Introduction
Glioma is one of the most common primary tumors of
the central nervous system in adults, accounting for ~31% of all
brain tumors (1). Although
multimodal treatments including surgery, chemotherapy and
radiotherapy have shown some efficacy, the outcome of patients with
malignant glioma remains poor. Recently, it was proven by a
randomized phase III clinical study that glioblastoma (GBM) patient
survival was improved when combining alkylating agent temozolomide
(TMZ) with radiotherapy. Overall patient survival was 27.2% at 2
years and 9.8% at 5 years in the combination group vs. 10.9% at 2
years and 1.9% at 5 years in the radiotherapy alone group (2,3).
However, the recurrence rate of malignant glioma patients remained
high even when they were sensitive to TMZ (4).
A high level of MGMT and the existence of glioma
stem-like cells (GSCs) are considered as the two main causes of
chemoresistance in malignant glioma patients. MGMT is a DNA repair
protein that is unique in its ability to remove DNA adducts and to
self-inactivate (5). Therefore, the
protein level of MGMT was found to be inversely related to the
chemosensitivity of gliomas to alkylating agents (6–9). GSCs
are known as tumor-initiating cells due to their stem cell-like
properties and the pivotal role in tumor development (10–12).
The expression levels of multiple drug resistance enzymes in GSCs
were also found to contribute to chemoresistance (13,14).
IFN-α and IFN-β, cytokines that elicit pleiotropic
biological effects, have been widely used either alone or in
combination with others in the treatment of malignant glioma
(15–19). Both IFN-α and IFN-β have been
reported to downregulate expression of MGMT and re-sensitize
resistant glioma cells to TMZ in vitro and in vivo.
However, how MGMT expression is regulated following IFN-α and IFN-β
treatment, and whether IFN-α and IFN-β help against GSCs have not
yet been addressed. In the present study, the effects of IFN-α and
IFN-β on the efficacy of TMZ therapy, as well as the possible
mechanism of this enhancement were investigated.
Materials and methods
Cell lines and GSC culture
Human glioma cell lines U251, SKMG-4 and U87 were
cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented
with 10% fetal calf serum (Invitrogen, New York, NY, USA) in a
humidified atmosphere containing 5% CO2 at 37°C.
GSCs were enriched from U251, SKMG-4 and U87 cell cultures using
serum-free DMEM/F12 (Invitrogen) supplemented with 2% B27 (Gibco,
Grand Island, NY, USA), 20 ng/ml EGF and 10 ng/ml bFGF (PeproTech,
Rocky Hill, NJ, USA) according to the procedure established by
Singh et al (11).
Cell proliferation analysis
GSCs, namely U251G (MGMT-positive), SKMG-4G
(MGMT-positive), and U87G (MGMT-negative), were plated at 5,000
cells/well in 96-well plates and incubated for 24 h. These GSCs
were then divided into 6 groups: i) the control group, in which the
inhibition of the growth rate of untreated cells was regarded as
0%; ii) TMZ (500 µM)-treated for 72 h; iii) IFN-α (100
U/ml)-treated for 72 h; iv) IFN-β (100 U/ml)-treated for 72 h; v)
IFN-α (100 U/ml)-treated for 24 h and then co-incubated with TMZ
(500 µM) for 48 h; vi) IFN-β (100 U/ml)-treated for 24 h and
then co-incubated with TMZ (500 µM) for 48 h. Four hours
prior to harvest, 10 µl/well of the reagent in the Cell
Counting Kit-8 (CCK-8; Dojindo, Japan) was added, and the cells
were incubated for 4 h at 37°C. The absorbance was measured
by Labsystems MK3 ELIASA (Thermo Fisher, Waltham, MA, USA) at a
wavelength of 490 nm. The percentage of cell survival (survival
rate) was calculated by dividing the absorbance value of the
treated sample by the absorbance value of the untreated control for
every group.
Subcutaneous xenograft tumor growth
inhibition experiments
BALB/c nude mice (male, 5–6 weeks of age) were
purchased from the SLAC Laboratory Animal Company (Shanghai,
China). The mice were housed in laminar flow cabinets under
specific pathogen-free conditions in the animal facility at our
institute. All animal studies were performed according to the
institutional ethical guidelines for experimental animal care and
were approved by the Medical Ethics Committee of Sun Yat-sen
University Cancer Center. U251G and SKMG-4G cells were harvested by
Accutase (Gibco) and injected (1×107 cells/mouse,
suspended in 100 µl PBS and 100 µl Matrigel)
subcutaneously into the right flank of the mice. When the
subcutaneous tumors had reached a volume of ~50 mm3, the
animals were randomly divided into groups: treated with saline, TMZ
only (TMZ 50 mg/kg), IFN-α only (IFN-α 2×105 IU), IFN-β
only (IFN-β 2×105 IU), TMZ+IFN-α (TMZ 50 mg/kg + IFN-α
2×105 IU) or TMZ + IFN-β (TMZ 50 mg/kg + IFN-β
2×105 IU). IFN-α or IFN-β was administered by
subcutaneous injection 6 h before an intraperitoneal injection of
TMZ. Treatments were repeated at 24-h intervals for a total of 5
doses. Subcutaneous tumors were measured once a week, and the tumor
growth inhibition rates were analyzed at week 5. Tumor volume was
calculated according to the following formula: Volume = length ×
width2/2. All animal experiments were approved by the
Institutional Animal Care and Use Committee of Sun Yat-Sen
University and in accordance with the national guidelines for the
care and maintenance of laboratory animals.
RNA preparation and RT-PCR
Total RNA from U251G and SKMG-4G cells (cell
cultures grouped as previously described) was extracted using
TRIzol reagent (Invitrogen) according to the manufacturer's
protocol. Reverse transcription was performed using 2 µg of
total RNA from each sample in a total volume of 20 µl using
the RevertAid™ First Strand cDNA Synthesis kit (Fermentas, USA).
GAPDH was used as a loading control. The sequences of the forward
and reverse primers for each gene are listed in Table I. Each PCR mixture (20 µl)
contained 1 µl cDNA, 10 µl Green GoTaq DNA polymerase
(Promega, USA) and 0.5 µl of each forward and reverse
primers (10 µM). The PCR program was carried out as follows:
94°C for 2 min for hot start; 94°C for 30 sec,
60°C for 30 sec and 72°C for 30 sec, for 35 cycles;
72°C for 2 min. The PCR products were separated on 2%
agarose gel, visualized by ethidium bromide staining and
photographed with Gel Doc XR (Bio-Rad, Hercules, CA, USA).
 | Table IPrimer sequences for PCR. |
Table I
Primer sequences for PCR.
| Gene name | Direction | Sequence
(5′-3′) | Product size
(bp) |
|---|
| MGMT | Forward |
GTTATGAATGTAGGAGCCCTTATG | 239 |
| Reverse |
TGACAACGGGAATGAAGTAATG | |
| NFκB | Forward |
TGTGGGTTTCCTGTGCTAATG | 208 |
| Reverse |
GAGACCAGCCTTTCTCCGTA | |
| GAPDH | Forward |
CGCTCTCTGCTCCTCCTGTTC | 108 |
| Reverse |
ATCCGTTGACTCCGACCTTCAC | |
Immunohistochemistry
Subcutaneous xenograft tumors were established as
previously described. When the subcutaneous tumors had reached a
volume ~50 mm3, the animals were randomly divided into 4
groups (6 animals per group) and treated as described: with saline
water, TMZ only (TMZ 50 mg/kg), TMZ+IFN-α (TMZ 50 mg/kg + IFN-α
2×105 IU), or TMZ+IFN-β (TMZ 50 mg/kg + IFN-β
2×105 IU). The treatments were repeated at 24-h
intervals for a total of 5 doses. Seven days after treatment, tumor
samples were harvested and flash frozen in liquid nitrogen. Half of
the tumor samples were used for histological analysis, while the
other half were used for western blot analysis. For histological
analysis, the tumor samples subjected to immunohistochemical
analysis were fixed in 4% paraformaldehyde and stained with
anti-human-MGMT (Invitrogen) and anti-human-NF-κB (BioVision, USA)
antibodies. MGMT and NF-κB protein quantification was performed by
counting the number of stained cells in 10 random fields at ×400
magnification for each tumor sample.
Western blotting
Tumor cells or tissues were washed with ice-cold PBS
and lysed on ice by RIPA buffer (Beyotime, China). The protein
concentration was measured using the BCA assay kit (Beyotime)
according to the manufacturer's protocol. Equal amounts (60
µg) of total protein from each sample were boiled at
95°C for 5 min, separated on 10% SDS-PAGE and transferred to
PVDF membranes (Millipore, USA). The membranes were then incubated
in 5% milk for 1.5 h. The following primary antibodies were probed
by overnight incubation at 4°C: anti-MGMT (Invitrogen),
anti-β-actin (Beyotime) and anti-NF-κB (BioVision). The next day,
the membranes were washed and incubated with the secondary antibody
for 1 h. Proteins were visualized using the ECL system (Millipore),
and the bands were exposed to BioMax MR film (Kodak, Japan).
Statistical analysis
Each experiment was repeated at least three times
and data are presented as mean ± SD. The difference was analyzed
using one-way ANOVA test. The correlations were determined by
bivariate correlation procedures (Spearman correlation
coefficient). All statistical analyses were performed using SPSS
16.0. The differences were considered as significant at
p<0.05.
Results
IFN-α/β enhances the sensitivity of
MGMT-positive GSCs to TMZ
Previous studies have shown that both IFN-α and
IFN-β enhance TMZ activity against MGMT-positive glioma cells
(20,21). However, their effects on GSCs remain
unknown. Therefore, whether IFN-α or IFN-β enhances the effect of
TMZ on MGMT-positive GSCs was initially assessed. Following
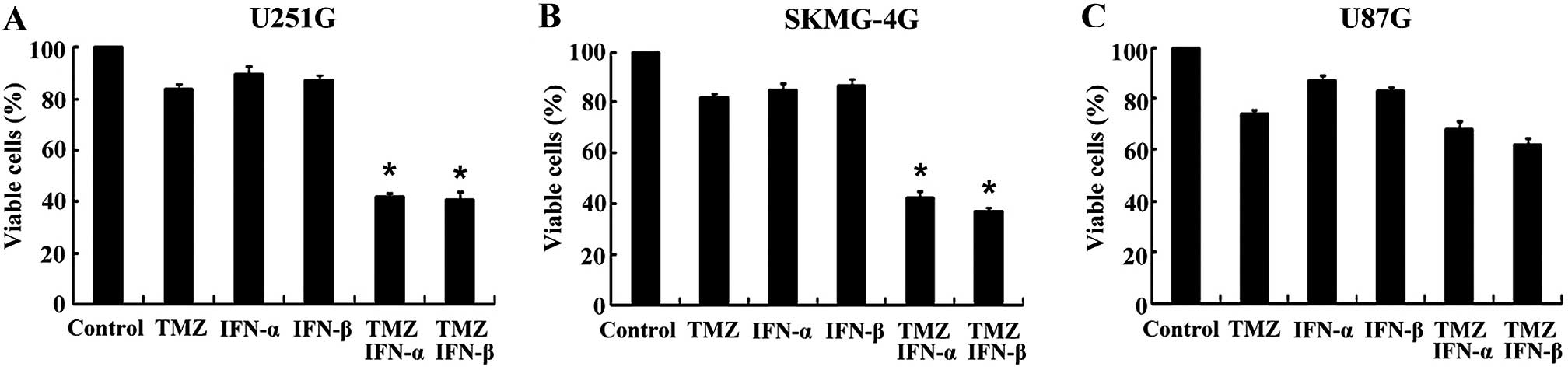
treatment with 100 IU/ml IFN-α or 100 IU/ml IFN-β, the viable cell
rates of the U251G cells were 42.05±1.54 and 40.86±2.83% compared
to 83.87±2.33% for the TMZ only group (p<0.05, Fig. 1A). In the SKMG-4G cells, the viable
cell rates of the combination groups were 42.26±2.91 and
37.38±1.55%, respectively, compared to 81.97±1.35% for the TMZ only
group (p<0.05, Fig. 1B).
However, in the MGMT-negative GSCs derived from U87G cells, the
viable cell rates were not markedly reduced in the combination
groups when compared with the TMZ only group (67.94±3.57 and
62.0±2.68 vs. 73.84±1.65%) (p>0.05, Fig. 1C). These results revealed that the
co-treatment of IFN-α/β significantly enhanced the sensitivity of
MGMT-positive GSCs to TMZ, while no marked elevation was found in
the MGMT-negative GSCs.
IFN-α/β enhances the antitumor efficacy
of TMZ in GSC xenografts
Subcutaneous xenografts were established via
implantation of MGMT-positive U251G and SKMG-4G GSCs in BALB/c nude
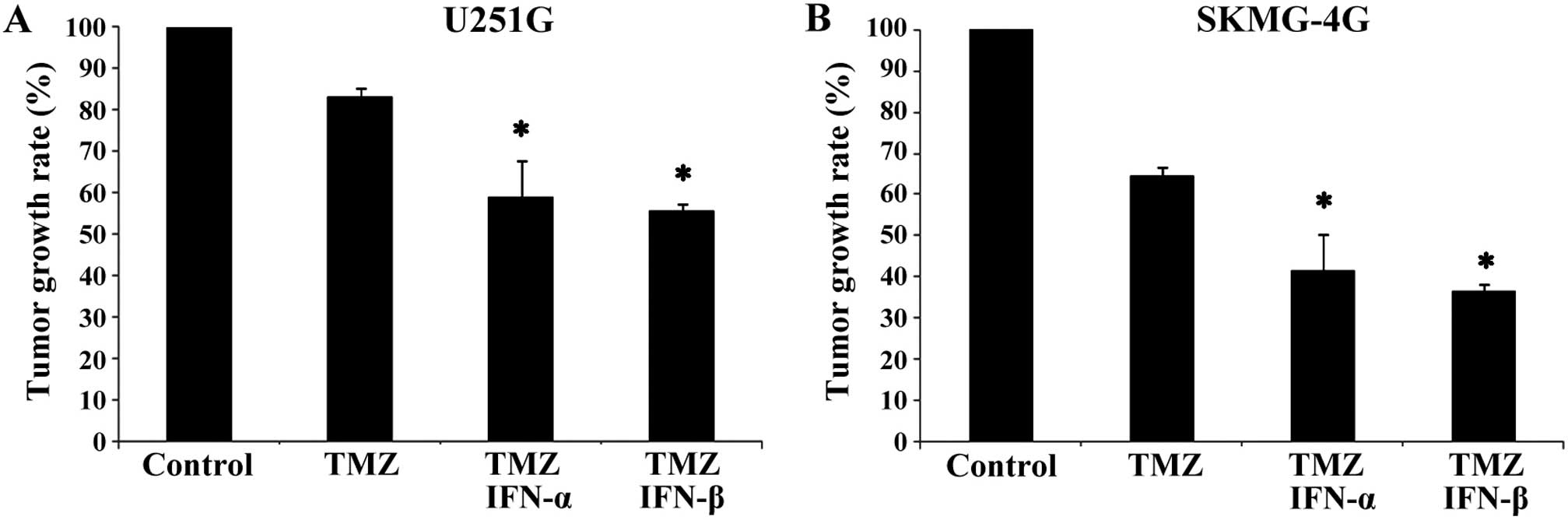
mice. After treatment for 5 weeks, the tumor growth rates of the
U251G xenografts in the combination groups (TMZ+IFN-α and
TMZ+IFN-β) were reduced when compared with the growth rate in the
TMZ only group (58.9±8.66 and 55.5±1.90 vs. 83.3±1.96%, p<0.05,
Fig. 2A). Similarly, the growth
rates of SKMG-4G xenografts in the TMZ+IFN-α and TMZ+IFN-β groups
were much lower than the rate in the TMZ only group (41.6±4.34 and
36.6±1.08 vs. 64.8±2.28%, p<0.05, Fig. 2B). These data demonstrated that
IFN-α/β enhanced the antitumor efficiency of TMZ in
vivo.
NF-κB and MGMT are downregulated by the
combination treatment
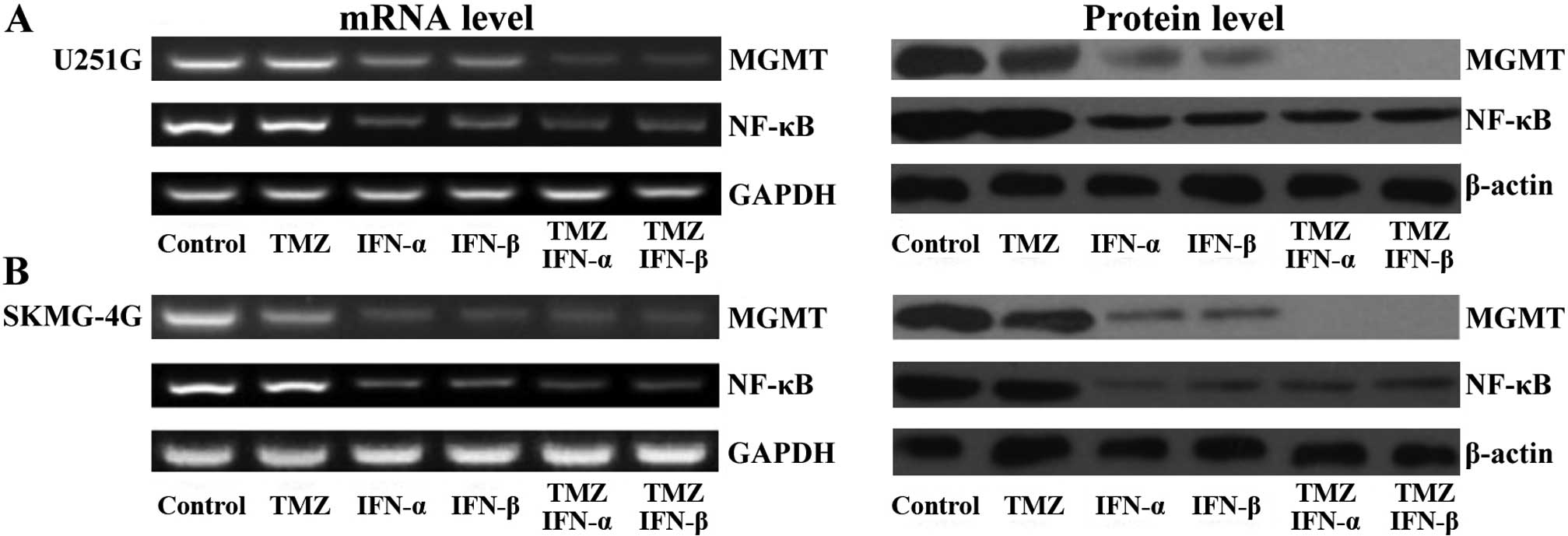
As a previous study showed that NF-κB regulates the
expression of MGMT in the process of DNA repair (22), we detected the levels of NF-κB and
MGMT by RT-PCR and western blot assays following the treatments.
Both the mRNA and protein levels of MGMT were reduced following
treatment with IFN-α/β and further decreased with the combination
treatment (Fig. 3A). NF-κB was
reduced following IFN-α/β treatment but remained at the same level
in the co-treatment cell lysates (Fig.
3A). The effect of TMZ on the level of MGMT was not marked. In
SKMG-4G cells, we obtained consistent data that IFN-α/β treatment
led to a reduction in both MGMT and NF-κB; the combined treatment
further decreased MGMT but not NF-κB (Fig. 3B). Our data indicated that IFN-α or
IFN-β sensitized MGMT-positive GSCs to TMZ, which may be through
the donwregulation of NF-κB leading to a reduction in MGMT.
IFN-α/β decreases NF-κB and MGMT protein
levels in the GSC xenografts
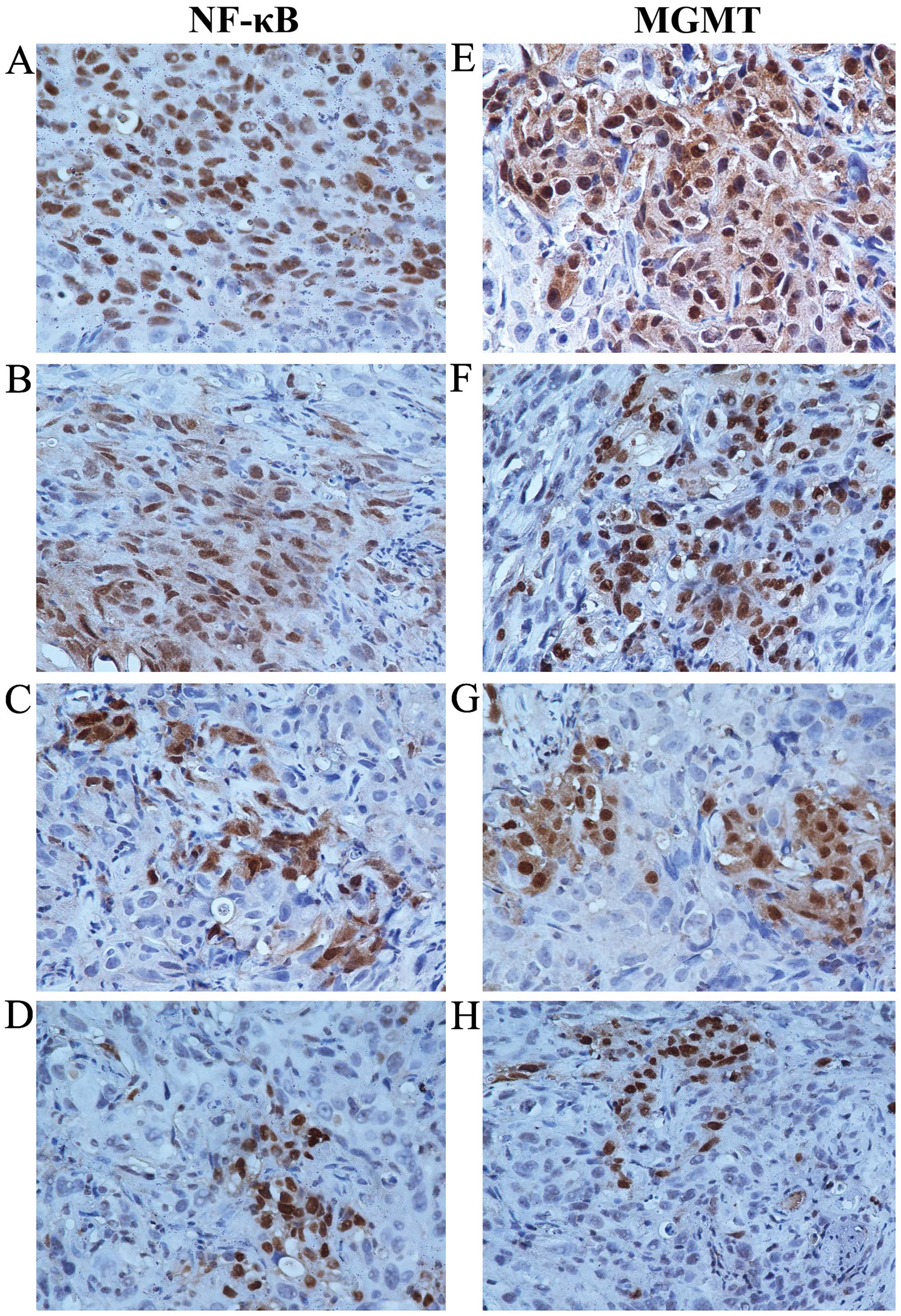
We collected U251G xenografts and performed
immunohistochemical staining following standard procedures. The
expression levels of NF-κB and MGMT in the control group were
considered as normal (Fig. 4A and
E). In the TMZ-treated group, the expression levels of NF-κB
and MGMT were slightly reduced when compared with these levels in
the control group (Fig. 4B and F),
which was further decreased in the xenografts co-treated with TMZ
and IFN-α or IFN-β (Fig. 4C and G, D
and H). Our data demonstrated that IFN-α or IFN-β decreased the
level of NF-κB. Consistent with previous reports, MGMT was reduced
accompanied by decreased NF-κB, which may be the potential
mechanism for the enhanced sensitivity of MGMT-positive GSCs to TMZ
by IFN-α/β.
MGMT levels are positively correlated
with NF-κB in the GSC xenografts
To confirm the results from IHC analysis, we
collected xenografts derived from U251G and SKMG-4G cells, and
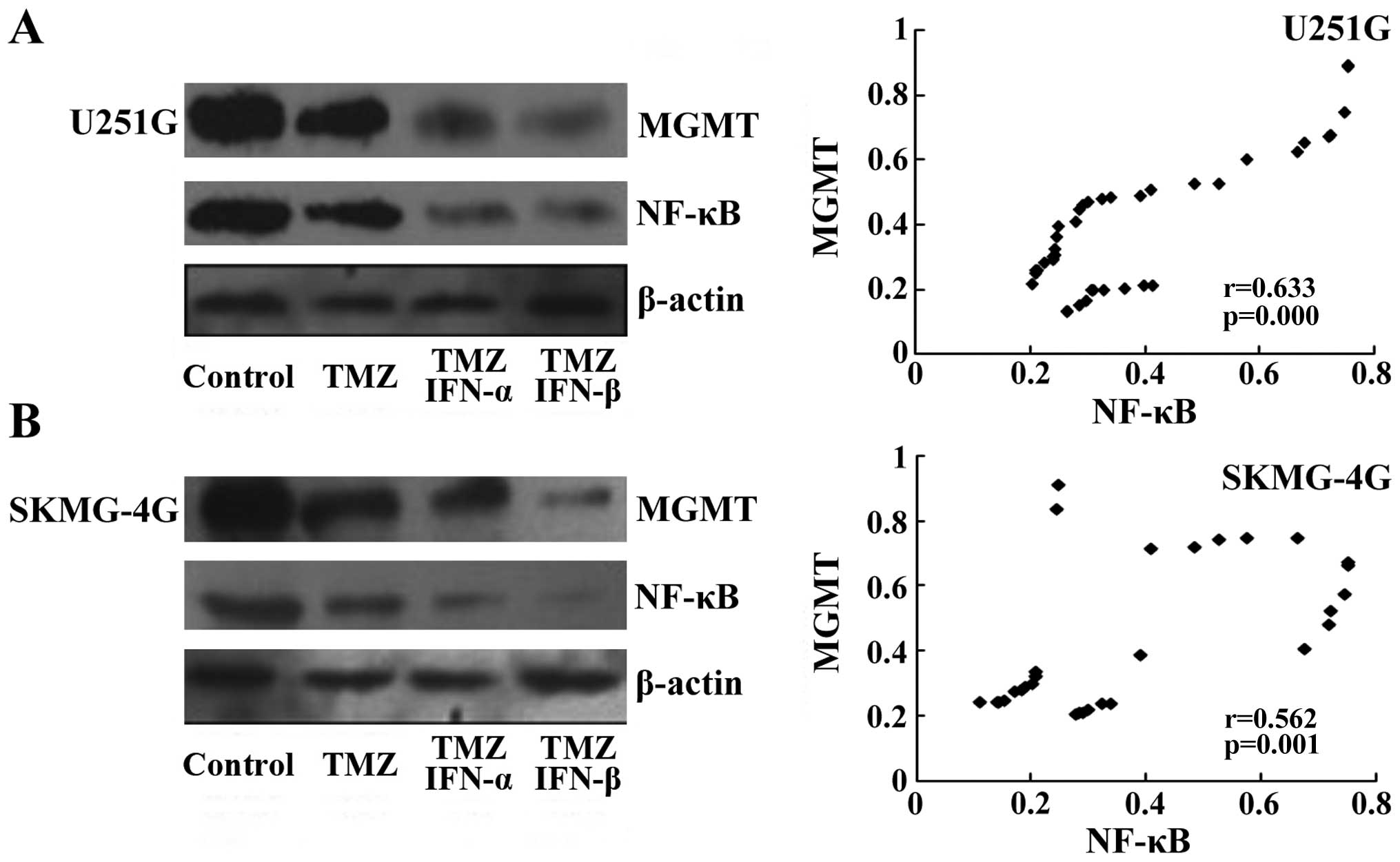
determined the levels of NF-κB and MGMT by western blot assay.
Levels of NF-κB and MGMT were slightly decreased following TMZ
treatment compared to levels in the untreated control group, while
NF-κB and MGMT levels were markedly reduced in the groups treated
with TMZ and IFN-α/β (Fig. 5A and
B, left panels). The protein levels of NF-κB and MGMT in each
xenograft were detected by western blotting, and then subjected to
Spearman correlation analysis. We found that a lower level of MGMT
was detected with a decrease in NF-κB. Statistical analysis also
confirmed that the MGMT level was positively associated with NF-κB
in both the U251G (r=0.633, p<0.05, Fig. 5A, right panel) and SKMG-4G
xenografts (r=0.562, p<0.05, Fig.
5B, right panel). Therefore, our data confirmed that the MGMT
level was correlated with NF-κB upon the combination treatment of
TMZ and IFN-α/β.
Discussion
The unique characteristics of GSCs, including
self-renewal, unlimited proliferation and differentiation
abilities, provide them with the ability to promote tumor
initiation, growth and recurrence. They also have altered signaling
pathways that are involved in the regulation of cell survival and
proliferation. GSCs have also been demonstrated to correlate with
chemo-resistance and poor survival since they express high levels
of multidrug resistance proteins and DNA repair enzymes (23). A previous study carried out by us
showed that MGMT was unmethylated, and the protein level was
increased in U251G and SKMG-4G cells when compared with their
parental U251 and SKMG-4 cells. In vitro experiments
revealed that U251G and SKMG-4G cells are more resistant to TMZ
than their parental cells with low level of MGMT expression
(24).
MGMT is a DNA repair enzyme that specifically
removes unfavorable methyl groups from the O6 position
of guanine, thereby restoring the nucleotide to its native form
without causing any DNA damage. It is a crucial protein both for
cancer prevention and treatment due to its ability to repair
mutagenic lesions in DNA and limit the effectiveness of alkylating
chemotherapies (25). Previous
studies have shown that high MGMT expression is correlated with
poor clinical outcome in glioma patients treated with
chloroethylnitrosoureas and alkylating agents (26–28).
In the clinical setting, the benefits of TMZ therapy are mainly
observed in those patients with methylated MGMT promoter for
whom TMZ-induced DNA damage is unable to be repaired. Patients with
unmethylated MGMT promoter fail to benefit from TMZ
chemotherapy. Even with methylated MGMT, gliomas are usually
sensitive to TMZ chemotherapy at the beginning and develop
resistance shortly after.
Natsume et al reported that IFN-β treatment
decreases MGMT levels in glioma cells via transcription inhibition.
Moreover, pre-treatment with IFN-β markedly enhances
chemosensitivity of glioma cells to TMZ (20,21).
Another study showed that IFN-β provides a survival benefit for
patients after radiation (29).
Recently, a multi-center study demonstrated that both the presence
of methylated MGMT and patient response to combination
treatment (IFN-β and TMZ) were independent favorable prognostic
indicators for GBM patients (30).
It was reported that the MGMT level is related with NF-κB activity,
since there are two putative NF-κB binding sites in the MGMT
promoter region that regulates MGMT expression. Thus, NF-κB plays
an important role in MGMT-mediated chemo-resistance (31,32). A
study by Lavon et al showed that cell lines with high
constitutive NF-κB activity are less sensitive to nitrosourea
treatment, and suppression of MGMT activity by small molecules
completely abolished the chemoresistance regulated by NF-κB
(22).
The present study aimed to explore whether GSCs also
contribute to TMZ-resistance in malignant glioma patients with
unmethylated MGMT and whether manipulating the MGMT level by
combination therapy will benefit glioma patients. We demonstrated
that the efficacy of TMZ on MGMT-positive GSCs was markedly
enhanced by the combination treatment with IFN-α/β when compared
with the TMZ single agent group, and MGMT expression was markedly
decreased at the same time. We also provided evidence that IFN-α/β
suppressed NF-κB activity, which further mediated the sensitization
of MGMT-positive GSCs to TMZ by decreasing the MGMT protein level.
Our data therefore suggest that inhibition of NF-κB may improve
clinical outcomes and prolong the survival of patients with
malignant gliomas.
Acknowledgments
The present study was funded by the National Natural
Science Foundation of China (30772551), the Science and Technology
Program of Guangdong Province (2011B031800178), the National
High-Technology Research and Development Program of China (863:
2012AA02A508), the National Science Research Program of China (973:
2015CB755500) and the Specialized Research Fund for the Doctoral
Program of Higher Education (no. 20110171110076).
References
|
1
|
CBTRUS: CBTRUS Statistical report: Primary
brain and central nervoussystem tumors diagnosed in the United
States in 2004–2007. Source: Central Brain Tumor Registry of the
United States. CBTRUS; Hindsdale, IL, USA: 2011, http://www.cbtrus.orgurisimplewww.cbtrus.org.
|
|
2
|
Stupp R, Mason WP, van den Bent MJ, et al
European Organisation for Research and Treatment of Cancer Brain
Tumor and Radiotherapy Groups; National Cancer Institute of Canada
Clinical Trials Group: Radiotherapy plus concomitant and adjuvant
temozolomide for glioblastoma. N Engl J Med. 352:987–996. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stupp R, Hegi ME, Mason WP, et al European
Organisation for Research and Treatment of Cancer Brain Tumour and
Radiation Oncology Groups; National Cancer Institute of Canada
Clinical Trials Group: Effects of radiotherapy with concomitant and
adjuvant temozolomide versus radiotherapy alone on survival in
glioblastoma in a randomised phase III study: 5-year analysis of
the EORTC-NCIC trial. Lancet Oncol. 10:459–466. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fukushima T, Takeshima H and Kataoka H:
Anti-glioma therapy with temozolomide and status of the DNA-repair
gene MGMT. Anticancer Res. 29:4845–4854. 2009.PubMed/NCBI
|
|
5
|
Sharma S, Salehi F, Scheithauer BW,
Rotondo F, Syro LV and Kovacs K: Role of MGMT in tumor development,
progression, diagnosis, treatment and prognosis. Anticancer Res.
29:3759–3768. 2009.PubMed/NCBI
|
|
6
|
Fruehauf JP, Brem H, Brem S, Sloan A,
Barger G, Huang W and Parker R: In vitro drug response and
molecular markers associated with drug resistance in malignant
gliomas. Clin Cancer Res. 12:4523–4532. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hermisson M, Klumpp A, Wick W, et al:
O6-methylguanine DNA methyltransferase and p53 status
predict temozolomide sensitivity in human malignant glioma cells. J
Neurochem. 96:766–776. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Esteller M, Garcia-Foncillas J, Andion E,
et al: Inactivation of the DNA-repair gene MGMT and the clinical
response of gliomas to alkylating agents. N Engl J Med.
343:1350–1354. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hegi ME, Diserens AC, Gorlia T, et al:
MGMT gene silencing and benefit from temozolomide in glioblastoma.
N Engl J Med. 352:997–1003. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ignatova TN, Kukekov VG, Laywell ED,
Suslov ON, Vrionis FD and Steindler DA: Human cortical glial tumors
contain neural stem-like cells expressing astroglial and neuronal
markers in vitro. Glia. 39:193–206. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Singh SK, Hawkins C, Clarke ID, et al:
Identification of human brain tumour initiating cells. Nature.
432:396–401. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kondo T, Setoguchi T and Taga T:
Persistence of a small subpopulation of cancer stem-like cells in
the C6 glioma cell line. Proc Natl Acad Sci USA. 101:781–786. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Eramo A, Ricci-Vitiani L, Zeuner A,
Pallini R, Lotti F, Sette G, Pilozzi E, Larocca LM, Peschle C and
De Maria R: Chemotherapy resistance of glioblastoma stem cells.
Cell Death Differ. 13:1238–1241. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Salmaggi A, Boiardi A, Gelati M, et al:
Glioblastoma-derived tumorospheres identify a population of tumor
stem-like cells with angiogenic potential and enhanced multidrug
resistance phenotype. Glia. 54:850–860. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jonasch E and Haluska FG: Interferon in
oncological practice: Review of interferon biology, clinical
applications, and toxicities. Oncologist. 6:34–55. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wakabayashi T, Natsume A, Hashizume Y,
Fujii M, Mizuno M and Yoshida J: A phase I clinical trial of
interferon-beta gene therapy for high-grade glioma: Novel findings
from gene expression profiling and autopsy. J Gene Med. 10:329–339.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Groves MD, Puduvalli VK, Gilbert MR, et
al: Two phase II trials of temozolomide with interferon-alpha2b
(pegylated and non-pegylated) in patients with recurrent
glioblastoma multi-forme. Br J Cancer. 101:615–620. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ohno M, Natsume A, Fujii M, Ito M and
Wakabayashi T: Interferon-beta, MCNU, and conventional radiotherapy
for pediatric patients with brainstem glioma. Pediatr Blood Cancer.
53:37–41. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Warren K, Bent R, Wolters PL, et al: A
phase 2 study of pegylated interferon α-2b (PEG-Intron®)
in children with diffuse intrinsic pontine glioma. Cancer.
118:3607–3613. 2012. View Article : Google Scholar :
|
|
20
|
Natsume A, Ishii D, Wakabayashi T, et al:
IFN-beta down-regulates the expression of DNA repair gene MGMT and
sensitizes resistant glioma cells to temozolomide. Cancer Res.
65:7573–7579. 2005.PubMed/NCBI
|
|
21
|
Natsume A, Wakabayashi T, Ishii D, et al:
A combination of IFN-beta and temozolomide in human glioma
xenograft models: Implication of p53-mediated MGMT downregulation.
Cancer Chemother Pharmacol. 61:653–659. 2008. View Article : Google Scholar
|
|
22
|
Lavon I, Fuchs D, Zrihan D, et al: Novel
mechanism whereby nuclear factor kappaB mediates DNA damage repair
through regulation of
O6-methylguanine-DNA-methyltransferase. Cancer Res.
67:8952–8959. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu G, Yuan X, Zeng Z, et al: Analysis of
gene expression and chemoresistance of CD133+ cancer
stem cells in glioblastoma. Mol Cancer. 5:672006. View Article : Google Scholar
|
|
24
|
Qiu ZK, Shen D, Chen YS, et al: Enhanced
MGMT expression contributes to temozolomide resistance in glioma
stem-like cells. Chin J Cancer. 33:115–122. 2014. View Article : Google Scholar :
|
|
25
|
Tubbs JL, Pegg AE and Tainer JA: DNA
binding, nucleotide flipping, and the helix-turn-helix motif in
base repair by O6-alkylguanine-DNA alkyltransferase and
its implications for cancer chemotherapy. DNA Repair. 6:1100–1115.
2007. View Article : Google Scholar
|
|
26
|
Chen ZP, Yarosh D, Garcia Y, et al:
Relationship between O6-methylguanine-DNA
methyltransferase levels and clinical response induced by
chloroethylnitrosourea therapy in glioma patients. Can J Neurol
Sci. 26:104–109. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Paz MF, Yaya-Tur R, Rojas-Marcos I, et al:
CpG island hyper-methylation of the DNA repair enzyme
methyltransferase predicts response to temozolomide in primary
gliomas. Clin Cancer Res. 10:4933–4938. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hegi ME, Liu L, Herman JG, et al:
Correlation of O6-methylguanine methyltransferase (MGMT)
promoter methylation with clinical outcomes in glioblastoma and
clinical strategies to modulate MGMT activity. J Clin Oncol.
26:4189–4199. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Colman H1, Berkey BA, Maor MH, et al
Radiation Therapy Oncology Group: Phase II Radiation Therapy
Oncology Group trial of conventional radiation therapy followed by
treatment with recombinant interferon-beta for supratentorial
glioblas-toma: Results of RTOG 9710. Int J Radiat Oncol Biol Phys.
66:818–824. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Motomura K, Natsume A, Kishida Y, et al:
Benefits of interferon-β and temozolomide combination therapy for
newly diagnosed primary glioblastoma with the unmethylated MGMT
promoter: A multicenter study. Cancer. 117:1721–1730. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Saccani S, Marazzi I, Beg AA and Natoli G:
Degradation of promoter-bound p65/RelA is essential for the prompt
termination of the nuclear factor kappaB response. J Exp Med.
200:107–113. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sethi G, Ahn KS, Sung B and Aggarwal BB:
Pinitol targets nuclear factor-kappaB activation pathway leading to
inhibition of gene products associated with proliferation,
apoptosis, invasion, and angiogenesis. Mol Cancer Ther.
7:1604–1614. 2008. View Article : Google Scholar : PubMed/NCBI
|