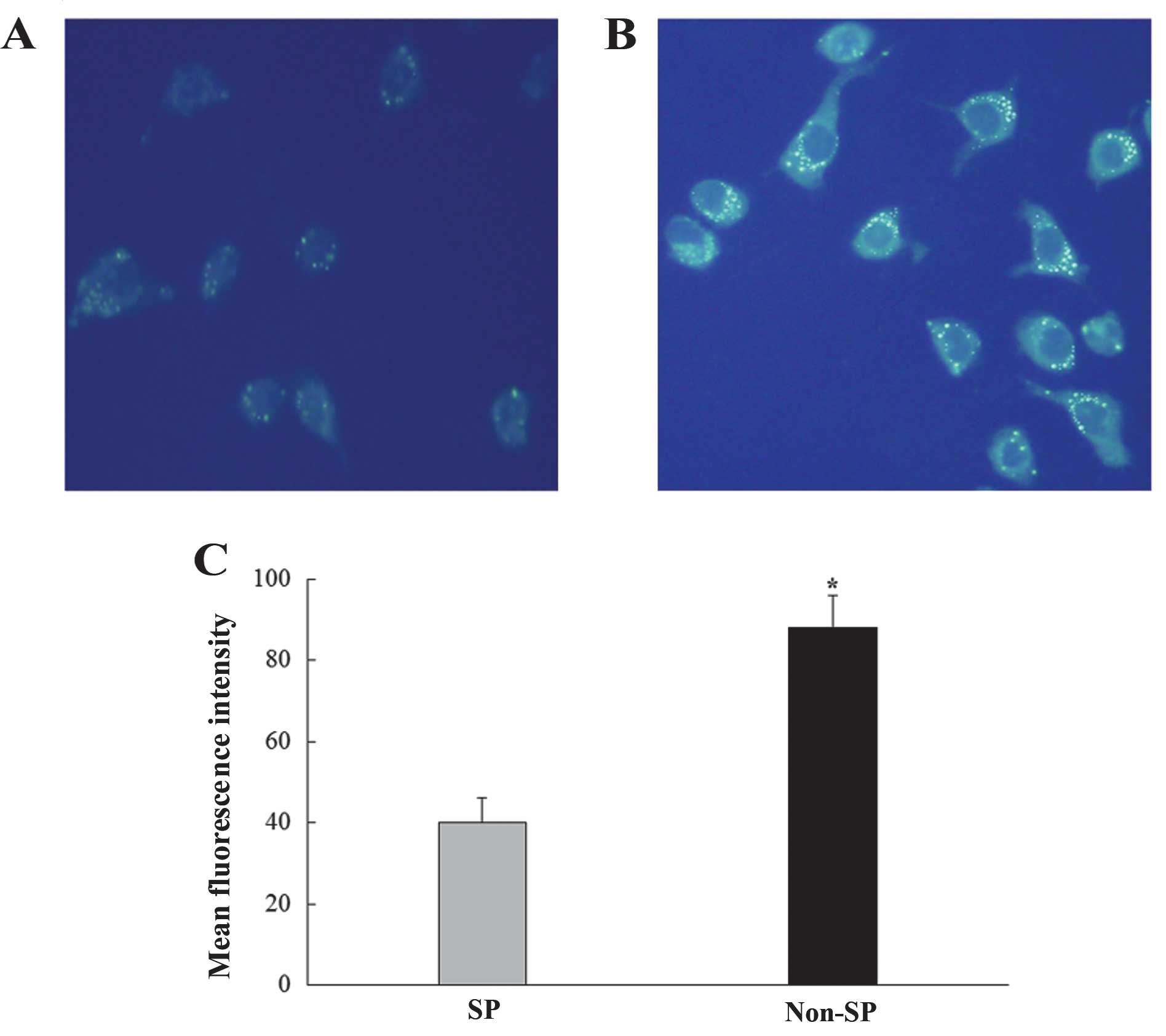
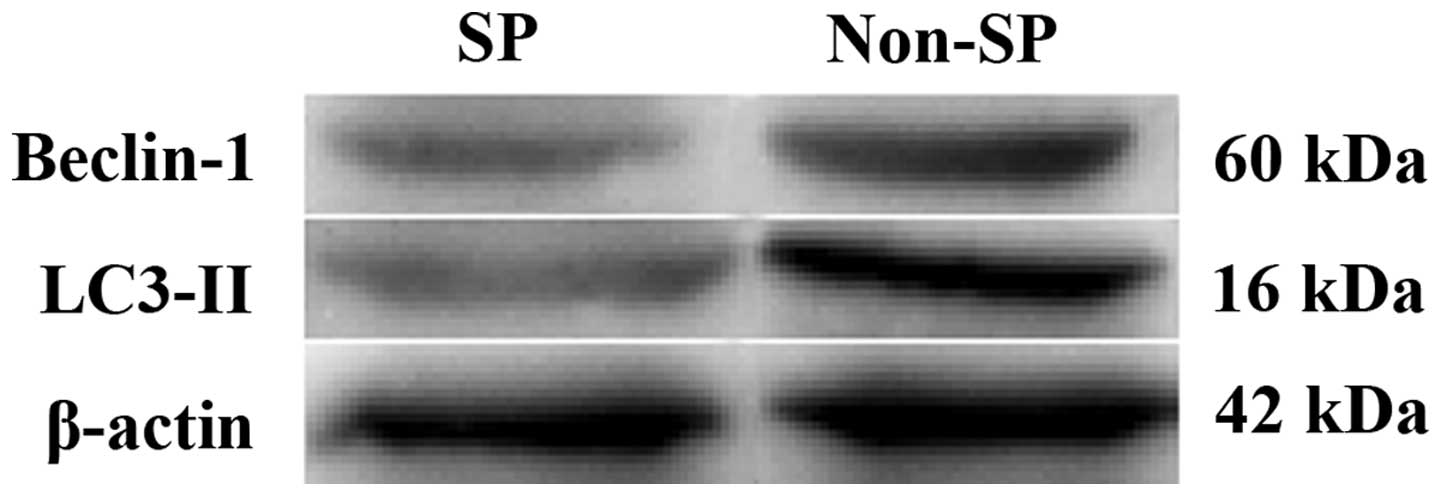
Autophagy expression in SP and non-SP
cells
MDC staining was performed to detect the
autophagolysosomes. We observed that the number of
autophagolysosomes in SP cells was less than that in non-SP cells
by confocal laser microscopy (Fig.
7). The expression of Beclin-1 and LC3-II, both of which were
involved in the autophagic process, was lower in SP cells than that
in non-SP cells (Fig. 8).
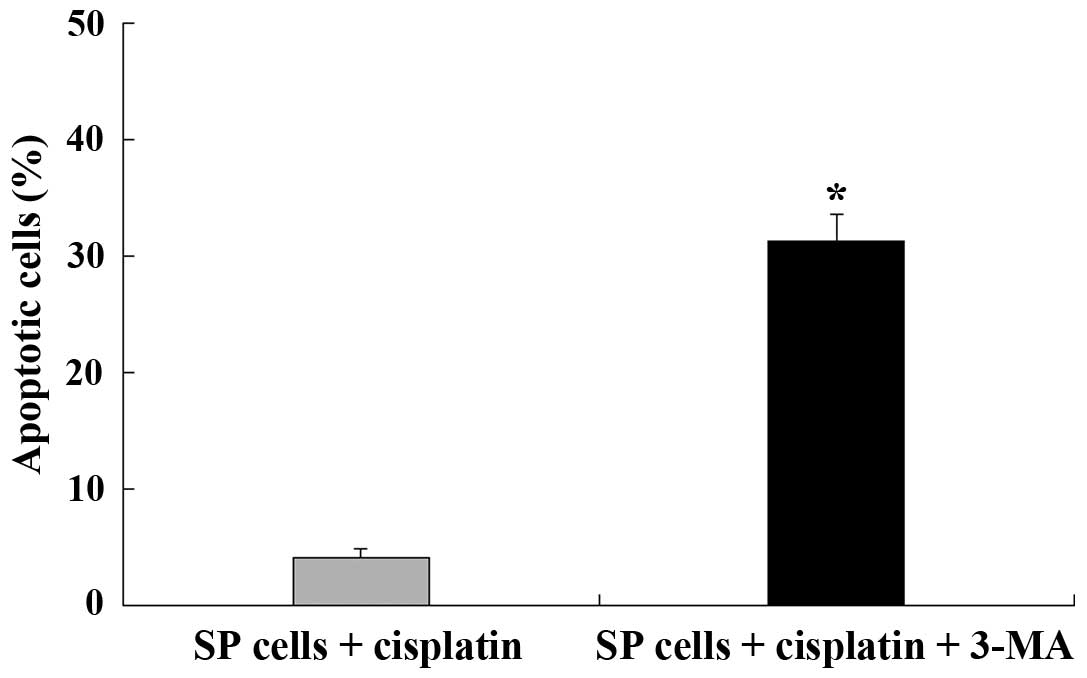
Autophagy provides resistance against
chemotherapy in SP cells
A significant increase in autophagic response
towards the chemotherapeutic drug, cisplatin, was observed in SP
cells. Autophagosomes were found to be markedly increased in the
presence of cisplatin. Compared to SP cells without treatment of
cisplatin, the expression of Beclin-1 and LC3-II in SP cells
treated with cisplatin was markedly upregulated. In addition, the
rate of apoptosis in SP cells with cisplatin was (4.2±0.7)%, while
it increased up to (31.4±2.3)% in the presence of 3-MA, which is an
inhibitor of autophagy (Fig.
9).
Discussion
The CSCs hypothesis suggests that cancer may be
maintained by CSCs that possess the properties of self-renewal,
multipotency and carcinogenesis (14). Recent evidence has shown that CSCs
are the root of recurrence, metastasis and resistance to the
chemotherapy of cancer (15,16).
Therefore, it is suggested that targeting CSCs in cancer provides a
novel effective therapeutic strategy to eradicate them completely.
Regarding the methods of isolating CSCs, identification of specific
markers for CSCs is the ideal method. It is reported that CSCs have
been identified by specific markers in several solid tumors,
including breast (17), prostate
(18) and colon cancer (19), and melanoma (20). However, specific markers of CSCs in
malignant tumors remain to be identified. Furthermore, the
so-called ‘CSCs’, which had differentiated surface markers, were
not considered real CSCs, as they had already differentiated
(21,22).
Due to the absence of specific markers of CSCs in
most malignant tumors, sorting SP cells using FCM by a dye Hoechst
33342 has become the main method of investigating CSCs. SP cells
efflux the dye Hoechst 33342 through an ABC membrane transporter
which is responsible for chemoresistance and is also thought to
possess stem cell characteristics in several types of cancer
(23). In the present study, we
isolated SP cells from the A549 lung cancer cell line by flow
cytometry and then verified the cancer stem cell-like properties of
SP cells in vitro and in vivo.
It is reported that CSCs have an
anchorage-independent growth ability and sphere formation assays
can be used to check the self-renewal ability of cells in
vitro (24). In the present
study, the formation of the primary and secondary floating sphere
showed that SP cells isolated from A549 lung cancer cells possessed
self-renewal ability. Infinite proliferation and tumorigenic
abilities are notable features of CSCs (25). Compared to non-SP cells, the fact
that single cells became anchorage-independent more rapidly and
formed colonies showed that SP cells had malignant and tumorigenic
abilities. These findings suggest that SP cells from lung cancer
cells possessed CSCs-like properties.
The expression of MDR1 and ABCG2, which belong to
the ABC transporter superfamily, were upregulated in SP cells when
compared with that in non-SP cells. This result was consistent with
the higher chemotherapeutic resistance of SP cells. OCT-4 is a
transcriptional activator belonging to the POU family, and it is
also an important regulator of self-renewal and differentiation in
stem cells. The overexpression of these representative stem cell
genes in SP cells suggested that the SP cells characterized the
properties of CSCs.
For the in vivo experiments, SP and non-SP
cells were injected into nude mice subcutaneously. SP cells formed
tumors after 8 weeks at densities of 103 and
104, whereas non-SP cells only formed tumors in some
mice at a density of 104, and the tumors were much
smaller than that of the SP group. Of note was that some non-SP
cells also formed tumors in some nude mice, suggesting that
although CSCs were enriched in SP cells, non-SP cells also contain
some CSCs.
Autophagy is a pivotal process for cell survival by
decomposing unessential proteins or impaired cell organelles and
utilizing them as energy when cells suffer from malnutrition,
exposure to toxic materials, hypoxia and virus infection (26). If the stresses continue, this
process leads to autophagic cell death (27). Notably, autophagy has two
contradictory aspects in that it acts as a mechanism of cell
survival and death (28). In a
previous experiment, using A549 lung cancer cells, we identified
that inhibition of autophagy was capable of promoting the apoptosis
induced by chemotherapeutics. In the present study, we found that
the expression of autophagosomes was much lower in SP cells than
that in non-SP cells. In addition, Beclin-1 and LC3-II, both of
which were involved in the autophagic process, also showed a lower
expression in SP cells compared to that in non-SP cells. However,
the autophagy level was found to be markedly increased in the
presence of cisplatin and inhibition of autophagy enhanced the
effects of apoptosis induced by the chemotherapeutic agent,
cisplatin, in lung cancer SP cells. Thus, autophagy is a potential
therapeutic target for lung cancer.
In conclusion, SP cells from the A549 lung cancer
cell line can be effectively sorted using FACS with Hoechst 33342.
SP cells possess the properties of CSC, such as increased
proliferation, higher clonogenicity, and stronger tumorigenicity.
Moreover, SP cells are more resistant to chemotherapy and
inhibition of autophagy enhances the effects of apoptosis induced
by cisplatin. Therefore, these findings collectively suggest that
SP cells from the A549 lung cancer cell line have cancer stem
cell-like properties and autophagy may be an attractive therapeutic
target for human lung cancer.
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
O’Flaherty JD, Barr M, Fennell D, Richard
D, Reynolds J, O’Leary J and O’Byrne K: The cancer stem-cell
hypothesis: Its emerging role in lung cancer biology and its
relevance for future therapy. J Thorac Oncol. 7:1880–1890. 2012.
View Article : Google Scholar
|
|
3
|
Richard V, Sebastian P, Nair MG, Nair SN,
Malieckal TT, Santhosh Kumar TR and Pillai MR: Multiple drug
resistant, tumorigenic stem-like cells in oral cancer. Cancer Lett.
338:300–316. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gillet JP, Efferth T and Remacle J:
Chemotherapy-induced resistance by ATP-binding cassette transporter
genes. Biochim Biophys Acta. 1775:237–262. 2007.PubMed/NCBI
|
|
5
|
Broadley KW, Hunn MK, Farrand KJ, Price
KM, Grasso C, Miller RJ, Hermans IF and McConnell MJ: Side
population is not necessary or sufficient for a cancer stem cell
phenotype in glioblastoma multiforme. Stem Cells. 29:452–461. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Van den Broeck A, Vankelecom H, Van Delm
W, Gremeaux L, Wouters J, Allemeersch J, Govaere O, Roskams T and
Topal B: Human pancreatic cancer contains a side population
expressing cancer stem cell-associated and prognostic genes. PLoS
One. 8:e739682013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huang B, Huang YJ, Yao ZJ, Chen X, Guo SJ,
Mao XP, Wang DH, Chen JX and Qiu SP: Cancer stem cell-like side
population cells in clear cell renal cell carcinoma cell line 769P.
PLoS One. 8:e682932013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hiraga T, Ito S and Nakamura H: Side
population in MDA- MB-231 human breast cancer cells exhibits cancer
stem cell-like properties without higher bone-metastatic potential.
Oncol Rep. 25:289–296. 2011.
|
|
9
|
Guo D, Xu BL, Zhang XH and Dong MM: Cancer
stem-like side population cells in the human nasopharyngeal
carcinoma cell line CNE-2 possess epithelial mesenchymal transition
properties in association with metastasis. Oncol Rep. 28:241–247.
2012.PubMed/NCBI
|
|
10
|
Golebiewska A, Brons NH, Bjerkvig R and
Niclou SP: Critical appraisal of the side population assay in stem
cell and cancer stem cell research. Cell Stem Cell. 8:136–147.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kato K: Stem cells in human normal
endometrium and endometrial cancer cells: Characterization of side
population cells. Kaohsiung J Med Sci. 28:63–71. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu F, Liu D, Yang Y and Zhao S: Effect of
autophagy inhibition on chemotherapy-induced apoptosis in A549 lung
cancer cells. Oncol Lett. 5:1261–1265. 2013.PubMed/NCBI
|
|
13
|
Ojha R, Jha V, Singh SK and Bhattacharyya
S: Autophagy inhibition suppresses the tumorigenic potential of
cancer stem cell enriched side population in bladder cancer.
Biochim Biophys Acta. 1842:2073–2086. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
O’Connor ML, Xiang D, Shigdar S, Macdonald
J, Li Y, Wang T, Pu C, Wang Z, Qiao L and Duan W: Cancer stem
cells: A contentious hypothesis now moving forward. Cancer Lett.
344:180–187. 2014. View Article : Google Scholar
|
|
15
|
Fitzgerald TL and McCubrey JA: Pancreatic
cancer stem cells: Association with cell surface markers,
prognosis, resistance, metastasis and treatment. Adv Biol Regul.
56:45–50. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kaur S, Singh G and Kaur K: Cancer stem
cells: An insight and future perspective. J Cancer Res Ther.
10:846–852. 2014. View Article : Google Scholar
|
|
17
|
Mannello F: Understanding breast cancer
stem cell heterogeneity: Time to move on to a new research
paradigm. BMC Med. 11:1692013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Garraway IP: Will identification of a
prostate cancer stem cell lead to its cure? Urol Oncol. 30:351–352.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gespach C: Stem cells and colon cancer:
The questionable cancer stem cell hypothesis. Gastroenterol Clin
Biol. 34:653–661. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chandrasekaran S and DeLouise LA:
Enriching and character-izing cancer stem cell sub-populations in
the WM115 melanoma cell line. Biomaterials. 32:9316–9327. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rahman M, Deleyrolle L, Vedam-Mai V, Azari
H, Abd-El-Barr M and Reynolds BA: The cancer stem cell hypothesis:
Failures and pitfalls. Neurosurgery. 68:531–545. 2011. View Article : Google Scholar
|
|
22
|
Sellheyer K: Basal cell carcinoma: Cell of
origin, cancer stem cell hypothesis and stem cell markers. Br J
Dermatol. 164:696–711. 2011. View Article : Google Scholar
|
|
23
|
Hadnagy A, Gaboury L, Beaulieu R and
Balicki D: SP analysis may be used to identify cancer stem cell
populations. Exp Cell Res. 312:3701–3710. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nishida S and Masumori N: Cancer stem
cells in prostate cancer. Nihon Rinsho. 72:2229–2233. 2014.In
Japanese. PubMed/NCBI
|
|
25
|
Blacking TM, Waterfall M and Argyle DJ:
CD44 is associated with proliferation, rather than a specific
cancer stem cell population, in cultured canine cancer cells. Vet
Immunol Immunopathol. 141:46–57. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Meijer AJ and Codogno P: Autophagy:
Regulation by energy sensing. Curr Biol. 21:R227–R229. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hwang MS and Baek WK: Glucosamine induces
autophagic cell death through the stimulation of ER stress in human
glioma cancer cells. Biochem Biophys Res Commun. 399:111–116. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mathew R and White E: Autophagy in
tumorigenesis and energy Shigdar S, Macdonald J, Li Y, Wang, foe by
night. Curr Opin Genet Dev. 21:113–119. 2011. View Article : Google Scholar : PubMed/NCBI
|