Introduction
Apoptosis represents one of the main types of
programmed cell death and is a tightly regulated cell suicide
response that facilitates the correct development and homeostasis
of multicellular organisms. Therefore, the susceptibility of cancer
cells to apoptosis is an important determinant of chemotherapy
efficacy (1,2). Moreover, during the past decade,
evidence suggests that many cancer chemotherapeutic agents kill
cancer cells by inducing apoptosis. In mammalian cells, two major
apoptosis pathways, the cell death receptor-mediated (extrinsic)
and mitochondrial-mediated apoptotic (intrinsic) pathways, have
been well-characterized. Both pathways are involved in an ordered
activation of a highly conserved family of cysteine proteases
called caspases, which in turn cleave cellular substrates,
resulting in the morphological and biochemical changes
characteristic of apoptosis (2,3). The
Bcl-2 family proteins also play a crucial role in the regulation of
apoptotic events in both signaling pathways (4). They contain anti-apoptotic members,
such as Bcl-2 and Bcl-xL, and pro-apoptotic members, such as Bax,
Bak and Bid. Overexpression of anti-apoptotic proteins results in
the prevention of apoptosis; however, overexpression of
pro-apoptotic proteins leads to an increase in cell susceptibility
to apoptotic signals (4,5).
Recently, the demand for more effective and safer
therapeutic agents for the chemoprevention of human cancer has
increased (6). In that respect,
fungi have been globally used as natural medicines and intensively
investigated for their antitumor properties with extemely low toxic
potential (7,8). Among them, Poria cocos Wolf. is
a saprophytic fungus in the Polyporaceae family that grows within
the diverse species of Pinus. Originally, its sclerotium has been
used as an important medicinal herb in several Asian countries,
particularly China, Japan and Korea, for its sedative, diuretic,
anti-depressant and tonic activities (9,10).
Recent studies have indicated that the extracts and components of
this fungus display a variety of biological activities, such as
anti-fungal and anti-bacterial (11), anti-oxidant (12,13),
neuroprotective (14),
anti-hypertonic (15),
anti-inflammatory (16–19), anti-angiogenic (20,21),
immunomodulatory (22,23) and anticancer effects (24,25).
Although several triterpenoids from P. cocos have been
isolated and were demonstrated to have a cytotoxic effect against a
number of human cancer cell types (26–29),
the efficacy and mechanism of the whole extract in cancer treatment
have not been systemically evaluated. In the present study, as a
part of our ongoing screening program to evaluate the anticancer
potential of medicinal fungi, we investigated the pro-apoptotic
properties of an ethanol extract of P. cocos (EEPC) and the
responsible underlying molecular mechanisms involved in the human
leukemia U937 cell line in vitro. Our data indicated that
EEPC exerts anti-proliferative effects on U937 cells through its
ability to induce apoptotic cell death.
Materials and methods
Preparation of EEPC
The dried sclerotium of P. cocos was supplied
by Dongeui University Oriental Hospital (Busan, Korea) and
authenticated by Professor S.H. Hong, Department of Biochemistry,
Dongeui University College of Korean Medicine. A voucher specimen
(accession no. DEU-27) was deposited at the Natural Resource Bank
of Dongeui University College of Korean Medicine. To prepare the
EEPC, the dried sclerotium of P. cocos was ground into
powder and extracted twice with 10 volumes of 80% ethanol at
85–90°C in a reflux condenser for 3 h. After being filtered through
a 0.2-µm filter, the extract was concentrated and
lyophilized by vacuum evaporation at 60°C. The solid form of the
extract was dissolved in dimethylsulfoxide (DMSO; Sigma-Aldrich,
St. Louis, MO, USA) prior to the experiment.
Cell culture
The human leukemia U937 cells and Chang liver cells
(an immortalized non-tumor cell line derived from normal liver
tissue) were purchased from the American Type Culture Collection
(ATCC; Manassas, VA, USA), and maintained at 37°C in a humidified
environment (95% air and 5% CO2), in RPMI-1640
supplemented with 10% heat-inactivated fetal bovine serum (FBS), 2
mM glutamine, 100 U/ml penicillin and 100 µg/ml streptomycin
(all from Gibco-BRL, Gaithersburg, MD, USA). Ectopic
Bcl-2-overexpressing U937 (U937/Bcl-2) cells were generously
provided by Professor T.K. Kwon (Department of Immunology, School
of Medicine, Keimyung University, Daegu, Korea) and were maintained
in a medium containing 0.7 µg/ml geneticin (G418 sulfate;
Calbiochem, San Diego, CA, USA).
Cell viability and growth assay
For the cell viability assay, cells were seeded at a
concentration of 1×105 cells/ml and were treated with
the indicated concentrations of EEPC for 24 h or with 90
µg/ml EEPC for the indicated times. After treatments,
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide
(MTT; Sigma-Aldrich) working solution was added to each culture
plate and continuously incubated at 37°C for 3 h. The culture
supernatant was removed from the wells, and DMSO was added to
completely dissolve the formazan crystals. The absorbance of each
well was measured at a wavelength of 540 nm with an enzyme-linked
immunosorbent assay (ELISA) plate reader (Molecular Devices,
Sunnyvale, CA, USA) (30). Cell
growth was assessed using the trypan blue dye exclusion assay. In
brief, the cells were trypsinized, and viable cells were counted by
trypan blue dye exclusion using a hemocytometer under an inverted
microscope (Carl Zeiss, Jena, Germany). The morphological changes
of cells incubated with or without EEPC for 24 h were examined
under an inverted microscope.
Nuclear staining with DAPI
For 4′,6-diamidino-2-phenylin-dole (DAPI;
Sigma-Aldrich) staining, the cells were washed with
phosphate-buffered saline (PBS) and fixed with 3.7%
paraformaldehyde (Sigma-Aldrich) in PBS for 10 min at room
temperature. The fixed cells were washed with PBS and stained with
2.5 µg/ml DAPI solution for 10 min at room temperature. The
cells were then washed twice with PBS and analyzed by fluorescence
microscopy (Carl Zeiss).
Flow cytometric analysis
The cells were fixed in 70% ethanol overnight at
4°C, washed in PBS and then resuspended in 1.12% sodium citrate
buffer (pH 8.4) together with 12.5 µg of RNase (DNase-free;
Sigma-Aldrich). Incubation was continued at 37°C for 30 min. The
cellular DNA was then stained by applying a propidium iodide (PI)
(10 µg/ml; Sigma-Aldrich) solution for 30 min at room
temperature in the dark. The stained cells were analyzed using a
FACSCalibur flow cytometer (Becton-Dickinson; San Jose, CA, USA).
The level of apoptotic cells containing sub-G1 DNA content was
determined as a percentage of the total number of cells (31).
Protein extraction and western blot
analysis
For the preparation of total cellular protein, the
cells were gently lysed with lysis buffer [40 mM Tris (pH 8.0), 120
mM, NaCl, 0.5% NP-40, 0.1 mM sodium orthovanadate, 2 µg/ml
aprotinin, 2 µg/ml leupeptin and 100 µg/ml
phenymethylsulfonyl fluoride] for 30 min. Supernatants were
collected and protein concentrations were determined using a
Bio-Rad protein assay kit (Bio-Rad, Hercules, CA, USA). In a
parallel experiment, the mitochondrial and cytosolic fractions were
isolated using a mitochondrial and cytosolic fractionation kit
(Active Motif, Carlsbad, CA, USA) according to the manufacturer's
protocol. Equal amounts of protein were separated on sodium dodecyl
sulfate (SDS)-polyacrylamide gels. Separated protein was
transferred to nitrocellulose membranes (Schleicher & Schuell,
Keene, NH, USA) and subsequently blocked with tris-buffered saline
(10 mM of Tris-Cl, pH 7.4) containing 0.5% Tween-20 and 5% non-fat
dry milk for 1 h at room temperature. The proteins were probed with
primary antibodies overnight at 4°C. After probing them with the
primary antibodies, the membranes were incubated with horseradish
peroxidase-conjugated anti-rabbit IgG as a secondary antibody
purchased from Amersham Corporation (Arlington Heights, IL, USA).
Using an enhanced chemiluminescence (ECL) detection system
(Amersham Corporation), immunoreactive bands were detected and
exposed to an X-ray film. Primary antibodies were purchased from
Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA), Cell
Signaling Technology, Inc. (Danvers, MA, USA) and Abcam (Cambridge,
UK).
In vitro caspase activity assay
The activities of the caspases were determined by
colorimetric assay kits (R&D Systems, Minneapolis, MN, USA),
which utilize synthetic tetrapeptides [Asp-Glu-Val-Asp (DEVD) for
caspase-3; Ile-Glu-Thr-Asp (IETD) for caspase-8; Leu-Glu-His-Asp
(LEHD) for caspase-9] labeled with p-nitroaniline (pNA). Briefly,
cells were lysed in the supplied lysis buffer according to the
manufacturer's protocol. The supernatants were collected and
incubated with the supplied reaction buffer and DEVD-pNA, IETD-pNA
or LEHD-pNA as substrates at 37°C. The reactions were measured by
changes in absorbance at 405 nm using an ELISA plate reader
(32).
Mitochondrial membrane potential (MMP)
assay
The MMP of intact cells was measured by a flow
cytometer using the lipophilic cationic probe
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetra-ethylbenzimidazolylcarbocyanine
iodide (JC-1; Calbiochem). U937 cells were collected and
resuspended in PBS and then incubated with 10 µM JC-1 for 20
min at 37°C. The cells were subsequently washed once with cold PBS,
suspended and subsequently analyzed using a flow cytometer
(33).
Statistical analysis
All data were derived from at least three
independent experiments. Statistical analyses were conducted using
SigmaPlot software, and values are presented as mean ± SD.
Significant differences between the groups were determined using
the unpaired Student's t-test.
Results
Induction of apoptosis in U937 cells
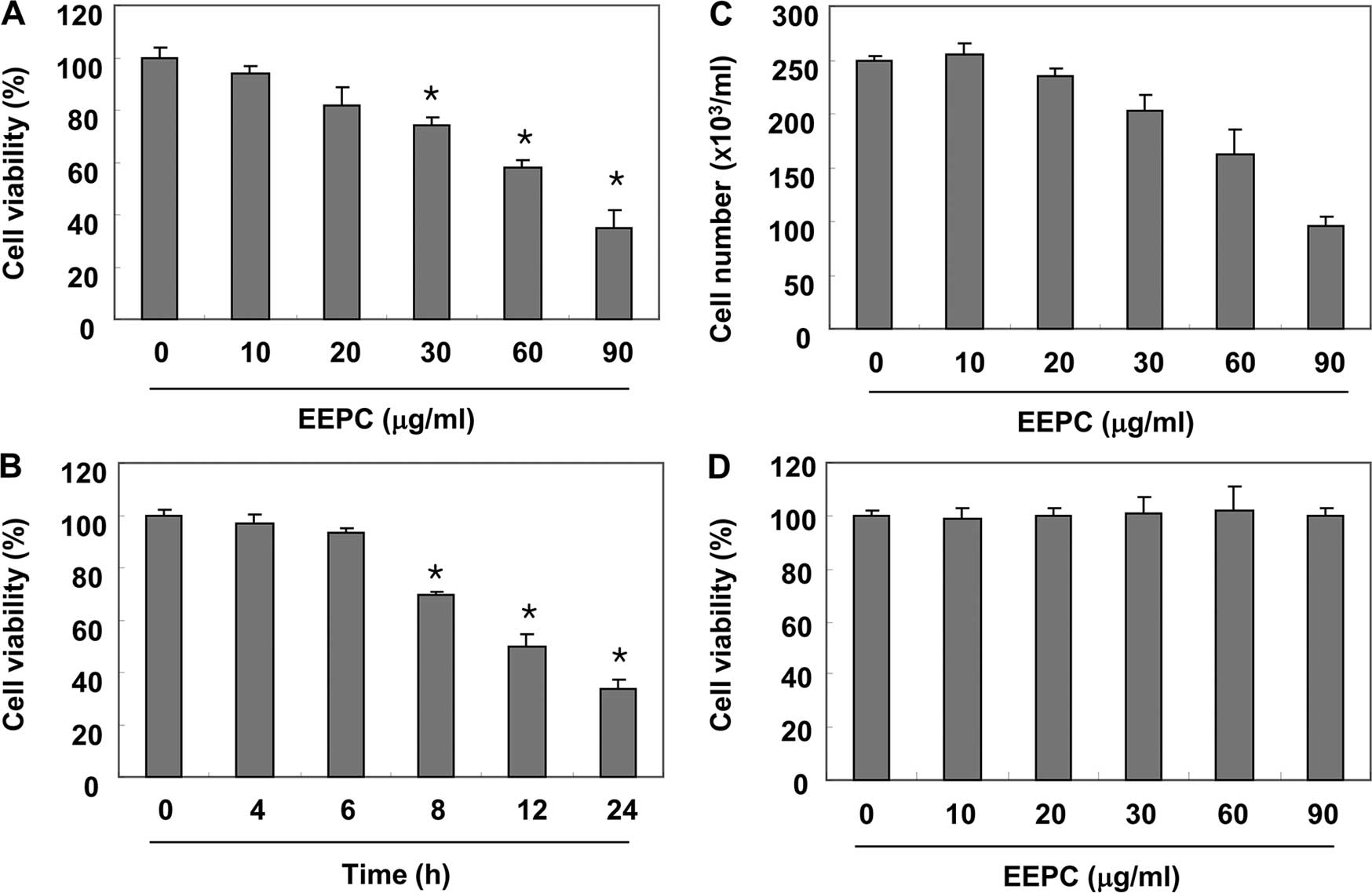
We first evaluated the cytotoxic effect of EEPC on
U937 cells using MTT assay and trypan blue exclusion method, and
found that EEPC significantly reduced the cell viability and
proliferation of U937 cells in a concentration- and time-dependent
manner (Fig. 1A–C). An additional
experiment was conducted using Chang liver cells in order to
examine the effect of EEPC on the viability of normal cells
(Fig. 1D). EEPC concentrations up
to 90 µg/ml did not induce cytotoxicity. Therefore,
experiments were performed to determine whether this inhibitory
effect of EEPC on U937 cell growth resulted from apoptotic cell
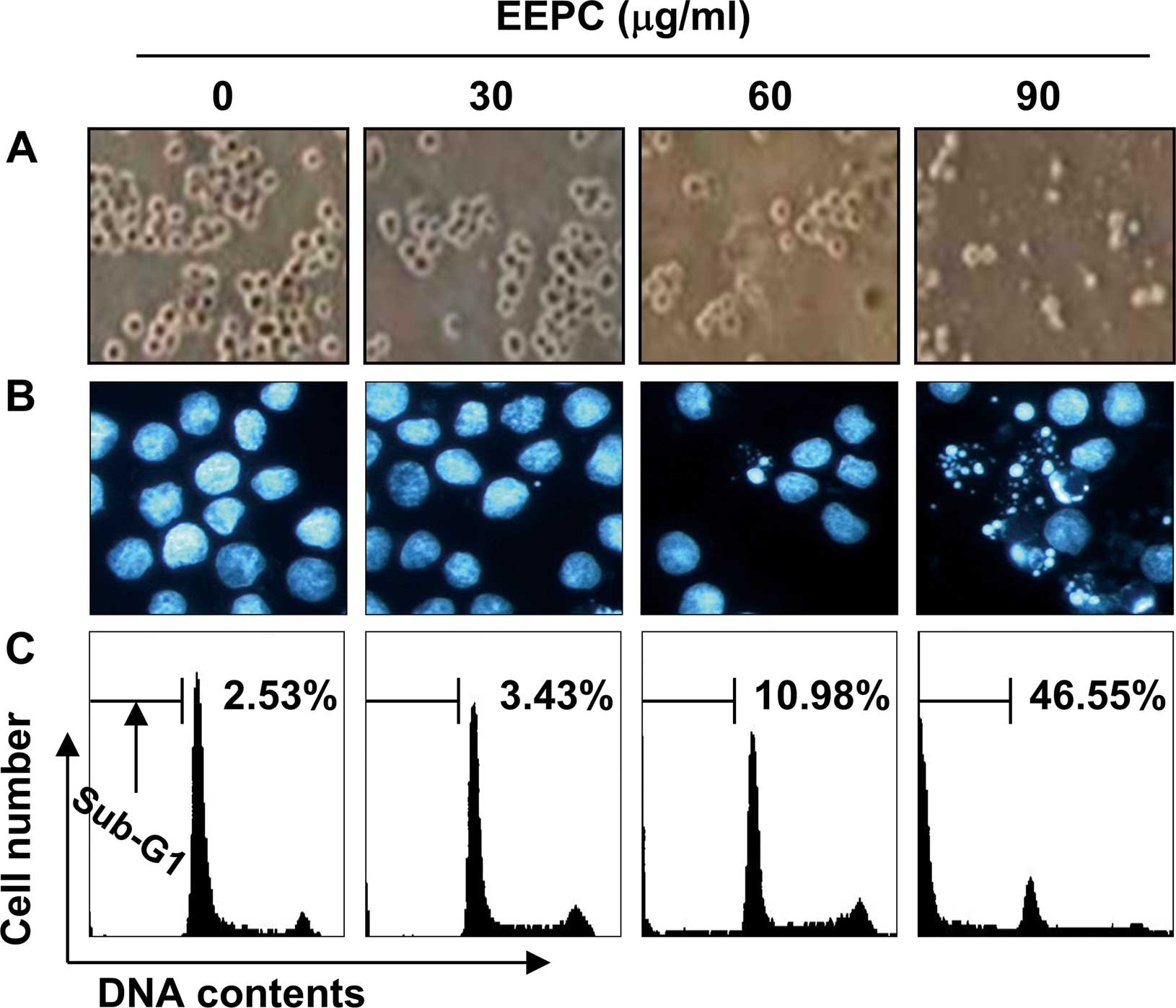
death. As shown in Fig. 2A and B,
EEPC induced U937 cell death with the characteristic apoptotic
features, including cell shrinkage, and chromatin condensation and
fragmentation in the nucleus as detected by DAPI staining. We next
quantified the apoptotic dead cells using flow cytometric analysis
to detect hypodiploid cell populations. As shown in Fig. 2C, treatment of U937 cells with EEPC
resulted in a markedly increased accumulation of sub-G1 phase
cells, and this response occurred in a concentration-dependent
manner. These results suggest an association between the growth
inhibition observed in response to EEPC and the induction of
apoptosis in U937 cells.
Activation of caspases by EEPC in U937
cells
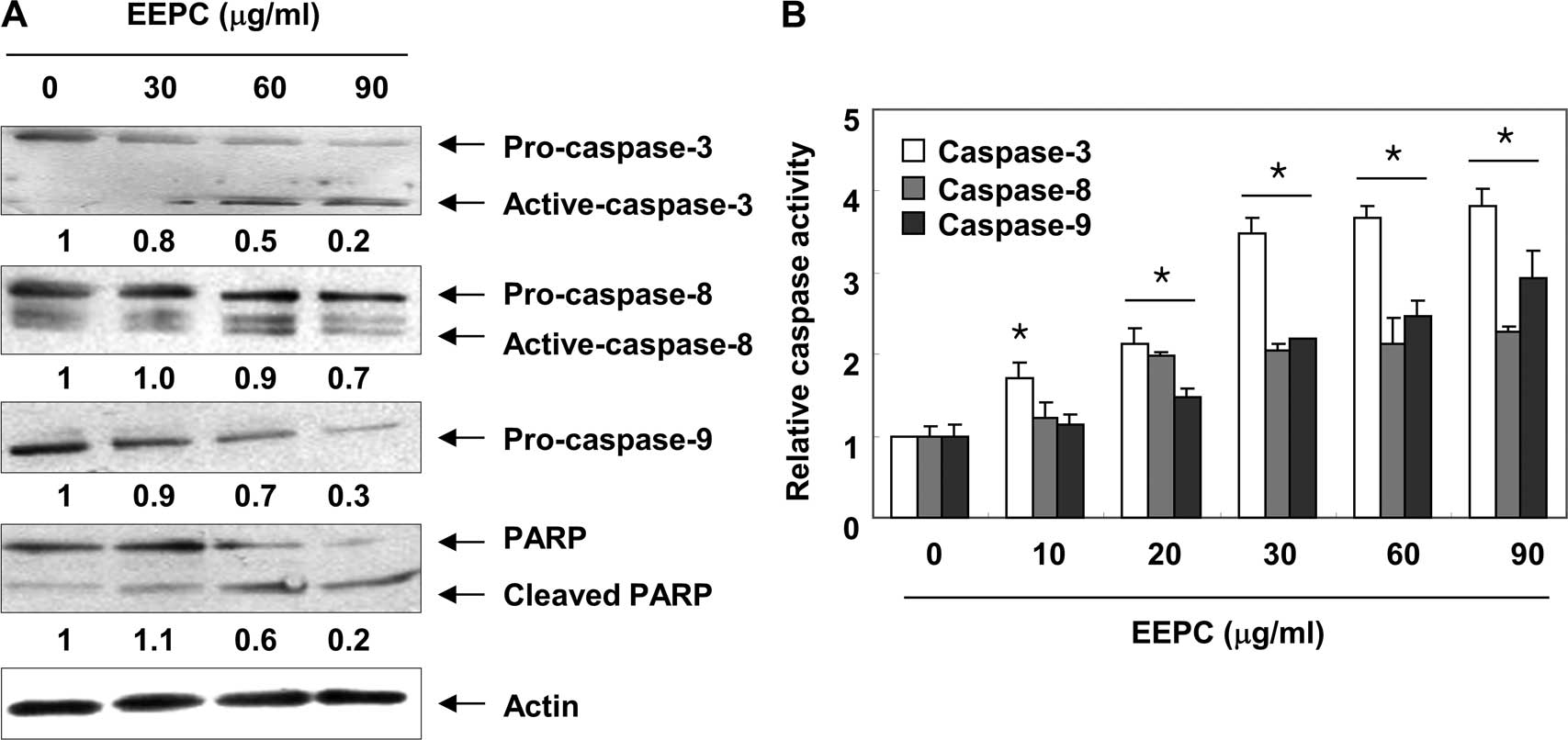
We next analyzed whether treatment with EEPC results
in the activation of caspases, a cardinal hallmark of apoptosis,
including two initiation caspases, caspase-8 and -9, and the
executioner caspase-3. As shown in Fig.
3A, the western blot analysis revealed that the expression
levels of pro-caspase-8, -9 and -3 were all decreased or the active
forms of caspase-8 and -3 were increased in a
concentration-dependent manner following EEPC treatment. In
addition, quantitative determinations of caspase activities by
colorimetric assays consistently showed that the activities of the
three caspases were significantly increased by EEPC treatment
(Fig. 3B). Furthermore, subsequent
immunoblot analysis revealed that progressive proteolytic cleavage
products of poly(ADP-ribose) polymerase (PARP), a downstream target
protein of activated caspase-3 (34), occurred in U937 cells treated with
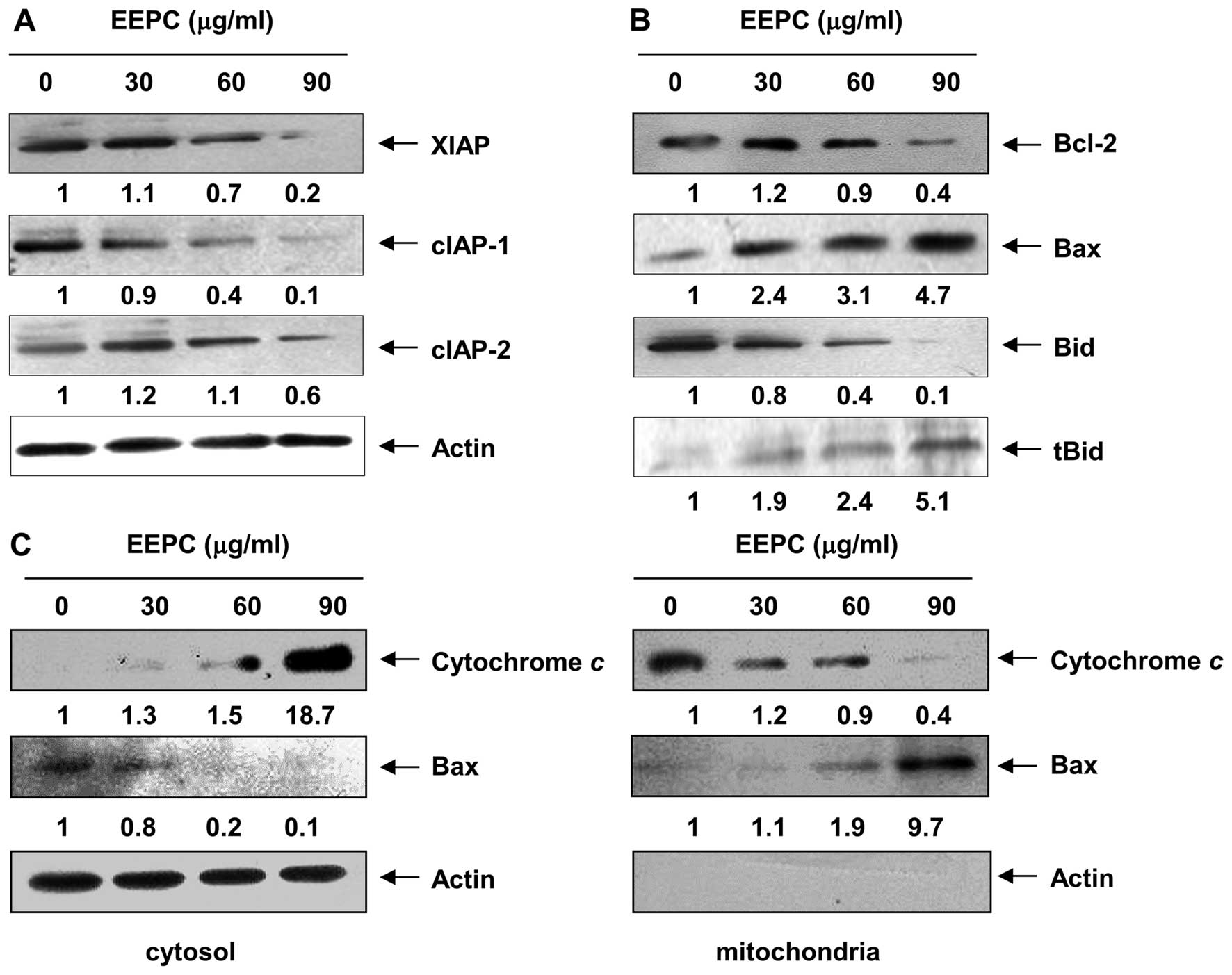
EEPC. Under the same conditions, levels of the anti-apoptotic
inhibitor of apoptosis proteins (IAP) family of proteins, such as
XIAP, cIAP-1 and cIAP-2 (Fig. 4A),
which bind to caspases and lead to their inactivation (35,36),
were markedly inhibited by EEPC treatment in a
concentration-dependent manner. These results indicated that EEPC
may trigger initiator caspase-8 and -9 initially and subsequently
activate the executioner caspase-3.
Modulation of Bcl-2 family members and
release of cytochrome c from mitochondria to the cytosol by EEPC in
U937 cells
Given that members of the Bcl-2 protein family are
primarily responsible for initiating mitochondrial-mediated
apoptosis, we next examined the levels of Bcl-2 family proteins
using western blot analysis. As shown in Fig. 4B, EEPC evoked a
concentration-dependent reduction in the level of anti-apoptotic
Bcl-2 expression, whereas the expression level of pro-apoptotic Bax
was markedly induced. The resultant alteration in Bcl-2 family
protein expression apparently lowered the ratio of the
anti-apoptotic to the pro-apoptotic Bcl-2 family proteins,
theoretically causing a disruption in mitochondrial membrane
integrity and consequent cytosolic release of cytochrome c
to initiate caspase-9 activation (37,38).
Therefore, we performed an immunoblot analysis using cytosolic and
mitochondrial fractions to examine the release of mitochondrial
cytochrome c in EEPC-treated cells and found that EEPC
treatment markedly induced a dose-dependent release of cytochrome
c into the cytoplasm (Fig.
4C).
Translocation of Bax from the cytosol to
mitochondria and Bid truncation by EEPC in U937 cells
Accumulating evidence has demonstrated that Bax
protein translocation to the mitochondria is a necessary step in
cell activating mitochondrial-mediated apoptosis (39,40).
As shown in Fig. 4C, the levels of
Bax in the cytosol declined in a concentration-dependent manner
after EEPC treatment. In contrast, the Bax levels in the
mitochondrial fraction increased, indicating that EEPC treatment
markedly led to Bax trans-location into the mitochondria from the
cytosol. Moreover, under the same experimental conditions, EEPC
caused a concentration-dependent cleavage of BH3-only Bid protein
and the formation of its truncated form of Bid, tBid (Fig. 4B), which could translocate to the
mitochondria to enhance the mitochondrial-mediated apoptosis
pathway (41,42). These results suggest that EEPC
reduces the Bcl-2/Bax ratio, inserts Bax from the cytosol into
mitochondria, and activates Bid, resulting in mitochondrial
dysfunction, release of cytochrome c to the cytosol and
apoptosis induction.
Effects of Bck-2 overexpression in
EEPC-induced U937 cell apoptosis
Since it has been well established that Bcl-2 plays
a critical role in the regulation of the mitochondrial-mediated
apoptotic pathway, U937 cells stably overexpressing Bcl-2 were
established and we determined the effect of overexpression of Bcl-2
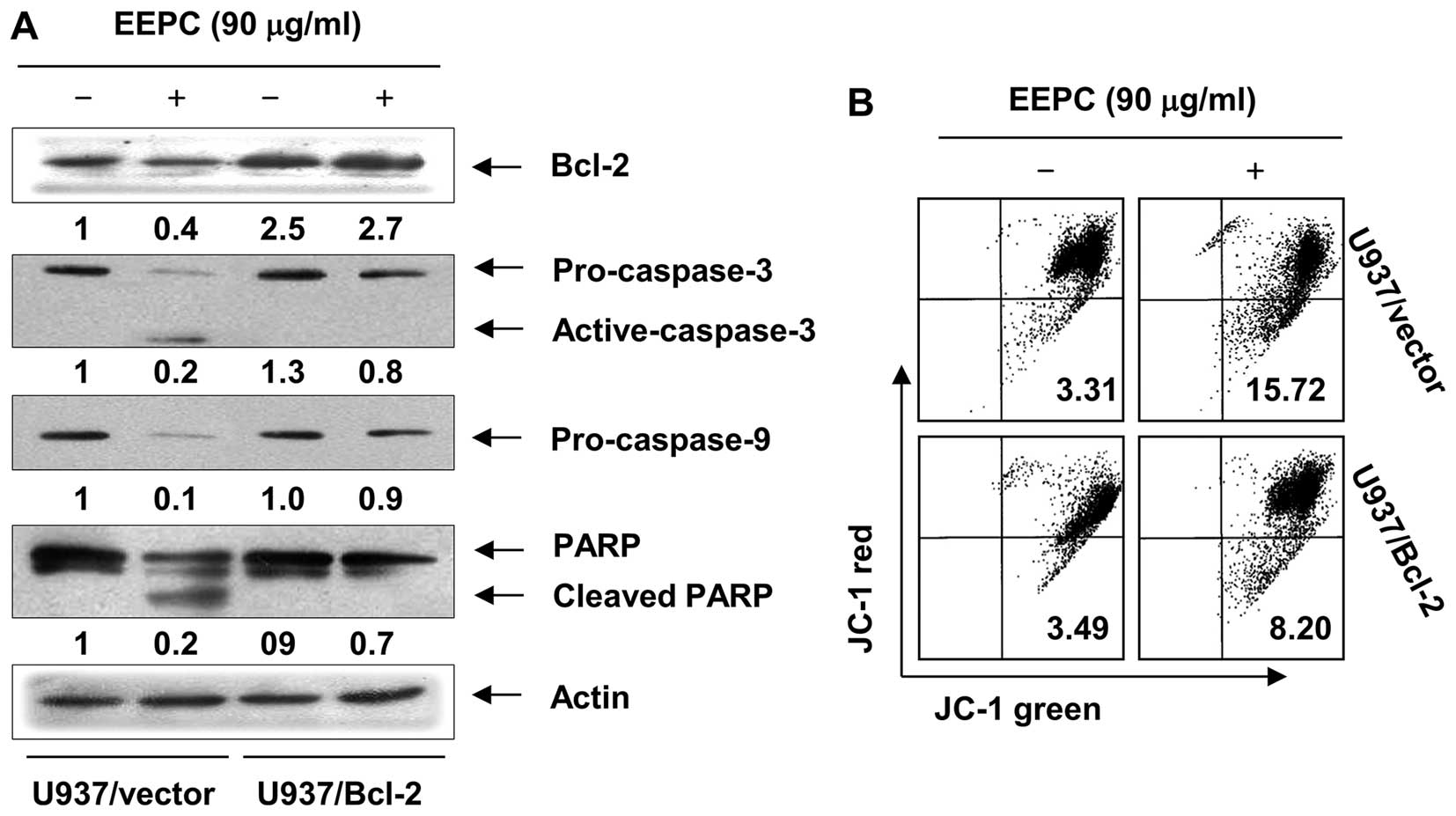
on EEPC-induced apoptosis. As shown in Fig. 5A, western blot analysis revealed
that Bcl-2 overexpression inhibited the EEPC-induced cleavage of
caspase-3, -9 and PARP. In addition, the role of mitochondria in
EEPC-induced apoptosis was further investigated by examining the
effect of EEPC on MMP levels. As shown in Fig. 5B, Bcl-2 overexpression was found to
significantly block cells from EEPC-induced MMP level reduction
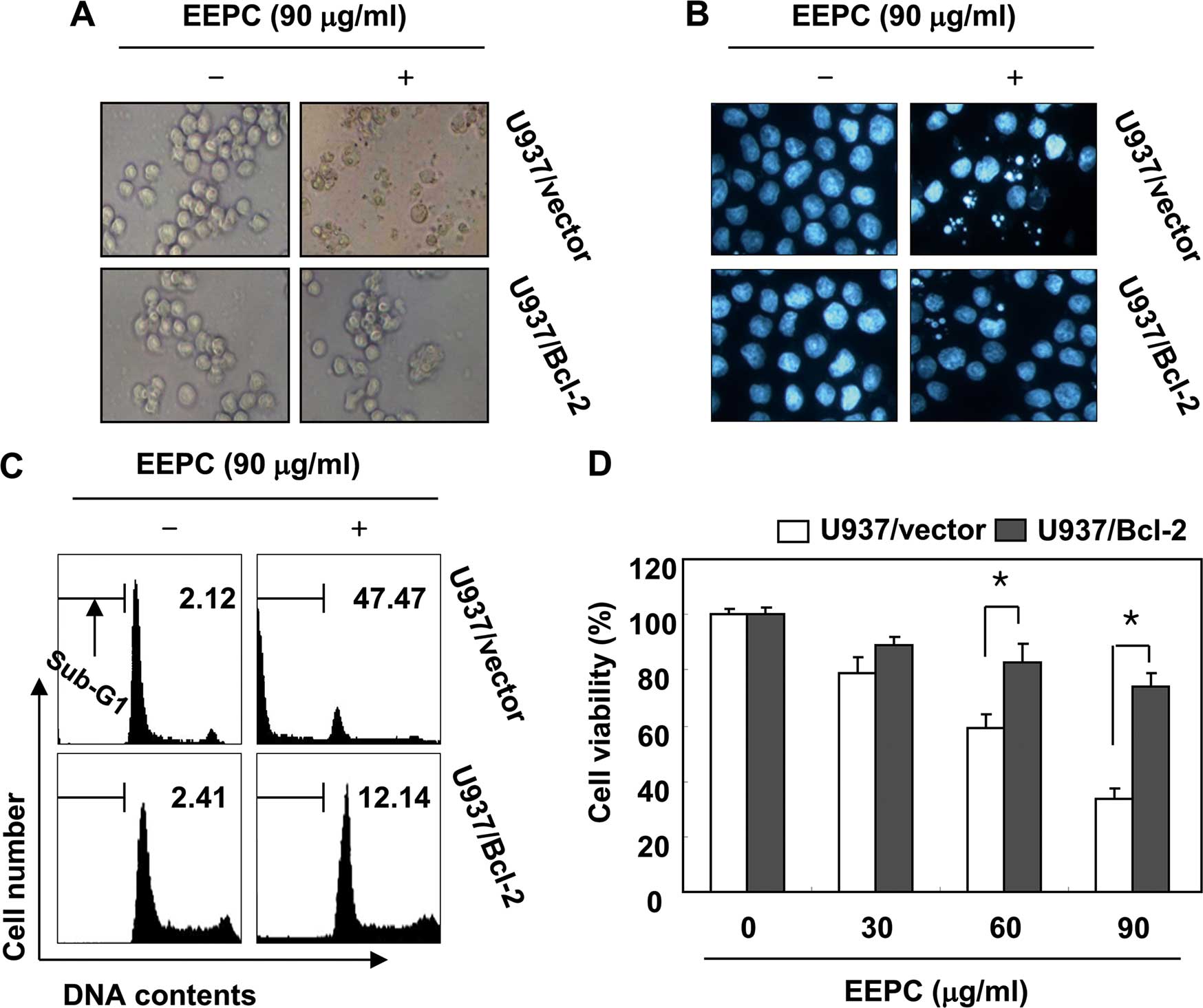
when compared to the U937/vector cells. Furthermore, Bcl-2
overexpression was found to significantly protect cells from
EEPC-induced morphological changes, accumulation of cells in the
sub-G1 phase and growth inhibition (Fig. 6) when compared to U937/vector cells.
Taken together, these results indicated that the ectopic expression
of Bcl-2 inhibits EEPC-induced apoptosis.
Discussion
As the induction of apoptosis is well recognized as
the primary cytotoxic mechanism for the anticancer effect of most
chemotherapeutic agents, the pro-apoptotic effect and its
underlying mechanisms of EEPC were herein investi gated. Our
results demonstrated that EEPC treatment led to the activation of
both initiator caspase-8 and -9 and effector caspase-3, and the
subsequent cleavage of PARP, in addition to the modulation of Bcl-2
and IAP family proteins. Furthermore, we found that EEPC induced
the truncation of BH3-only Bid protein, translocation of Bax to the
mitochondria, disruption of mitochondrial function with the loss of
MMP and the release of cytochrome c into the cytosol. On the
other hand, Bcl-2 overexpression significantly abolished
EEPC-induced cell death.
Caspases, which are a family of cysteine acid
proteases, exist as pro-enzymes in the cytosol of cells and are the
central regulators for the execution of cell death in response to
various apoptotic stimuli (43,44).
Among two main apoptotic pathways, the death receptor pathway
involves the engagement of a set of ligands and their corresponding
receptors and then transmission of the apoptotic signal in the
cytoplasm by a number of caspases such as caspase-8, forming the
death-inducing signaling complex (3). This recruitment leads to the
downstream activation of executioner caspase-3 and -7, and
induction of apoptosis (45).
Caspase-8 is involved in the activation of the mitochondrial
pathway via the truncation of Bid, a BH3 domain containing a
pro-apoptotic Bcl-2 family member (41,42).
The mitochondrial pathway can be activated by various stimuli, such
as DNA damage and many types of chemotherapeutic agents (3,46). In
this pathway, the mitochondrial integrity is disrupted as a result
of the loss of MMP, leading to the release of cytochrome c
from the mitochondria to the cytosol where it can associate with
apoptotic peptidase activating factor 1 (Apaf1) and pro-caspase-9,
leading to the activation of caspase-9 (37,38).
Activated caspase-9 directly cleaves and activates the executioner
caspases, which leads to the cleavage of several target proteins
including PARP, chromatin condensation, DNA laddering and the
formation of apoptotic bodies (34). Our data showed that EEPC promoted
the activation of caspase-8 and -3, and concomitant PARP cleavage
in U937 cells (Fig. 3). We also
found the activation of caspase-9, an initiator caspase of the
intrinsic pathway, and a sharp decline in Bid-complete levels,
together with a rise in tBid in the EEPC-treated U937 cells
(Fig. 4B). Therefore, EEPC appears
to induce apoptosis primarily via caspase activation-mediated
intrinsic and extrinsic apoptotic pathways in U937 cells.
Activation of caspases may also be regulated by a variety of
proteins, including members of the IAP family, which promote cell
survival after a wide variety of apoptotic stimuli elicited via
intrinsic as well as extrinsic pathways through selectively binding
with caspases, and as a result, inhibit caspase activity and
apoptosis (35,36). Our results revealed that EEPC
treatment decreased the expression level of IAP family proteins
(Fig. 4A), indicating that the
downregulation of IAPs may be involved in the activation of
caspases in EEPC-induced apoptosis in U937 cells.
The mitochondrial membrane integrity is tightly
regulated by members of the Bcl-2 protein family, such as
anti-apoptotic Bcl-2 and Bcl-xL, in addition to pro-apoptotic Bax
and Bak (4,47). Overexpression of anti-apoptotic
Bcl-2 members results in the prevention of apoptosis; however,
overexpression of pro-apoptotic Bcl-2 members leads to an increase
in cell susceptibility to apoptotic signals (37,47).
In particular, a decrease in the ratio of anti-apoptotic to
pro-apoptotic Bcl-2 family proteins leads to a disruption in the
mitochondrial outer membrane and the consequent cytosolic release
of cyto chrome c for caspase-9 activation (37,38).
Moreover, it has been suggested that following initiation of the
apoptotic cascade, Bax translocates from the cytoplasm to the
mitochondria, and both the Bax and Bak proteins change their
conformation and form homo-oligomers (5,40). On
the outer membrane of the mitochondria, they may form a channel or
membrane pore, thus allowing the release of cytochrome c
(46,48). In addition to the activation of
caspases, EEPC treatment resulted in a significant increase in Bax
expression and a decrease in Bcl-2 expression (Fig. 4B). Accompanying modulation of Bcl-2
family proteins, Bax translocation by EEPC could be related to the
mitochondrial response to the generation of tBid, leading to
mitochondrial disturbance and releasing cytochrome c, and
ultimately activating caspase-9 to intensify the initial apoptotic
response. Taken together, these findings indicate that a
mitochondrial amplification step is required for complete
activation of the effector caspases and apoptosis induction by EEPC
in U937 cells to occur.
We further investigated the finding that Bcl-2
overexpression confers protection against EEPC-induced apoptosis in
U937 cells, and Bcl-2 overexpression was found to block the
EEPC-induced cleavage of caspase-3 and -9, as well as the cleavage
of PARP, a caspase-3 substrate (Fig.
5A). Additionally, Bcl-2 overexpression blocked the
EEPC-induced loss of MMP in U937 cells, which indicates that Bcl-2
has a regulatory function upstream of the mitochondria (Fig. 5B). Furthermore, Bcl-2 overexpression
was found to significantly protect cells from EEPC-induced
apoptosis and growth inhibition, compared to U937/vector cells
(Fig. 6), indicating that Bcl-2
plays a critical role in the EEPC-induced apoptosis in U937
cells.
In summary, our results demonstrated that
EEPC-induced apoptosis in human leukemia U937 cells is associated
with the activation of caspases at the initiative and executive
stages via mitochondrial-mediated and extrinsic pathways. The
results of the present study demonstrated that EEPC treatment
requires a mitochondrial amplification step to enable the full
activation of the caspase cascade and apoptosis induction in U937
cells to occur. In addition, our results demonstrated that Bcl-2
overexpression inhibits EEPC-induced apoptosis by exerting effects
at the mitochondrial level, as well as downstream to the
mitochondria. Although additional in vivo studies are needed
to establish the role of EEPC as a chemopreventive and/or
therapeutic agent for leukemia and other cancers, these findings
provide further elucidation of the mechanisms involved in
EEPC-induced apoptosis.
Acknowledgments
The present study was supported by the Basic Science
Research Program through the National Research Foundation of Korea
(NRF) grant funded by the Korea government
(2015R1A2A2A01004633).
References
|
1
|
Lavrik IN: Systems biology of apoptosis
signaling networks. Curr Opin Biotechnol. 21:551–555. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hassan M, Watari H, AbuAlmaaty A, Ohba Y
and Sakuragi N: Apoptosis and molecular targeting therapy in
cancer. Biomed Res Int. 2014:1508452014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fulda S and Debatin KM: Extrinsic versus
intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene.
25:4798–4811. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ola MS, Nawaz M and Ahsan H: Role of Bcl-2
family proteins and caspases in the regulation of apoptosis. Mol
Cell Biochem. 351:41–58. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Karbowski M, Norris KL, Cleland MM, Jeong
SY and Youle RJ: Role of Bax and Bak in mitochondrial
morphogenesis. Nature. 443:658–662. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Khuda-Bukhsh AR, Das S and Saha SK:
Molecular approaches toward targeted cancer prevention with some
food plants and their products: Inflammatory and other signal
pathways. Nutr Cancer. 66:194–205. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bultman SJ: Emerging roles of the
microbiome in cancer. Carcinogenesis. 35:249–255. 2014. View Article : Google Scholar :
|
|
8
|
Song FQ, Liu Y, Kong XS, Chang W and Song
G: Progress on understanding the anticancer mechanisms of medicinal
mushroom: Inonotus obliquus. Asian Pac J Cancer Prev. 14:1571–1578.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ríos JL: Chemical constituents and
pharmacological properties of Poria cocos. Planta Med. 77:681–691.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sun Y: Biological activities and potential
health benefits of polysaccharides from Poria cocos and their
derivatives. Int J Biol Macromol. 68:131–134. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang L, Ravipati AS, Koyyalamudi SR,
Jeong SC, Reddy N, Bartlett J, Smith PT, de la Cruz M, Monteiro MC,
Melguizo A, et al: Anti-fungal and anti-bacterial activities of
ethanol extracts of selected traditional Chinese medicinal herbs.
Asian Pac J Trop Med. 6:673–681. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Park YH, Son IH, Kim B, Lyu YS, Moon HI
and Kang HW: Poria cocos water extract (PCW) protects PC12 neuronal
cells from beta-amyloid-induced cell death through antioxidant and
antiapoptotic functions. Pharmazie. 64:760–764. 2009.
|
|
13
|
Zhou L, Zhang Y, Gapter LA, Ling H,
Agarwal R and Ng KY: Cytotoxic and anti-oxidant activities of
lanostane-type triterpenes isolated from Poria cocos. Chem Pharm
Bull. 56:1459–1462. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chung TW, Koo BS, Choi EG, Kim MG, Lee IS
and Kim CH: Neuroprotective effect of a chuk-me-sun-dan on neurons
from ischemic damage and neuronal cell toxicity. Neurochem Res.
31:1–9. 2006.PubMed/NCBI
|
|
15
|
Lee SM, Lee YJ, Yoon JJ, Kang DG and Lee
HS: Effect of Poria cocos on hypertonic stress-induced water
channel expression and apoptosis in renal collecting duct cells. J
Ethnopharmacol. 141:368–376. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cuellar MJ, Giner RM, Recio MC, Just MJ,
Mañez S and Rios JL: Effect of the basidiomycete Poria cocos on
experimental dermatitis and other inflammatory conditions. Chem
Pharm Bull. 45:492–494. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fuchs SM, Heinemann C, Schliemann-Willers
S, Härtl H, Fluhr JW and Elsner P: Assessment of anti-inflammatory
activity of Poria cocos in sodium lauryl sulphate-induced irritant
contact dermatitis. Skin Res Technol. 12:223–227. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jeong JW, Lee HH, Han MH, Kim GY, Hong SH,
Park C and Choi YH: Ethanol extract of Poria cocos reduces the
production of inflammatory mediators by suppressing the NF-kappaB
signaling pathway in lipopolysaccharide-stimulated RAW 264.7
macrophages. BMC Complement Altern Med. 14:1012014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yasukawa K, Kaminaga T, Kitanaka S, Tai T,
Nunoura Y, Natori S and Takido M: 3β-p-hydroxybenzoyldehydrotumul
osic acid from Poria cocos, and its anti-inflammatory effect.
Phytochemistry. 48:1357–1360. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yance DR Jr and Sagar SM: Targeting
angiogenesis with integrative cancer therapies. Integr Cancer Ther.
5:9–29. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sagar SM, Yance D and Wong RK: Natural
health products that inhibit angiogenesis: A potential source for
investigational new agents to treat cancer-Part 1. Curr Oncol.
13:14–26. 2006.
|
|
22
|
Lu YT, Kuan YC, Chang HH and Sheu F:
Molecular cloning of a Poria cocos protein that activates Th1
immune response and allays Th2 cytokine and IgE production in a
murine atopic dermatitis model. J Agric Food Chem. 62:2861–2871.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chang HH, Yeh CH and Sheu F: A novel
immunomodulatory protein from Poria cocos induces Toll-like
receptor 4-dependent activation within mouse peritoneal
macrophages. J Agric Food Chem. 57:6129–6139. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cheng S, Eliaz I, Lin J, Thyagarajan-Sahu
A and Sliva D: Triterpenes from Poria cocos suppress growth and
invasiveness of pancreatic cancer cells through the downregulation
of MMP-7. Int J Oncol. 42:1869–1874. 2013.PubMed/NCBI
|
|
25
|
Kikuchi T, Uchiyama E, Ukiya M, Tabata K,
Kimura Y, Suzuki T and Akihisa T: Cytotoxic and apoptosis-inducing
activities of triterpene acids from Poria cocos. J Nat Prod.
74:137–144. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Feng YL, Lei P, Tian T, Yin L, Chen DQ,
Chen H, Mei Q, Zhao YY and Lin RC: Diuretic activity of some
fractions of the epidermis of Poria cocos. J Ethnopharmacol.
150:1114–1118. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ling H, Zhang Y, Ng KY and Chew EH:
Pachymic acid impairs breast cancer cell invasion by suppressing
nuclear factor-κB-dependent matrix metalloproteinase-9 expression.
Breast Cancer Res Treat. 126:609–620. 2011. View Article : Google Scholar
|
|
28
|
Ling H, Zhou L, Jia X, Gapter LA, Agarwal
R and Ng KY: Polyporenic acid C induces caspase-8-mediated
apoptosis in human lung cancer A549 cells. Mol Carcinog.
48:498–507. 2009. View
Article : Google Scholar
|
|
29
|
Gapter L, Wang Z, Glinski J and Ng KY:
Induction of apoptosis in prostate cancer cells by pachymic acid
from Poria cocos. Biochem Biophys Res Commun. 332:1153–1161. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee SJ, Hwang SO, Noh EJ, Kim DU, Nam M,
Kim JH, Nam JH and Hoe KL: Transactivation of bad by
vorinostat-induced acetylated p53 enhances doxorubicin-induced
cytotoxicity in cervical cancer cells. Exp Mol Med. 46:e762014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kim YS, Li XF, Kang KH, Ryu B and Kim SK:
Stigmasterol isolated from marine microalgae Navicula incerta
induces apoptosis in human hepatoma HepG2 cells. BMB Rep.
47:433–438. 2014. View Article : Google Scholar :
|
|
32
|
Park C, Park S, Chung YH, Kim GY, Choi YW,
Kim BW and Choi YH: Induction of apoptosis by a hexane extract of
aged black garlic in the human leukemic U937 cells. Nutr Res Pract.
8:132–137. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Seo K, Ki SH and Shin SM: Methylglyoxal
induces mitochondrial dysfunction and cell death in liver. Toxicol
Res. 30:193–198. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Duriez PJ and Shah GM: Cleavage of
poly(ADP-ribose) polymerase: A sensitive parameter to study cell
death. Biochem Cell Biol. 75:337–349. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Danson S, Dean E, Dive C and Ranson M:
IAPs as a target for anticancer therapy. Curr Cancer Drug Targets.
7:785–794. 2007. View Article : Google Scholar
|
|
36
|
de Graaf AO, de Witte T and Jansen JH:
Inhibitor of apoptosis proteins: New therapeutic targets in
hematological cancer? Leukemia. 18:1751–1759. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kadenbach B, Arnold S, Lee I and Hüttemann
M: The possible role of cytochrome c oxidase in stress-induced
apoptosis and degenerative diseases. Biochim Biophys Acta.
1655:400–408. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Scorrano L and Korsmeyer SJ: Mechanisms of
cytochrome c release by proapoptotic BCL-2 family members. Biochem
Biophys Res Commun. 304:437–444. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Stankiewicz AR, Lachapelle G, Foo CP,
Radicioni SM and Mosser DD: Hsp70 inhibits heat-induced apoptosis
upstream of mitochondria by preventing Bax translocation. J Biol
Chem. 280:38729–38739. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Degli Esposti M and Dive C: Mitochondrial
membrane permeabilisation by Bax/Bak. Biochem Biophys Res Commun.
304:455–461. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Shamas-Din A, Brahmbhatt H, Leber B and
Andrews DW: BH3-only proteins: Orchestrators of apoptosis. Biochim
Biophys Acta. 1813:508–520. 2011. View Article : Google Scholar
|
|
42
|
Fennell DA and Chacko A: Exploiting BH3
only protein function for effective cancer therapy. Front Biosci.
13:6682–6692. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
43
|
Fiandalo MV and Kyprianou N: Caspase
control: Protagonists of cancer cell apoptosis. Exp Oncol.
34:165–175. 2012.PubMed/NCBI
|
|
44
|
Hensley P, Mishra M and Kyprianou N:
Targeting caspases in cancer therapeutics. Biol Chem. 394:831–843.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Walczak H and Krammer PH: The CD95
(APO-1/Fas) and the TRAIL (APO-2L) apoptosis systems. Exp Cell Res.
256:58–66. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Asakura T and Ohkawa K: Chemotherapeutic
agents that induce mitochondrial apoptosis. Curr Cancer Drug
Targets. 4:577–590. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Tomek M, Akiyama T and Dass CR: Role of
Bcl-2 in tumour cell survival and implications for pharmacotherapy.
J Pharm Pharmacol. 64:1695–1702. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Jourdain A and Martinou JC: Mitochondrial
outer-membrane permeabilization and remodelling in apoptosis. Int J
Biochem Cell Biol. 41:1884–1889. 2009. View Article : Google Scholar : PubMed/NCBI
|